Volume 14, Number 8—August 2008
Dispatch
Pathogenicity of Highly Pathogenic Avian Influenza Virus (H5N1) in Adult Mute Swans
Abstract
Adult, healthy mute swans were experimentally infected with highly pathogenic avian influenza virus A/Cygnus cygnus/Germany/R65/2006 subtype H5N1. Immunologically naive birds died, whereas animals with preexisting, naturally acquired avian influenza virus–specific antibodies became infected asymptomatically and shed virus. Adult mute swans are highly susceptible, excrete virus, and can be clinically protected by preexposure immunity.
Since 2002, highly pathogenic avian influenza (HPAI) subtype H5N1 viruses have spread from endemically infected areas of Southeast Asia to Europe and Africa and infected poultry and wild birds. Especially in Europe, swans proved to be the most frequently affected wild bird species (1). Recently, Brown et al. (2) inoculated juvenile mute swans and confirmed that they are the most likely swan species to transmit HPAI (H5N1). Nevertheless, it remains unclear if an age-related susceptibility exists as it does for ducks (3). In addition, a more detailed knowledge regarding the role of preinfection with low-pathogenicity avian influenza virus is required. Our study was designed to answer these questions by experimental infection of adult mute swans (Cygnus olor).
All experiments with HPAI virus A/Cygnus cygnus/Germany/R65/2006 subtype H5N1 (4) were conducted under Biosaftey Level (BSL) 3+ conditions (trial approval LVL M-V/TSD/7221.3-1.1-003/07). The immunologically naive mute swans, 1–4 years of age, were divided into 2 groups. The high-dose group was inoculated oculo-oronasally with 106 50% egg infectious dose (EID)50/animal (n = 4); 1 additional naive contact swan was included in this group. The low-dose group received 104 EID50/animal (n = 5); 2 additional swans were in contact with this group. Two additional animals had preexposure avian influenza virus–specific antibody titers and were included in the high-dose group; 1 was also dedicated as contact animal.
In most birds the clinical signs were inconspicuous after HPAI virus (H5N1) inoculation. However, 3 swans exhibited severe neurologic disorders, including opisthotonus, torticollis, and ataxia. In addition, 3 animals died suddenly without any clinical signs developing. The incubation period for the high-dose group was at least 4 days. All swans of this group died or had to be humanely killed 5–9 days postinoculation (DPI) (Figure 1, panels A, B). The minimum incubation period of the low-dose group was 7 days (Figure 1, panel A). Only 1 animal of the low-dose group survived until the end of the trial (21 DPI), and all other swans of the group succumbed between 8 and 14 DPI.
Oropharyngeal and cloacal swab samples were collected daily in Dulbecco modified Eagle medium supplemented with 5% fetal calf serum and antimicrobial drugs. All individual swabs were tested by real-time reverse transcription–PCR (5) specific for subtype H5N1, and the genomic load was semiquantified by the cycle threshold (Ct) value. Infectivity titers of swab samples were calculated as the 50% tissue culture infectious dose/mL on Madin-Darby canine kidney (MDCK) cells (collection of cell lines in veterinary medicine, Friedrich-Loeffler-Institut, Südufer Insel Riems, RIE83).
Viral RNA as well as replicating virus could be detected from 1 until 6 DPI in oropharyngeal swabs of the high-dose group (Figure 1, panel C). The Ct values ranged from 17 to 33. The swans of the low-dose group excreted infectious virus from 3 until 11 DPI in oropharyngeal swabs (Figure 1, panel D), and real-time reverse transcription–PCR detected viral RNA in this group from 3 until 14 DPI. Virus excretion from cloacal swabs was demonstrated from 2 to 6 DPI in the high-dose group (Figure 1, panel C) and 4–10 DPI in the low-dose group (Figure 1, panel D). Viral RNA detection from cloacal swabs were positive 3 days longer than titration in cell culture (low-dose group; Figure 1, panel D). Maximum duration of viral shedding per individual swan was 6 days in both groups for cloacal and tracheal swab samples. Virus excretion of the contact animals was delayed, but the quantities of excreted virus were similar to those of the inoculated animals. One adult male swan of the low-dose group was the only surviving naive swan; his excretion pattern was delayed and shortened compared to other swans, which received the same virus dosage.
Sera were collected at 0, 7, 14, and 21 DPI from surviving swans as well as on the day of euthanasia. Serum samples were heat inactivated at 56°C for 30 min and subsequently nucleoprotein antibody ELISA, serum neutralization test (SNT), and hemagglutination-inhibition (HI) test were performed. Results of serologic tests from the day of euthanasia or the last day of serum collection before death are shown in Table 1. The comparison of the 3 serologic tests showed that the nucleoprotein-specific ELISA was the most sensitive assay, showing positive results as early as 5 DPI. All but 4 swan sera were positive in the nucleoprotein antibody ELISA after HPAI virus (H5N1) inoculation. Six of 8 ELISA-positive serum samples also exhibited neutralizing antibodies, whereas only 4 of 8 ELISA-positive serum samples exhibited positive HI titers against the challenge virus antigen. However, even postinfection antibody titers >400 SNT or 128 HI, respectively, did not protect swans from dying (Table 1). The 1 surviving swan developed antibody titers >1,000 SNT or 500 HI at the end of the experiment.
Two mute swans showed positive or questionable results in a nucleoprotein-specific ELISA before inoculation. The neutralizing activity of sera of both animals against HPAI virus (H5N1) was low, with titers of 10 and 3, respectively; no specific HI titers were detected. After HPAI virus (H5N1) inoculation, both swans survived without any clinical signs. When these swans were compared with the inoculated naïve swans, viral shedding was delayed and had a shorter duration with reduced viral loads (Table 2).
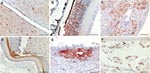
Gross pathology showed widespread hemorrhages as predominant lesions in both infected groups. Ecchymoses were especially present within the myocardium, submeningeally in the brain, in the peritracheal connective tissue, and within the lungs. Petechiae were seen in the pancreas, liver, and subcutis and on serosal surfaces. Only 2 swans exhibited multifocal to coalescent foci of coagulative necrosis in the pancreas. Table 1 summarizes semiquantified viral RNA loads in comparison to immunohistochemical detection of avian influenza virus nucleoprotein in different tissues. Immunostaining for avian influenza virus antigen was positive in 11 of 14 animals and confined to 3 locations: neuronal, epithelial, and endothelial (Figure 2, Table 1). In all animals, a strong neuronal infection could be observed with viral antigen in the cytoplasm and nuclei of neurons, glial cells, and ependymal cells in the brain, spinal cord, and eye (Table 1, Figure 2, panels A, B). Peripheral nerves, e.g., innervating the adrenal glands, the ovary, or area located adjacent to the cecal tonsil also stained positive. Some swans showed immunoreactivity in epithelial cells, e.g., of the pancreas, adrenal glands, ovaries, liver (Figure 2, panel C), feather follicles, and nasal cavity (Figure 2, panels D, E). Endothelia of different organs stained strongly positive only in 3 swans (Figure 2, panel F); in addition, these animals exhibited very high loads of viral genome in all tissue samples (Table 1).
We demonstrated that few adult mute swans might have the ability to survive infection with HPAI virus (H5N1). Surviviors would most likely be older swans in good health infected with a low dosage (e.g., <104 EID50/animal). However, because of viral shedding for several days without showing severe clinical symptoms, adult mute swans could play a key role in the spread of HPAI virus (H5N1), a conclusion that contradicts those of other investigators (8–10). Gross and histologic lesions in infected swans were independent of dosage, age, or sex of infected swans. Two parallel courses of pathogenesis with predominantly endothelial (n = 3) or epithelial/neuronal (n = 9) infections were distinguishable. Both forms have been described (11). However, normally only 1 dominant type was observed, depending on the experimental conditions (e.g., species, age of the animals, virus strain). At least 2 swans with the endotheliotropic course of infection were negative for avian influenza virus–specific antibodies. Thus, failure to mount an early antibody response might be responsible for or promote infection of the vasculature. In contrast, preexisting avian influenza virus–specific antibodies can be an efficient modulator of the outcome of an infection with HPAI virus (H5N1).
Dr Kalthoff is a veterinarian at the Institute of Diagnostic Virology, Friedrich-Loeffler-Institut, Greifswald-Insel Riems. Her research interests are focused on pathogenesis and vaccine development.
Acknowledgments
We thank M. Grawe and G. Busch for expert technical assistance, T. Arnold and G. Bauer for excellent animal care, M. Durban for sample preparation, and R. Wäckerlin for training in swan handling.
This study was funded by the Federal Ministry of Food, Agriculture and Consumer Protection (BMELV), Germany (FSI project no. 1-4.1).
References
- Terregino C, Milani A, Capua I, Marino AM, Cavaliere N. Highly pathogenic avian influenza H5N1 subtype in mute swans in Italy. Vet Rec. 2006;158:491.PubMedGoogle Scholar
- Brown JD, Stallknecht DE, Swayne DE. Experimental infection of swans and geese with highly pathogenic avian influenza virus (H5N1) of Asian lineage. Emerg Infect Dis. 2008;14:136–42. DOIPubMedGoogle Scholar
- Swayne DE, Pantin-Jackwood M. Pathogenicity of avian influenza viruses in poultry. Dev Biol (Basel). 2006;124:61–7.PubMedGoogle Scholar
- Weber S, Harder T, Starick E, Beer M, Werner O, Hoffmann B, Molecular analysis of highly pathogenic avian influenza virus of subtype H5N1 isolated from wild birds and mammals in northern Germany. J Gen Virol. 2007;88:554–8. DOIPubMedGoogle Scholar
- Hoffmann B, Harder T, Starick E, Depner K, Werner O, Beer M. Rapid and highly sensitive pathotyping of avian influenza A H5N1 virus by using real-time reverse transcription-PCR. J Clin Microbiol. 2007;45:600–3. DOIPubMedGoogle Scholar
- Rowe T, Abernathy RA, Hu-Primmer J, Thompson WW, Lu X, Lim W, Detection of antibody to avian influenza A (H5N1) virus in human serum by using a combination of serologic assays. J Clin Microbiol. 1999;37:937–43.PubMedGoogle Scholar
- World Organization for Animal Health. Manual of diagnostic tests for terrestrial animals, 5th edition. 2004 [cited 2007 Aug 27]. Available from http://www.oie.int/eng/normes/mmanual/a_00037.htm
- Nagy A, Machova J, Hornickova J, Tomci M, Nagl I, Horyna B, Highly pathogenic avian influenza virus subtype H5N1 in mute swans in the Czech Republic. Vet Microbiol. 2007;120:9–16. DOIPubMedGoogle Scholar
- Pálmai N, Erdélyi K, Bálint A, Márton L, Dán A, Deim Z, Pathobiology of highly pathogenic avian influenza virus (H5N1) infection in mute swans (Cygnus olor). Avian Pathol. 2007;36:245–9. DOIPubMedGoogle Scholar
- Weber TP, Stilianakis NI. Ecologic immunology of avian influenza (H5N1) in migratory birds. Emerg Infect Dis. 2007;13:1139–43.PubMedGoogle Scholar
- Swayne DE. Understanding the complex pathobiology of high pathogenicity avian influenza viruses in birds. Avian Dis. 2007;51(Suppl):242–9. DOIPubMedGoogle Scholar
Figures
Tables
Cite This ArticleTable of Contents – Volume 14, Number 8—August 2008
| EID Search Options |
|---|
|
|
|
|
|
|

Please use the form below to submit correspondence to the authors or contact them at the following address:
Martin Beer, Institute of Diagnostic Virology, Friedrich-Loeffler-Institut, Südufer 10, 17493 Greifswald-Insel Riems, Germany;
Top