Volume 9, Number 5—May 2003
Research
Pandemic Influenza and Healthcare Demand in the Netherlands: Scenario Analysis
Abstract
In accordance with World Health Organization guidelines, the Dutch Ministry of Health, Welfare and Sports designed a national plan to minimize effects of pandemic influenza. Within the scope of the Dutch pandemic preparedness plan, we were asked to estimate the magnitude of the problem in terms of the number of hospitalizations and deaths during an influenza pandemic. Using scenario analysis, we also examined the potential effects of intervention options. We describe and compare the scenarios developed to understand the potential impact of a pandemic (i.e., illness, hospitalizations, deaths), various interventions, and critical model parameters. Scenario analysis is a helpful tool for making policy decisions about the design and planning of outbreak control management on a national, regional, or local level.
In 1997, avian influenzavirus was shown to infect humans directly when an influenza virus A/H5N1 infected 18 people in Hong Kong; of those, six died (1,2). After this event, experts predicted that another influenza pandemic is highly likely, if not inevitable (3,4). The impact of a pandemic depends on factors such as the virulence of the pandemic virus and the availability of a vaccine. Because development is time-consuming, the vaccine would likely not be available in the early stages of a pandemic, and a major vaccine shortage would be expected (5). An influenza virus pandemic would likely cause substantial social disruption because of high rates of illness, sick leave, hospitalization, and death. Therefore, pandemic planning is essential to minimize influenza-related illness, death, and social disruption (5,6).
In accordance with World Health Organization guidelines, the Dutch Ministry of Health, Welfare and Sports developed a national plan to minimize or avert effects of pandemic influenza. Within the scope of the Dutch pandemic preparedness plan, we were asked to estimate the magnitude of the problem in terms of the expected number of hospitalizations and deaths during an influenza pandemic. We also estimated the potential effects of intervention options, including the use of the relatively new antiviral drugs, neuraminidase inhibitors (7,8).
One published study (9) has estimated the economic effects of an influenza pandemic. Meltzer et al. examined the possible effects of influenza vaccine-based interventions in terms of outpatient visits, hospitalizations, deaths, and related costs during a pandemic in the United States. More recently, different strategies for the control of interpandemic influenza for the elderly population in three European countries (England and Wales, France, and Germany) have been evaluated (10). Our objective was to examine the potential impact of pandemic influenza in the Netherlands and to analyze the effects of several (other than influenza vaccine–based) possible interventions in terms of hospitalizations and deaths.
Predicting when the next influenza pandemic will occur and how it will evolve is impossible, and the same is true for forecasting the number of persons who will become ill, be hospitalized, or die. Because of the many uncertainties, we performed a scenario analysis (11) that included consulting of experts and modeling. At a meeting of experts held to discuss an influenza pandemic in the Netherlands, specialists on influenza (virology, epidemiology, and surveillance) and on controlling epidemics and disasters gave their opinions about the formulated intervention scenarios, the assumptions made, and the value of critical parameters (12). A model was used to estimate the number of hospitalizations and deaths in the Netherlands for different scenarios. We also compared the number of expected hospitalizations and deaths for each of the different intervention scenarios to the number expected for the nonintervention scenario.
Scenarios
Various scenarios are possible, depending on whether influenza vaccine, pneumococcal vaccine, or antiviral drugs are available (among other factors). In all scenarios, we assumed a gross attack rate of 30%; we also assumed age-specific attack, hospitalization, and death rates and healthcare utilization (e.g., antibiotic drug prescription) as in a regular epidemic. Table 1 shows the base-case assumptions in the various scenarios. Following are descriptions of the scenarios considered relevant and sufficiently realistic by the specialists who participated in the meeting of experts.
Nonintervention Scenario
The nonintervention scenario is a “worst case” situation in which no intervention is possible. The scenario includes a pandemic influenza for which no vaccine is available and only regular care and regularly prescribed antibiotic drugs are provided. In the base case, we assume a gross attack rate of 30%; an age-specific attack; and hospitalization, death rates, and healthcare utilization as in a regular epidemic.
Influenza Vaccination Scenario
In this scenario, when an influenza vaccine becomes available, two possible strategies are considered: 1) vaccination of risk groups including persons >65 years of age (n = 2.78×106) and healthcare workers (n = 0.80×106) and 2) vaccination of the total population (n = 15.6×106). In the base case, influenza vaccination is assumed to be 56% effective in preventing hospitalizations and deaths in persons >65 years of age (15), and 80% effective in those <64 years of age (Table 1) (13,14).
Pneumococcal Vaccination Scenario
In the absence of a vaccine available at the beginning of a pandemic, the Dutch Health Council recommends providing influenza risk groups (including those >65 years of age; n = 2.78×106) with pneumococcal vaccination (18), which is a 23-valent vaccine assumed to prevent invasive infections caused by Streptococcus pneumoniae, one of the possible complications of influenza. For the base case, we assumed that 50% of hospitalizations and deaths from influenza-related pneumonia are caused by invasive pneumococcal infection and that pneumococcal vaccination prevents 80% of invasive infections caused by vaccine serotypes (Table 1) (16,17). In the Netherlands, 80% of serotypes involved in invasive pneumococcal infections are covered by the 23-valent vaccine, which results in a vaccine effectiveness of 64% against invasive pneumococcal infections.
Therapeutic Use of Neuraminidase Inhibitors Scenario
This scenario includes the use of neuraminidase inhibitors. When taken within 48 hours after onset of symptoms and continued for 5 days, neuraminidase inhibitors (zanamivir and oseltamivir) (19) reduce the duration and seriousness of influenza by 1 to 2 days for adults (20–24), children (22,25,26), and persons at high risk (22,27–29). However, the effectiveness of neuraminidase inhibitors for preventing hospitalizations and deaths (our outcome parameters) is unknown. Therefore, we assumed that 25% to 75% of the hospitalizations and deaths attributed to influenza would be avoided by therapeutic use of neuraminidase inhibitors (12) in this scenario (each person with an influenzalike illness begins the medication within 48 hours after the first symptoms). An advantage of therapeutic use of neuraminidase inhibitors is that antibodies are formed (26) because infection is not prevented; thus protection against an infection resulting from the same virus is built up, as in an untreated infection.
Although neuraminidase inhibitors have proven to be effective prophylactically (27,30–32), the specialists were unanimous in their opinion that using neuraminidase inhibitors prophylactically on a large scale in a pandemic is not feasible because they need to be taken as long as the threat of influenza virus infection lasts. The medication would therefore need to be taken for at least several weeks to several months in a pandemic. An enormous stockpile of neuraminidase inhibitors would be required for the Dutch population; compliance, in the course of time, would likely diminish. In this scenario, using this medication for prophylactic purposes might merely postpone the pandemic, and the disease might emerge at the moment that most of the population stops the prophylaxis unless an effective and safe vaccine is available in sufficient amount at that time.
The specialists considered neuraminidase inhibitors to be more suitable than previous antiviral medicines (amantadine and rimantadine), which lead to viral resistance, have serious side effects, and are only effective against influenza A (7,8,14). Neuraminidase inhibitors are effective against influenza A and B and have not generated much resistance thus far (19,33,34); they appear to be safe and have seldom caused serious side effects (34–36).
Model and Data
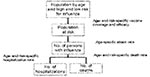
Building a mathematical model of influenza spread is difficult because of yearly differences in virus transmission and virulence, lack of understanding of the factors affecting the spread of influenza, and shortage of population-based data (9,37). We used a static model (12) that estimates the numbers of hospitalizations and deaths in the Netherlands by using data from earlier influenza epidemics and literature review. The model was implemented by using an Excel spreadsheet (Microsoft Corp., Redmond, CA) (Figure 1). In the model, we distinguished three age groups (<19 years, 20–64 years, and >65 years) by low or high risk (susceptibility to the complications of hospitalization and death) for influenza. The population not protected against influenza depends on vaccination coverage and vaccine and neuraminidase efficacy; all can be different in each scenario. We calculated the number of influenza cases in each age group at low or high risk for influenza by multiplying numbers not protected against influenza and attack rates. We calculated the absolute number of hospitalizations and deaths in each age group at low or high risk for influenza by multiplying the calculated number of influenza cases and the influenza-specific complication (hospitalization or death) rates. The case-specific complication rates in each age group at low or high risk for influenza are computed from general population–specific complication rates, current vaccination degree, and vaccine efficacy by assuming that during a regular epidemic 10% of the population becomes ill (12). The age distribution of the influenza cases in the general population is assumed to be equal to the age distribution of persons consulting their general practitioner for influenzalike illness. Table 2 shows the values of the basic input variables.
Sensitivity Analyses
Sensitivity analyses were performed on the gross attack rate, age-specific attack, hospitalization and death rates, and on efficacy of vaccines and neuraminidase inhibitors. Table 1 describes assumptions used in sensitivity analysis.
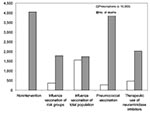
Results are shown in terms of number of hospitalizations and deaths (prevented) in relation to doses of vaccines or antiviral drugs needed. During a regular influenza epidemic in the Netherlands, approximately 1,900 hospitalizations and 800 deaths related to influenza occur. The nonintervention scenario of an influenza pandemic with a gross attack rate of 30% and no interventions available could lead to as many as 10,000 influenza-related hospitalizations and >4,000 deaths (Figures 2 and 3).
The influenza vaccination scenario could prevent >6,000 (>60%) of hospitalizations and >2,200 (>55%) of deaths. Vaccination of the total population requires 15.6 million doses of vaccine; vaccination only of risk groups for influenza (including persons >65 years of age and healthcare workers) requires 3.6 million vaccines. The pneumoccoccal vaccination scenario, which requires 2.8 million doses of vaccine, could prevent 2,600 (25%) of the hospitalizations and 140 (3.5%) of the deaths. The therapeutic use of neuraminidase inhibitors scenario could prevent 5,000 hospitalizations and 2,000 deaths (assuming 50% efficacy) and would require 4.7 million prescriptions of neuraminidase inhibitors.
A decrease (increase) in the gross attack rate to 10% (to 50%) shows a similar decrease (increase) in the absolute number of expected hospitalizations and deaths. Assuming different gross attack rates does not change the percentage of hospitalizations and deaths that might be avoided in the different scenarios (Table 3). By using a range of age-specific attack rates (Table 4) for the nonintervention scenario, we estimated that the number of hospitalizations ranged from 7,500 to >19,000 and the number of deaths from 2,700 to approximately 9,000 (Table 5). The variation in the number of hospitalizations and deaths in each of the scenarios is substantial. However, assuming different age-specific attack rates leads to little difference in the percentage of hospitalizations and deaths that might be avoided by a certain intervention.
If one assumes that complication (i.e., hospitalization and death) rates for low-risk persons are equal to the complication rates for high-risk persons, the number of hospitalizations and deaths increases dramatically. In the nonintervention scenario, we estimated >64,000 hospitalizations (>10,000 in the base case) and approximately 10,000 deaths (approximately 4,000 in the base case). The number of avoided hospitalizations ranges from almost 6,000 in the pneumococcal vaccination scenario to >45,000 in the influenza vaccination (of the total population) scenario, and the number of avoided deaths ranges from 1,000 to >6,000 (Table 6). In the scenario with influenza vaccination of risk groups, this assumption leads to a decrease in the percentage of hospitalizations and deaths that might be avoided, 21% (base case 61%) and 47% (base case 56%), respectively. In the scenario with pneumococcal vaccination of risk groups, the percentage of hospitalizations and deaths that might be avoided decreases to 9% (base case 31%) and 1% (base case 3%), respectively.
Low and high levels for age-specific influenza vaccine efficacy show that the number of expected hospitalizations varies from almost 2,000 to >6,900 and the number of deaths varies from almost 800 to >2,800 (Table 7). These numbers are equal to a range of 30% to 80% in the percentage of the number of hospitalizations and deaths that might be avoided (base case 55% to 60%).
For the pneumococcal vaccine scenario, we tested two parameters: the percentage of complications (25% to 75%) to be prevented by pneumococcal vaccination and the pneumococcal vaccine efficacy (also 25% to 75%). Our results showed that the number of expected hospitalizations varies from 5,400 to 8,950, the number of deaths varies from >3,800 to 4,000 (Table 8). These values are equal to a range of 12% to 47% (base case 31%) and 1% to 5% (base case 3%) in the percentage of the number of hospitalizations and deaths that might be avoided. When assuming 25% to 75% effectiveness for the neuraminidase inhibitors scenario, we also estimated that between 25% and 75% of the number of hospitalizations and deaths can be avoided.
The nonintervention scenario describes a pandemic situation in which no interventions are available; such an influenza pandemic, with a gross attack rate of 30%, would result in five times as many influenza-related hospitalizations and deaths as in a regular influenza epidemic with the current degree of vaccination, mostly in persons >65 years of age. Sensitivity analysis shows that varying the gross attack rate does not change the percentage of hospitalizations and deaths that might be avoided in the different scenarios. Varying the age-specific attack, hospitalization, and death rates has a large impact on the estimated number of hospitalizations and deaths. However, the impact is less in terms of the percentage of the number of hospitalizations and deaths that might be avoided by the various interventions.
Influenza vaccination may prevent many hospitalizations and deaths. The influenza vaccination scenario suggests that when assuming the age-specific complication rates of a regular epidemic, vaccination of the total population compared to vaccination of healthcare workers and the groups at risk for influenza would do little to avert hospitalizations and deaths. However, sensitivity analysis shows this result to be quite sensitive to the assumptions of the complication rates by age. As a consequence of higher complication rates in lower age and risk groups, the percentage of averted hospitalizations and deaths substantially decreases in the scenario’s pneumococcal and influenza vaccination of risk groups for influenza.
Only a pandemic itself can provide better estimates of the age-specific attack and complication rates, but these analyses show a range of what might be expected. While the likelihood of an available influenza vaccine in the beginning of a pandemic is low, the next best option seems to be the therapeutic use of neuraminidase inhibitors. However, this option has three major considerations: 1) effective use of neuraminidase inhibitors depends greatly on the assumption of 50% effectiveness to prevent hospitalizations and deaths; 2) every patient with influenzalike illness must begin medication within 48 hours after onset of symptoms (a logistically complicated task); and 3) a sufficient stock of neuraminidase inhibitors must be available, which is currently not the case. In our current approach, we probably underestimated the effect of influenza vaccination and the therapeutic use of neuraminidase inhibitors because we did not take into account the specific features of influenza as an infectious transmissible disease.
Pneumococcal vaccination could prevent 31% of the hospitalizations and 3.4% of the deaths. This intervention is the least effective because pneumococcal vaccination prevents only one complication of influenza (i.e., invasive pneumococcal infections). In contrast to hospitalizations, few deaths might be prevented by pneumococcal vaccination because relatively more excess hospitalizations than deaths are attributable to influenza-related pneumonia. An advantage of this intervention is that pneumococcal vaccination can be done before the pandemic starts since the vaccine is effective in preventing invasive pneumococcal infections for approximately 5 years (15). As expected, sensitivity analysis showed that lower vaccine effectiveness results in less hospitalizations and deaths prevented. In the next pandemic, if pneumoccocal infections occur more often as a complication of influenza than in the base case, using this intervention would prevent increased hospitalizations and deaths.
The objective of our study was to examine the potential impact (in terms of hospitalizations and deaths) of pandemic influenza in the Netherlands and to analyze the effects of several possible interventions. Ideally, after a pandemic has started, the influenza vaccine should be available and administered as quickly as possible following a prioritized scheme. In the Netherlands, developing this scheme is a governmental task. The scheme may be dependent on the actual (observed) age-specific attack and complication rates. However, at the start of the pandemic, no vaccine is expected to be available. Based on our analysis and assumptions, we conclude that a combined strategy of pneumococcal vaccination of risk groups for influenza together with the therapeutic use of neuraminidase inhibitors for all patients with influenzalike illness (within 48 hours after onset of symptoms) is the best strategy in preventing hospitalizations and deaths.
This recommendation is not valid if therapeutic use of neuraminidase inhibitors is shown to be ineffective in preventing influenza-related hospitalizations and deaths. Also, if the next pandemic shows that invasive pneumococcal infections are not a complication of influenza, pneumococcal vaccination is no longer a valid intervention. Because these questions are still unanswered, we also recommend ongoing research in the field of vaccine production techniques.
To prepare effectively for the next pandemic, the Dutch government will continue to investigate stockpiling neuraminidase inhibitors and securing influenza vaccine supply during a pandemic.
Our scenario analysis provides information about reducing the effects of a pandemic to a minimum, both regionally and nationally, to those who must prepare for the control of an actual pandemic. The insights from the scenario analysis provide a possible order of magnitude for providing healthcare (regional data were also calculated; data not shown). Furthermore, by using a model and a set of assumptions, we compared the effects of various interventions on the demand for care. Scenario analysis provided insight into which parameters have the most influence on the outcome variables (the age-specific attack and complication rates). If outbreaks of a new, potentially pandemic, influenza virus occur abroad and if these outbreaks yield real information about the attack and complication rates by age group, we can use these values in our model to update the estimate of the demand for care that can be expected in the Netherlands, nationally and regionally. Other countries might also use a similar approach to support their pandemic preparedness planning.
References
- Yuen KY, Chan PKS, Peiris M, Tsang DNC, Que TL, Shortridge KF, Clinical features and rapid viral diagnosis of human disease associated with avian influenza A H5N1 virus. Lancet. 1998;351:467–71. DOIPubMedGoogle Scholar
- Claas ECJ, Osterhaus ADME, van Beek R, de Jong JC, Rimmelzwaan GF, Senne DA, Human influenza A H5N1 virus related to a highly pathogenic avian influenza virus. Lancet. 1998;351:472–7. DOIPubMedGoogle Scholar
- Patriarca PA, Cox NJ. Influenza pandemic preparedness plan for the United States. J Infect Dis. 1997;176(Suppl 1):S4–7. DOIPubMedGoogle Scholar
- Belshe RB. Influenza as a zoonosis: how likely is a pandemic? [Commentary]. Lancet. 1998;351:460–1. DOIPubMedGoogle Scholar
- World Health Organization, Department of Communicable Disease Surveillance and Response. Influenza pandemic plan. The role of the WHO and guidelines for national and regional planning. 1999 Apr. Available from: URL: www.who.int/emc-documents/influenza
- Snacken R, Kendal AP, Haaheim LR, Wood JM. The next influenza pandemic: lessons from Hong Kong, 1997. Emerg Infect Dis. 1999;5:195–9. DOIPubMedGoogle Scholar
- Osterhaus ADME, de Jong JC. The control of influenza: antivirals as an adjunct to vaccines. Vaccine. 1999;18:779–80. DOIPubMedGoogle Scholar
- Centers for Disease Control and Prevention. Neuraminidase inhibitors for treatment of influenza A and B infections. MMWR Recomm Rep. 1999;48:1–9.PubMedGoogle Scholar
- Meltzer MI, Cox NJ, Fukuda K. The economic impact of pandemic influenza in the United States: priorities for intervention. Emerg Infect Dis. 1999;5:659–71. DOIPubMedGoogle Scholar
- Scuffham PA, West PA. Economic evaluation of strategies for the control and management of influenza in Europe. Vaccine. 2002;20:2562–78. DOIPubMedGoogle Scholar
- van Genugten MLL, Rutten FFH, Jager JC. Scenario development and costing in health care. Methodological accomplishments and practical guidelines. Utrecht (Netherlands): International Books; 1996.
- van Genugten MLL, Heijnen MLA, Jager JC. Scenario analysis of the expected number of hospitalizations and deaths due to pandemic influenza in the Netherlands. Bilthoven (Netherlands): National Institute of Public Health and the Environment; 2002. RIVM report 282701002/2002. Available from: URL: www.rivm.nl/bibliotheek/rapporten/282701002.pdf
- Couch RB. Prevention and treatment of influenza. N Engl J Med. 2000;343:1778–87. DOIPubMedGoogle Scholar
- Gross PA, Hermogenes AW, Sacks HS, Lau JL, Levandowski RA. The efficacy of influenza vaccine in elderly persons. A meta-analysis and review of the literature. Ann Intern Med. 1995;123:518–27.PubMedGoogle Scholar
- Postma MJ, Heijnen MLA, Jager JC. Cost-effectiveness analysis of pneumococcal vaccination for elderly individuals in the Netherlands. Pharmacoeconomics. 2001;19:215–22. DOIPubMedGoogle Scholar
- Shapiro ED, Berg AT, Austrian R, Schroeder D, Parcells V, Margolis A, The protective efficacy of polyvalent pneumococcal polysaccharide vaccine. N Engl J Med. 1991;325:1453–60. DOIPubMedGoogle Scholar
- Health Council. Commissie vaccinatie tegen influenza. Vaccinatie bij een griep pandemie. Den Haag: Gezondheidsraad; 2000. Publication no. 2000/1.
- Gubareva LV, Kaiser L, Hayden FG. Influenza virus neuraminidase inhibitors. Lancet. 2000;355:827–35. DOIPubMedGoogle Scholar
- Hayden FG, Osterhaus ADME, Treanor JJ, Fleming DM, Aoki FY, Nicholson KG, Efficacy and safety of the neuraminidase inhibitor Zanamivir in the treatment of influenza virus infections. N Engl J Med. 1997;337:874–80. DOIPubMedGoogle Scholar
- MIST study group. Randomised trial of efficacy and safety of inhaled Zanamivir in treatment of influenza A and B virus infections. Lancet. 1998;352:1877–81. DOIPubMedGoogle Scholar
- Mäkelä MJ, Pauksens K, Rostila T, Fleming DM, Man CY, Keene ON, Clinical efficacy and safety of the orally inhaled neuaraminadase inhibitor Zanamivir in the treatment of influenza: a randomized, double-blind, placebo-controlled European study. J Infect. 2000;40:42–8. DOIPubMedGoogle Scholar
- Treanor JJ, Hayden FG, Vrooman PS, Barbarash R, Bettis R, Riff D, Efficacy and safety of the oral neuraminidase inhibitor oseltamivir in treating acute influenza: a randomized controlled trial. JAMA. 2000;283:1016–24. DOIPubMedGoogle Scholar
- Nicholson KG, Aoki FY, Osterhaus ADME, Trottier S, Carewicz O, Mercier CH, Efficacy and safety of oseltamivir in treatment of acute influenza: a randomised controlled trial. Lancet. 2000;355:1845–50. DOIPubMedGoogle Scholar
- Hedrick JA, Barzilai A, Behre U, Henderson FW, Hammond J, Reilly L, Zanamivir for treatment of symptomatic influenza A and B infection in children five to twelve years of age: a randomized controlled trial. Pediatr Infect Dis J. 2000;19:410–7. DOIPubMedGoogle Scholar
- Whitley RJ, Hayden FG, Reisinger KS, Young N, Dutkowski R, Ipe D, Oral oseltamivir treatment of influenza in children. Pediatr Infect Dis J. 2001;20:127–33. DOIPubMedGoogle Scholar
- Monto AS, Robinson DP, Herlocher ML, Hinson JM, Elliott MJ, Crisp A. Zanamivir in the prevention of influenza among healthy adults: a randomized controlled trial. JAMA. 1999;282:31–5. DOIPubMedGoogle Scholar
- Murphy KR, Eivindson A, Pauksens K, Stein WJ, Tellier G, Watts R, Efficacy and safety of inhaled Zanamivir for the treatment of influenza in patients with asthma or COPD–a double-blind, randomized, placebo-controlled, multicentre study. Clin Drug Investig. 2000;20:337–49. DOIGoogle Scholar
- Lalezari J, Campion K, Keene O, Silagy C. Zanamivir for the treatment of influenza A and B infection in high risk patients. Arch Intern Med. 2001;161:212–7. DOIPubMedGoogle Scholar
- Hayden FG, Gubareva LV, Monto AS, Klein TC, Elliott MJ, Hammond JM, Inhaled Zanamivir for the prevention of influenza in families. N Engl J Med. 2000;343:1282–9. DOIPubMedGoogle Scholar
- Welliver R, Monto AS, Carewicz O, Schatteman E, Hassman M, Hedrick J, Effectiveness of oseltamivir in preventing influenza in household contacts: a randomized clinical trial. JAMA. 2001;285:748–54. DOIPubMedGoogle Scholar
- Hayden FG, Atmar RL, Schilling M, Johnson C, Poretz D, Paar D, Use of the selective oral neuraminidase inhibitor to prevent influenza. N Engl J Med. 1999;341:1336–43. DOIPubMedGoogle Scholar
- Gubareva LV, Matrosovich MN, Brenner MK, Bethell RC, Webster RG. Evidence for zanamivir resistance in an immunocompromised child infected with influenza B virus. J Infect Dis. 1998;178:1257–62. DOIPubMedGoogle Scholar
- McNichol IR, McNichol JJ. Neuraminidase inhibitors: zanamivir and oseltamivir. Ann Pharmacother. 2001;35:57–70. DOIPubMedGoogle Scholar
- MacDonald L. New influenza drugs Zanamivir (Relenza) and oseltamivir (Tamiflu): unexpected serious reactions. CMAJ. 2000;163:879–81.PubMedGoogle Scholar
- Williamson JC. Respiratory distress associated with zanamivir. N Engl J Med. 2000;342:661–2. DOIPubMedGoogle Scholar
- Cliff AD. Statistical modeling of measles and influenza outbreaks. Stat Methods Med Res. 1993;2:43–73. DOIPubMedGoogle Scholar
- van Weel C. William Pickles Lecture 1992: what our practices teach us. Br J Gen Pract. 1992;42:206–9.PubMedGoogle Scholar
- Knottnerus JA, Metsemakers J, Höppener P, Limonard C. Chronic illness in the community and the concept of ‘social prevalence’. Fam Pract. 1992;9:15–21. DOIPubMedGoogle Scholar
- Lamberts H, Hofmans-Okkes I. Episode of care: a core concept in family practice. J Fam Pract. 1996;42:161–7.PubMedGoogle Scholar
- Bartelds AIM. Continue morbiditeits registration peilstations Nederland 1999. Utrecht (Netherlands): Netherlands Institute of Health Services Research; 2000.
- Tacken M, Braspenning J, van Paassen J, van den Hoogen H, de Bakker D, Grol R. Nine years of influenza vaccination in the Netherlands in general practice [in Dutch]. Huisarts Wet. 2000;43:566–7.
- Vademecum gezondheidsstatistiek, 1999 [in Dutch]. Statistics Netherlands, Heerlen (Netherlands): Voorburg; 1999.
- Baltussen RMPM, Reinders A, Sprenger MJW, Postma MJ, Jager JC, Ament AJHA, Estimating influenza related hospitalization in the Netherlands. Epidemiol Infect. 1998;121:129–38. DOIPubMedGoogle Scholar
- Sprenger MJW, Mulder PGH, Beyer WEP, van Strik R, Masurel N. Impact of influenza on mortality in relation to age and underlying disease, 1967–1989. Int J Epidemiol. 1993;22:334–40. DOIPubMedGoogle Scholar
Figures
Tables
Cite This ArticleTable of Contents – Volume 9, Number 5—May 2003
| EID Search Options |
|---|
|
|
|
|
|
|

Please use the form below to submit correspondence to the authors or contact them at the following address:
Address for correspondence: Marianne L.L. van Genugten, Department for Health Services Research, National Institute for Public Health and the Environment, P.O. Box 1, 3720 BA Bilthoven, the Netherlands; fax: +31 30 2744466
Top