Abstract
Assessment of the immune response to tumors is growing in importance as the prognostic implications of this response are increasingly recognized, and as immunotherapies are evaluated and implemented in different tumor types. However, many different approaches can be used to assess and describe the immune response, which limits efforts at implementation as a routine clinical biomarker. In part 1 of this review, we have proposed a standardized methodology to assess tumor infiltrating lymphocytes (TILs) in solid tumors, based on the International Immuno-Oncology Biomarkers Working Group guidelines for invasive breast carcinoma. In part 2 of this review, we discuss the available evidence for the prognostic and predictive value of TILs in common solid tumors, including carcinomas of the lung, gastrointestinal tract, genitourinary system, gynecological system, and head and neck, as well as primary brain tumors, mesothelioma and melanoma. The particularities and different emphases in TIL assessment in different tumor types are discussed. The standardized methodology we propose can be adapted to different tumor types and may be used as a standard against which other approaches can be compared. Standardization of TIL assessment will help clinicians, researchers and pathologists to conclusively evaluate the utility of this simple biomarker in the current era of immunotherapy.
Keywords: Lymphocytes, tumor-infiltrating, Biomarkers, Cancer, Immunotherapy, Pathology
Introduction
Assessment of tumor infiltrating lymphocytes (TILs) is growing in importance as evidence emerges of the prognostic and potentially predictive significance of TILs in many different tumor types, and as immunotherapies show exciting results in clinical trials and clinical practice. A significant research effort is underway to identify reliable biomarkers to select patients with the highest likelihood of responding to immunotherapeutic agents. As discussed in part 1 of this review, TIL assessment on H&E sections has shown clinical validity as a prognostic marker in invasive breast carcinoma [1], and is reproducible [2], affordable and widely available. However, many different approaches are used to assess the immune infiltrate in tumors with highly variable requirements, costs and complexity. In part 1 of this review, we proposed a standardized methodology for TIL assessment in solid tumors, based on the International Immuno-Oncology Biomarkers Working Group guidelines for TIL assessment in invasive breast carcinoma [1]. In part 2, we discuss the TILs literature in different tumor types, and suggest ways in which the proposed methodology may be applied to these tumor types and adapted as required based on the available evidence and expert opinion.
TILs in melanoma
Reporting of TILs in primary cutaneous melanoma has long been routine in histopathology practice, following the early recognition of their prognostic significance [3–5]. The host immune response to melanoma has been highlighted by the recent impressive results of immune checkpoint inhibitor therapy, which is now standard of care in metastatic melanoma [6]. The immune infiltrate in melanomas is therefore of great interest to clinicians and researchers, both as a prognostic marker and as a potential predictive marker of response to immunotherapy. TILs in melanoma have been well studied, and the current body of literature is discussed below with regard to scoring methodologies, prognostic value, predictive value of sentinel node positivity, and scoring in metastatic deposits.
The prognostic value of TILs in primary cutaneous melanoma has been debated in the literature over the past few decades. Initial reports established simple H&E based scoring criteria [4,5], classifying the immune infiltrate as brisk (“TILs present throughout the substance of the vertical growth phase or present and infiltrating across the entire base of the vertical growth phase”), non-brisk (“TILs noted in one or more foci of the vertical growth phase”) or absent (entirely absent from the tumor or present but not infiltrating the melanoma cell nests). The immune infiltrate as classified by this system was found to be an independent prognostic factor, with an adjusted odds ratio for survival of 11.3 for a brisk infiltrate and 3.5 for a non-brisk infiltrate [5]. This study established strict guidelines to define a “TIL” – the lymphocytes must infiltrate and disrupt the tumor cell nests, that is, stromal lymphocytes are not included in the assessment [5]. Clark’s TIL scoring system is reproducible amongst pathologists [7] and has subsequently been validated in studies involving over 5000 patients [8–11], all reporting that TILs are an independent prognostic factor in multivariate analyses. In 2012, a group at the Melanoma Institute of Australia (MIA) proposed a modification to the system described by Clark et al, introducing a grade based on the density (absent/mild/moderate/marked, score 0–3) and distribution (absent/focal/multifocal/diffuse, score 0–3) of the immune infiltrate [12]. The possible combinations were collapsed into four TILs grades as follows: grade 0 = absent; grade 1 = mild or moderate focal infiltrate, or mild multifocal infiltrate; grade 2 = marked focal, moderate or marked multifocal, or mild diffuse infiltrate; grade 3 = moderate or marked diffuse infiltrate [12]. In a cohort of 1865 melanomas over 0.75mm thick, this scheme was an independent predictor of melanoma specific survival, with a 5-year survival of 100% seen in the patients with grade 3 TILs [12], however it remains to be validated in an independent cohort.
Despite this body of evidence, a number of studies have also been published that report a lack of independent prognostic value using Clark’s scoring system [13–15]. Rao et al found that the difference in overall survival across the three TILs groups was not statistically significant but a significant difference was observed when the absent TILs group was compared with those with TILs present, brisk or non-brisk [16]. In a recent population based study of over 4000 patients [17], Eriksson et al used a TIL scoring system of absent-to-sparse/moderate/marked based on H&E assessment, which was approximated to the absent/non-brisk/brisk system described by Clark et al [5]. This TILs score was not found to be an independent prognostic factor [17].
The discrepant results from these studies may be in part due to differing patient populations, in particular, differences in melanoma thickness and growth phase. Studies including a large proportion of thin melanomas in which only the radial growth phase is present appear more likely to report an absence of an association between TILs and survival [15,17], however a significant association was found in the study by Thomas et al in which 82% of cases were <1.0mm thick [9]. A meta-analysis of high quality published studies may be of value to resolve the issue. Fortunately, as many of these studies have used a standard TILs scoring method, combination in a meta-analysis should have validity.
Studies using IHC to delineate and quantify TIL subsets help to demonstrate the importance of the host immune response in melanoma. CD69+ activated lymphocytes [18], CD20+ B cells [19], and cytotoxic T cells identified by granzyme B [20] have been shown to correlate with improved survival. In contrast, FOXP3+ Tregs negatively impacted survival [21]. Multispectral immunohistochemistry can be used reliably even in the presence of heavy melanin pigmentation [22] and has been used to predict the yield of TILs generated for adoptive cell transfer [23]. Importantly, Weiss et al found no added prognostic benefit to quantifying lymphocyte subsets by IHC over a three-tiered H&E assessment [11].
Although the therapeutic benefit of sentinel lymph node biopsy in melanoma is still being debated [24], it is well established as an important prognostic factor [25]. Recent studies have shown that the TIL score in the primary tumor is inversely correlated with sentinel node involvement [12,14,15,26,27]. This has been demonstrated both with the scoring system described by Clark et al [14,15,27] and the modified MIA system [12]. Wong et al examined a cohort of patients with thin melanomas (<1mm) who underwent sentinel lymph node biopsy and found no association between TILs and sentinel lymph node positivity, however numbers were small and did not represent the usual patient population undergoing sentinel lymph node biopsy [28]. Interestingly, although finding a significant association between TILs and sentinel lymph node positivity, and between sentinel lymph node positivity and survival, two studies did not find TILs to be an independent prognostic factor for survival [14,15].
While most research in other solid tumors has focused on the primary lesion, evaluation of TILs in metastatic sites has also been the focus of investigation in melanoma. In 1996, Mihm et al showed that the TILs scoring method of Clark et al could be slightly modified and applied to metastatic tumor deposits in regional nodes, likening the expansive proliferation of malignant cells to the vertical growth phase of the primary tumor [29]. The lymphocytic infiltrate within the metastatic tumor nests (carefully excluding the surrounding lymphoid stroma from the assessment) was found to be an independent prognostic factor [29]. Similar results were demonstrated by Bogunovic et al [30] and Kakavand et al [31], using both H&E TIL assessment and semi-quantitative scoring of immunohistochemical stained sections. Recently, as part of The Cancer Genome Atlas project, a modified TILs scoring system was used to correlate histological assessment of TILs with RNA-based gene expression profiling and survival [32]. The majority of samples submitted for this study were from metastatic sites [32]. This system scored lymphocyte density (score 0–3) and distribution (score 0–3) score to produce a seven-tiered L-score, which was found to be significantly associated with the “immune” subclass of melanomas identified to be rich in immune-related transcripts on mRNA expression profiling [32]. Whilst more information is potentially provided by this more detailed scoring system, it remains to be validated in an independent cohort and compared with the traditional three-tiered system of Clark et al [5].
Assessing TILs in metastatic deposits within lymph nodes is clearly complicated by the presence of pre-existing lymphoid stroma. As per the guidelines established by Clark et al [5] and Mihm et al [29], only lymphocytes in direct contact with melanoma cells and disrupting melanoma cell nests should be included in the assessment. As this recommendation is the same for the primary lesion, little modification is required to adapt the scoring system to the metastatic setting. As discussed further in part 1 of this review, how to assess TILs in metastases, particularly lymph nodes, is less clear for other tumor types. For example, as the guidelines for TILs assessment in invasive breast cancer recommend only assessing the stromal compartment [1], modification of the protocol will be required to investigate the impact of TILs in lymph node metastases. For further discussion of TIL assessment in metastatic deposits, including those in lymph nodes, the reader is referred to part 1 of this review.
Further investigation of the potential prognostic importance of stromal TILs in melanoma would be of interest. The tumor stroma, that is, the stroma within the borders of the invasive tumor, between the tumor nests, is altered relative to the adjacent stroma and has important interactions with the tumor cells [33,34]. We consider this stroma to be an integral part of the tumor, and hence stromal TILs should also be assessed as they may also play an important role in melanoma. Following Clark’s initial definition of TILs [5], lymphocytes within the stromal compartment have been largely excluded from analysis in melanoma.
Another area of interest is the potential importance of peri-tumoral lymphocytes. Less is known about the potential prognostic effect of a marked lymphocytic infiltrate in this compartment. Ladanyi et al have considered peri-tumoral and intra-tumoral lymphocytes separately in a series of small immunohistochemical studies, and have found that high numbers of peri-tumoral activated T helper cells, B cells and mature dendritic cells are associated with improved survival [35–37]. In contrast, Hillen et al found no significant association between survival and the density of peri-tumoral lymphocytes subsets defined by immunohistochemistry [18]. A recent study attracting much interest showed that the CD8+ T cell density at the invasive margin of melanoma metastases was able to consistently predict response to immune checkpoint inhibitor therapy with pembrolizumab, performing better than CD8+ T cell density within the tumor and better than CD4+, PD-1+ and PD-L1+ cell densities within tumor or at the invasive margin [38]. The invasive margin was defined as an area outside the tumor nests, which were delineated by S100 IHC [38]. This important study has renewed interest in the potential importance of peri-tumoral lymphocytes, given the ongoing search for a reliable biomarker able to predict response to immune checkpoint inhibitor therapy.
As there is significant prognostic information available from examining both stromal TILs and intra-tumoral TILs in other tumor types, it would be of interest to further examine the stromal compartment as well as the peri-tumoral area in melanoma. As such, standardized definitions of these compartments would be of value to pathologists and researchers, as illustrated in part 1 of this review. The definition of “invasive margin” used by Galon and colleagues [39] is preferred, and we suggest this will be applicable to most solid tumor types as well as colorectal carcinoma. This defines the invasive margin as the region centered on the border separating the host tissue from the malignant nests, with an extent of 1mm [39]. Tissue within this region is considered central tumor and beyond this region is considered peri-tumor. While lacking specific supporting evidence, this is a pragmatic, easily applied and widely applicable definition. In invasive breast carcinoma, there is currently no evidence to suggest a functional difference between lymphocytes at the invasive margin and within the central tumor stroma, and it is recommended that these areas are combined in daily practice [1]. However, in melanoma, colorectal carcinoma (discussed below) and potentially other tumor types, there does appear to be value in separating the two areas, at least in the research setting. With clear definitions such as these in place, it is hoped that data from future studies can be combined and compared in a valid manner. A suggested approach to applying the proposed standardized methodology to TIL assessment in melanoma is illustrated in Figure 1 and detailed further in a tutorial available online in Supplementary File 1.
Figure 1.
Applying the proposed standardized methodology to evaluate TILs in melanoma. Although traditional scoring systems have only considered intra-tumoral TILs in melanoma, both stromal and intra-tumoral TILs may be evaluated in the research setting. Areas of necrosis or ulceration are excluded.
TILs in colorectal carcinoma
Recognition of the prognostic impact of TILs in colorectal carcinoma dates back to the 1930s [40]. Much evidence has accumulated in the intervening years, such that proposals have been made to include an assessment of the immune infiltrate in the traditional Tumor-Node-Metastasis staging system [41]. Early interest in colorectal cancer TILs revolved around their association with cancers showing sporadic or familial microsatellite instability (MSI). In recent years, attention has shifted to the prognostic value of TILs assessment, different scoring methodologies and the ability of TILs scoring to predict response to neoadjuvant therapy in rectal cancer.
Colorectal adenocarcinoma arises through genetically distinct pathways – the traditional stepwise adenoma-carcinoma sequence characterized by mutations in APC, TP53 and KRAS and chromosomal instability, the CpG island methylator phenotype (CIMP) in which high levels of promoter methylation lead to silencing of tumor suppressor genes, and the MSI pathway characterized by deficient DNA mismatch repair (dMMR) [42,43]. dMMR/MSI-high cancers often have a distinctive morphology, and these histological features including TILs, Crohn-like lymphocytic reaction, mucinous/signet-ring cell differentiation and medullary growth pattern form part of the 2004 revised Bethesda criteria to select patients for further MSI testing [44]. Many studies have shown the value of a histological assessment of TILs in predicting MSI status, alone or as part of a predictive model [45–49]. A count of intra-epithelial lymphocytes on H&E alone can predict MSI status with a sensitivity of 21 – 93% and a specificity of 62 – 97%, with cut-offs ranging from 2–5 intraepithelial lymphocytes per high power field [45–48]. Joost et al [49] compared five different predictive models incorporating an assessment of TILs [46,48,50–52], with sensitivities ranging from 78 – 97% and specificities of 46 – 93%. However, National Comprehensive Cancer guidelines now recommend universal screening of all colorectal carcinomas for MMR/MSI status to identify Lynch syndrome [53], and the prognostic stratification of all patients based on MMR/MSI status is increasingly being recommended [54–56]. Universal screening has become routine practice in many histopathology laboratories, with a panel of two or four immunohistochemical stains reliably identifying dMMR colorectal carcinomas [57,58]. As such, the imperfect sensitivity and specificity of TILs and other histological features is no longer considered sufficient to identify these cases in practice. It has recently been recognized that, although rare, POLE proofreading domain mutations are present in a small subset of colorectal carcinomas, and result in an ultramutated, highly immunogenic phenotype with improved prognosis [59]. As is discussed further below in the section “Endometrial Carcinoma”, the high level of TILs in these tumors may prove to have diagnostic and therapeutic importance.
In addition to identifying dMMR/MSI-high colorectal carcinomas, TILs have also been shown to have important prognostic value in all colorectal carcinomas, regardless of MSI status [60]. Both semi-quantitative H&E-based scoring and digital quantitation of TILs on IHC have received much attention in the literature. In 1986, Jass et al published a semi-quantitative H&E assessment of TILs in rectal cancer which was found to be an independent prognostic factor [61]. The lymphocytic infiltrate was described as predominating in the “delicate connective tissue lamina at the growing tissue margin”, that is, in the stromal compartment, and scored as little/none, moderate or pronounced [61]. Semi-quantitative H&E assessment also formed the basis of the Klintrup-Mäkinen score developed in 2005, where the immune infiltrate was scored from 0–3, with score 0 = no increase in inflammatory cells; score 1 = a patchy increase of inflammatory cells at the invasive margin, but no destruction of invading cancer cell islets; score 2 = a band-like infiltrate at the invasive margin with some destruction of cancer cell islets; and score 3 = a very prominent inflammatory reaction, forming a cup-like zone at the invasive margin, and frequent and invariable destruction of cancer cell islets [62]. Whilst again focusing on the invasive margin, the importance of intra-tumoral TILs disrupting cancer cell nests is also emphasized. Scores were collapsed into low-grade inflammation (score 0–1) and high grade inflammation (score 2–3) and inter-observer and intra-observer agreement was good [62]. High grade inflammation at the invasive margin was found to be a strong independent prognostic factor for survival in Dukes A and B colorectal carcinomas [62]. The prognostic value of the Klintrup-Mäkinen score has subsequently been validated in independent cohorts [63–67].
A comprehensive and detailed approach to TIL scoring in colorectal carcinoma developed by Galon and colleagues has attracted international attention [68,69]. An initial study by this group in 2005 confirmed the prognostic value of a semi-quantitative H&E assessment of TILs in colorectal cancer and performed a detailed analysis of the lymphocyte subsets involved using IHC-based digital quantitation, flow cytometry and mRNA profiling [70]. Subsequent demonstration of the marked prognostic impact of a CD3+ T cell infiltrate in colorectal cancer [71] was followed by development of the “Immunoscore®” which uses CD8 IHC to mark cytotoxic T cells and CD45RO to mark memory T cells, which are scored in hotspots selected from the invasive margin and central tumor [72]. This Immunoscore® was strongly predictive of disease free, disease specific and overall survival, and found to be superior to the traditional Tumor-Node-Metastasis staging system of the AJCC/UICC [72], confirmed in further studies by the same research group [73]. More recently, on the basis of antibody performance, the Immunoscore® was modified to include CD3 rather than CD45RO [41], which has also been demonstrated to be predictive of distant metastasis [39], and show superior prognostic value to MSI status [74]. Recently, initial results were presented in abstract form from a worldwide taskforce established to prospectively validate the Immunoscore® [75]. The assay was reportedly reproducible across the 23 participating institutions, and the primary endpoints of the study were reached, demonstrating a significantly longer time to recurrence in patients with a high Immunoscore® at the invasive margin [75]. Full results of this important international collaboration are eagerly awaited. It is emphasized that this review provides a framework for TIL-assessment and should be considered complementary to all other important evolutions such as the Immunoscore®.
The relative benefits and limitations of semi-quantitative H&E assessment compared with digital quantitation of IHC stained slides in colorectal carcinoma have begun to be addressed [65–67]. The digital image analysis software used by Galon et al was developed in-house and is not publically available for independent validation and comparison with other methodologies [76]. However, largely concordant results have been reported using other image analysis software (reviewed in [77]). Väyrynen et al performed digital quantitation of IHC-defined lymphocyte subsets and a manual Klintrup-Mäkinen score of TILs, and found strong correlation between all IHC–based counts and the Klintrup-Mäkinen grade, supporting an overall immune assessment [66]. Richards et al compared a manual semi-quantitative approximation of the Galon Immunoscore® with the Klintrup-Mäkinen grade, and found similar prognostic information was provided by both methodologies [65]. However, a recent comparison by the same group showed additional prognostic information was provided by the manual semi-quantitative approximation of the Galon Immunoscore® when compared to the Klintrup-Mäkinen score in whole sections of 246 stage 1 to 3 colorectal cancers [67]. A detailed cost-benefit analysis of any additional prognostic information provided by digital IHC-based scoring is needed to justify the additional time and resources required to perform the immunohistochemical assays, implement slide scanning capabilities and develop image analysis software. Furthermore, additional direct comparison of the Galon Immunoscore® by independent groups using different technologies, manual approximation, or Klintrup-Mäkinen grading may allow definition of the most appropriate balance between simplicity and depth of information. As has been performed in invasive breast cancer and lung cancer, TILs assessment as part of a treatment-related randomized controlled trial can provide high level evidence of prognostic and predictive value, and could be considered in colorectal carcinoma.
In addition to prognostic information, TILs assessment in rectal cancer may help to predict the degree of response to neoadjuvant chemoradiotherapy. This has been investigated in a number of studies, all using IHC to delineate different lymphocyte subsets [78–83]. Yasuda et al found high numbers of CD8+ TILs in the pre-treatment biopsy to be predictive of high histological regression grade following chemoradiotherapy in multivariate analysis [81], while Shinto et al found the CD8/FOXP3 ratio to be predictive of treatment response [83]. Similar results were found in a univariate analyses [78–80]. McCoy et al found a low stromal Treg count to be associated with tumor regression, but not with overall survival [82]. To the best of our knowledge, this question has not been comprehensively addressed using semi-quantitative H&E based scoring such as the Klintrup-Mäkinen grade.
TILs in upper gastrointestinal tract carcinomas
As a group, carcinomas of the stomach, pancreas, and liver are relatively common, and also account for a disproportionately high number of cancer deaths [84]. Chronic inflammation due to infection and other causes is at least partly responsible for many cases. Treatment of metastatic disease with chemo-radiotherapy generally has modest effects, and initial trials of immunotherapy agents in this group of tumors have reported mixed success [85,86], suggesting the need for predictive biomarkers and a personalized approach to the use of these agents.
Gastric carcinoma
Gastric cancer is notable for its associations with infection (Helicobacter pylori, Epstein-Barr virus (EBV)), chronic inflammation, and genomic instability (both microsatellite instability and chromosomal instability) [87]. EBV-associated gastric carcinoma typically shows a particularly high immune infiltrate and accounts for a high proportion of the histological subtype known as lymphoepithelioma-like carcinoma or gastric carcinoma with lymphoid stroma [88]. It is thought that the improved prognosis seen in EBV-associated gastric cancer may be related to the high proportion of lymphoepithelioma-like carcinoma in this group, rather than the presence of EBV itself [89]. Similar to colorectal carcinomas, gastric carcinomas with microsatellite instability due to deficiencies in DNA mismatch repair (dMMR/MSI-high) contain high mutational loads, display a prominent lymphocytic infiltrate, and are associated with improved prognosis [88].
Assessment of gastric cancer TILs on H&E sections has been reported in few studies. Kang et al [90] assessed the prognostic value of TILs amongst EBV-associated gastric carcinomas using a modification of the International Immuno-Oncology Biomarkers Working Group guidelines for breast carcinoma [1]. sTILs were found to be an independent prognostic factor for recurrence free survival but not overall survival, while iTILs were not significantly associated with either recurrence free or overall survival [90]. Giamperi et al used a similar approach and found that sTILs were significantly associated with dMMR status, but that both sTILs and dMMR status were independent favorable prognostic factors in a multivariate model [91].
Studies of immunohistochemical markers of immune cells have been reported more often, singly and in combination. Infiltration by CD3+ and CD8+ T cells [92,93], CD20+ B cells [94] and expression of the chemokine receptor CXCR3 [95] have been reported to correlate with improved prognosis. Conflicting results have been seen for FOXP3+ Tregs, which in some studies are associated with improved prognosis [96,97], and in others, a worse prognosis [98,99]. Expression of the chemokine receptor CCR7 [99] and immune checkpoint molecule PD-L1 [100] have also been associated with worse prognosis in gastric cancer. These studies, reviewed by Solinas et al [101], although predominantly small and retrospective with varying methodologies, appear to support a favorable prognostic role of an active cytotoxic immune response, and an unfavorable role of an exhausted or suppressive immune response in gastric carcinoma.
Both dMMR/MSI-high and EBV-associated gastric cancers have been proposed as candidates for immune checkpoint inhibitor therapy [102,103], as both types are associated with prominent host immune responses. The high mutational load in dMMR/MSI-high cancers is thought to result in high immunogenicity [104], as discussed further below. EBV-associated gastric cancers show extreme levels of hypermethylation, causing epigenetic silencing of many tumor suppressor genes [103]. In addition, PD-L1 expression in gastric carcinoma appears to correlate with high immune cell infiltration, MMR deficiency and the EBV-associated subtype [100,105,106]. Further evaluation in large clinical trials is needed to confirm the prognostic value of TILs in gastric cancer, and to assess the potential value of TILs as a biomarker for immune checkpoint inhibition and other immunotherapy approaches.
Pancreatic carcinoma
Pancreatic ductal adenocarcinomas typically have abundant desmoplastic stroma, which contributes to tumorigenesis [107], interacts with immune and inflammatory cells [108], and will be important to consider in the histological assessment of TILs. Despite the recent proposal of an “immunogenic” subtype of pancreatic ductal adenocarcinoma identified through integrated genomic analysis [109], TILs in pancreatic cancer have received relatively little attention. There are few published studies examining TILs in pancreatic adenocarcinoma on H&E sections and prognosis. The presence of intra-tumoral tertiary lymphoid structures, as judged by pathologists, was associated with longer overall and disease free survival in one study [110]. Hart et al scored intra-tumoral lymphocytes as high or low on H&E sections in 63 patients, but found no association between TILs and survival [111].
Immunohistochemical studies of the tumor microenvironment in pancreatic carcinoma have found that high levels of tumor associated M2 macrophages marked by CD68, CD163 and CD204, neutrophils marked by CD66b, and FOXP3+ Tregs correlated with worse prognosis [112,113]. Improved prognosis was seen with high levels of infiltration by CD3+, CD8+ or CD4+ T cells and CD20+ B cells [113–115]. Further histological characterization of the proposed immunogenic subtype of pancreatic ductal adenocarcinoma will be of value to inform forthcoming immunotherapy clinical trials.
Hepatocellular carcinoma
Many cases of hepatocellular carcinoma are causally linked to chronic inflammation, with viral hepatitis, alcohol, and non-alcoholic steatohepatitis as major underlying contributors. Chronic inflammation can be seen as the persistence of an ineffective immune response, and is associated with the development of an immunosuppressive environment with high expression of immune checkpoint molecules, impaired antigen presentation, and the presence of Tregs [116]. Hepatocellular carcinomas have highly variable amounts of tumor stroma, with some cases having very little, which will need to be factored into the assessment of stromal TILs. Marked inflammatory cell infiltration on H&E assessment of hepatocellular carcinomas was reported to be associated with improved survival [117]. Infiltration by FOXP3+ Tregs was again found to be associated with a worse prognosis [118], whereas an improved prognosis was seen with cytotoxic T cell and B cell infiltration [119,120]. Initial results of immunotherapy trials show moderate responses of hepatocellular carcinoma to immune checkpoint inhibition, and further investigation of combination approaches is underway [116].
TILs in non-small cell lung carcinoma
The immune microenvironment in non-small cell lung cancer has been extensively studied and detailed descriptions exist in the literature [121,122]. Similar to colorectal cancer, inclusion of an IHC-based “Immunoscore” into the traditional Tumor-Node-Metastasis staging system has been proposed, following evidence of a significant prognostic impact of TILs in non-small cell lung carcinoma [123]. Like melanoma, non-small cell lung carcinoma often contains a high somatic mutation burden and immune checkpoint inhibitors are a promising therapeutic advance [124–128]. The immune system clearly plays an important role in the development, progression and treatment of non-small cell lung cancer, and assessment of the immune infiltrate is of great interest to clinicians and researchers.
An excellent and extensive review of the prognostic impact of different innate and adaptive immune cell subsets in non-small cell lung cancer, as well as tertiary lymphoid structures and immune checkpoint molecule expression, can be found in Remark et al [121]. In summary, cytotoxic T cells, natural killer cells, mature dendritic cells and M1 macrophages have largely been associated with improved prognosis, Tregs have been associated with poorer prognosis, and inconclusive results have been found for neutrophils and B cells [121]. Geng et al have performed a meta-analysis of studies investigating the prognostic impact of TILs in lung cancer patients, including 29 studies involving 8600 patients with non-small cell lung carcinoma [129]. Only three included studies addressed “generalized TILs” based on H&E assessment [130–132], discussed further below. The majority of included studies used IHC to define T cell subsets, including CD3, CD8, CD4 and FOXP3 [129]. Overall, CD8+ cell density in sTILs and in iTILs were associated with improved overall survival, with similar results seen for CD3+ cell density [129]. CD4+ T cells were only associated with overall survival when assessed in the tumor stroma rather than the tumor cell nests, while FOXP3+ Tregs in the tumor stroma were associated with poorer progression free survival [129]. Sixteen of these studies, along with an additional eight studies, were included in a meta-analysis by Zeng et al [133]. These authors concluded that high levels of CD8+, CD3+ and CD4+ TILs had prognostic significance for both overall survival and recurrence [133]. Of note is the moderate to significant heterogeneity identified in both of these meta-analyses, as well as the retrospective nature of the included studies, many of which had incomplete data with regard to important prognostic factors [129,133]. In addition, the lack of standardized TILs assessment and arbitrary cut-points may reduce the validity of combining data in this manner.
In contrast to the Immunoscore® developed for colorectal carcinoma, many of the studies of IHC-based TIL subset assessment in non-small cell lung carcinoma use a manual semi-quantitative approach developed in studies of tissue microarrays by Al-Shibli and colleagues [134], in which the percentage of the nucleated cells showing positive staining for the marker in question is estimated [134–144]. Different cut-offs are used to define “low” and “high” for each marker, and for the epithelial/tumor nest compartment and the stromal compartment, according to the staining distribution [134]. Other studies have used an absolute count of positive cells per mm2, determined by digital image analysis [145–150] or manual counting [151–157]. Based on their previous work, Donnem et al recently demonstrated that the stromal CD8+ T cell density, scored on a manual semi-quantitative four-point scale, has independent prognostic value and can stratify patients within each Tumor-Node-Metastasis stage [138]. This paper was followed by a proposal to introduce an IHC-based “TNM-Immune” staging system into clinical use for non-small cell lung cancer, as has been proposed for colorectal cancer [123].
In contrast to this IHC based method, Brambilla et al recently reported a large analysis of the prognostic impact of TILs in non-small cell lung carcinoma, performed as part of the LACE-Bio pool of four randomized clinical trials [158]. Samples from discovery and internal validation cohorts were evaluated for lymphocytic infiltration by H&E assessment and scored on a four-point semi-quantitative scale which was collapsed into binary “intense” and “non-intense” categories, where an intense infiltrate was defined as mimicking a lymph node involved by cancer metastasis [158]. Overall agreement was good when binary classification was used [158]. Intense lymphocytic infiltration was found to be an independent prognostic variable for overall survival and disease free survival on multivariate analysis in both the discovery and validation sets [158]. Neither tumor histology nor treatment showed significant interactions with degree of lymphocytic infiltration and TILs did not predict benefit to platinum-based adjuvant chemotherapy [158]. Three studies have used a similar semi-quantitative H&E based scale and found high lymphocytic infiltration to correlate with recurrence free survival [130,131,159], however no prognostic impact was found in a two separate studies using different subjective H&E assessments [132,160]. Mignon et al recently presented results demonstrating that scoring percentage stromal TILs on H&E was highly reproducible and showed prognostic significance, particularly in KRAS mutant non-small cell lung cancer [161]. However, scoring percentage iTILs on H&E was not reproducible between pathologists and failed to demonstrate prognostic significance [161].
Further research may be required to clarify the potential importance of separating the stromal and intra-epithelial compartments, as advocated by Donnem et al [123], compared with an overall assessment as preferred by Brambilla et al [158]. Issues of reproducibility in iTILs scoring must also be taken into consideration. In addition, consideration of potential differences at the invasive margin and in the central tumor has not been extensively studied in non-small cell lung carcinoma in contrast to colorectal carcinoma. A prospective evaluation of the lung Immunoscore, potentially using digital image analysis, has been proposed [123]. Quantitative immunofluorescence has also been used to demonstrate the prognostic value of TIL subsets in this setting [132]. An important aspect of the immune response to tumors, the formation of tertiary lymphoid structures, may not be captured by an overall H&E based TILs assessment or by IHC-based hotspot assessment. The prognostic importance of tertiary lymphoid structures has been well studied in non-small cell lung cancer [121,147,162], and their presence correlates with an activated cytotoxic T cell response, indicating an important role of these local organized lymphoid structures in coordinating the immune response to tumors [147]. Head-to-head comparison of the IHC-based Immunoscore and the overall H&E assessment, with consideration of the inclusion of additional features such as tertiary lymphoid structures, would also be of value in progressing towards a consensus for TILs assessment in non-small cell lung cancer. An approach to features particular to lung carcinoma such as lepidic growth and aerogenous spread is found in the accompanying tutorial, available in Supplementary File 2. Pre-existing alveolar macrophages would be excluded from a TIL assessment in non-small cell lung carcinoma, while the sTILs compartment would include the fibrovascular cores of papillary structures (Figure 2).
Figure 2.

When assessing TILs in non-small cell lung carcinoma, include lymphocytes in the fibrovascular cores of papillary structures (marked sTILs), and exclude alveolar macrophages.
Assessment of the immune response to non-small cell lung carcinoma is of particular interest following accumulating evidence of the efficacy of immune checkpoint inhibitor therapy for this indication [124–128]. Whilst studies of PD-1 inhibitors nivolumab and pembrolizumab have focused on tumor cell PD-L1 expression as a predictive biomarker [124,125,127], studies of the PD-L1 inhibitor atezolizumab have also shown predictive value of PD-L1 expression on tumor infiltrating immune cells [126]. Response to atezolizumab also correlated with high expression of effector T-cell and interferon-γ associated gene signatures in tumor tissue [126]. Whilst further discussion of the issues surrounding PD-L1 IHC is beyond the scope of this article, it is clear that a standardized method of assessing and quantifying the immune infiltrate in tumors is needed to then reliably assess immune cell PD-L1 expression.
Less is known about the immune microenvironment of pleural malignant mesothelioma, but the potential effect of TILs on mesothelioma prognosis is beginning to be described. Early IHC-based studies suggested a favorable prognostic effect of TILs in mesothelioma [163,164]. More recently, in a cohort of 329 pleural malignant mesothelioma cases comprising video-assisted thoracoscopic surgery pleurodesis biopsies and resection specimens, Russell, Thapa and John (manuscript in preparation) assessed the percentage of stromal TILs and the presence or absence of tertiary lymphoid structures. Interestingly, the sTILs score was strongly negatively correlated with survival, however the presence of tertiary lymphoid structures was independently associated with better survival in multivariate analysis. A tutorial demonstrating the proposed approach to TILs assessment in pleural malignant mesothelioma is available online in Supplementary File 3.
TILs in gynecological carcinomas
Endometrial carcinoma
Relatively little is known about the prognostic significance of TILs in endometrial carcinoma, with many studies focusing instead on the value of TILs to predict MSI status. With increasing recognition of the frequency of Lynch syndrome amongst women with endometrial cancer and the advent of reliable immunohistochemical screening, assessment of TILs is receiving less attention. However, interest has been renewed following identification of the ultramutated POLE subtype of endometrial carcinoma and the potential utility of immune checkpoint inhibitors.
Approximately 10–20% of endometrial carcinomas display microsatellite instability due to either epigenetic silencing or germline mutations in DNA mismatch repair genes (Lynch syndrome) [165,166]. Similar to colorectal carcinoma, initial screening guidelines incorporated clinical and pathological features (reviewed in [167]). Shia et al found high TILs counts and the presence of peri-tumoral lymphocytes to be predictive of deficient MMR in endometrial carcinomas, with a sensitivity of 85% and specificity of 46% [168]. Only lymphocytes within tumor cell nests (i.e. intraepithelial) were counted as TILs, and an absolute count of 10 H&E stained high power fields was obtained [168]. This method and the proposed cut-off of 40 lymphocytes per 10hpf were used in subsequent studies, which showed similar results [169–172]. As for colorectal cancer, the reported sensitivities of even multivariate models are now considered insufficient for screening for MMR defects [167,173].
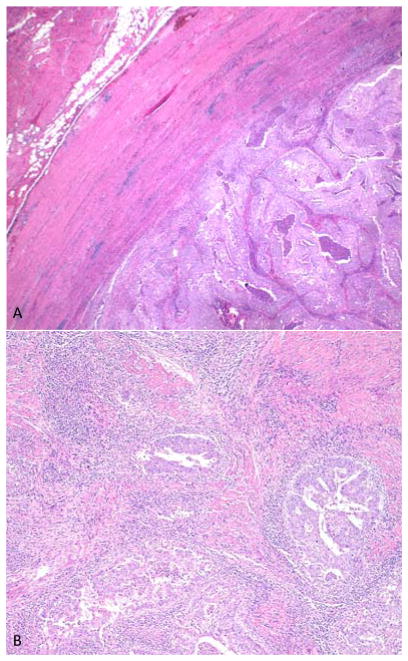
A recently recognized subgroup of endometrial cancers has a very high mutation rate due to deficiencies in DNA proofreading [174]. POLE mutations are found in approximately 7% of endometrial carcinomas, resulting in loss of DNA polymerase epsilon and ineffective DNA proofreading [175]. This group of POLE ultramutated endometrial carcinomas appears to have an improved prognosis, particularly in high-grade cancers, and shows particular histological features as illustrated in Figure 3 [174,176,177]. Howitt et al found that POLE-mutated endometrial carcinomas had very high predicted neoantigen loads, and that POLE and MSI tumors had higher TILs and higher PD-L1 expression on immune cells than microsatellite stable tumors [178]. Similar histological findings were reported by Hussein et al [179] and van Gool et al [180]. Given the initial positive results of immune checkpoint inhibition in MSI tumors [102], similar responses may be predicted in POLE ultramutated tumors, although this has not yet been clinically tested to our knowledge. The recognition of POLE tumors has prognostic value and potential predictive value, and as there is not yet a widely available immunohistochemical marker to screen cases, histological features including TILs may prove useful to identify cases for further testing.
Figure 3.
An example of an ultramutated endometrial carcinoma with POLE mutation. Characteristic histological features include expansile growth with a pushing border, solid areas and serous-like morphology (panel A), as well as high FIGO grade and prominent stromal and intra-tumoral lymphoid infiltrate (panel B).
Robust evidence of the clinical validity of TILs assessment for prognosis in endometrial carcinoma remains to be demonstrated. Intra-epithelial CD8+ T cell density has shown independent prognostic significance in initial studies [181–183], and stromal CD3+ T cells may also have prognostic value [184]. Workel and colleagues demonstrated that CD103, a marker discussed further below, defined intra-epithelial CD8+ PD-1+ lymphocytes, and that high numbers of these iTILs were associated with improved prognosis in patients with high-risk endometrial adenocarcinoma [185]. High Treg counts were associated with poorer disease free survival in the study of Yamagami et al [186]. Further work in large, high quality studies is needed to confirm the prognostic significance of TILs in endometrial carcinoma, which may have significant interactions with MSI and POLE status. A tutorial outlining the proposed approach to scoring TILs in endometrial cancer is available online in Supplementary File 4.
Ovarian carcinoma
A seminal paper published in 2003 by Zhang et al established the prognostic significance of TILs in ovarian carcinoma [187]. In the intervening years, evidence has accumulated largely in support of this finding and has refined the clinicopathological features associated with a robust TIL response.
In their study of 186 ovarian carcinomas, Zhang et al found that the presence of any intra-epithelial T cells, as assessed on CD3 IHC, was an independent favorable prognostic factor on multivariate analysis, with striking differences in progression free and overall survival between the two groups [187]. Both manual counting and digital image analysis were used to determine the number of T cells per high power field, averaged from a total of 15 to 20 high power fields [187]. Both iTILs and sTILs were assessed, however only results for iTILs were reported [187]. While some studies have since reported no association between TILs and prognosis in ovarian carcinoma [188,189], a recent meta-analysis of 10 studies involving 1815 patients found women with ovarian cancers lacking intra-epithelial TILs had a risk of dying 1.53x that of women with tumors containing CD8+ TILs (95%CI 1.22–1.93) [190]. This pooled hazard ratio was higher, up to 2.67 (95%CI 2.02–3.53), when studies using a higher cut-off to define “TIL negative” were analyzed [190]. The authors concluded that >5 CD8+ TILs per 200x high power field should be used to define “TIL positive” in ovarian carcinoma [190]. To the best of our knowledge, no study has formally compared the information gained from quantitative IHC based assessment to that potentially obtained from semi-quantitative H&E based scoring.
As has been reported in other tumor types, different lymphocyte subsets may have different impacts on the progression and prognosis of ovarian carcinoma. In the meta-analysis by Hwang et al, CD8 was found to have a more consistent and stronger association with overall survival than CD3 [190]. Cytotoxic T cells marked by TIA-1 and granzyme B have also been shown to positively correlate with survival [191]. Interestingly, the presence of CD20+ B cells and plasma cells co-localized with T cells appears to increase the positive prognostic effect of TILs above that seen with CD8+ T cells alone [192,193]. Plasma cells were predominantly seen in the stromal compartment, often in association with organized tertiary lymphoid structures [193], which have been shown to correlate with improved prognosis in many tumor types [194]. In contrast, conflicting results have been seen with regard to intra-epithelial FOXP3+ Tregs, with studies showing both positive [191,195] and negative [196–198] effects on survival in ovarian carcinoma.
The location of TILs may have particular significance in ovarian carcinoma. Following the original description of intra-epithelial TILs in ovarian carcinoma [187], most studies have focused on the epithelial compartment. Stumpf et al reported that high numbers of iTILs, but not sTILs, were associated with improved survival [199]. Similar findings were reported by Darb-Esfahani and colleagues [200]. Webb et al have demonstrated that the integrin CD103, which binds to E-cadherin expressed by epithelial cells, is highly expressed by intra-epithelial effector T cells in ovarian carcinoma, and suggested that it is these CD103+ T cells that contribute to the improved prognosis seen with high levels of TILs [201]. Similarly, Bosmuller et al found CD103 added prognostic value to conventional T cell markers in the assessment of TILs in ovarian carcinoma [202]. However, two studies have reported important prognostic value of TILs in the stromal compartment, similar to that seen in the epithelial compartment [203,204]. In addition, the important interaction between T cells, B cells and plasma cells described by Nielsen and Kroeger [192,193], and the formation of tertiary lymphoid structures, take place predominantly in the tumor stroma. Therefore although intra-epithelial TILs clearly have well-demonstrated prognostic significance in ovarian carcinoma, the stromal compartment should also be considered when evaluating TILs in these tumors. A tutorial on TILs assessment in ovarian carcinoma is available in Supplementary File 5. Figure 4 illustrates a range of sTILs percentages in ovarian carcinoma as a reference.
Figure 4.
Examples of a range of stromal TILs percentages in high-grade serous ovarian carcinoma.
Neoadjuvant chemotherapy for ovarian carcinoma is a controversial area, and selection of patients who should receive neoadjuvant chemotherapy rather than undergo primary debulking surgery is currently challenging [205–207]. Pre-treatment biopsies matched with post-treatment resection specimens provide valuable opportunities for examining modulation of the immune response by chemotherapy and evaluating potential predictors of response to neoadjuvant chemotherapy. Early work suggests that TILs increase following neoadjuvant chemotherapy, both cytotoxic and regulatory T cells [208,209]. Importantly, many TILs post-neoadjuvant chemotherapy express CTLA-4, PD-1 and PD-L1 [208], and tumor cell expression of PD-L1 may also be induced by neoadjuvant chemotherapy. These molecules may be considered markers of T cell exhaustion in the appropriate context, and expression of these markers may support the use of immune checkpoint inhibitors following chemotherapy to reawaken the immune response [208]. Lo et al also described an increase in immune infiltration following neoadjuvant chemotherapy in those tumors showing some degree of baseline TILs [210]. However, TIL negative tumors tended to remain TIL negative following neoadjuvant chemotherapy, suggesting that assessment of TILs in pre-treatment biopsies may help to identify “immunologically inert” tumors, which would be unlikely to respond to immunotherapy approaches [210]. Inclusion of TILs as planned biomarker analyses in future randomized clinical trials, as has been performed in breast carcinoma, may help to reveal any value of TILs in predicting response to neoadjuvant chemotherapy.
The majority of ovarian malignancies are high grade serous carcinomas which often present at high stage and have a dismal prognosis [211]. The prognostic impact of TILs appears limited to high grade serous ovarian carcinomas [191], however numbers of other subtypes including endometrioid, clear cell and mucinous carcinomas were small. Interestingly, the association between TILs and MMR status seen in endometrial carcinoma does not appear to be recapitulated in Lynch-syndrome associated endometrioid ovarian carcinoma [212]. Approximately half of high grade serous ovarian carcinomas will show defects in the homologous recombination pathway, most commonly BRCA1 inactivation through germline or sporadic mutation or methylation [213]. Higher levels of TILs are seen in BRCA1 mutated ovarian carcinomas [214–217], which also correlate with the immunoreactive molecular subtype as defined by The Cancer Genome Atlas [213,218]. TILs are included in a set of histological criteria suggested to predict BRCA1 mutations in high grade serous ovarian carcinomas [219], which demonstrated a high negative predictive value but low positive predictive value. Conflicting results have been obtained regarding the level of TILs in BRCA2 mutated ovarian carcinomas and those showing other defects in the homologous recombination pathway [214,216,218]. Parallels may be drawn to BRCA1/2 associated breast carcinoma in which higher lymphocytic infiltrates are seen in BRCA1 mutated cancers, while BRCA2 associated breast carcinomas do not have a characteristic morphology [220,221]. Why BRCA1 associated tumors show high TILs while tumors with other homologous recombination-pathway defects including BRCA2 do not, is yet to be conclusively explained. The unique function of BRCA1 as a transcription factor, links between BRCA1 mutations and copy number alterations, or potential cancer-testis antigen expression, may warrant further investigation in this context [222].
TILs in head and neck squamous cell carcinoma
Head and neck squamous cell carcinoma encompasses a heterogeneous group of tumors arising in different sub-sites within the upper aerodigestive tract, including the oral cavity, the oropharynx, larynx and hypopharynx. While many cancers are etiologically related to the traditional risk factors of tobacco and alcohol, others are associated with human papilloma virus (HPV) infection. These HPV-related tumors typically arise in the oropharynx, are found in younger, never-smokers and are associated with better prognosis. Following Wolf’s description in 1986 of improved outcome in tumors with increased lymphocyte infiltration in a small cohort of patients with oral cavity squamous cell carcinoma [223], many studies have described immune cell infiltrates in head and neck squamous cell carcinoma and correlated these with outcome [224–226]. While the presence of TILs has generally been associated with improved prognosis, differences have been reported according to anatomic sub-site, tumor compartment (intra-tumoral vs. stromal) and importantly in HPV-positive (HPV+) compared to HPV-negative (HPV−) head and neck squamous cell carcinomas.
Biological and immunological differences exist between tumors arising in different anatomic sub-sites of the head and neck. Oropharyngeal squamous cell carcinomas, which arise from the squamous epithelium associated with the lymphoid tissue of the tonsils and base of tongue, have higher numbers of infiltrating iTILs and sTILs compared with other sub-sites [227]. The pre-existing background lymphoid stroma will clearly complicate TIL assessment in these tumors, and an approach similar to that used to assess metastatic deposits in lymph nodes is recommended, that is, to discount any established lymphoid stroma and focus on iTILs in this setting if no desmoplastic stroma is present. Alternatively, it has been suggested that this pre-existing lymphoid stroma is not simply a bystander, but may contribute to the improved prognosis of oropharyngeal squamous cell carcinomas compared to squamous cell carcinomas in other regions of the head and neck [228]. This interesting question requires further investigation.
Importantly, within the subset of oropharyngeal tumors, there are significant genomic and immunologic differences between HPV+ and HPV− tumors. Many studies have reported a higher number of TILs, in particular CD8+ T cells within tumor and stroma, in HPV+ tumors compared with HPV− tumors [229–233]. Increased numbers of FOXP3+ Tregs, PD-1+ T cells and CD20+ B cells within immune infiltrates in HPV+ tumors have also been described [234,235]. Using gene expression analysis, Wood et al found increased expression of genes encoding PD-1, CTLA-4, and TIM3 in HPV+ tumors, indicating an exhausted immune response [236]. Interestingly, a B cell signature distinguished HPV+ from HPV− tumors, suggesting B cells rather than T cells account for the increased TILs seen in HPV+ tumors [236]. Immunohistochemical studies have shown CD8+ and FOXP3+ TILs to significantly correlate with improved prognosis in oropharyngeal HPV+ tumors [229–236] and probably also HPV− tumors [233,236].
Genomic analyses have also highlighted the importance of immune cell infiltrates in head and neck squamous cell carcinomas. Using unsupervised clustering of gene expression data, Keck et al identified immune mesenchymal subtypes of HPV+ and HPV– head and neck squamous cell carcinomas, which were associated with increased expression of immune markers, increased CD8+ TILs and improved outcomes [237]. Mandal et al, in an analysis of transcriptome data from 280 head and neck squamous cell carcinomas from The Cancer Genome Atlas, found that HPV+ tumors had higher levels of T cell and overall immune gene signatures, together with higher expression of markers of immune activation such as granzyme B and perforin [238]. Patients with tumors showing high immune gene expression had superior overall survival, and when controlled for HPV status, CD8+ T cells were significantly associated with survival. Tregs and CD56dim NK cells were also associated with favorable prognosis [238].
Studies including tumors from other sub-sites have also identified improved outcome with higher immune cell infiltrates. In tumors of the oral cavity, a three-tiered qualitative assessment of the lymphocytic infiltrate on H&E sections was found to be an independent prognostic factor for local recurrence and overall survival in multivariate analysis [239]. Similar favorable findings were seen with increased intra-tumoral or stromal CD8+ cells [240], while increased numbers of FOXP3+ Tregs may have detrimental effects oral cavity squamous cell carcinoma outcome [241]. Vassilakopoulou et al and Wang et al applied the consensus TILs scoring guidelines from the International Immuno-Oncology Biomarkers Working Group [1] to laryngeal squamous cell carcinoma, and both studies found that the sTILs score was an independent prognostic factor for disease free and overall survival [242,243].
Studies using semi-quantitative scoring of IHC to describe TILs in head and neck squamous cell carcinomas have suggested a potential predictive role of infiltrating CD3+ and CD8+ T cells [244,245]. High levels of CD3+ and CD8+ iTILs correlated with improved outcome following definitive chemoradiotherapy, while sTILs showed no significant association [244]. In a subsequent cohort of 161 patients treated with surgery followed by adjuvant chemoradiation, CD3+ and CD8+ TILs in the stromal, intra-tumoral and tumor periphery compartments were all associated with improved outcome [245]. Large prospective clinical trials of treatment modalities would be an ideal setting to further investigate the potential role of TILs in predicting the efficacy of chemoradiation in head and neck squamous cell carcinomas.
As discussed in a recent review [224], TILs in head and neck squamous cell carcinomas may not yet be ready for implementation as a clinical biomarker. Studies evaluating TILs in head and neck squamous cell carcinomas have been limited by small cohort sizes, retrospective approaches, inclusion of heterogeneous populations, univariate analyses, and lack of standardized methodology for TIL quantification. This argues for the need for larger studies with prospective validation that take into account factors such as tumor site and HPV status, and which also determine the relationship between immune infiltrates and immune regulatory markers such as PD-L1. This will be particularly important in the context of predicting response to immune therapies such as PD-1 or PD-L1 inhibitors that are showing promising efficacy in head and neck squamous cell carcinomas [246,247]. It is encouraging that different research groups have been able to adapt the consensus guidelines for TILs assessment in breast cancer to head and neck squamous cell carcinomas, and demonstrate significant results with regards to prognosis. A standardized methodology will help to overcome many of the barriers to clinical implementation.
TILs in genitourinary carcinomas
Urothelial carcinoma
The successful introduction of intravesical bacillus Calmette-Guerin (BCG) therapy for high-risk non-muscle invasive urothelial carcinoma over the past decades can be regarded as early proof of the potential effectiveness of immunotherapy in urothelial carcinoma [248,249]. The induction of inflammation and a Th1 cytotoxic immune response by BCG administration can control in situ carcinoma and prevent progression to invasive disease [249]. Further support for immunotherapy in bladder cancer has been sparked by the recent FDA approval of the PD-L1 inhibitor atezolizumab for use in advanced urothelial carcinoma [250] and encouraging results from early trials of PD-1 inhibitor pembrolizumab [251]. Response is correlated with the expression of PD-L1 on stromal immune cells [250,251], hence the presence of an immune infiltrate in urothelial carcinoma is critical for the effectiveness of these novel treatments. The presence of TILs in urothelial carcinoma has gained more and more interest during the last few decades. Currently, we can discriminate two types of studies on TILs in UC: those focusing on the prognostic relevance of TILs and those focusing on TILs as predictors of treatment response.
Studies of the prognostic relevance of TILs in urothelial carcinoma, largely based on immunohistochemical quantification of different TIL subsets, have returned somewhat conflicting results. Several decades ago it was reported that the presence of TILs in urothelial carcinoma was associated with a favorable prognosis [252,253]. In a retrospective study on a cohort of 69 UC cases, Sharma et al found that high numbers of CD8+ intra-tumoral T cells in urothelial carcinoma correlated with improved disease free and overall survival [254]. The presence of FOXP3+ TILs in urothelial carcinoma and the presence of CD3+ TILs in non-muscle invasive urothelial carcinoma have also been associated with a better prognosis [255,256]. In contrast, others have reported that CD3+ and CD8+ TILs are predictive of disease recurrence in patients with solitary low grade non-muscle invasive urothelial carcinoma and that CD4+ T cells are associated with a poor prognosis in this setting [257,258]. Another study showed that high CD3+ and CD8+ T cell infiltrates demonstrated trends towards better prognosis, but that high FOXP3/CD3 and FOXP3/CD8 ratios were correlated with poor outcomes [259]. Similar observations were made by Parodi and coworkers, who reported that the intra-tumoral T effector/Treg cell ratio in urothelial carcinoma patients with disease recurrence is invariably less than one, while it is always greater than one in patients without recurrence [260]. Interestingly, PD-L1 expression on TILs was shown to be significantly associated with better overall survival in urothelial carcinoma patients who subsequently developed metastatic disease and received platinum-based chemotherapy [261]. Studies investigating TILs as a prognostic marker in urothelial carcinoma have been largely retrospective in relatively small cohorts, with variable definitions of TILs, inclusion of iTILs and/or sTILs, and the scoring methodology employed. These inconsistencies hamper comparisons across studies and extrapolation of findings to clinical settings. Large studies investigating the potential prognostic value of TILs as assessed on H&E are lacking.
Platinum-based combination chemotherapy remains the standard first-line treatment for patients with metastatic urothelial carcinoma [262]. In the second-line setting, many drugs have been tested, but none have become established as a standard of care because of a low frequency of response. In the context of the success of BCG immunotherapy, and the recent development of immune checkpoint inhibitors as an exciting option for second-line systemic therapy, the potential predictive value of TILs has earned significant scientific interest. Intravesical BCG therapy induces an immune response, with a significant increase of CD3+, CD4+ and CD8+ T-cells in tissue specimens observed after treatment, although no significant differences between responders and non-responders have been found [263]. Several studies confirmed that a large number of tumor associated macrophages and an increased cancer cell-to-lamina propria tumor associated macrophage ratio were associated with a poor oncologic outcome after BCG [264–266]. These macrophages play important roles in coordinating polarization of the immune response to either protect or attack the tumor. Pichler et al reported similar findings of an inverse correlation between tumor associated macrophages, Tregs and T-bet+ T-cells and disease free survival following BCG therapy [267]. High levels of CD4+ and GATA-3+ T cells were associated with improved disease free survival [267].
The hypothesis that the immune system also plays a role in the response of urothelial carcinoma to systemic chemotherapy is supported by a recent study by Baras et al, in which ratio of CD8+ TILs to CD25+ Tregs was strongly associated with response to platinum-based neoadjuvant chemotherapy [268]. As mentioned above, it appears that in urothelial carcinoma the expression of checkpoint molecule PD-L1 on the cells of the immune infiltrate is more relevant in predicting response to PD-1 and PD-L1 inhibitors than expression on tumor cells [250,251]. Of note is the definition of immune cell positivity used in these clinical trials, scoring the percentage of tumor stroma that is covered by PD-L1 positive immune cells, with a cut-off of 1% [250,251]. Scoring sTILs on H&E as a percentage of tumor area is therefore easily comparable with, and translatable into, immune cell scoring on PD-L1 IHC. The expression of PD-L1 on stromal immune cells correlated with CD8+ T cell infiltration assessed on IHC, which also correlated with response to atezolizumab [250]. Up-regulation of immune checkpoint molecules is linked to the activation of T cells and is dependent on their presence in the tumor microenvironment [269]. As these two parameters are strongly correlated, TIL assessment alone may prove valuable in predicting response to immune checkpoint inhibition, as more novel agents targeting different or multiple checkpoint or stimulatory pathways are developed. An illustrated tutorial for TILs assessment in urothelial carcinoma is available in Supplementary File 6.
Prostate carcinoma
Traditionally, prostate cancer has not been associated with a florid immune response and the potential of prostate cancer to respond to immunotherapy is still questioned [270]. Most reports on TILs in prostate cancer have focused on the prognostic relevance of TILs, with fewer studies investigating the predictive value to drug therapies. Reports on the composition of TILs in prostate cancer are heterogeneous and sometimes conflicting. One study found that TILs in prostate cancer are predominantly CD8+ T-lymphocytes [271], whilst other studies reported opposite findings with predominant populations of CD4+ T lymphocytes and sparse CD8+ T cells [272]. A pronounced presence of CD25+ and FOXP3+ Tregs in the TIL-infiltrate has been reported [272–275]. Another study found that a high proportion of CD8+ TILs in prostate cancer showed expression of PD-1 and had undergone a clonal expansion to an as yet unidentified antigen [276].
In prostate cancer the relationship between TILs and survival is still unclear, although surprisingly, most reports show evidence for a correlation between TILs and poor prognosis. A high TIL infiltrate has been associated with increased risk of recurrence [277–279], metastasis [280], and poor cancer specific survival [281]. Flammiger and co-workers have published the largest cohort on the prognostic effect of TILs in prostate cancer to date and concluded that patients with either a high or very low number of CD3+ lymphocytes in tumor epithelial areas had a shorter time to biochemical recurrence [282]. They did not, however, investigate how the different subsets of T lymphocytes contributed to the clinical outcome. Ness et al showed that the negative prognostic effect may be mediated primarily through CD8+ lymphocytes rather than the overall density of T lymphocytes as measured by CD3 positivity [283], while Davidsson et al attributed the poor prognostic effect to Tregs in the prostate cancer microenvironment [284]. Others have reported a correlation between FOXP3+ TILs in prostate cancer and biochemical recurrence [285], though Vesalainen et al. reported that tumors with dense TILs were associated with higher survival rates than tumors with absent or decreased TILs [286]. In a recent study it was found that higher CD8+ and lower PD-1+ TIL scores correlated to a longer biochemical-recurrence free survival in patients subjected to salvage radiotherapy after biochemical relapse [287]. The contribution of B cells to clinicopathological features of prostate cancer, recurrence and survival is also unclear [278,282,288–290] and requires further investigation. This apparent association between high TILs and poor prognosis in prostate cancer contrasts with most other solid tumors, as discussed in other sections, and requires validation in large cohorts using a standardized methodology.
Androgen deprivation therapy is the mainstay of systemic treatment for prostate cancer, and has been shown to have immunomodulatory effects, triggering an influx of CD4+ and CD8+ TILs [291,292]. Despite the traditional view of prostate cancer as a poorly immunogenic tumor, sipuleucel-T, an autologous dendritic cell vaccine, became the first cancer vaccine to receive FDA approval in 2010 [293]. Following the development of more effective chemotherapy for metastatic castrate resistant prostate cancer, sipuleucel-T currently has a limited role in this setting [294]. Trials of immune checkpoint inhibition in prostate cancer have been largely disappointing [295–297], however a small phase II trial of pembrolizumab in enzalutamide-resistant prostate cancer found tumors with T cell infiltrates and PD-L1 expression may show more promising results [298]. Further exploration of the immune contexture of prostate cancer, its association with prognosis and potential as an immunotherapy biomarker will be of great interest to clinicians and researchers.
Renal cell carcinoma
Prior to the introduction of tyrosine kinase inhibitors with anti-angiogenic actions such as sunitinib, and mTOR inhibitors such as everolimus, immunotherapy was the mainstay of systemic treatment for metastatic renal cell carcinoma [299]. High dose IL-2 therapy could result in durable complete responses but significant toxicity limited its application [299]. Recently, excitement has grown over the potential of immune checkpoint inhibitor therapy, with anti-PD-1 agent nivolumab receiving FDA approval for advanced renal cell carcinoma in 2015 [300]. These clinical successes suggest that the immune system plays an important role in the control or progression of renal cell carcinoma, however the prognostic and predictive value of TILs in this setting remains under investigation.
Early reports showed that TILs in renal cell carcinoma are predominantly T cells and natural killer cells with minor populations of B-cells [301–305]. T lymphocytes in renal cell carcinoma were found to be enriched in functional CD4+ cells of the Th1 lineage and in effector memory CD8+ cells [306]. Additionally, several populations of CD4+ and CD8+ Tregs were identified that may synergize to locally dampen antitumor T cell responses [306–308]. Several studies have investigated the relation between TILs and clinical outcome in renal cell carcinoma. Interestingly, increased TILs, both CD4+ and CD8+ T cells, appear to correlate with tumor recurrence and poor prognosis in renal cell carcinoma [305,309–312]. Potentially of most relevance was the ability of some studies to differentiate between the effector cytotoxic CD8+ T-cells and their exhausted counterparts [309]. When dichotomized in such a way, Giraldo et al were able to clearly demonstrate good prognosis with the former CD8+ T-cell population, and poor prognosis with the latter [313]. Similarly, Nakano et al showed that TILs with high CD8+ T cell content that exhibited high proliferative activity were associated with improved survival among patients with advanced renal cell carcinoma [311]. High numbers of FOXP3+ Tregs, both in the tumor microenvironment and the peripheral blood, have been associated with metastasis, short disease-free survival, and poor prognosis [308,314–317]. The presence of CD4+CD25+FOXP3− Tregs in renal cell carcinoma was also significantly associated with poor outcome [311]. In clear cell renal cell carcinoma, PD-1+ TILs were independent prognostic indicators for overall survival [315,318], however no significant association was found in non-clear cell renal cell carcinoma [319]. Others have reported on the independent prognostic values of concomitant quantification of densities of CD8+, PD-1+, and LAG-3+ lymphocytes in addition to PD-L1/PD-L2 expression by tumor cells [313]. These somewhat confusing results from retrospective observational studies using different techniques require validation and clarification.
Early data suggest that anti-angiogenic targeted therapies in renal cell carcinoma may have immunomodulatory effects. For example, pre-treatment with sunitinib increased the ability of the investigators to expand TILs from the tumor ex-vivo [320]. In a phase 1 clinical trial, the anti-angiogenic drug bevacizumab increased immune cell infiltration and Th1 gene expression when combined with atezolizumab in metastatic renal cell carcinoma [321]. Data on the potential predictive value of TILs in this setting are limited, however in one study, higher intra-tumor CD8+ T cell counts were independently associated with shorter overall survival in patients receiving tyrosine kinase inhibitor therapy [322]. Studies of immune checkpoint inhibitors in renal cell carcinoma have largely focused on PD-L1 expression on tumor cells as a potential predictive biomarker [300,323], however data from other tumor types such as urothelial carcinoma suggest that the immune infiltrate may also have predictive value in this setting and this should be investigated further.
TILs in brain tumors
The central nervous system has long been considered an immune-privileged organ, but this view is being increasingly challenged as it has become clear that the immune system is active and important in many central nervous system disorders, including neoplastic disease. A prognostic role of TIL infiltration has been shown in some small and retrospective studies for gliomas and brain metastases (reviewed by Bienkowski et al [324]). The principles of TIL assessment described for the respective primary tumor types in this review (for example breast cancer, lung cancer, melanoma, etc.) are also likely to be applicable in brain metastases (discussed further in part 1 of this review). However, the histomorphology of most primary brain tumors is unique and distinct from other solid cancers and therefore specific TIL assessment algorithms may need to be developed. Nevertheless, here, we summarize the current knowledge on the most common primary brain tumor types, namely gliomas and meningiomas.
Gliomas
TILs are frequently present in gliomas, although in most cases at relatively low densities [325]. TILs in gliomas are often found in perivascular areas or show perivascular accentuation when infiltrating the tumor tissue. In addition, TILs may be observed in the invasive edge of the tumor, an area much larger and less definable than the invasive margin of epithelial tumors. Immunohistochemical studies in gliomas have identified microglia/macrophages and various lymphocyte subsets including CD8+, CD4+, FOXP3+, and CD45R0+ lymphocytes among others [326–328]. Rutledge et al used a simple H&E based three-tiered scoring system to assess TILs in glioblastomas and identified significant associations with the sarcomatous, gemistocytic, epithelioid, and giant cell histological subtypes, which cluster within the mesenchymal molecular class [327]. A number of studies have addressed the prognostic role of TILs in gliomas, however, the strength of these studies is generally limited by sample size issues, retrospective design, and non-standardized assessment of lymphocytic infiltration. As a result the studies are inconsistent with some showing a prognostic role of certain TIL subsets and others showing no association with patient outcome (reviewed by Bienkowski et al [324]). Adequately designed studies investigating a predictive role of TILs for response to immunotherapies in gliomas are not yet available.
As the methodology of TIL assessment in gliomas varies between studies no clear recommendations on preferred assessment algorithms can be made. Visualization of TIL subsets may require immunohistochemical staining for specific immune cell markers to facilitate clear separation from other cell types of the glioma microenvironment, such as pre-existing or neoplastic small astrocytic or oligodendroglial cells. However, there are no systematic studies investigating the optimal method of TIL enumeration or classification and there is great variability in the techniques used in published studies, including estimation of TIL content by visual impression, manual counting, and computer-assisted evaluation. Another area of uncertainty is the tumor compartment in which TILs should be assessed as areas of interest include perivascular spaces, intratumoral areas, perinecrotic areas, the invasive edge, and, where present, the tumor stroma compartment.
The exact role of immune cells in glioma and their potential as clinically relevant biomarkers informing patient management and treatment decisions also remains unclear. Currently, many immunotherapeutic clinical trials enrolling glioma patients are ongoing and investigation of immune cell infiltration as prognostic or predictive markers should be systematically analyzed in translational companion projects of these studies. An important issue that needs to be addressed in this context is the definition of standardized assessment algorithms of TIL infiltrates for gliomas. Given the specific architecture and histomorphology of gliomas, our proposed guidelines for other tumor types such as carcinomas may not be directly applicable to these tumors.
Meningiomas
Lymphocyte infiltration of variable extent is commonly found in meningiomas. In most cases TILs are not very prominent, but rare cases display striking amounts of intratumoral immune cells, for example, the lymphoplasmacyte-rich meningioma subtype [329]. Some studies have reported higher TIL amounts in atypical and malignant meningiomas as compared with benign meningiomas, while other studies have observed lower TIL numbers with higher meningioma grade [324,325,330]. TILs in meningiomas can be present in the perivascular area or intratumorally, and may include CD8+, CD4+, CD45+, and CD20+ lymphocytes, natural killer cells, and others. The biological and clinical significance of TILs in meningioma is unclear and further study is required. Of note, immunotherapy with immune checkpoint inhibitors of the PD-1/PD-L1 axis has been suggested as a potential therapeutic option in recurrent meningioma, and TIL infiltration may be a candidate biomarker that should be investigated in such studies [331]. However, as for gliomas, the methodology of TIL assessment is non-standardized and needs to be elaborated in systematic investigations.
Discussion
In this review, we have focused on common solid tumors in which data about the potential significance of TILs is available or emerging. The importance of TILs and host-tumor immune interactions is clearly not limited to these tumor types, and further exploration of less common or less well-studied tumors such as cervical carcinoma, sarcomas, and pediatric malignancies is anticipated with interest. As can be seen from the above discussion, approaches to TIL scoring have varied both between and within tumor types. Through the efforts of researchers, clinicians and pathologists, the significance of TILs is gaining increasing recognition. However, a standardized methodology would help to increase the quantity and quality of comparable evidence and enable the implementation of TIL scoring in large-scale clinical trials and routine histopathological practice. The consensus methodology we propose has limitations and many open questions remain, as discussed in part 1 of this review. These methods should be considered a tool for further research, to investigate both the significance of TILs in solid tumors and how we can best assess and describe the host immune response to tumors. They can be used as a reference against which other methods can be tested, and should be thoroughly validated for reproducibility and utility. Significant results have already been demonstrated using this methodology in diverse tumor types including gastric cancer, lung cancer and laryngeal squamous cell carcinoma. However, these recommendations may not be translatable into other tumor types, such as gliomas, for which further work is required to develop a standardized, reproducible and valid methodology. As more evidence becomes available, recommendations will be reviewed, expanded and clarified. It is hoped this proposed standardized methodology will help clinicians, researchers and pathologists to evaluate the utility of TILs assessment with a view to both prognosis and prediction of response to treatment.
Supplementary Material
TILs assessment in melanoma tutorial.
TILs assessment in lung carcinoma tutorial.
TILs assessment in mesothelioma tutorial.
TILs assessment in endometrial carcinoma tutorial.
TILs assessment in ovarian carcinoma tutorial.
TILs assessment in urothelial carcinoma tutorial.
Acknowledgments
Funding sources:
Roberto Salgado is supported by a grant from the Breast Cancer Research Foundation (BRCF).
Federico Rojo is supported by ISCiii/FEDER (CIBERONCO, PI15/00934).
Douglas B. Johnson receives funding from the NIH (NCI K23 CA204726).
Footnotes
The authors have no conflicts of interest to disclose.
No specific funding was obtained for this project.
References
- 1.Salgado R, Denkert C, Demaria S, et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann Oncol. 2015;26:259–271. doi: 10.1093/annonc/mdu450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Denkert C, Wienert S, Poterie A, et al. Standardized evaluation of tumor-infiltrating lymphocytes in breast cancer: results of the ring studies of the international immuno-oncology biomarker working group. Mod Pathol. 2016;29:1155–1164. doi: 10.1038/modpathol.2016.109. [DOI] [PubMed] [Google Scholar]
- 3.McGovern VJ, Mihm MC, Jr, Bailly C, et al. The Classification of Malignant Melanoma and its Histologic Reporting. Cancer. 1973;32:1446–1457. doi: 10.1002/1097-0142(197312)32:6<1446::aid-cncr2820320623>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 4.Elder DE, DuPont G, Van Horn M, et al. The Role of Lymph Node Dissection for Clinical Stage I Malignant Melanoma of Intermediate Thickness (1.51–3. 99 mm) Cancer. 1985;56:413–418. doi: 10.1002/1097-0142(19850715)56:2<413::aid-cncr2820560234>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 5.Clark WH, Jr, Elder DE, DuPont G, et al. Model Predicting Survival in Stage I Melanoma Based on Tumor Progression. J Natl Cancer Inst. 1989;81:1893–1904. doi: 10.1093/jnci/81.24.1893. [DOI] [PubMed] [Google Scholar]
- 6.Dummer R, Hauschild A, Lindenblatt N, et al. Cutaneous melanoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26(Suppl 5):v126–132. doi: 10.1093/annonc/mdv297. [DOI] [PubMed] [Google Scholar]
- 7.Busam KJ, Antonescu CR, Marghoob AA, et al. Histologic Classification of Tumor-Infiltrating Lymphocytes in Primary Cutaneous Malignant Melanoma: A Study of Interobserver Agreement. Am J Clin Pathol. 2001;115:856–860. doi: 10.1309/G6EK-Y6EH-0LGY-6D6P. [DOI] [PubMed] [Google Scholar]
- 8.Clemente CG, Mihm MC, Jr, Bufalino R, et al. Prognostic Value of Tumor Infiltrating Lymphocytes in the Vertical Growth Phase of Primary Cutaneous Melanoma. Cancer. 1996;77:1303–1310. doi: 10.1002/(SICI)1097-0142(19960401)77:7<1303::AID-CNCR12>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 9.Thomas NE, Busam KJ, From L, et al. Tumor-infiltrating lymphocyte grade in primary melanomas is independently associated with melanoma-specific survival in the population-based genes, environment and melanoma study. J Clin Oncol. 2013;31:4252–4259. doi: 10.1200/JCO.2013.51.3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tuthill RJ, Unger JM, Liu PY, et al. Risk Assessment in Localized Primary Cutaneous Melanoma: A Southwest Oncology Group Study Evaluating Nine Factors and a Test of the Clark Logistic Regression Prediction Model. Am J Clin Pathol. 2002;118:504–511. doi: 10.1309/WBF7-N8KH-71KT-RVQ9. [DOI] [PubMed] [Google Scholar]
- 11.Weiss SA, Han SW, Lui K, et al. Immunologic heterogeneity of tumor-infiltrating lymphocyte composition in primary melanoma. Hum Pathol. 2016;57:116–125. doi: 10.1016/j.humpath.2016.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Azimi F, Scolyer RA, Rumcheva P, et al. Tumor-infiltrating lymphocyte grade is an independent predictor of sentinel lymph node status and survival in patients with cutaneous melanoma. J Clin Oncol. 2012;30:2678–2683. doi: 10.1200/JCO.2011.37.8539. [DOI] [PubMed] [Google Scholar]
- 13.Barnhill RL, Fine JA, Roush GC, et al. Predicting Five-Year Outcome for Patients with Cutaneous Melanoma in a Population-Based Study. Cancer. 1996;78:427–432. doi: 10.1002/(SICI)1097-0142(19960801)78:3<427::AID-CNCR8>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 14.Taylor RC, Patel A, Panageas KS, et al. Tumor-infiltrating lymphocytes predict sentinel lymph node positivity in patients with cutaneous melanoma. J Clin Oncol. 2007;25:869–875. doi: 10.1200/JCO.2006.08.9755. [DOI] [PubMed] [Google Scholar]
- 15.Mandala M, Imberti GL, Piazzalunga D, et al. Clinical and histopathological risk factors to predict sentinel lymph node positivity, disease-free and overall survival in clinical stages I-II AJCC skin melanoma: outcome analysis from a single-institution prospectively collected database. Eur J Cancer. 2009;45:2537–2545. doi: 10.1016/j.ejca.2009.05.034. [DOI] [PubMed] [Google Scholar]
- 16.Rao UN, Lee SJ, Luo W, et al. Presence of tumor-infiltrating lymphocytes and a dominant nodule within primary melanoma are prognostic factors for relapse-free survival of patients with thick (t4) primary melanoma: pathologic analysis of the e1690 and e1694 intergroup trials. Am J Clin Pathol. 2010;133:646–653. doi: 10.1309/AJCPTXMEFOVYWDA6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eriksson H, Frohm-Nilsson M, Jaras J, et al. Prognostic factors in localized invasive primary cutaneous malignant melanoma: results of a large population-based study. Br J Dermatol. 2015;172:175–186. doi: 10.1111/bjd.13171. [DOI] [PubMed] [Google Scholar]
- 18.Hillen F, Baeten CI, van de Winkel A, et al. Leukocyte infiltration and tumor cell plasticity are parameters of aggressiveness in primary cutaneous melanoma. Cancer Immunol Immunother. 2008;57:97–106. doi: 10.1007/s00262-007-0353-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martinez-Rodriguez M, Thompson AK, Monteagudo C. A significant percentage of CD20-positive TILs correlates with poor prognosis in patients with primary cutaneous malignant melanoma. Histopathology. 2014;65:726–728. doi: 10.1111/his.12437. [DOI] [PubMed] [Google Scholar]
- 20.van Houdt IS, Sluijter BJ, Moesbergen LM, et al. Favorable outcome in clinically stage II melanoma patients is associated with the presence of activated tumor infiltrating T-lymphocytes and preserved MHC class I antigen expression. Int J Cancer. 2008;123:609–615. doi: 10.1002/ijc.23543. [DOI] [PubMed] [Google Scholar]
- 21.Miracco C, Mourmouras V, Biagioli M, et al. Utility of tumour-infiltrating CD25+FOXP3+ regulatory T cell evaluation in predicting local recurrence in vertical growth phase cutaneous melanoma. Oncology Reports. 2007;18:1115–1122. [PubMed] [Google Scholar]
- 22.Vasaturo A, Di Blasio S, Verweij D, et al. Multispectral imaging for highly accurate analysis of tumour-infiltrating lymphocytes in primary melanoma. Histopathology. 2016 doi: 10.1111/his.13070. [DOI] [PubMed] [Google Scholar]
- 23.Robinson D, Van Allen EM, Wu YM, et al. Integrative clinical genomics of advanced prostate cancer. Cell. 2015;161:1215–1228. doi: 10.1016/j.cell.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pasquali S, Spillane A. Contemporary controversies and perspectives in the staging and treatment of patients with lymph node metastasis from melanoma, especially with regards positive sentinel lymph node biopsy. Cancer Treat Rev. 2014;40:893–899. doi: 10.1016/j.ctrv.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 25.Balch CM, Gershenwald JE, Soong SJ, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27:6199–6206. doi: 10.1200/JCO.2009.23.4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Duprat JP, Brechtbulh ER, Costa de Sa B, et al. Absence of Tumor-Infiltrating Lymphocyte Is a Reproducible Predictive Factor for Sentinel Lymph Node Metastasis: A Multicenter Database Study by the Brazilian Melanoma Group. PLoS One. 2016;11:e0148160. doi: 10.1371/journal.pone.0148160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kruper LL, Spitz FR, Czerniecki BJ, et al. Predicting sentinel node status in AJCC stage I/II primary cutaneous melanoma. Cancer. 2006;107:2436–2445. doi: 10.1002/cncr.22295. [DOI] [PubMed] [Google Scholar]
- 28.Wong SL, Brady MS, Busam KJ, et al. Results of sentinel lymph node biopsy in patients with thin melanoma. Ann Surg Oncol. 2006;13:302–309. doi: 10.1245/ASO.2006.02.021. [DOI] [PubMed] [Google Scholar]
- 29.Mihm MC, Jr, Clemente CG, Cascinelli N. Tumor Infiltrating Lymphocytes in Lymph Node Melanoma Metastases: A Histopathologic Prognostic Indicator and an Expression of Local Immune Response. Laboratory Investigation. 1996;74:43–47. [PubMed] [Google Scholar]
- 30.Bogunovic D, O’Neill DW, Belitskaya-Levy I, et al. Immune profile and mitotic index of metastatic melanoma lesions enhance clinical staging in predicting patient survival. Proc Natl Acad Sci U S A. 2009;106:20429–20434. doi: 10.1073/pnas.0905139106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kakavand H, Vilain RE, Wilmott JS, et al. Tumor PD-L1 expression, immune cell correlates and PD-1+ lymphocytes in sentinel lymph node melanoma metastases. Mod Pathol. 2015;28:1535–1544. doi: 10.1038/modpathol.2015.110. [DOI] [PubMed] [Google Scholar]
- 32.Cancer Genome Atlas N. Genomic Classification of Cutaneous Melanoma. Cell. 2015;161:1681–1696. doi: 10.1016/j.cell.2015.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bremnes RM, Donnem T, Al-Saad S, et al. The Role of Tumor Stroma in Cancer Progression and Prognosis; Emphasis on Carcinoma-associated Fibroblasts and Non-Small Cell Lung Cancer. J Thorac Oncol. 2011;6:209–217. doi: 10.1097/JTO.0b013e3181f8a1bd. [DOI] [PubMed] [Google Scholar]
- 34.Ruiter D, Bogenrieder T, Elder D, et al. Melanoma–stroma interactions: structural and functional aspects. Lancet Oncol. 2002;3:35–43. doi: 10.1016/s1470-2045(01)00620-9. [DOI] [PubMed] [Google Scholar]
- 35.Ladanyi A, Somlai B, Gilde K, et al. T-Cell Activation Marker Expression on Tumor-Infiltrating Lymphocytes As Prognostic Factor in Cutaneous Malignant Melanoma. Clin Cancer Res. 2004;10:521–530. doi: 10.1158/1078-0432.ccr-1161-03. [DOI] [PubMed] [Google Scholar]
- 36.Ladanyi A, Kiss J, Somlai B, et al. Density of DC-LAMP(+) mature dendritic cells in combination with activated T lymphocytes infiltrating primary cutaneous melanoma is a strong independent prognostic factor. Cancer Immunol Immunother. 2007;56:1459–1469. doi: 10.1007/s00262-007-0286-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ladanyi A, Kiss J, Mohos A, et al. Prognostic impact of B-cell density in cutaneous melanoma. Cancer Immunol Immunother. 2011;60:1729–1738. doi: 10.1007/s00262-011-1071-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tumeh PC, Harview CL, Yearley JH, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515:568–571. doi: 10.1038/nature13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mlecnik B, Bindea G, Kirilovsky A, et al. The tumor microenvironment and Immunoscore are critical determinants of dissemination to distant metastasis. Sci Transl Med. 2015;8:327ra326. doi: 10.1126/scitranslmed.aad6352. [DOI] [PubMed] [Google Scholar]
- 40.MacCarty WC. Principles of prognosis in cancer. Journal of the American Medical Association. 1931;96:30–33. [Google Scholar]
- 41.Galon J, Mlecnik B, Bindea G, et al. Towards the introduction of the ‘Immunoscore’ in the classification of malignant tumours. J Pathol. 2014;232:199–209. doi: 10.1002/path.4287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nowak JA, Hornick JL. Molecular Evaluation of Colorectal Adenocarcinoma: Current Practice and Emerging Concepts. Surg Pathol Clin. 2016;9:427–439. doi: 10.1016/j.path.2016.04.007. [DOI] [PubMed] [Google Scholar]
- 43.Ogino S, Goel A. Molecular classification and correlates in colorectal cancer. J Mol Diagn. 2008;10:13–27. doi: 10.2353/jmoldx.2008.070082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Asad Umar C, Boland R, Terdiman JP, et al. Revised Bethesda Guidelines for Hereditary Nonpolyposis Colorectal Cancer (Lynch Syndrome) and Microsatellite Instability. J Natl Cancer Inst. 2004;96:261–268. doi: 10.1093/jnci/djh034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Alexander J, Watanabe T, Wu T, et al. Histopathological Identification of Colon Cancer with Microsatellite Instability. Am J Pathol. 2001;158:527–535. doi: 10.1016/S0002-9440(10)63994-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Greenson JK, Bonner JD, Ben-Yzhak O, et al. Phenotype of Microsatellite Unstable Colorectal Carcinomas: Well-differentiated and Focally Mucinous Tumors and the Absence of Dirty Necrosis Correlate with Microsatellite Instability. Am J Surg Pathol. 2003;27:563–570. doi: 10.1097/00000478-200305000-00001. [DOI] [PubMed] [Google Scholar]
- 47.Smyrk TC, Watson P, Kaul K, et al. Tumor-Infiltrating Lymphocytes Are a Marker for Microsatellite Instability in Colorectal Carcinoma. Cancer. 2001;91:2417–2422. [PubMed] [Google Scholar]
- 48.Jenkins MA, Hayashi S, O’Shea AM, et al. Pathology features in Bethesda guidelines predict colorectal cancer microsatellite instability: a population-based study. Gastroenterology. 2007;133:48–56. doi: 10.1053/j.gastro.2007.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Joost P, Bendahl P, Halvarsson B, et al. Efficient and reproducible identification of mismatch repair deficient colon cancer: validation of the MMR index and comparison with other predictive models. BMC Clinical Pathology. 2013;13:33. doi: 10.1186/1472-6890-13-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Halvarsson B, Anderson H, Domanska K, et al. Clinicopathologic factors identify sporadic mismatch repair-defective colon cancers. Am J Clin Pathol. 2008;129:238–244. doi: 10.1309/0PP5GDRTXUDVKAWJ. [DOI] [PubMed] [Google Scholar]
- 51.Roman R, Verdu M, Calvo M, et al. Microsatellite instability of the colorectal carcinoma can be predicted in the conventional pathologic examination. A prospective multicentric study and the statistical analysis of 615 cases consolidate our previously proposed logistic regression model. Virchows Arch. 2010;456:533–541. doi: 10.1007/s00428-010-0896-6. [DOI] [PubMed] [Google Scholar]
- 52.Hyde A, Fontaine D, Stuckless S, et al. A Histology-Based Model for Predicting Microsatellite Instability in Colorectal Cancers. Am J Surg Pathol. 2010;34:1820–1829. doi: 10.1097/PAS.0b013e3181f6a912. [DOI] [PubMed] [Google Scholar]
- 53.Network NCC. [Accessed January 19, 2017];Genetic/Familial High-Risk Assessment: Colorectal (Version 2.2016) Available from: https://www.nccn.org/professionals/physician_gls/pdf/genetics_colon.pdf.
- 54.Sinicrope FA, Foster NR, Thibodeau SN, et al. DNA mismatch repair status and colon cancer recurrence and survival in clinical trials of 5-fluorouracil-based adjuvant therapy. J Natl Cancer Inst. 2011;103:863–875. doi: 10.1093/jnci/djr153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Popat S, Hubner R, Houlston RS. Systematic review of microsatellite instability and colorectal cancer prognosis. J Clin Oncol. 2005;23:609–618. doi: 10.1200/JCO.2005.01.086. [DOI] [PubMed] [Google Scholar]
- 56.Guastadisegni C, Colafranceschi M, Ottini L, et al. Microsatellite instability as a marker of prognosis and response to therapy: a meta-analysis of colorectal cancer survival data. Eur J Cancer. 2010;46:2788–2798. doi: 10.1016/j.ejca.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 57.Lindor NM, Burgart LJ, Leontovich O, et al. Immunohistochemistry Versus Microsatellite Instability Testing in Phenotyping Colorectal Tumors. J Clin Oncol. 2002;20:1043–1048. doi: 10.1200/JCO.2002.20.4.1043. [DOI] [PubMed] [Google Scholar]
- 58.Shia J, Tang LH, Vakiani E, et al. Immunohistochemistry as First-line Screening for Detecting Colorectal Cancer Patients at Risk for Hereditary Nonpolyposis Colorectal Cancer Syndrome: A 2-antibody Panel May be as Predictive as a 4-antibody Panel. Am J Surg Pathol. 2009;33:1639–1645. doi: 10.1097/PAS.0b013e3181b15aa2. [DOI] [PubMed] [Google Scholar]
- 59.Glaire MA, Domingo E, Vermeulen L, et al. POLE proofreading domain mutation defines a subset of immunogenic colorectal cancers with excellent prognosis. Ann Oncol. 2016;27:460O. [Google Scholar]
- 60.Rozek LS, Schmit SL, Greenson JK, et al. Tumor-Infiltrating Lymphocytes, Crohn’s-Like Lymphoid Reaction, and Survival From Colorectal Cancer. J Natl Cancer Inst. 2016:108. doi: 10.1093/jnci/djw027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jass JR. Lymphocytic infiltration and survival in rectal cancer. J Clin Pathol. 1986;39:585–589. doi: 10.1136/jcp.39.6.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Klintrup K, Makinen JM, Kauppila S, et al. Inflammation and prognosis in colorectal cancer. Eur J Cancer. 2005;41:2645–2654. doi: 10.1016/j.ejca.2005.07.017. [DOI] [PubMed] [Google Scholar]
- 63.Huh JW, Lee JH, Kim HR. Prognostic Significance of Tumor-Infiltrating Lymphocytes for Patients With Colorectal Cancer. Archives of Surgery. 2012;147:366–371. doi: 10.1001/archsurg.2012.35. [DOI] [PubMed] [Google Scholar]
- 64.Richards CH, Flegg KM, Roxburgh CS, et al. The relationships between cellular components of the peritumoural inflammatory response, clinicopathological characteristics and survival in patients with primary operable colorectal cancer. Br J Cancer. 2012;106:2010–2015. doi: 10.1038/bjc.2012.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Richards CH, Roxburgh CS, Powell AG, et al. The clinical utility of the local inflammatory response in colorectal cancer. Eur J Cancer. 2014;50:309–319. doi: 10.1016/j.ejca.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 66.Vayrynen JP, Vornanen JO, Sajanti S, et al. An improved image analysis method for cell counting lends credibility to the prognostic significance of T cells in colorectal cancer. Virchows Arch. 2012;460:455–465. doi: 10.1007/s00428-012-1232-0. [DOI] [PubMed] [Google Scholar]
- 67.Park JH, McMillan DC, Edwards J, et al. Comparison of the prognostic value of measures of the tumor inflammatory cell infiltrate and tumor-associated stroma in patients with primary operable colorectal cancer. OncoImmunology. 2016;5:e1098801. doi: 10.1080/2162402X.2015.1098801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Galon J, Pages F, Marincola FM, et al. Cancer classification using the Immunoscore: a worldwide task force. J Transl Med. 2012;10:205. doi: 10.1186/1479-5876-10-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Broussard EK, Disis ML. TNM Staging in Colorectal Cancer: T is for T Cell and M is for Memory. J Clin Oncol. 2011;29:601–603. doi: 10.1200/JCO.2010.32.9078. [DOI] [PubMed] [Google Scholar]
- 70.Pages F, Berger A, Camus M, et al. Effector Memory T Cells, Early Metastasis, and Survival in Colorectal Cancer. N Engl J Med. 2005;353:2654–2666. doi: 10.1056/NEJMoa051424. [DOI] [PubMed] [Google Scholar]
- 71.Galon J, Costes A, Sanchez-Cabo F, et al. Type, Density and Location of Immune Cells Within Human Colorectal Tumors Predict Clinical Outcome. Science. 2006;313:1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 72.Pages F, Kirilovsky A, Mlecnik B, et al. In situ cytotoxic and memory T cells predict outcome in patients with early-stage colorectal cancer. J Clin Oncol. 2009;27:5944–5951. doi: 10.1200/JCO.2008.19.6147. [DOI] [PubMed] [Google Scholar]
- 73.Mlecnik B, Tosolini M, Kirilovsky A, et al. Histopathologic-based prognostic factors of colorectal cancers are associated with the state of the local immune reaction. J Clin Oncol. 2011;29:610–618. doi: 10.1200/JCO.2010.30.5425. [DOI] [PubMed] [Google Scholar]
- 74.Mlecnik B, Bindea G, Angell HK, et al. Integrative Analyses of Colorectal Cancer Show Immunoscore Is a Stronger Predictor of Patient Survival Than Microsatellite Instability. Immunity. 2016;44:698–711. doi: 10.1016/j.immuni.2016.02.025. [DOI] [PubMed] [Google Scholar]
- 75.Galon J, Mlecnik B, Marliot F, et al. Validation of the Immunoscore (IM) as a prognostic marker in stage I/II/III colon cancer: Results of a worldwide consortium-based analysis of 1,336 patients. J Clin Oncol. 2016;34(suppl) Abstract 3500. [Google Scholar]
- 76.Hermitte F. Biomarkers immune monitoring technology primer: Immunoscore(R) Colon. J Immunother Cancer. 2016;4:57. doi: 10.1186/s40425-016-0161-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mei Z, Liu Y, Liu C, et al. Tumour-infiltrating inflammation and prognosis in colorectal cancer: systematic review and meta-analysis. Br J Cancer. 2014;110:1595–1605. doi: 10.1038/bjc.2014.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Anitei MG, Zeitoun G, Mlecnik B, et al. Prognostic and predictive values of the immunoscore in patients with rectal cancer. Clin Cancer Res. 2014;20:1891–1899. doi: 10.1158/1078-0432.CCR-13-2830. [DOI] [PubMed] [Google Scholar]
- 79.Teng F, Mu D, Meng X, et al. Tumor infiltrating lymphocytes (TILs) before and after neoadjuvant chemoradiotherapy and its clinical utility for rectal cancer. Am J Cancer Res. 2015;5:2064–2074. [PMC free article] [PubMed] [Google Scholar]
- 80.Teng F, Meng X, Kong L, et al. Tumor-infiltrating lymphocytes, forkhead box P3, programmed death ligand-1, and cytotoxic T lymphocyte-associated antigen-4 expressions before and after neoadjuvant chemoradiation in rectal cancer. Transl Res. 2015;166:721–732. e721. doi: 10.1016/j.trsl.2015.06.019. [DOI] [PubMed] [Google Scholar]
- 81.Yasuda K, Nirei T, Sunami E, et al. Density of CD4(+) and CD8(+) T lymphocytes in biopsy samples can be a predictor of pathological response to chemoradiotherapy (CRT) for rectal cancer. Radiation Oncology. 2011;6:49. doi: 10.1186/1748-717X-6-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.McCoy MJ, Hemmings C, Miller TJ, et al. Low stromal Foxp3+ regulatory T-cell density is associated with complete response to neoadjuvant chemoradiotherapy in rectal cancer. Br J Cancer. 2015;113:1677–1686. doi: 10.1038/bjc.2015.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shinto E, Hase K, Hashiguchi Y, et al. CD8+ and FOXP3+ tumor-infiltrating T cells before and after chemoradiotherapy for rectal cancer. Ann Surg Oncol. 2014;21(Suppl 3):S414–421. doi: 10.1245/s10434-014-3584-y. [DOI] [PubMed] [Google Scholar]
- 84.Torre LA, Bray F, Siegel RL, et al. Global cancer statistics 2012. CA: A Cancer Journal for Clinicians. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 85.Kunk PR, Bauer TW, Slingluff CL, et al. From bench to bedside a comprehensive review of pancreatic cancer immunotherapy. J Immunother Cancer. 2016;4:14. doi: 10.1186/s40425-016-0119-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.El-Khoueiry AB, Sangro B, Yau TC, et al. Phase I/II safety and antitumor activity of nivolumab (nivo) in patients (pts) with advanced hepatocellular carcinoma (HCC): Interim analysis of the CheckMate-040 dose escalation study. J Clin Oncol. 2016;34 Abstr 4012. [Google Scholar]
- 87.Wang M, Busuttil RA, Pattison S, et al. Immunological battlefield in gastric cancer and role of immunotherapies. World J Gastroenterol. 2016;22:6373–6384. doi: 10.3748/wjg.v22.i28.6373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bosman FT, Carneiro F, Hruban RH, et al. WHO Classification of Tumours of the Digestive System. 4. IARC; Lyon: 2010. [Google Scholar]
- 89.Song HJ, Srivastava A, Lee J, et al. Host inflammatory response predicts survival of patients with Epstein-Barr virus-associated gastric carcinoma. Gastroenterology. 2010;139:84–92. e82. doi: 10.1053/j.gastro.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 90.Kang BW, Seo AN, Yoon S, et al. Prognostic value of tumor-infiltrating lymphocytes in Epstein-Barr virus-associated gastric cancer. Ann Oncol. 2016;27:494–501. doi: 10.1093/annonc/mdv610. [DOI] [PubMed] [Google Scholar]
- 91.Giampieri R, Maccaroni E, Mandolesi A, et al. Mismatch repair deficiency may affect clinical outcome through immune response activation in metastatic gastric cancer patients receiving first-line chemotherapy. Gastric Cancer. 2017;20:156–163. doi: 10.1007/s10120-016-0594-4. [DOI] [PubMed] [Google Scholar]
- 92.Lee HE, Chae SW, Lee YJ, et al. Prognostic implications of type and density of tumour-infiltrating lymphocytes in gastric cancer. Br J Cancer. 2008;99:1704–1711. doi: 10.1038/sj.bjc.6604738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chiaravalli AM, Feltri M, Bertolini V, et al. Intratumour T cells, their activation status and survival in gastric carcinomas characterised for microsatellite instability and Epstein–Barr virus infection. Virchows Arch. 2005;448:344–353. doi: 10.1007/s00428-005-0066-4. [DOI] [PubMed] [Google Scholar]
- 94.Hennequin A, Derangere V, Boidot R, et al. Tumor infiltration by Tbet+ effector T cells and CD20+ B cells is associated with survival in gastric cancer patients. Oncoimmunology. 2015;5:e1054598. doi: 10.1080/2162402X.2015.1054598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Li K, Zhu Z, Luo J, et al. Impact of chemokine receptor CXCR3 on tumor-infiltrating lymphocyte recruitment associated with favorable prognosis in advanced gastric cancer. Int J Clin Exp Pathol. 2015;8:14725–14732. [PMC free article] [PubMed] [Google Scholar]
- 96.Haas M, Dimmler A, Hohenberger W, et al. Stromal regulatory T-cells are associated with a favourable prognosis in gastric cancer of the cardia. BMC Gastroenterology. 2009;9:65. doi: 10.1186/1471-230X-9-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kim K-J, Lee KS, Cho HJ, et al. Prognostic implications of tumor-infiltrating FoxP3+ regulatory T cells and CD8+ cytotoxic T cells in microsatellite-unstable gastric cancers. Hum Pathol. 2014;45:285–293. doi: 10.1016/j.humpath.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 98.Liu K, Yang K, Wu B, et al. Tumor-Infiltrating Immune Cells Are Associated With Prognosis of Gastric Cancer. Medicine (Baltimore) 2015;94:e1631. doi: 10.1097/MD.0000000000001631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhou S, Xu S, Tao H, et al. CCR7 Expression and Intratumoral FOXP3+ Regulatory T Cells are Correlated with Overall Survival and Lymph Node Metastasis in Gastric Cancer. PLoS ONE. 2013;8:e74430–74437. doi: 10.1371/journal.pone.0074430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Thompson ED, Zahurak M, Murphy A, et al. Patterns of PD-L1 expression and CD8 T cell infiltration in gastric adenocarcinomas and associated immune stroma. Gut. 2016 doi: 10.1136/gutjnl-2015-310839. Epub: gutjnl-2015–310839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Solinas C, Pusole G, Demurtas L, et al. Tumor infiltrating lymphocytes in gastrointestinal tumors: Controversies and future clinical implications. Crit Rev Oncol Hematol. 2017;110:106–116. doi: 10.1016/j.critrevonc.2016.11.016. [DOI] [PubMed] [Google Scholar]
- 102.Le DT, Uram JN, Wang H, et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med. 2015;372:2509–2520. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cancer Genome Atlas Research N. Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513:202–209. doi: 10.1038/nature13480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rooney MS, Shukla SA, Wu CJ, et al. Molecular and genetic properties of tumors associated with local immune cytolytic activity. Cell. 2015;160:48–61. doi: 10.1016/j.cell.2014.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Dai C, Geng R, Wang C, et al. Concordance of immune checkpoints within tumor immune contexture and their prognostic significance in gastric cancer. Mol Oncol. 2016;10:1551–1558. doi: 10.1016/j.molonc.2016.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kawazoe A, Kuwata T, Kuboki Y, et al. Clinicopathological features of programmed death ligand 1 expression with tumor-infiltrating lymphocyte, mismatch repair, and Epstein-Barr virus status in a large cohort of gastric cancer patients. Gastric Cancer. 2016 doi: 10.1007/s10120-10016-10631-10123. Epub. [DOI] [PubMed] [Google Scholar]
- 107.Erkan M, Hausmann S, Michalski CW, et al. The role of stroma in pancreatic cancer: diagnostic and therapeutic implications. Nat Rev Gastroenterol Hepatol. 2012;9:454–467. doi: 10.1038/nrgastro.2012.115. [DOI] [PubMed] [Google Scholar]
- 108.Nielsen MFB, Mortensen MB, Detlefsen S. Key players in pancreatic cancer-stroma interaction: Cancer-associated fibroblasts, endothelial and inflammatory cells. World J Gastroenterol. 2016;22:2678–2700. doi: 10.3748/wjg.v22.i9.2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bailey P, Chang DK, Nones K, et al. Genomic analyses identify molecular subtypes of pancreatic cancer. Nature. 2016;531:47–52. doi: 10.1038/nature16965. [DOI] [PubMed] [Google Scholar]
- 110.Hiraoka N, Ino Y, Yamazaki-Itoh R, et al. Intratumoral tertiary lymphoid organ is a favourable prognosticator in patients with pancreatic cancer. Br J Cancer. 2015;112:1782–1790. doi: 10.1038/bjc.2015.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hart PA, Smyrk TC, Bamlet WR, et al. Impact of Intratumoral Inflammation on Survival After Pancreatic Cancer Resection. Pancreas. 2016;45:123–126. doi: 10.1097/MPA.0000000000000423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tang Y, Xu X, Guo S, et al. An Increased Abundance of Tumor-Infiltrating Regulatory T Cells Is Correlated with the Progression and Prognosis of Pancreatic Ductal Adenocarcinoma. PLoS ONE. 2014;9:e91551–91559. doi: 10.1371/journal.pone.0091551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ino Y, Yamazaki-Itoh R, Shimada K, et al. Immune cell infiltration as an indicator of the immune microenvironment of pancreatic cancer. Br J Cancer. 2013;108:914–923. doi: 10.1038/bjc.2013.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Fukunaga A, Miyamoto M, Cho Y, et al. CD8+ tumor-infiltrating lymphocytes together with CD4+ tumor-infiltrating lymphocytes and dendritic cells improve the prognosis of patients with pancreatic adenocarcinoma. Pancreas. 2004;28:e26–31. doi: 10.1097/00006676-200401000-00023. [DOI] [PubMed] [Google Scholar]
- 115.Tewari N, Zaitoun AM, Arora A, et al. The presence of tumour-associated lymphocytes confers a good prognosis in pancreatic ductal adenocarcinoma: an immunohistochemical study of tissue microarrays. BMC Cancer. 2013;13:436. doi: 10.1186/1471-2407-13-436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Harding JJ, El Dika I, Abou-Alfa GK. Immunotherapy in hepatocellular carcinoma: Primed to make a difference? Cancer. 2016;122:367–377. doi: 10.1002/cncr.29769. [DOI] [PubMed] [Google Scholar]
- 117.Wada Y, Nakashima O, Kutami R, et al. Clinicopathological study on hepatocellular carcinoma with lymphocytic infiltration. Hepatology. 1998;27:407–414. doi: 10.1002/hep.510270214. [DOI] [PubMed] [Google Scholar]
- 118.Gao Q, Qiu SJ, Fan J, et al. Intratumoral Balance of Regulatory and Cytotoxic T Cells Is Associated With Prognosis of Hepatocellular Carcinoma After Resection. J Clin Oncol. 2007;25:2586–2593. doi: 10.1200/JCO.2006.09.4565. [DOI] [PubMed] [Google Scholar]
- 119.Gabrielson A, Wu Y, Wang H, et al. Intratumoral CD3 and CD8 T-cell Densities Associated with Relapse-Free Survival in HCC. Cancer Immunol Res. 2016;4:419–430. doi: 10.1158/2326-6066.CIR-15-0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Garnelo M, Tan A, Her Z, et al. Interaction between tumour-infiltrating B cells and T cells controls the progression of hepatocellular carcinoma. Gut. 2017;66:342–351. doi: 10.1136/gutjnl-2015-310814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Remark R, Becker C, Gomez JE, et al. The non-small cell lung cancer immune contexture. A major determinant of tumor characteristics and patient outcome. Am J Respir Crit Care Med. 2015;191:377–390. doi: 10.1164/rccm.201409-1671PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Carbone DP, Gandara DR, Antonia SJ, et al. Non-Small-Cell Lung Cancer: Role of the Immune System and Potential for Immunotherapy. J Thorac Oncol. 2015;10:974–984. doi: 10.1097/JTO.0000000000000551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Donnem T, Kilvaer TK, Andersen S, et al. Strategies for clinical implementation of TNM-Immunoscore in resected nonsmall-cell lung cancer. Ann Oncol. 2016;27:225–232. doi: 10.1093/annonc/mdv560. [DOI] [PubMed] [Google Scholar]
- 124.Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med. 2015;373:1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Brahmer JR, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non–Small-Cell Lung Cancer. N Engl J Med. 2015;373:123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Fehrenbacher L, Spira A, Ballinger M, et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. The Lancet. 2016;387:1837–1846. doi: 10.1016/S0140-6736(16)00587-0. [DOI] [PubMed] [Google Scholar]
- 127.Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372:2018–2028. doi: 10.1056/NEJMoa1501824. [DOI] [PubMed] [Google Scholar]
- 128.Rizvi NA, Mazières J, Planchard D, et al. Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a phase 2, single-arm trial. The Lancet Oncology. 2015;16:257–265. doi: 10.1016/S1470-2045(15)70054-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Geng Y, Shao Y, He W, et al. Prognostic Role of Tumor-Infiltrating Lymphocytes in Lung Cancer: a Meta-Analysis. Cell Physiol Biochem. 2015;37:1560–1571. doi: 10.1159/000438523. [DOI] [PubMed] [Google Scholar]
- 130.Horne ZD, Jack R, Gray ZT, et al. Increased levels of tumor-infiltrating lymphocytes are associated with improved recurrence-free survival in stage 1A non-small-cell lung cancer. J Surg Res. 2011;171:1–5. doi: 10.1016/j.jss.2011.03.068. [DOI] [PubMed] [Google Scholar]
- 131.Kilic A, Landreneau RJ, Luketich JD, et al. Density of tumor-infiltrating lymphocytes correlates with disease recurrence and survival in patients with large non-small-cell lung cancer tumors. J Surg Res. 2011;167:207–210. doi: 10.1016/j.jss.2009.08.029. [DOI] [PubMed] [Google Scholar]
- 132.Schalper KA, Brown J, Carvajal-Hausdorf D, et al. Objective measurement and clinical significance of TILs in non-small cell lung cancer. J Natl Cancer Inst. 2015:107. doi: 10.1093/jnci/dju435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zeng D, Yu Y, Ou Q, et al. Prognostic and predictive value of tumor-infiltrating lymphocytes for clinical therapeutic research in patients with non-small cell lung cancer. Oncotarget. 2016;7:13765–13781. doi: 10.18632/oncotarget.7282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Al-Shibli KI, Donnem T, Al-Saad S, et al. Prognostic effect of epithelial and stromal lymphocyte infiltration in non-small cell lung cancer. Clin Cancer Res. 2008;14:5220–5227. doi: 10.1158/1078-0432.CCR-08-0133. [DOI] [PubMed] [Google Scholar]
- 135.Ameratunga M, Asadi K, Lin X, et al. PD-L1 and Tumor Infiltrating Lymphocytes as Prognostic Markers in Resected NSCLC. PLoS One. 2016;11:e0153954. doi: 10.1371/journal.pone.0153954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Al-Shibli K, Al-Saad S, Andersen S, et al. The prognostic value of intraepithelial and stromal CD3-, CD117- and CD138-positive cells in non-small cell lung carcinoma. APMIS. 2010;118:371–382. doi: 10.1111/j.1600-0463.2010.02609.x. [DOI] [PubMed] [Google Scholar]
- 137.Al-Shibli K, Al-Saad S, Donnem T, et al. The prognostic value of intraepithelial and stromal innate immune system cells in non-small cell lung carcinoma. Histopathology. 2009;55:301–312. doi: 10.1111/j.1365-2559.2009.03379.x. [DOI] [PubMed] [Google Scholar]
- 138.Donnem T, Hald SM, Paulsen EE, et al. Stromal CD8+ T-cell Density-A Promising Supplement to TNM Staging in Non-Small Cell Lung Cancer. Clin Cancer Res. 2015;21:2635–2643. doi: 10.1158/1078-0432.CCR-14-1905. [DOI] [PubMed] [Google Scholar]
- 139.Paulsen EE, Kilvaer T, Khanehkenari MR, et al. CD45RO(+) Memory T Lymphocytes--a Candidate Marker for TNM-Immunoscore in Squamous Non-Small Cell Lung Cancer. Neoplasia. 2015;17:839–848. doi: 10.1016/j.neo.2015.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Pelletier MP, Edwardes MD, Michel RP, et al. Prognostic markers in resectable non-small cell lung cancer: a multivariate analysis. Canadian Journal of Surgery. 2001;44:180–188. [PMC free article] [PubMed] [Google Scholar]
- 141.Petersen RP, Campa MJ, Sperlazza J, et al. Tumor infiltrating Foxp3+ regulatory T-cells are associated with recurrence in pathologic stage I NSCLC patients. Cancer. 2006;107:2866–2872. doi: 10.1002/cncr.22282. [DOI] [PubMed] [Google Scholar]
- 142.Ruffini E, Asioli S, Filosso PL, et al. Clinical significance of tumor-infiltrating lymphocytes in lung neoplasms. Ann Thorac Surg. 2009;87:365–371. doi: 10.1016/j.athoracsur.2008.10.067. discussion 371–362. [DOI] [PubMed] [Google Scholar]
- 143.Suzuki K, Kadota K, Sima CS, et al. Clinical impact of immune microenvironment in stage I lung adenocarcinoma: tumor interleukin-12 receptor beta2 (IL-12Rbeta2), IL-7R, and stromal FoxP3/CD3 ratio are independent predictors of recurrence. J Clin Oncol. 2013;31:490–498. doi: 10.1200/JCO.2012.45.2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Tokito T, Azuma K, Kawahara A, et al. Predictive relevance of PD-L1 expression combined with CD8+ TIL density in stage III non-small cell lung cancer patients receiving concurrent chemoradiotherapy. Eur J Cancer. 2016;55:7–14. doi: 10.1016/j.ejca.2015.11.020. [DOI] [PubMed] [Google Scholar]
- 145.Alifano M, Mansuet-Lupo A, Lococo F, et al. Systemic inflammation, nutritional status and tumor immune microenvironment determine outcome of resected non-small cell lung cancer. PLoS One. 2014;9:e106914. doi: 10.1371/journal.pone.0106914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Carus A, Ladekarl M, Hager H, et al. Tumor-associated neutrophils and macrophages in non-small cell lung cancer: no immediate impact on patient outcome. Lung Cancer. 2013;81:130–137. doi: 10.1016/j.lungcan.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 147.Goc J, Germain C, Vo-Bourgais TK, et al. Dendritic cells in tumor-associated tertiary lymphoid structures signal a Th1 cytotoxic immune contexture and license the positive prognostic value of infiltrating CD8+ T cells. Cancer Res. 2014;74:705–715. doi: 10.1158/0008-5472.CAN-13-1342. [DOI] [PubMed] [Google Scholar]
- 148.Ilie M, Hofman V, Ortholan C, et al. Predictive clinical outcome of the intratumoral CD66b-positive neutrophil-to-CD8-positive T-cell ratio in patients with resectable nonsmall cell lung cancer. Cancer. 2012;118:1726–1737. doi: 10.1002/cncr.26456. [DOI] [PubMed] [Google Scholar]
- 149.Kim MY, Koh J, Kim S, et al. Clinicopathological analysis of PD-L1 and PD-L2 expression in pulmonary squamous cell carcinoma: Comparison with tumor-infiltrating T cells and the status of oncogenic drivers. Lung Cancer. 2015;88:24–33. doi: 10.1016/j.lungcan.2015.01.016. [DOI] [PubMed] [Google Scholar]
- 150.Kinoshita T, Muramatsu R, Fujita T, et al. Prognostic value of tumor-infiltrating lymphocytes differs depending on histological type and smoking habit in completely resected non-small-cell lung cancer. Ann Oncol. 2016;27:2117–2123. doi: 10.1093/annonc/mdw319. [DOI] [PubMed] [Google Scholar]
- 151.Djenidi F, Adam J, Goubar A, et al. CD8+CD103+ tumor-infiltrating lymphocytes are tumor-specific tissue-resident memory T cells and a prognostic factor for survival in lung cancer patients. J Immunol. 2015;194:3475–3486. doi: 10.4049/jimmunol.1402711. [DOI] [PubMed] [Google Scholar]
- 152.Hiraoka K, Miyamoto M, Cho Y, et al. Concurrent infiltration by CD8+ T cells and CD4+ T cells is a favourable prognostic factor in non-small-cell lung carcinoma. Br J Cancer. 2006;94:275–280. doi: 10.1038/sj.bjc.6602934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kawai O, Ishii G, Kubota K, et al. Predominant infiltration of macrophages and CD8(+) T Cells in cancer nests is a significant predictor of survival in stage IV nonsmall cell lung cancer. Cancer. 2008;113:1387–1395. doi: 10.1002/cncr.23712. [DOI] [PubMed] [Google Scholar]
- 154.Kayser G, Schulte-Uentrop L, Sienel W, et al. Stromal CD4/CD25 positive T-cells are a strong and independent prognostic factor in non-small cell lung cancer patients, especially with adenocarcinomas. Lung Cancer. 2012;76:445–451. doi: 10.1016/j.lungcan.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 155.Liu H, Zhang T, Ye J, et al. Tumor-infiltrating lymphocytes predict response to chemotherapy in patients with advance non-small cell lung cancer. Cancer Immunol Immunother. 2012;61:1849–1856. doi: 10.1007/s00262-012-1231-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Tao H, Mimura Y, Aoe K, et al. Prognostic potential of FOXP3 expression in non-small cell lung cancer cells combined with tumor-infiltrating regulatory T cells. Lung Cancer. 2012;75:95–101. doi: 10.1016/j.lungcan.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 157.Wakabayashi O, Yamazaki K, Oizumi S, et al. CD4+ T cells in cancer stroma, not CD8+ T cells in cancer cell nests, are associated with favorable prognosis in human non-small cell lung cancers. Cancer Sci. 2003;94:1003–1009. doi: 10.1111/j.1349-7006.2003.tb01392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Brambilla E, Le Teuff G, Marguet S, et al. Prognostic Effect of Tumor Lymphocytic Infiltration in Resectable Non-Small-Cell Lung Cancer. J Clin Oncol. 2016;34:1223–1230. doi: 10.1200/JCO.2015.63.0970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Feng W, Li Y, Shen L, et al. Prognostic value of tumor-infiltrating lymphocytes for patients with completely resected stage IIIA(N2) non-small cell lung cancer. Oncotarget. 2016;7:7227–7240. doi: 10.18632/oncotarget.6979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Johnson SK, Kerr KM, Chapman AD, et al. Immune cell infiltrates and prognosis in primary carcinoma of the lung. Lung Cancer. 2000;27:27–35. doi: 10.1016/s0169-5002(99)00095-1. [DOI] [PubMed] [Google Scholar]
- 161.Mignon S, Willard-Gallo K, Van Den Eynden G, et al. P3. 02c-087 The Relationship of TILs and PD-L1 Expression in NSCLC Adenocarcinoma in Little to Non-Smokers with Driver Mutations and Outcome Parameters. J Thorac Oncol. 2017;12:S1331. [Google Scholar]
- 162.Dieu-Nosjean MC, Antoine M, Danel C, et al. Long-term survival for patients with non-small-cell lung cancer with intratumoral lymphoid structures. J Clin Oncol. 2008;26:4410–4417. doi: 10.1200/JCO.2007.15.0284. [DOI] [PubMed] [Google Scholar]
- 163.Yamada N, Oizumi S, Kikuchi E, et al. CD8+ tumor-infiltrating lymphocytes predict favorable prognosis in malignant pleural mesothelioma after resection. Cancer Immunol Immunother. 2010;59:1543–1549. doi: 10.1007/s00262-010-0881-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Anraku M, Cunningham KS, Yun Z, et al. Impact of tumor-infiltrating T cells on survival in patients with malignant pleural mesothelioma. J Thorac Cardiovasc Surg. 2008;135:823–829. doi: 10.1016/j.jtcvs.2007.10.026. [DOI] [PubMed] [Google Scholar]
- 165.Salvesen HB, MacDonald N, Ryan P, et al. Methylation of hMLH1 in a Population-based Series of Endometrial Carcinomas. Clin Cancer Res. 2000;6:3607–3613. [PubMed] [Google Scholar]
- 166.Hampel H, Frankel W, Panescu J, et al. Screening for Lynch syndrome (hereditary nonpolyposis colorectal cancer) among endometrial cancer patients. Cancer Res. 2006;66:7810–7817. doi: 10.1158/0008-5472.CAN-06-1114. [DOI] [PubMed] [Google Scholar]
- 167.Mills AM, Longacre TA. Lynch Syndrome Screening in the Gynecologic Tract: Current State of the Art. Am J Surg Pathol. 2016;40:e35–e44. doi: 10.1097/PAS.0000000000000608. [DOI] [PubMed] [Google Scholar]
- 168.Shia J, Black D, Hummer AJ, et al. Routinely assessed morphological features correlate with microsatellite instability status in endometrial cancer. Hum Pathol. 2008;39:116–125. doi: 10.1016/j.humpath.2007.05.022. [DOI] [PubMed] [Google Scholar]
- 169.Garg K, Leitao MM, Kauff ND, et al. Selection of Endometrial Carcinomas for DNA Mismatch Repair Protein Immunohistochemistry Using Patient Age and Tumor Morphology Enhances Detection of Mismatch Repair Abnormalities. Am J Surg Pathol. 2009;33:925–933. doi: 10.1097/PAS.0b013e318197a046. [DOI] [PubMed] [Google Scholar]
- 170.Ferguson SE, Aronson M, Pollett A, et al. Performance characteristics of screening strategies for Lynch syndrome in unselected women with newly diagnosed endometrial cancer who have undergone universal germline mutation testing. Cancer. 2014;120:3932–3939. doi: 10.1002/cncr.28933. [DOI] [PubMed] [Google Scholar]
- 171.Rabban JT, Calkins SM, Karnezis AN, et al. Association of Tumor Morphology With Mismatch-repair Protein Status in Older Endometrial Cancer Patients: Implications for Universal Versus Selective Screening Strategies for Lynch Syndrome. Am J Surg Pathol. 2014;38:793–800. doi: 10.1097/PAS.0000000000000177. [DOI] [PubMed] [Google Scholar]
- 172.Ryan P, Mulligan AM, Aronson M, et al. Comparison of clinical schemas and morphologic features in predicting Lynch syndrome in mutation-positive patients with endometrial cancer encountered in the context of familial gastrointestinal cancer registries. Cancer. 2012;118:681–688. doi: 10.1002/cncr.26323. [DOI] [PubMed] [Google Scholar]
- 173.Mills AM, Liou S, Ford JM, et al. Lynch Syndrome Screening Should Be Considered for All Patients With Newly Diagnosed Endometrial Cancer. Am J Surg Pathol. 2014;38:1501–1509. doi: 10.1097/PAS.0000000000000321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Cancer Genome Atlas Research N. Kandoth C, Schultz N, et al. Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497:67–73. doi: 10.1038/nature12113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Church DN, Briggs SE, Palles C, et al. DNA polymerase epsilon and delta exonuclease domain mutations in endometrial cancer. Hum Mol Genet. 2013;22:2820–2828. doi: 10.1093/hmg/ddt131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Church DN, Stelloo E, Nout RA, et al. Prognostic significance of POLE proofreading mutations in endometrial cancer. J Natl Cancer Inst. 2015;107:402. doi: 10.1093/jnci/dju402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Meng B, Hoang LN, McIntyre JB, et al. POLE exonuclease domain mutation predicts long progression-free survival in grade 3 endometrioid carcinoma of the endometrium. Gynecol Oncol. 2014;134:15–19. doi: 10.1016/j.ygyno.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 178.Howitt BE, Shukla SA, Sholl LM, et al. Association of Polymerase e-Mutated and Microsatellite-Instable Endometrial Cancers With Neoantigen Load, Number of Tumor-Infiltrating Lymphocytes, and Expression of PD-1 and PD-L1. JAMA Oncol. 2015;1:1319–1323. doi: 10.1001/jamaoncol.2015.2151. [DOI] [PubMed] [Google Scholar]
- 179.Hussein YR, Weigelt B, Levine DA, et al. Clinicopathological analysis of endometrial carcinomas harboring somatic POLE exonuclease domain mutations. Mod Pathol. 2015;28:505–514. doi: 10.1038/modpathol.2014.143. [DOI] [PubMed] [Google Scholar]
- 180.van Gool IC, Eggink FA, Freeman-Mills L, et al. POLE Proofreading Mutations Elicit an Antitumor Immune Response in Endometrial Cancer. Clin Cancer Res. 2015;21:3347–3355. doi: 10.1158/1078-0432.CCR-15-0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Kondratiev S, Sabo E, Yakirevich E, et al. Intratumoral CD8+ T Lymphocytes as a Prognostic Factor of Survival in Endometrial Carcinoma. Clin Cancer Res. 2004;10:4450–4456. doi: 10.1158/1078-0432.CCR-0732-3. [DOI] [PubMed] [Google Scholar]
- 182.Cermakova P, Melichar B, Tomsova M, et al. Prognostic significance of CD3+ tumor-infiltrating lymphocytes in patients with endometrial carcinoma. Anticancer Research. 2014;34:5555–5562. [PubMed] [Google Scholar]
- 183.de Jong RA, Leffers N, Boezen HM, et al. Presence of tumor-infiltrating lymphocytes is an independent prognostic factor in type I and II endometrial cancer. Gynecol Oncol. 2009;114:105–110. doi: 10.1016/j.ygyno.2009.03.022. [DOI] [PubMed] [Google Scholar]
- 184.Ino K, Yamamoto E, Shibata K, et al. Inverse correlation between tumoral indoleamine 2,3-dioxygenase expression and tumor-infiltrating lymphocytes in endometrial cancer: its association with disease progression and survival. Clin Cancer Res. 2008;14:2310–2317. doi: 10.1158/1078-0432.CCR-07-4144. [DOI] [PubMed] [Google Scholar]
- 185.Workel HH, Komdeur FL, Wouters MC, et al. CD103 defines intraepithelial CD8+ PD1+ tumour-infiltrating lymphocytes of prognostic significance in endometrial adenocarcinoma. Eur J Cancer. 2016;60:1–11. doi: 10.1016/j.ejca.2016.02.026. [DOI] [PubMed] [Google Scholar]
- 186.Yamagami W, Susumu N, Tanaka H, et al. Immunofluorescence-detected infiltration of CD4+FOXP3+ regulatory T cells is relevant to the prognosis of patients with endometrial cancer. Int J Gynecol Cancer. 2011;21:1628–1634. doi: 10.1097/IGC.0b013e31822c271f. [DOI] [PubMed] [Google Scholar]
- 187.Zhang L, Conejo-Garcia JR, Katsaros D, et al. Intratumoral T Cells, Recurrence, and Survival in Epithelial Ovarian Cancer. N Engl J Med. 2003;348:203–213. doi: 10.1056/NEJMoa020177. [DOI] [PubMed] [Google Scholar]
- 188.Preston CC, Maurer MJ, Oberg AL, et al. The ratios of CD8+ T cells to CD4+CD25+ FOXP3+ and FOXP3- T cells correlate with poor clinical outcome in human serous ovarian cancer. PLoS One. 2013;8:e80063. doi: 10.1371/journal.pone.0080063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Shah CA, Allison KH, Garcia RL, et al. Intratumoral T cells, tumor-associated macrophages, and regulatory T cells: association with p53 mutations, circulating tumor DNA and survival in women with ovarian cancer. Gynecol Oncol. 2008;109:215–219. doi: 10.1016/j.ygyno.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 190.Hwang WT, Adams SF, Tahirovic E, et al. Prognostic significance of tumor-infiltrating T cells in ovarian cancer: a meta-analysis. Gynecol Oncol. 2012;124:192–198. doi: 10.1016/j.ygyno.2011.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Milne K, Kobel M, Kalloger SE, et al. Systematic analysis of immune infiltrates in high-grade serous ovarian cancer reveals CD20, FoxP3 and TIA-1 as positive prognostic factors. PLoS One. 2009;4:e6412. doi: 10.1371/journal.pone.0006412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Nielsen JS, Sahota RA, Milne K, et al. CD20+ tumor-infiltrating lymphocytes have an atypical CD27- memory phenotype and together with CD8+ T cells promote favorable prognosis in ovarian cancer. Clin Cancer Res. 2012;18:3281–3292. doi: 10.1158/1078-0432.CCR-12-0234. [DOI] [PubMed] [Google Scholar]
- 193.Kroeger DR, Milne K, Nelson BH. Tumor-Infiltrating Plasma Cells Are Associated with Tertiary Lymphoid Structures, Cytolytic T-Cell Responses, and Superior Prognosis in Ovarian Cancer. Clin Cancer Res. 2016;22:3005–3015. doi: 10.1158/1078-0432.CCR-15-2762. [DOI] [PubMed] [Google Scholar]
- 194.Dieu-Nosjean MC, Goc J, Giraldo NA, et al. Tertiary lymphoid structures in cancer and beyond. Trends Immunol. 2014;35:571–580. doi: 10.1016/j.it.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 195.Santoiemma PP, Reyes C, Wang LP, et al. Systematic evaluation of multiple immune markers reveals prognostic factors in ovarian cancer. Gynecol Oncol. 2016;143:120–127. doi: 10.1016/j.ygyno.2016.07.105. [DOI] [PubMed] [Google Scholar]
- 196.Hermans C, Anz D, Engel J, et al. Analysis of FoxP3+ T-regulatory cells and CD8+ T-cells in ovarian carcinoma: location and tumor infiltration patterns are key prognostic markers. PLoS One. 2014;9:e111757. doi: 10.1371/journal.pone.0111757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Curiel TJ, Coukos G, Zou L, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 198.Kryczek I, Wei S, Zhu G, et al. Relationship between B7-H4, regulatory T cells, and patient outcome in human ovarian carcinoma. Cancer Res. 2007;67:8900–8905. doi: 10.1158/0008-5472.CAN-07-1866. [DOI] [PubMed] [Google Scholar]
- 199.Stumpf M, Hasenburg A, Riener MO, et al. Intraepithelial CD8-positive T lymphocytes predict survival for patients with serous stage III ovarian carcinomas: relevance of clonal selection of T lymphocytes. Br J Cancer. 2009;101:1513–1521. doi: 10.1038/sj.bjc.6605274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Darb-Esfahani S, Kunze CA, Kulbe H, et al. Prognostic impact of programmed cell death-1 (PD-1) and PD-ligand 1 (PD-L1) expression in cancer cells and tumor-infiltrating lymphocytes in ovarian high grade serous carcinoma. Oncotarget. 2016;7:1486–1499. doi: 10.18632/oncotarget.6429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Webb JR, Milne K, Watson P, et al. Tumor-infiltrating lymphocytes expressing the tissue resident memory marker CD103 are associated with increased survival in high-grade serous ovarian cancer. Clin Cancer Res. 2014;20:434–444. doi: 10.1158/1078-0432.CCR-13-1877. [DOI] [PubMed] [Google Scholar]
- 202.Bosmuller HC, Wagner P, Peper JK, et al. Combined Immunoscore of CD103 and CD3 Identifies Long-Term Survivors in High-Grade Serous Ovarian Cancer. Int J Gynecol Cancer. 2016;26:671–679. doi: 10.1097/IGC.0000000000000672. [DOI] [PubMed] [Google Scholar]
- 203.Hagemann AR, Hagemann IS, Cadungog M, et al. Tissue-based immune monitoring II. Cancer Biology & Therapy. 2014;12:367–377. doi: 10.4161/cbt.12.4.16908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Barnett JC, Bean SM, Whitaker RS, et al. Ovarian cancer tumor infiltrating T-regulatory (T(reg)) cells are associated with a metastatic phenotype. Gynecol Oncol. 2010;116:556–562. doi: 10.1016/j.ygyno.2009.11.020. [DOI] [PubMed] [Google Scholar]
- 205.Wright AA, Bohlke K, Armstrong DK, et al. Neoadjuvant Chemotherapy for Newly Diagnosed, Advanced Ovarian Cancer: Society of Gynecologic Oncology and American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2016;34:3460–3473. doi: 10.1200/JCO.2016.68.6907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Dizon DS. Neoadjuvant chemotherapy for newly diagnosed ovarian cancer: It’s all about selection. Gynecol Oncol. 2017;144:241–242. doi: 10.1016/j.ygyno.2016.12.020. [DOI] [PubMed] [Google Scholar]
- 207.Narod S, Sopik V. Neoadjuvant chemotherapy for advanced-stage ovarian cancer: Are the ASCO and SGO recommendations warranted? Gynecol Oncol. 2017;144:238–240. doi: 10.1016/j.ygyno.2016.11.046. [DOI] [PubMed] [Google Scholar]
- 208.Bohm S, Montfort A, Pearce OM, et al. Neoadjuvant Chemotherapy Modulates the Immune Microenvironment in Metastases of Tubo-Ovarian High-Grade Serous Carcinoma. Clin Cancer Res. 2016;22:3025–3036. doi: 10.1158/1078-0432.CCR-15-2657. [DOI] [PubMed] [Google Scholar]
- 209.Mesnage SJL, Auguste A, Genestie C, et al. Neoadjuvant chemotherapy (NACT) increases immune infiltration and programmed death-ligand 1 (PD-L1) expression in epithelial ovarian cancer (EOC) Ann Oncol. 2016 doi: 10.1093/annonc/mdw625. mdw625 (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 210.Lo CS, Sanii S, Kroeger DR, et al. Neoadjuvant Chemotherapy of Ovarian Cancer Results in Three Patterns of Tumor-Infiltrating Lymphocyte Response with Distinct Implications for Immunotherapy. Clin Cancer Res. 2016 doi: 10.1158/1078-0432.CCR-16-1433. [DOI] [PubMed] [Google Scholar]
- 211.Kurman RJ, Carcangiu ML, Herrington CS, et al. WHO Classification of Tumours of Female Reproductive Organs. 4. IARC; Lyon: 2014. [Google Scholar]
- 212.Aysal A, Karnezis A, Medhi I, et al. Ovarian Endometrioid Adenocarcinoma: Incidence and Clinical Significance of the Morphologic and Immunohistochemical Markers of Mismatch Repair Protein Defects and Tumor Microsatellite Instability. Am J Surg Pathol. 2012;36:163–172. doi: 10.1097/PAS.0b013e31823bc434. [DOI] [PubMed] [Google Scholar]
- 213.Cancer Genome Atlas Research N. Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474:609–615. doi: 10.1038/nature10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Clarke B, Tinker AV, Lee CH, et al. Intraepithelial T cells and prognosis in ovarian carcinoma: novel associations with stage, tumor type, and BRCA1 loss. Mod Pathol. 2009;22:393–402. doi: 10.1038/modpathol.2008.191. [DOI] [PubMed] [Google Scholar]
- 215.McAlpine JN, Porter H, Kobel M, et al. BRCA1 and BRCA2 mutations correlate with TP53 abnormalities and presence of immune cell infiltrates in ovarian high-grade serous carcinoma. Mod Pathol. 2012;25:740–750. doi: 10.1038/modpathol.2011.211. [DOI] [PubMed] [Google Scholar]
- 216.Soslow RA, Han G, Park KJ, et al. Morphologic patterns associated with BRCA1 and BRCA2 genotype in ovarian carcinoma. Mod Pathol. 2012;25:625–636. doi: 10.1038/modpathol.2011.183. [DOI] [PubMed] [Google Scholar]
- 217.Strickland KC, Howitt BE, Shukla SA, et al. Association and prognostic significance of BRCA1/2-mutation status with neoantigen load, number of tumor-infiltrating lymphocytes and expression of PD-1/PD-L1 in high grade serous ovarian cancer. Oncotarget. 2016;7:13587–13598. doi: 10.18632/oncotarget.7277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.George J, Alsop K, Etemadmoghadam D, et al. Nonequivalent gene expression and copy number alterations in high-grade serous ovarian cancers with BRCA1 and BRCA2 mutations. Clin Cancer Res. 2013;19:3474–3484. doi: 10.1158/1078-0432.CCR-13-0066. [DOI] [PubMed] [Google Scholar]
- 219.Fujiwara M, McGuire VA, Felberg A, et al. Prediction of BRCA1 Germline Mutation Status in Women With Ovarian Cancer Using Morphology-based Criteria: Identification of a BRCA1 Ovarian Cancer Phenotype. Am J Surg Pathol. 2012;36:1170–1177. doi: 10.1097/PAS.0b013e31825d9b8d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Lakhani SR, Jacquemier J, Sloane JP, et al. Multifactorial Analysis of Differences Between Sporadic Breast Cancers and Cancers Involving BRCA1 and BRCA2 Mutations. Journal of the National Cancer Institute. 1998;90:1138–1145. doi: 10.1093/jnci/90.15.1138. [DOI] [PubMed] [Google Scholar]
- 221.Lakhani SR, Gusterson BA, Jacquemier J, et al. The Pathology of Familial Breast Cancer: Histological Features of Cancers in Families Not Attributable to Mutations in BRCA1 or BRCA2. Clin Cancer Res. 2000;6:782–789. [PubMed] [Google Scholar]
- 222.Nelson BH. New insights into tumor immunity revealed by the unique genetic and genomic aspects of ovarian cancer. Curr Opin Immunol. 2015;33:93–100. doi: 10.1016/j.coi.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 223.Wolf GT, Hudson JL, Peterson KA, et al. Lymphocyte subpopulations infiltrating squamous carcinomas of the head and neck: Correlations with extent of tumor and prognosis. Otolaryngol Head Neck Surg. 1986;95:142–152. doi: 10.1177/019459988609500203. [DOI] [PubMed] [Google Scholar]
- 224.De Meulenaere A, Vermassen T, Aspeslagh S, et al. TILs in Head and Neck Cancer: Ready for Clinical Implementation and Why (Not)? Head Neck Pathol. 2016 doi: 10.1007/s12105-016-0776-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Uppaluri R, Dunn GP, Lewis JS. Focus on TILs: Prognostic significance of tumor infiltrating lymphocytes in head and neck cancers. Cancer Immunity. 2008;8:16–26. [PMC free article] [PubMed] [Google Scholar]
- 226.Wallis SP, Stafford ND, Greenman J. Clinical relevance of immune parameters in the tumor microenvironment of head and neck cancers. Head Neck. 2015;37:449–459. doi: 10.1002/hed.23736. [DOI] [PubMed] [Google Scholar]
- 227.Green VL, Michno A, Stafford ND, et al. Increased prevalence of tumour infiltrating immune cells in oropharyngeal tumours in comparison to other subsites: relationship to peripheral immunity. Cancer Immunol Immunother. 2013;62:863–873. doi: 10.1007/s00262-013-1395-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Economopoulou P, Agelaki S, Perisanidis C, et al. The promise of immunotherapy in head and neck squamous cell carcinoma. Ann Oncol. 2016;27:1675–1685. doi: 10.1093/annonc/mdw226. [DOI] [PubMed] [Google Scholar]
- 229.Ward MJ, Thirdborough SM, Mellows T, et al. Tumour-infiltrating lymphocytes predict for outcome in HPV-positive oropharyngeal cancer. Br J Cancer. 2014;110:489–500. doi: 10.1038/bjc.2013.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Oguejiofor K, Hall J, Slater C, et al. Stromal infiltration of CD8 T cells is associated with improved clinical outcome in HPV-positive oropharyngeal squamous carcinoma. Br J Cancer. 2015;113:886–893. doi: 10.1038/bjc.2015.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Nordfors C, Grun N, Tertipis N, et al. CD8+ and CD4+ tumour infiltrating lymphocytes in relation to human papillomavirus status and clinical outcome in tonsillar and base of tongue squamous cell carcinoma. Eur J Cancer. 2013;49:2522–2530. doi: 10.1016/j.ejca.2013.03.019. [DOI] [PubMed] [Google Scholar]
- 232.Nasman A, Romanitan M, Nordfors C, et al. Tumor infiltrating CD8+ and Foxp3+ lymphocytes correlate to clinical outcome and human papillomavirus (HPV) status in tonsillar cancer. PLoS One. 2012;7:e38711. doi: 10.1371/journal.pone.0038711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Matlung SE, van Kempen PMW, Bovenschen N, et al. Differences in T-cell infiltrates and survival between HPV+ and HPV- oropharyngeal squamous cell carcinoma. Future Science. 2016;2:FSO88. doi: 10.4155/fso.15.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Russell SM, Angell TE, Lechner MG, et al. Immune cell infiltration patterns and survival in head and neck squamous cell carcinoma. Head Neck Oncol. 2013;5:24. [PMC free article] [PubMed] [Google Scholar]
- 235.Badoual C, Hans S, Merillon N, et al. PD-1-expressing tumor-infiltrating T cells are a favorable prognostic biomarker in HPV-associated head and neck cancer. Cancer Res. 2013;73:128–138. doi: 10.1158/0008-5472.CAN-12-2606. [DOI] [PubMed] [Google Scholar]
- 236.Wood O, Woo J, Seumois G, et al. Gene expression analysis of TIL rich HPV-driven head and neck tumors reveals a distinct B-cell signature when compared to HPV independent tumors. Oncotarget. 2016;7:56781–56797. doi: 10.18632/oncotarget.10788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Keck MK, Zuo Z, Khattri A, et al. Integrative analysis of head and neck cancer identifies two biologically distinct HPV and three non-HPV subtypes. Clin Cancer Res. 2015;21:870–881. doi: 10.1158/1078-0432.CCR-14-2481. [DOI] [PubMed] [Google Scholar]
- 238.Mandal R, Senbabaoglu Y, Desrichard A, et al. The head and neck cancer immune landscape and its immunotherapeutic implications. JCI Insight. 2016;1:e89829. doi: 10.1172/jci.insight.89829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Brandwein-Gensler M, Teixeira MS, Lewis CM, et al. Oral squamous cell carcinoma: histologic risk assessment, but not margin status, is strongly predictive of local disease-free and overall survival. Am J Surg Pathol. 2005;29:167–178. doi: 10.1097/01.pas.0000149687.90710.21. [DOI] [PubMed] [Google Scholar]
- 240.Watanabe Y, Katou F, Ohtani H, et al. Tumor-infiltrating lymphocytes, particularly the balance between CD8(+) T cells and CCR4(+) regulatory T cells, affect the survival of patients with oral squamous cell carcinoma. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;109:744–752. doi: 10.1016/j.tripleo.2009.12.015. [DOI] [PubMed] [Google Scholar]
- 241.Liang YJ, Liu HC, Su YX, et al. Foxp3 expressed by tongue squamous cell carcinoma cells correlates with clinicopathologic features and overall survival in tongue squamous cell carcinoma patients. Oral Oncol. 2011;47:566–570. doi: 10.1016/j.oraloncology.2011.04.017. [DOI] [PubMed] [Google Scholar]
- 242.Vassilakopoulou M, Avgeris M, Velcheti V, et al. Evaluation of PD-L1 Expression and Associated Tumor-Infiltrating Lymphocytes in Laryngeal Squamous Cell Carcinoma. Clin Cancer Res. 2016;22:704–713. doi: 10.1158/1078-0432.CCR-15-1543. [DOI] [PubMed] [Google Scholar]
- 243.Wang J, Wang S, Song X, et al. The prognostic value of systemic and local inflammation in patients with laryngeal squamous cell carcinoma. Onco Targets Ther. 2016;9:7177–7185. doi: 10.2147/OTT.S113307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Balermpas P, Michel Y, Wagenblast J, et al. Tumour-infiltrating lymphocytes predict response to definitive chemoradiotherapy in head and neck cancer. Br J Cancer. 2014;110:501–509. doi: 10.1038/bjc.2013.640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Balermpas P, Rodel F, Rodel C, et al. CD8+ tumour-infiltrating lymphocytes in relation to HPV status and clinical outcome in patients with head and neck cancer after postoperative chemoradiotherapy: A multicentre study of the German cancer consortium radiation oncology group (DKTK-ROG) Int J Cancer. 2016;138:171–181. doi: 10.1002/ijc.29683. [DOI] [PubMed] [Google Scholar]
- 246.Seiwert TY, Burtness B, Mehra R, et al. Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-012): an open-label, multicentre, phase 1b trial. The Lancet Oncology. 2016;17:956–965. doi: 10.1016/S1470-2045(16)30066-3. [DOI] [PubMed] [Google Scholar]
- 247.Ferris RL, Blumenschein G, Jr, Fayette J, et al. Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. N Engl J Med. 2016;375:1856–1867. doi: 10.1056/NEJMoa1602252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Morales A, Eidinger D, Bruce AW. Intracavitary bacillus Calmette-Guerin in the treatment of superficial bladder tumors. J Urol. 1976;116:180–183. doi: 10.1016/s0022-5347(17)58737-6. [DOI] [PubMed] [Google Scholar]
- 249.Shelley M, Court JB, Kynaston H, et al. Intravesical Bacillus Calmette-Guérin in Ta and T1 bladder cancer. Cochrane Database of Systematic Reviews. 2000;4 doi: 10.1002/14651858.CD001986. Art. No.: CD001986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Rosenberg JE, Hoffman-Censits J, Powles T, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. The Lancet. 2016;387:1909–1920. doi: 10.1016/S0140-6736(16)00561-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Plimack ER, Bellmunt J, Gupta S, et al. Safety and activity of pembrolizumab in patients with locally advanced or metastatic urothelial cancer (KEYNOTE-012): a non-randomised, open-label, phase 1b study. The Lancet Oncology. 2017;18:212–220. doi: 10.1016/S1470-2045(17)30007-4. [DOI] [PubMed] [Google Scholar]
- 252.Mostofi FK, Sesterhenn I. Plenary lecture: lymphocytic infiltration in relationship to urologic tumors. Natl Cancer Inst Monogr. 1978:133–141. [PubMed] [Google Scholar]
- 253.Lipponen PK, Eskelinen MJ, Jauhiainen K, et al. Tumour infiltrating lymphocytes as an independent prognostic factor in transitional cell bladder cancer. Eur J Cancer. 1992;29A:69–75. doi: 10.1016/0959-8049(93)90579-5. [DOI] [PubMed] [Google Scholar]
- 254.Sharma P, Shen Y, Wen S, et al. CD8 tumor-infiltrating lymphocytes are predictive of survival in muscle-invasive urothelial carcinoma. Proc Natl Acad Sci USA. 2007:104. doi: 10.1073/pnas.0611618104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Winerdal ME, Marits P, Winerdal M, et al. FOXP3 and survival in urinary bladder cancer. BJU Int. 2011:108. doi: 10.1111/j.1464-410X.2010.10020.x. [DOI] [PubMed] [Google Scholar]
- 256.Otto W, Denziger S, Wieland WF, et al. First analysis of immune cell infiltration in stage pT1 urothelial bladder carcinoma: CD3 positivity as a prognostic marker for cancer-specific survival. World J Urol. 2012:30. doi: 10.1007/s00345-012-0974-2. [DOI] [PubMed] [Google Scholar]
- 257.Krpina K, Babarovic E, Jonjic N. Correlation of tumor-infiltrating lymphocytes with bladder cancer recurrence in patients with solitary low-grade urothelial carcinoma. Virchows Archiv. 2015;467:443–448. doi: 10.1007/s00428-015-1808-6. [DOI] [PubMed] [Google Scholar]
- 258.Zhang Q, Hao C, Cheng G, et al. High CD4+ T cell density is associated with poor prognosis in patients with non-muscle-invasive bladder cancer. Int J Clin Exp Pathol. 2015;8:11510–11516. [PMC free article] [PubMed] [Google Scholar]
- 259.Horn T, Laus J, Seitz AK, et al. The prognostic effect of tumour-infiltrating lymphocytic subpopulations in bladder cancer. World J Urol. 2016;34:181–187. doi: 10.1007/s00345-015-1615-3. [DOI] [PubMed] [Google Scholar]
- 260.Parodi A, Traverso P, Kalli F, et al. Residual tumor micro-foci and overwhelming regulatory T lymphocyte infiltration are the causes of bladder cancer recurrence. Oncotarget. 2016;7:6424–6435. doi: 10.18632/oncotarget.7024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Bellmunt J, Mullane SA, Werner L, et al. Association of PD-L1 expression on tumor-infiltrating mononuclear cells and overall survival in patients with urothelial carcinoma. Ann Oncol. 2015:26. doi: 10.1093/annonc/mdv009. [DOI] [PubMed] [Google Scholar]
- 262.Dash A, Galsky MD, Vickers AJ, et al. Impact of renal impairment on eligibility for adjuvant cisplatin-based chemotherapy in patients with urothelial carcinoma of the bladder. Cancer. 2006;107:506–513. doi: 10.1002/cncr.22031. [DOI] [PubMed] [Google Scholar]
- 263.Honda S, Sakamoto Y, Fujime M, et al. Immunohistochemical Study of Tumor-Infiltrating Lymphocytes Before and After Intravesical Bacillus Calmette-Guérin Treatment for Superficial Bladder Cancer. Int J Urol. 1997;4:68–73. doi: 10.1111/j.1442-2042.1997.tb00143.x. [DOI] [PubMed] [Google Scholar]
- 264.Suriano F, Santini D, Perrone G, et al. Tumor associated macrophages polarization dictates the efficacy of BCG instillation in non-muscle invasive urothelial bladder cancer. J Exp Clin Cancer Res. 2013:32. doi: 10.1186/1756-9966-32-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Ajili F, Kourda N, Dariouche A, et al. Prognostic value of tumor-associated macrophages count in human non-muscle-invasive bladder cancer treated by BCG immunotherapy. Ultrastruct Pathol. 2013;37:56–61. doi: 10.3109/01913123.2012.728688. [DOI] [PubMed] [Google Scholar]
- 266.Takayama H, Nishimura K, Tsujimura A, et al. Increased infiltration of tumor associated macrophages is associated with poor prognosis of bladder carcinoma in situ after intravesical bacillus Calmette-Guerin instillation. J Urol. 2009:181. doi: 10.1016/j.juro.2008.11.090. [DOI] [PubMed] [Google Scholar]
- 267.Pichler R, Fritz J, Zavadil C, et al. Tumor-infiltrating immune cell subpopulations influence the oncologic outcome after intravesical Bacillus Calmette-Guérin therapy in bladder cancer. Oncotarget. 2016;7:39916–39930. doi: 10.18632/oncotarget.9537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Baras AS, Drake CG, Liu J-J, et al. The ratio of CD8 to Treg tumor-infiltrating lymphocytes is associated with response to cisplatin-based neoadjuvant chemotherapy in patients with muscle invasive urothelial carcinoma of the bladder. OncoImmunology. 2016;5:e1134412. doi: 10.1080/2162402X.2015.1134412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Spranger S, Spaapen RM, Zha Y, et al. Up-Regulation of PD-L1, IDO, and Tregs in the Melanoma Tumor Microenvironment Is Driven by CD8+ T Cells. Sci Transl Med. 2013;5:ra116. doi: 10.1126/scitranslmed.3006504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Slovin SF. Immunotherapy for prostate cancer: is prostate an immune responsive tumor? Curr Opin Urol. 2016;26:529–534. doi: 10.1097/MOU.0000000000000334. [DOI] [PubMed] [Google Scholar]
- 271.Bronte V, Kasic T, Gri G, et al. Boosting antitumor responses of T lymphocytes infiltrating human prostate cancers. J Exp Med. 2005;201:1257–1268. doi: 10.1084/jem.20042028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Ebelt K, Babaryka G, Figel AM, et al. Dominance of CD4+ lymphocytic infiltrates with disturbed effector cell characteristics in the tumor microenvironment of prostate carcinoma. The Prostate. 2008:68. doi: 10.1002/pros.20661. [DOI] [PubMed] [Google Scholar]
- 273.Kiniwa Y, Miyahara Y, Wang HY, et al. CD8+ Foxp3+ Regulatory T Cells Mediate Immunosuppression in Prostate Cancer. Clin Cancer Res. 2007:13. doi: 10.1158/1078-0432.CCR-07-0842. [DOI] [PubMed] [Google Scholar]
- 274.Miller AM, Lundberg K, Ozenci V, et al. CD4+CD25high T Cells Are Enriched in the Tumor and Peripheral Blood of Prostate Cancer Patients. J Immunol. 2006:177. doi: 10.4049/jimmunol.177.10.7398. [DOI] [PubMed] [Google Scholar]
- 275.Sfanos KS, Bruno TC, Maris CH, et al. CD4+CD25high T Cells Are Enriched in the Tumor and Peripheral Blood of Prostate Cancer Patients. Clin Cancer Res. 2008;14:3254–3261. [Google Scholar]
- 276.Sfanos KS, Bruno TC, Meeker AK, et al. Human prostate-infiltrating CD8+ T lymphocytes are oligoclonal and PD-1+ The Prostate. 2009;69:1694–1703. doi: 10.1002/pros.21020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Irani J, Goujon J-M, Ragni E, et al. High-grade inflammation in prostate cancer as a prognostic factor for biochemical recurrence after radical prostatectomy. Urology. 1999;54:467–472. doi: 10.1016/s0090-4295(99)00152-1. [DOI] [PubMed] [Google Scholar]
- 278.Karja V, Aaltomaa S, Lipponen PK, et al. Tumour-infiltrating Lymphocytes: A Prognostic Factor of PSA-free Survival in Patients with Local Prostate Carcinoma Treated by Radical Prostatectomy. Anticancer Res. 2005;25:4435–4438. [PubMed] [Google Scholar]
- 279.Zeigler-Johnson C, Morales KH, Lal P, et al. The Relationship between Obesity, Prostate Tumor Infiltrating Lymphocytes and Macrophages, and Biochemical Failure. PLoS ONE. 2016;11:e0159109. doi: 10.1371/journal.pone.0159109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Richardsen E, Uglehaus RD, Due J, et al. The prognostic impact of M-CSF, CSF-1 receptor, CD68 and CD3 in prostatic carcinoma. Histopathology. 2008;53:30–38. doi: 10.1111/j.1365-2559.2008.03058.x. [DOI] [PubMed] [Google Scholar]
- 281.McArdle PA, Canna K, McMillan DC, et al. The relationship between T-lymphocyte subset infiltration and survival in patients with prostate cancer. Br J Cancer. 2004;91:541–543. doi: 10.1038/sj.bjc.6601943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Flammiger A, Bayer F, Cirugeda-Kuhnert A, et al. Intratumoral T but not B lymphocytes are related to clinical outcome in prostate cancer. APMIS. 2012;120:901–908. doi: 10.1111/j.1600-0463.2012.02924.x. [DOI] [PubMed] [Google Scholar]
- 283.Ness N, Andersen S, Valkov A, et al. Infiltration of CD8+ lymphocytes is an independent prognostic factor of biochemical failure-free survival in prostate cancer. The Prostate. 2014;74:1452–1461. doi: 10.1002/pros.22862. [DOI] [PubMed] [Google Scholar]
- 284.Davidsson S, Ohlson A-L, Andersen S-O, et al. CD4 helper T cells, CD8 cytotoxic T cells, and FOXP3+ regulatory T cells with respect to lethal prostate cancer. Mod Pathol. 2013;26:448–455. doi: 10.1038/modpathol.2012.164. [DOI] [PubMed] [Google Scholar]
- 285.Comperat E, Egevad L, Camparo P, et al. Clinical significance of intratumoral CD8+ regulatory T cells in prostate carcinoma. Anal Quant Cytol Histol. 2010;32:39–44. [PubMed] [Google Scholar]
- 286.Vesalainen S, Lipponen PK, Talja M, et al. Histological grade, perineural infiltration, tumour-infiltrating lymphocytes and apoptosis as determinants of long-term prognosis in prostatic adenocarcinoma. Eur J Cancer. 1994;30:1797–1803. doi: 10.1016/0959-8049(94)e0159-2. [DOI] [PubMed] [Google Scholar]
- 287.Nardone V, Botta C, Caraglia M, et al. Tumor infiltrating T lymphocytes expressing FoxP3, CCR7 or PD-1 predict the outcome of prostate cancer patients subjected to salvage radiotherapy after biochemical relapse. Cancer Biol Ther. 2016:17. doi: 10.1080/15384047.2016.1235666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Hussein M-RA, Al-Assiri M, Musalam AO. Phenotypic characterization of the infiltrating immune cells in normal prostate, benign nodular prostatic hyperplasia and prostatic adenocarcinoma. Exp Mod Pathol. 2009;86:108–113. doi: 10.1016/j.yexmp.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 289.Fujii T, Shimada K, Asai O, et al. Immunohistochemical Analysis of Inflammatory Cells in Benign and Precancerous Lesions and Carcinoma of the Prostate. Pathobiology. 2013;80:119–126. doi: 10.1159/000342396. [DOI] [PubMed] [Google Scholar]
- 290.Woo JR, Liss MA, Muldong MT, et al. Tumor infiltrating B-cells are increased in prostate cancer tissue. J Transl Med. 2014;12:30. doi: 10.1186/1479-5876-12-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Mercader M, Bodner BK, Moser MT, et al. T cell infiltration of the prostate induced by androgen withdrawal in patients with prostate cancer. Proc Natl Acad Sci USA. 2001;98:14565–14570. doi: 10.1073/pnas.251140998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Gannon PO, Poisson AO, Delvoye N, et al. Characterization of the intra-prostatic immune cell infiltration in androgen-deprived prostate cancer patients. J Immunol Methods. 2009;348:9–17. doi: 10.1016/j.jim.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 293.Kantoff P, Higano CS, Shore ND, et al. Sipuleucel-T Immunotherapy for Castration-Resistant Prostate Cancer. N Engl J Med. 2010;363:411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 294.McNeel DG, Bander NH, Beer TM, et al. The Society for Immunotherapy of Cancer consensus statement on immunotherapy for the treatment of prostate carcinoma. J Immunother Cancer. 2016;4:92. doi: 10.1186/s40425-016-0198-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Kwon ED, Drake CG, Scher HI, et al. Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184-043): a multicentre, randomised, double-blind, phase 3 trial. The Lancet Oncology. 2014;15:700–712. doi: 10.1016/S1470-2045(14)70189-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Beer TM, Kwon ED, Drake CG, et al. Randomized, Double-Blind, Phase III Trial of Ipilimumab Versus Placebo in Asymptomatic or Minimally Symptomatic Patients With Metastatic Chemotherapy-Naive Castration-Resistant Prostate Cancer. J Clin Oncol. 2017;35:40–47. doi: 10.1200/JCO.2016.69.1584. [DOI] [PubMed] [Google Scholar]
- 297.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, Activity, and Immune Correlates of Anti–PD-1 Antibody in Cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Graff JN, Alumkal JJ, Drake CG, et al. Early evidence of anti-PD-1 activity in enzalutamide-resistant prostate cancer. Oncotarget. 2016;7:52810–52817. doi: 10.18632/oncotarget.10547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Rini BI, McDermott DF, Hammers H, et al. Society for Immunotherapy of Cancer consensus statement on immunotherapy for the treatment of renal cell carcinoma. J Immunother Cancer. 2016;4:81. doi: 10.1186/s40425-016-0180-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. N Engl J Med. 2015;373:1803–1813. doi: 10.1056/NEJMoa1510665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Angevin E, Kremer F, Gaudin C, et al. Analysis of T-cell immune response in renal cell carcinoma: Polarization to type 1-like differentiation pattern, clonal T-cell expansion and tumor-specific cytotoxicity. Int J Cancer. 1997;72:431–440. doi: 10.1002/(sici)1097-0215(19970729)72:3<431::aid-ijc10>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 302.Schleypen JS, Baur N, Kammerer R, et al. Cytotoxic Markers and Frequency Predict Functional Capacity of Natural Killer Cells Infiltrating Renal Cell Carcinoma. Clin Cancer Res. 2006;12:718–725. doi: 10.1158/1078-0432.CCR-05-0857. [DOI] [PubMed] [Google Scholar]
- 303.Van Den Hove LE, Van Gool SW, Van Poppel H, et al. Phenotype, cytokine production and cytolytic capacity of fresh (uncultured) tumour-infiltrating T lymphocytes in human renal cell carcinoma. Clin Exp Immunol. 1997:109. doi: 10.1046/j.1365-2249.1997.4771375.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Banner BF, Burnham JA, Bahnson RR, et al. Immunophenotypic markers in renal cell carcinoma. Mod Pathol. 1990;3:129–134. [PubMed] [Google Scholar]
- 305.Kolbeck PC, Kaveggia FF, Johansson SL, et al. The relationships among tumor-infiltrating lymphocytes, histopathologic findings, and long-term clinical follow-up in renal cell carcinoma. Mod Pathol. 1992;5:420–425. [PubMed] [Google Scholar]
- 306.Attig S, Hennenlotter J, Pawelec G, et al. Simultaneous Infiltration of Polyfunctional Effector and Suppressor T Cells into Renal Cell Carcinomas. Cancer Res. 2009;69:8412–8419. doi: 10.1158/0008-5472.CAN-09-0852. [DOI] [PubMed] [Google Scholar]
- 307.Siddiqui SA, Frigola X, Bonne-Annee S, et al. Tumor-Infiltrating Foxp3−CD4+CD25+ T Cells Predict Poor Survival in Renal Cell Carcinoma. Clin Cancer Res. 2007;13:2075–2081. doi: 10.1158/1078-0432.CCR-06-2139. [DOI] [PubMed] [Google Scholar]
- 308.Griffiths RW, Elkord E, Gilham DE, et al. Frequency of regulatory T cells in renal cell carcinoma patients and investigation of correlation with survival. Cancer Immunol Immunother. 2007;56:1743–1753. doi: 10.1007/s00262-007-0318-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Bromwich EJ, McArdle PA, Canna K, et al. The relationship between T-lymphocyte infiltration, stage, tumour grade and survival in patients undergoing curative surgery for renal cell cancer. Br J Cancer. 2003;89:1906–1908. doi: 10.1038/sj.bjc.6601400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Remark R, Alifano M, Cremer I, et al. Characteristics and Clinical Impacts of the Immune Environments in Colorectal and Renal Cell Carcinoma Lung Metastases: Influence of Tumor Origin. Clin Cancer Res. 2013;19:4079–4091. doi: 10.1158/1078-0432.CCR-12-3847. [DOI] [PubMed] [Google Scholar]
- 311.Nakano O, Sato M, Naito Y, et al. Proliferative Activity of Intratumoral CD8+ T-Lymphocytes As a Prognostic Factor in Human Renal Cell Carcinoma. Cancer Res. 2001;61:5132–5136. [PubMed] [Google Scholar]
- 312.Hotta K, Sho M, Fujimoto K, et al. Prognostic significance of CD45RO+ memory T cells in renal cell carcinoma. Br J Cancer. 2011;105:1191–1196. doi: 10.1038/bjc.2011.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Giraldo NA, Becht E, Pages F, et al. Orchestration and Prognostic Significance of Immune Checkpoints in the Microenvironment of Primary and Metastatic Renal Cell Cancer. Clin Cancer Res. 2015;21:3031–3040. doi: 10.1158/1078-0432.CCR-14-2926. [DOI] [PubMed] [Google Scholar]
- 314.Polimeno M, Napolitano M, Costantini S, et al. Regulatory T cells, interleukin (IL)-6, IL-8, Vascular endothelial growth factor (VEGF), CXCL10, CXCL11, epidermal growth factor (EGF) and hepatocyte growth factor (HGF) as surrogate markers of host immunity in patients with renal cell carcinoma. BJU Int. 2013;112:686–696. doi: 10.1111/bju.12068. [DOI] [PubMed] [Google Scholar]
- 315.Kang MJ, Kim KM, Bae JS, et al. Tumor-infiltrating PD1-Positive Lymphocytes and FoxP3-Positive Regulatory T Cells Predict Distant Metastatic Relapse and Survival of Clear Cell Renal Cell Carcinoma. Transl Oncol. 2013;6:282–289. doi: 10.1593/tlo.13256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Liotta F, Gacci M, Frosali F, et al. Frequency of regulatory T cells in peripheral blood and in tumour-infiltrating lymphocytes correlates with poor prognosis in renal cell carcinoma. BJU Int. 2011;107:1500–1506. doi: 10.1111/j.1464-410X.2010.09555.x. [DOI] [PubMed] [Google Scholar]
- 317.Li JF, Chu YW, Wang GM, et al. The prognostic value of peritumoral regulatory T cells and its correlation with intratumoral cyclooxygenase-2 expression in clear cell renal cell carcinoma. BJU Int. 2009;103:399–405. doi: 10.1111/j.1464-410X.2008.08151.x. [DOI] [PubMed] [Google Scholar]
- 318.Thompson RH, Dong H, Lohse CM, et al. PD-1 Is Expressed by Tumor-Infiltrating Immune Cells and Is Associated with Poor Outcome for Patients with Renal Cell Carcinoma. Clin Cancer Res. 2007:13. doi: 10.1158/1078-0432.CCR-06-2599. [DOI] [PubMed] [Google Scholar]
- 319.Abbas M, Steffens S, Bellut M, et al. Do programmed death 1 (PD-1) and its ligand (PD-L1) play a role in patients with non-clear cell renal cell carcinoma? Med Oncol. 2016;33:59. doi: 10.1007/s12032-016-0770-8. [DOI] [PubMed] [Google Scholar]
- 320.Guislain A, Gadiot J, Kaiser A, et al. Sunitinib pretreatment improves tumor-infiltrating lymphocyte expansion by reduction in intratumoral content of myeloid-derived suppressor cells in human renal cell carcinoma. Cancer Immunol Immunother. 2015;64:1241–1250. doi: 10.1007/s00262-015-1735-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Wallin JJ, Bendall JC, Funke R, et al. Atezolizumab in combination with bevacizumab enhances antigen-specific T-cell migration in metastatic renal cell carcinoma. Nat Comm. 2016;7:12624. doi: 10.1038/ncomms12624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Choueiri TK, Figueroa DJ, Fay AP, et al. Correlation of PD-L1 Tumor Expression and Treatment Outcomes in Patients with Renal Cell Carcinoma Receiving Sunitinib or Pazopanib: Results from COMPARZ, a Randomized Controlled Trial. Clin Cancer Res. 2015;21:1071–1077. doi: 10.1158/1078-0432.CCR-14-1993. [DOI] [PubMed] [Google Scholar]
- 323.Rodriguez-Vida A, Strijbos M, Hutson T. Predictive and prognostic biomarkers of targeted agents and modern immunotherapy in renal cell carcinoma. ESMO Open. 2016;1:e000013. doi: 10.1136/esmoopen-2015-000013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Bienkowski M, Preusser M. Prognostic role of tumour-infiltrating inflammatory cells in brain tumours: literature review. Curr Opin Neurol. 2015;28:647–658. doi: 10.1097/WCO.0000000000000251. [DOI] [PubMed] [Google Scholar]
- 325.Domingues P, Gonzalez-Tablas M, Otero A, et al. Tumor infiltrating immune cells in gliomas and meningiomas. Brain Behav Immun. 2016;53:1–15. doi: 10.1016/j.bbi.2015.07.019. [DOI] [PubMed] [Google Scholar]
- 326.Berghoff AS, Kiesel B, Widhalm G, et al. Programmed death ligand 1 expression and tumor-infiltrating lymphocytes in glioblastoma. Neuro Oncol. 2015;17:1064–1075. doi: 10.1093/neuonc/nou307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Rutledge WC, Kong J, Gao J, et al. Tumor-infiltrating lymphocytes in glioblastoma are associated with specific genomic alterations and related to transcriptional class. Clin Cancer Res. 2013;19:4951–4960. doi: 10.1158/1078-0432.CCR-13-0551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Han S, Zhang C, Li Q, et al. Tumour-infiltrating CD4(+) and CD8(+) lymphocytes as predictors of clinical outcome in glioma. Br J Cancer. 2014;110:2560–2568. doi: 10.1038/bjc.2014.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Zhu H-D, Xie Q, Gong Y, et al. Lymphoplasmacyte-rich meningioma: our experience with 19 cases and a systematic literature review. Int J Clin Exp Med. 2013;6:504–515. [PMC free article] [PubMed] [Google Scholar]
- 330.Fang L, Lowther DE, Meizlish ML, et al. The immune cell infiltrate populating meningiomas is composed of mature, antigen-experienced T and B cells. Neuro Oncol. 2013;15:1479–1490. doi: 10.1093/neuonc/not110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Bi WL, Wu WW, Santagata S, et al. Checkpoint inhibition in meningiomas. Immunotherapy. 2016;8:721–731. doi: 10.2217/imt-2016-0017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
TILs assessment in melanoma tutorial.
TILs assessment in lung carcinoma tutorial.
TILs assessment in mesothelioma tutorial.
TILs assessment in endometrial carcinoma tutorial.
TILs assessment in ovarian carcinoma tutorial.
TILs assessment in urothelial carcinoma tutorial.