Preprint
Article
Preliminary Report of Nationwide COVID-19 Vaccine Compensation in Taiwan
Altmetrics
Downloads
121
Views
60
Comments
0
A peer-reviewed article of this preprint also exists.
This version is not peer-reviewed
Preprints on COVID-19 and SARS-CoV-2
Abstract
The potential adverse effects of coronavirus disease 2019 (COVID-19) vaccinations raise public concerns. Data from Taiwan’s Vaccine Injury Compensation Program (VICP) can provide valuable insights. This study analyzed preliminary application data for COVID-19 vaccine compensation in Taiwan’s VICP, focusing on applicants receiving vaccines between March 2021 and June 2022. Among the 2941 adverse events, 113 cases (3.8%) were deemed causally associated with vaccination, 313 (10.6%) were indeterminate, and 2515 (85.5%) had no causal association. Nearly half (47.6%) of applicants were over 60 years old, and 76.6% had a history of preexisting chronic dis-eases. Among the 426 vaccine-associated or indeterminate cases, the most common causes were hematological diseases and thrombosis. There were 920 mortality cases reported, and 97.4% were unassociated with vaccination. Only five deaths were judged to be associated with the COVID-19 vaccination, all involving the adenovirus vector vaccine and thrombosis with thrombocytopenia syndrome. In conclusion, most compensation applications were not causally linked to vaccination. Compared to other countries, the number of applications in Taiwan’s VICP is relatively high. These findings may indicate a need to adjust the application requirements for compensation in Taiwan’s program.
Keywords:
Subject: Public Health and Healthcare - Primary Health Care
1. Introduction
The coronavirus disease 2019 (COVID-19) is a global epidemic characterized by severe outbreaks, and vaccination represents the most effective strategy to mitigate and ultimately end the pandemic. A variety of COVID-19 vaccines have received emergency use authorization to combat the infection and achieve widespread immunity across the entire population. Among these vaccines, one adenovirus vector COVID-19 vaccine, ChAdOx1 nCoV-19 (Oxford/AstraZeneca, AZ), two mRNA vaccines, mRNA-1273 (Moderna, MDN) and BNT162b2 (Pfizer/BioNTech, BNT), and one protein-based vaccine, MVC-COV1901 (Medigen, MVC), all have received authorization for emergency use in Taiwan since February 2021 [1]. AZ was the first vaccine available in Taiwan, starting in March 2021 [1]. Subsequently, the MDN vaccine was introduced in June 2021, followed by the MVC vaccine in August 2021, and the BNT vaccine in September 2021 [2]. Billions of doses of COVID-19 vaccines have been administered worldwide in the fight against severe acute respiratory syndrome coronavirus 2. While many have lauded the efficiency of these newly developed vaccines, a considerable number of individuals remain skeptical. This skepticism has been exacerbated by a surge of reports on vaccine-related complications [3,4]. Vaccine refusal could be attributable to distrust in health authorities, low confidence in vaccines, misconceptions, and political beliefs [5]. In Taiwan, applications for the Vaccine Injury Compensation Program (VICP) are free and easy to access, leading to a relatively high number of applications compared to other countries. The study aimed to provide clinicians with an overview of the preliminary data regarding the demographics, clinical manifestations, and symptoms of COVID-19 vaccine Adverse Events Following Immunization (AEFI) reported to Taiwan’s VICP.
2. Materials and Methods
We have compiled data on VICP judgments from 2021 to June 2023 for COVID-19 vaccination compensation claims. For each patient, the following data were collected: vaccination types, vaccination dosage, age, gender, preexisting chronic diseases, symptoms experienced, onset timing of symptoms, final diagnoses of symptoms after vaccination, prognosis, and VICP’s decision on the application. Preexisting chronic diseases were categorized into cardiovascular diseases (such as hypertension, coronary artery diseases, cardiac arrhythmia, congenital cardiac malformation, etc.), endocrine/metabolic diseases (such as diabetes mellitus, hyperlipidemia, thyroid disorders, etc.), urinary system diseases (such as renal failure on dialysis, hydronephrosis, renal stones, renal tumors, etc.), neurological diseases (such as stroke, cerebral hemorrhage, epilepsy, dementia, Parkinsonism, psychiatric disorders, etc.), and others (such as hematological disorders, gastrointestinal and hepatobiliary disorders, chronic respiratory tract diseases, ophthalmic disorders, ear, nose, and throat disorders, dermatological disorders, orthopedic disorders, gynecological diseases, etc.). The compensation claim diagnoses were classified into categories, such as cardiovascular diseases, neurological diseases, infectious diseases, hematological diseases, dermatological diseases, anaphylaxis, and others. Apart from anaphylaxis, the final diagnoses of symptoms following vaccination applications were grouped into five main categories: hematological, dermatological, neurological, cardiovascular diseases, and others. This study was approved by the Mackay Hospital Institutional Review Board (21MMHIS408e), and all participant’s informed consent are waived following the Helsinki Declaration.
The VICP committee determined the association between adverse events and COVID-19 vaccines by reviewing patients’ medical charts and vaccination histories. Compensation was awarded based on the established association and the severity of the health damage incurred. Based on VICP’s judgments, the association between adverse events and the vaccines was categorized into three groups: causally associated, indeterminate, and unassociated. Cases deemed associated or indeterminate required special attention, and they were grouped together for further analysis compared to the unassociated cases. For fatal cases, additional analyses were conducted, including the examination of autopsy results. Statistical analysis was performed using the Statistical Package for the Social Sciences (Version 26.0, SPSS, Inc., Chicago, Illinois, USA). The chi-squared test was used, as appropriate, to assess differences between categorical variables. A p-value of less than 0.05 was considered statistically significant.
3. Results
3.1. Demographics of the Applicants
From 2021 to June 2023, a total of 2941 cases inoculated with COVID-19 vaccines between March 2021 and June 2022 applied for the VICP. The distribution of cases by vaccine type was as follows: 1590 (54.1%) after AZ vaccine, 782 (26.6%) after MDN vaccine, 426 (14.5%) after BNT vaccine, and 143 (4.9%) after MVC vaccine (Figure S1). Among the applicants, 1547 (52.6%) were male, and 1394 (47.4%) were female. The most common age group ranged from 40 to 79 years old, with nearly half (47.6%) of cases being over 60 years old (Figure 1). Out of the total cases, 2202 individuals (74.8%) had complete previous three-year medical records available in the VICP data. Preexisting chronic diseases were identified in more than three-fourths (76.6%) of these individuals. Cardiovascular diseases were the most prevalent preexisting condition, affecting 51.3% of individuals, followed by endocrine/metabolic diseases (40.2%), neurological diseases (30.0%), and urinary system diseases (11.9%). Comorbidities were common, with only 23.3% of cases having a single preexisting disease, while the majority had multiple preexisting chronic conditions.
3.2. Adverse Events Following Vaccination
Approximately one-third (31.6%) of AEFI occurred within one day after the administration of the COVID-19 vaccine, with 42.9% occurring between one day and one week, 23.5% between two and six weeks, and 2.1% more than six weeks postvaccination (Figure S2). Figure 2 summarizes the diagnoses for compensation claims and the associated vaccine types. The most common claimed problems were related to the cardiovascular system, accounting for 24.7% (758 cases) of reported issues. This category included 379 cases (12.3%) of arrhythmia or heart failure, 286 cases (9.3%) of coronary artery disease or myocardial infarction, 55 cases (1.8%) of myocarditis or pericarditis, and 38 cases (1.2%) of aortic dissection. Manifestations related to the nervous system amounted to 691 cases (22.5%), including 477 cases (15.5%) of stroke, cerebral hemorrhage, or hypoxic brain lesions; 34 cases (1.1%) of Guillain–Barre syndrome (GBS); 12 cases (0.4%) of encephalitis; and 168 cases (5.5%) of peripheral nervous system disorders, such as facial palsy, hearing loss, or dizziness. Infection-related manifestations amounted to 389 cases (12.7%), including 260 cases (8.5%) of pneumonia or sepsis and 129 cases (4.2%) of local cellulitis. Thrombocytopenia and thrombosis were reported in 210 cases (6.8%), urticaria and other skin allergic reactions in 92 cases (3%), and anaphylaxis in six cases (0.2%).
3.3. Vaccine Types and Associations
Of the reported AEFI, 113 cases (3.8%) were classified as causally associated with vaccination, 313 cases (10.6%) were indeterminate, and 2515 cases (85.5%) were deemed unassociated. Table 1 summarizes the demographic and clinical characteristics between the combined associated/indeterminate cases and unassociated cases. The combined associated/indeterminate group had more female and younger patients with fewer preexisting chronic diseases and lower mortality rates, but no significant differences in vaccine-symptom onset intervals. On the contrary, the unassociated group had higher MDN vaccination rates and lower AZ vaccination rates.
Among the 426 associated/indeterminate cases, the AZ vaccine accounted for 259 cases, with 91.9% (238 cases) following the first dose and 8.1% (21 cases) following the second dose. However, the MDN vaccine accounted for 82 cases, with 61.0% (50 cases) following the first dose, 35.4% (29 cases) following the second dose, and 3.6% (three cases) following the third dose. Furthermore, the BNT vaccine accounted only for 65 cases, with 81.5% (53 cases) following the first dose, 17% (11 cases) following the second dose, and 1.5% (one case) following the third dose. Finally, the MVC vaccine accounted for 20 cases, with 70.0% (14 cases) following the first dose and 30.0% (six cases) following the second dose.
Among the associated/indeterminate group, hematological diseases were the most common diagnoses (75 cases, 17.2%), followed by dermatological diseases (62 cases, 14.3%), neurological diseases (57 cases, 13.1%), and cardiovascular diseases (52 cases, 12%) (Figure 3, Figures S3 and S4). All cases of hematological diseases were inoculated with AZ vaccine, with 94.7% occurring after the first dose. Twenty-five cases of thrombosis with thrombocytopenia syndrome (TTS) were diagnosed, five of which resulted in death. The time intervals between vaccine administration and symptom onset were between one and 15 days in these five patients. Most dermatological cases (58, 93.5%) were diagnosed as urticaria. GBS was reported in 43, with 36 occurring after the AZ vaccination and seven after the MDN vaccination. Similarly, 35 cases of myocarditis/pericarditis were identified, with 15 occurring after the BNT vaccination, 12 after the MDN vaccination, and eight after the AZ vaccination. Anaphylaxis occurred in six cases, including one case after AZ vaccination, three cases after MDN vaccination, and two cases after MVC vaccination.
3.4. Fatal Cases
There were 920 fatal cases, including 357 (38.8%) over 80 years old, 367 (39.9%) between 60–79 years, 149 (16.2%) between 40–59 years, 39 (4.2%) between 21–39 years, and eight (0.9%) less than 20 years (Figure S5). Cardiovascular diseases were the leading cause of death (473 cases, 49.7%), followed by infection diseases (203 cases, 21.3%), neurological diseases (107 cases, 11.2%), and hematological diseases (26 cases, 2.7%) (Figure S6). Most of the fatal cases (896 cases, 97.4%) were unassociated with vaccination, while five cases (0.5%, four females and one male) were judged as causally associated with vaccination. All five cases occurred after the first dose of the AZ vaccine and were diagnosed with TTS. There were 19 cases (2.1%) judged as having an indeterminate association with vaccination, including 15 cases of cardiovascular diseases (heart failure [n = 9], myocarditis [n = 5], and acute myocardial infarction [n = 1]), three cases of hematological diseases (subdural hemorrhage due to thrombocytopenia [n = 2] and idiopathic thrombocytopenia purpura [n = 1]), and one case of anaphylactic shock.
3.5. Autopsy Cases
Autopsies were performed on 198 fatal cases, with none linked to vaccination. Among these, 162 cases (81.8%) had preexisting diseases, and 95 cases (47.9%) had new, unrelated conditions identified. Cardiovascular diseases were the most common newly identified condition (38.9%), followed by neurological diseases (15.8%), pulmonary diseases (12.6%), renal disease (1.1%), and others (14.7%). In 16 autopsy cases (16.8%), deaths were classified as unnatural due to chocking, trauma, intoxication, and other causes.
4. Discussion
Vaccination is a cornerstone in controlling the spread of severe acute respiratory syndrome coronavirus 2 and saving lives. However, like any medication, AEFI can occur. To compensate individuals who experience serious AEFI potentially linked to COVID-19 vaccines, many countries have established VICPs. Due to the rapid development of the pandemic, COVID-19 vaccines were swiftly authorized for emergency use, further raising doubts about their safety [5]. Taiwan’s VICP stands out for its unique features, offering free and easy application processes and providing subsidies for autopsies in suspected vaccine-related deaths. These factors contribute to a high volume of applications, potentially overwhelming the review process. To quickly gain insight into the association between AEFI and COVID-19 vaccines, we analyzed preliminary data from Taiwan’s VICP.
The number of vaccine compensation applicants is likely correlated with the number of vaccine doses administered. This study primarily focused on cases involving early recipients, resulting in a significantly higher proportion of applications for first doses compared to subsequent doses. The AZ vaccine was the first COVID-19 vaccine authorized in Taiwan [1]. A higher number of vaccine compensation applicants in this study does not necessarily equate to a higher risk of adverse events. People are more likely to attribute health problems to vaccination if symptoms appear soon after inoculation. In this study, nearly one-third of AEFI occurred within one day of vaccination, and almost two-thirds occurred within one week.
Elderly individuals with preexisting health conditions are more susceptible to experiencing AEFI. The presence of comorbidities further complicates the assessment of causality. Patients with conditions such as hypertension, poorly controlled diabetes, or end-stage renal disease on dialysis are more prone to sudden health deterioration, constituting a significant proportion of applicants. In Taiwan, the National Health Insurance System provides access to medical records for the past three years, which the VICP committee uses to review vaccines’ medical histories. However, limitations in recordkeeping may result in underreporting of preexisting conditions.
Acute symptoms from cardiovascular or neurological conditions can deteriorate rapidly and are more likely to be misattributed to vaccination, leading to a higher rate of compensation claims. Cardiovascular diseases account for approximately one-quarter and neurological conditions for over one-fifth of the applications in this study. Infectious diseases, logically unrelated to vaccination, still constitute one-eighth of reports, possibly due to postvaccination fever raising suspicion among applicants. Beyond the higher number of people initially receiving the AZ vaccine, another factor contributing to a higher rate of compensation claims from AZ vaccine recipients is likely the public awareness of possible thrombosis events after AZ vaccination, which was heavily publicized during the program’s rollout.
Standards for assessing the association between COVID-19 vaccines and AEFI vary globally [6,7]. Our findings revealed that the majority of AEFI (85.5%) were not attributed to the vaccine, suggesting a relatively high safety profile. Many cases were deemed indeterminate due to symptom timing, clinical presentation, or insufficient supporting data. Since more population-based studies on the relationship between vaccination and AEFI have been published, these judgments may be revised.
A higher proportion of females were observed in the associated or indeterminate group, which may be linked to AZ vaccines with hematological complications. Older individuals with underlying health conditions are more likely to have non-vaccine-related reasons for AEFI, resulting in most cases being unassociated. In this study, thrombosis events are predominantly associated with the AZ vaccine, consistent with previous research [8,9]. AEFI complaints were more common after the first dose of the AZ vaccine, aligning with existing literature [10]. Despite reports of higher AEFI rates after the second dose of mRNA vaccines [2], our study found more AEFI after the first dose of mRNA vaccines. This discrepancy may be due to our focus on the initial data from the COVID-19 vaccine compensation cases. Nonetheless, even within this limited timeframe, a trend toward a higher rate of AEFI after the second dose of the MDN vaccine compared to the AZ vaccine was observed in this study.
The most documented reactions associated with COVID-19 vaccines include thrombosis/TTS [11,12], myocarditis/pericarditis [13,14], GBS [14,15], and anaphylaxis [16]. TTS, a rare but serious adverse event linked mostly to the AZ vaccine, is characterized by low platelet counts and blood clots in large blood vessels [5]. Previous studies suggest two main mechanisms: the action of anti-platelet factor 4 antibodies and direct interaction between the adenovirus vector and platelets [17,18]. In our study, myocarditis and pericarditis are more commonly associated with BNT and MDN vaccines, consistent with the higher proportion of cardiovascular complications, including myocarditis and pericarditis, reported with mRNA vaccines [3,19,20]. The suspected mechanisms for COVID-19 mRNA vaccine-induced myocarditis involve hyperimmunity, potentially due to mRNA immune reactivity, the production of antibodies to SARS-CoV-2 spike glycoproteins cross-reacting with myocardial contractile proteins, and hormonal differences [21,22]. Neurological disorders such as GBS or encephalitis can be challenging to link to the vaccine [23]. Diagnosis often relies on the timing of onset and the exclusion of other diagnoses, leading to some cases being categorized as indeterminate [24]. Similarly, urticaria and certain skin allergic reactions can fall into this category [25]. Anaphylaxis is diagnosed based on clinical presentation and occurs rarely with COVID-19 vaccines, typically without lasting effects [26].
The vast majority of death cases reviewed are not linked to the COVID-19 vaccination. Older individuals with preexisting health conditions have a higher mortality rate, and most deaths are unrelated to vaccination, often attributed to the exacerbation of the preexisting illnesses. Autopsies have confirmed these findings and sometimes uncovered preexisting health problems. Occasionally, autopsies may reveal unnatural causes of death. Taiwan’s VICP offers funeral subsidies to promote autopsies, a unique feature of the program.
Our study has several limitations. The VICP is a passive reporting system, which may result in underreporting. In Taiwan, the government encourages people to report adverse events following the COVID-19 vaccination through a free and convenient reporting system. However, retrospective medical records may have missing data, affecting the accuracy of determining the association between the vaccine and adverse events. We have only analyzed approximately one-third of the total applicants, so our findings are preliminary and may not represent the full scope of the situation. As new data on COVID-19 vaccines continue to emerge, decisions made by the VICP may change based on updated evidence from published literature.
5. Conclusions
This is a preliminary report, and more data and time are required to investigate additional cases before drawing a definitive conclusion. Based on the current information from Taiwan’s VICP, severe adverse reactions associated with COVID-19 vaccines are rare. Therefore, the COVID-19 vaccination can be considered safe. The VICP is currently overwhelmed by a large volume of applications, which cannot be resolved in a short time frame. It may be advisable to consider implementing more stringent regulations for compensation applications, especially for cases that are clearly unrelated.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org, Figure S1: Types and doses of COVID-19 vaccines applied for compensation; Figure S2: Time interval from vaccine administration to symptom onset and their association with the vaccination; Figure S3: Diagnoses of problems and vaccine types in associated cases; Figure S4: Diagnoses of problems and vaccine types in indeterminate cases; Figure S5: Age distribution of fatal cases; Figure S6: Diagnoses of problems in fatal cases.
Author Contributions
Conceptualization, N.C.C.; methodology, N.C.C. and Y.A.L.; software, Y.A.L.; validation, H.F.Y., C.H. and C.Y.L.; formal analysis, N.C.C and Y.A.L.; resources, N.C.C, H.F.Y. and C.H.; writing—Y.A.L. and N.C.C.; visualization, Y.A.L and N.C.C.; supervision, N.C.C.; project administration, N.C.C. All authors have read and agreed to the published version of the manuscript.”
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board of MacKay Memorial Hospital (protocol code 21MMHIS408e, approved on March 17, 2022)
Informed Consent Statement
When the applicant submits an application for VICP, they have already consented to the relevant medical records being used within the scope of the application. Our data are anonymized. Thus, patient consent was waived.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Acknowledgments
We thank the members of the Taiwan Vaccine Injury Compensation Program committee for their evaluation of the association between the COVID-19 vaccine and possible adverse reactions.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Food and Drug Administration, M.o.H.a.W. TFDA granted emergency use authorization (EUA) for four COVID-19 vaccines in Taiwan. 2021. Available online: https://www.mohw.gov.tw/cp-4745-64023-2.html (accessed on 30 March 2024).
- Su, W.J.; Arnold Chan, K.; Chuang, J.H.; Wang, T.A.; Chen, S.F.; Chang, Y.C.; Chen, M.Y.; Chang, C.C.; Yang, C.H. Acute reactions after a homologous primary COVID-19 vaccination series: Analysis of Taiwan V-Watch data. Vaccine 2023, 41, 2853–2859. [Google Scholar] [CrossRef] [PubMed]
- Goddard, K.; Lewis, N.; Fireman, B.; Weintraub, E.; Shimabukuro, T.; Zerbo, O.; Boyce, T.G.; Oster, M.E.; Hanson, K.E.; Donahue, J.G.; et al. Risk of myocarditis and pericarditis following BNT162b2 and mRNA-1273 COVID-19 vaccination. Vaccine 2022, 40, 5153–5159. [Google Scholar] [CrossRef] [PubMed]
- Rosenblum, H.G.; Gee, J.; Liu, R.; Marquez, P.L.; Zhang, B.; Strid, P.; Abara, W.E.; McNeil, M.M.; Myers, T.R.; Hause, A.M.; et al. Safety of mRNA vaccines administered during the initial 6 months of the US COVID-19 vaccination programme: An observational study of reports to the Vaccine Adverse Event Reporting System and v-safe. Lancet Infect Dis 2022, 22, 802–812. [Google Scholar] [CrossRef] [PubMed]
- Lun, P.; Ning, K.; Wang, Y.; Ma, T.S.W.; Flores, F.P.; Xiao, X.; Subramaniam, M.; Abdin, E.; Tian, L.; Tsang, T.K.; et al. COVID-19 Vaccination Willingness and Reasons for Vaccine Refusal. JAMA Netw Open 2023, 6, e2337909. [Google Scholar] [CrossRef] [PubMed]
- Schönborn, L.; Pavord, S.; Chen, V.M.Y.; Pai, M.; Gwarzo, D.H.; Buttery, J.; Munoz, F.M.; Tran, H.; Greinacher, A.; Law, B. Thrombosis with thrombocytopenia syndrome (TTS) and vaccine-induced immune thrombocytopenia and thrombosis (VITT): Brighton Collaboration case definitions and guidelines for data collection, analysis, and presentation of immunisation safety data. Vaccine 2024, 42, 1799–1811. [Google Scholar] [CrossRef] [PubMed]
- Sexson Tejtel, S.K.; Munoz, F.M.; Al-Ammouri, I.; Savorgnan, F.; Guggilla, R.K.; Khuri-Bulos, N.; Phillips, L.; Engler, R.J.M. Myocarditis and pericarditis: Case definition and guidelines for data collection, analysis, and presentation of immunization safety data. Vaccine 2022, 40, 1499–1511. [Google Scholar] [CrossRef] [PubMed]
- Bayas, A.; Menacher, M.; Christ, M.; Behrens, L.; Rank, A.; Naumann, M. Bilateral superior ophthalmic vein thrombosis, ischaemic stroke, and immune thrombocytopenia after ChAdOx1 nCoV-19 vaccination. Lancet 2021, 397, e11. [Google Scholar] [CrossRef] [PubMed]
- Schultz, N.H.; Sørvoll, I.H.; Michelsen, A.E.; Munthe, L.A.; Lund-Johansen, F.; Ahlen, M.T.; Wiedmann, M.; Aamodt, A.H.; Skattør, T.H.; Tjønnfjord, G.E.; et al. Thrombosis and Thrombocytopenia after ChAdOx1 nCoV-19 Vaccination. N Engl J Med 2021, 384, 2124–2130. [Google Scholar] [CrossRef] [PubMed]
- Eterafi, M.; Fouladi, N.; Golizadeh, M.; Shaker, H.; Matin, S.; Safarzadeh, E. Reported side-effects following Oxford/AstraZeneca COVID-19 vaccine in the north-west province, Iran: A cross-sectional study. PLoS ONE 2024, 19, e0296669. [Google Scholar] [CrossRef] [PubMed]
- Marcucci, R.; Marietta, M. Vaccine-induced thrombotic thrombocytopenia: The elusive link between thrombosis and adenovirus-based SARS-CoV-2 vaccines. Intern Emerg Med 2021, 16, 1113–1119. [Google Scholar] [CrossRef] [PubMed]
- Günther, A.; Brämer, D.; Pletz, M.W.; Kamradt, T.; Baumgart, S.; Mayer, T.E.; Baier, M.; Autsch, A.; Mawrin, C.; Schönborn, L.; et al. Complicated Long Term Vaccine Induced Thrombotic Immune Thrombocytopenia-A Case Report. Vaccines (Basel) 2021, 9. [Google Scholar] [CrossRef] [PubMed]
- Weintraub, E.S.; Oster, M.E.; Klein, N.P. Myocarditis or Pericarditis Following mRNA COVID-19 Vaccination. JAMA Network Open 2022, 5, e2218512. [Google Scholar] [CrossRef]
- Faksova, K.; Walsh, D.; Jiang, Y.; Griffin, J.; Phillips, A.; Gentile, A.; Kwong, J.C.; Macartney, K.; Naus, M.; Grange, Z.; et al. COVID-19 vaccines and adverse events of special interest: A multinational Global Vaccine Data Network (GVDN) cohort study of 99 million vaccinated individuals. Vaccine 2024. [Google Scholar] [CrossRef]
- Abara, W.E.; Gee, J.; Marquez, P.; Woo, J.; Myers, T.R.; DeSantis, A.; Baumblatt, J.A.G.; Woo, E.J.; Thompson, D.; Nair, N.; et al. Reports of Guillain-Barré Syndrome After COVID-19 Vaccination in the United States. JAMA Netw Open 2023, 6, e2253845. [Google Scholar] [CrossRef] [PubMed]
- Shimabukuro, T.T.; Cole, M.; Su, J.R. Reports of Anaphylaxis After Receipt of mRNA COVID-19 Vaccines in the US-December 14, 2020-January 18, 2021. Jama 2021, 325, 1101–1102. [Google Scholar] [CrossRef] [PubMed]
- Rzymski, P.; Perek, B.; Flisiak, R. Thrombotic Thrombocytopenia after COVID-19 Vaccination: In Search of the Underlying Mechanism. Vaccines (Basel) 2021, 9. [Google Scholar] [CrossRef] [PubMed]
- Stone, D.; Liu, Y.; Shayakhmetov, D.; Li, Z.Y.; Ni, S.; Lieber, A. Adenovirus-platelet interaction in blood causes virus sequestration to the reticuloendothelial system of the liver. J Virol 2007, 81, 4866–4871. [Google Scholar] [CrossRef] [PubMed]
- Oster, M.E.; Shay, D.K.; Su, J.R.; Gee, J.; Creech, C.B.; Broder, K.R.; Edwards, K.; Soslow, J.H.; Dendy, J.M.; Schlaudecker, E.; et al. Myocarditis Cases Reported After mRNA-Based COVID-19 Vaccination in the US From December 2020 to August 2021. JAMA 2022, 327, 331–340. [Google Scholar] [CrossRef]
- Alami, A.; Krewski, D.; Farhat, N.; Mattison, D.; Wilson, K.; Gravel, C.A.; Farrell, P.J.; Crispo, J.A.G.; Haddad, N.; Perez-Lloret, S.; et al. Risk of myocarditis and pericarditis in mRNA COVID-19-vaccinated and unvaccinated populations: A systematic review and meta-analysis. BMJ Open 2023, 13, e065687. [Google Scholar] [CrossRef]
- Vojdani, A.; Kharrazian, D. Potential antigenic cross-reactivity between SARS-CoV-2 and human tissue with a possible link to an increase in autoimmune diseases. Clin Immunol 2020, 217, 108480. [Google Scholar] [CrossRef]
- Heymans, S.; Eriksson, U.; Lehtonen, J.; Cooper, L.T., Jr. The Quest for New Approaches in Myocarditis and Inflammatory Cardiomyopathy. J Am Coll Cardiol 2016, 68, 2348–2364. [Google Scholar] [CrossRef]
- Woo, E.J.; Mba-Jonas, A.; Dimova, R.B.; Alimchandani, M.; Zinderman, C.E.; Nair, N. Association of Receipt of the Ad26.COV2.S COVID-19 Vaccine With Presumptive Guillain-Barré Syndrome, February-July 2021. Jama 2021, 326, 1606–1613. [Google Scholar] [CrossRef]
- Garg, R.K.; Paliwal, V.K. Spectrum of neurological complications following COVID-19 vaccination. Neurol Sci 2022, 43, 3–40. [Google Scholar] [CrossRef] [PubMed]
- Magen, E.; Yakov, A.; Green, I.; Israel, A.; Vinker, S.; Merzon, E. Chronic spontaneous urticaria after BNT162b2 mRNA (Pfizer-BioNTech) vaccination against SARS-CoV-2. Allergy Asthma Proc 2022, 43, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Jaggers, J.; Wolfson, A.R. mRNA COVID-19 Vaccine Anaphylaxis: Epidemiology, Risk Factors, and Evaluation. Curr Allergy Asthma Rep 2023, 23, 195–200. [Google Scholar] [CrossRef] [PubMed]
Figure 1.
Age distribution of applicants and their association with COVID-19 vaccines.
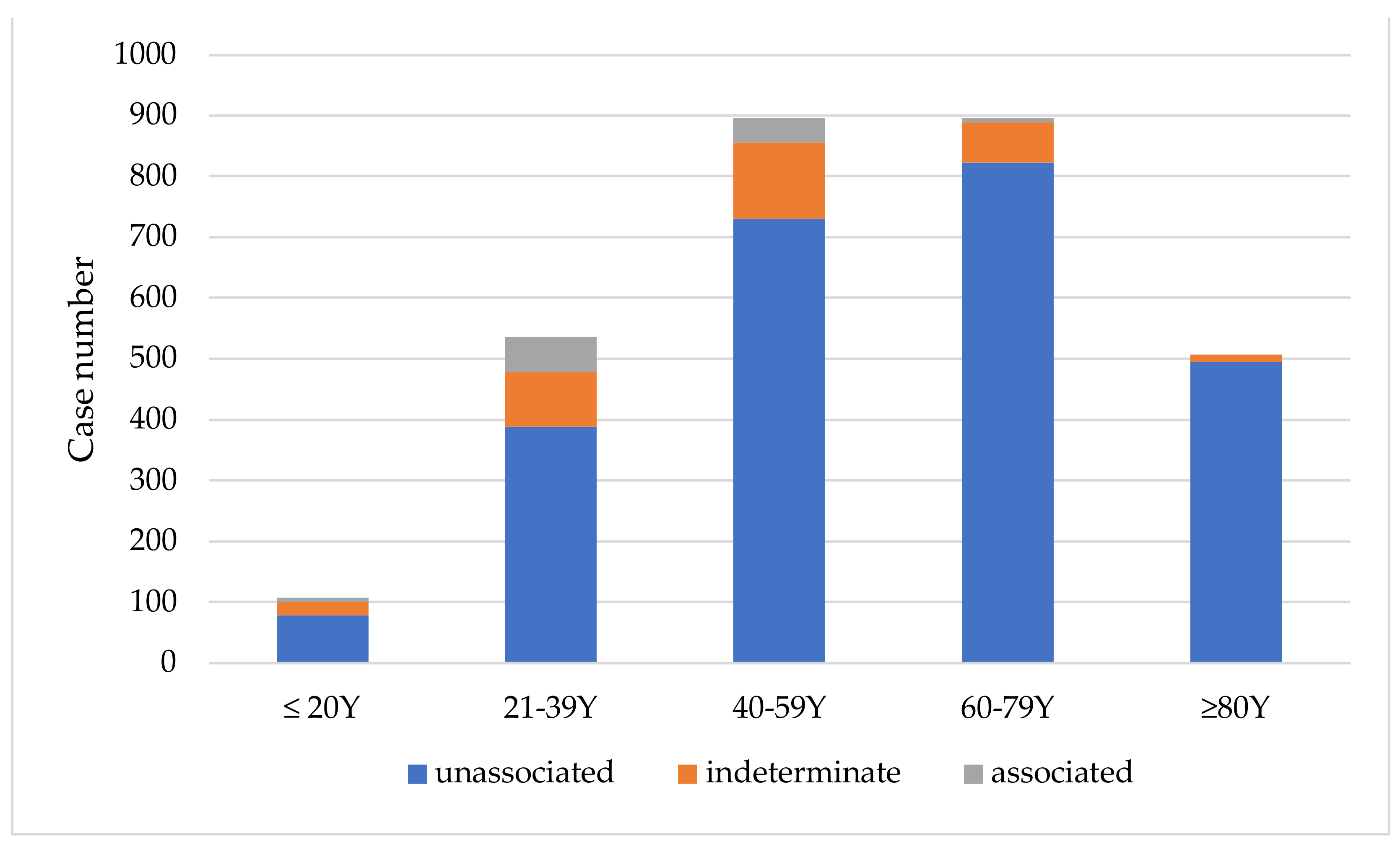
Figure 2.
Diagnoses associated with compensation claims by vaccine type.

Figure 3.
Diagnoses of Adverse Events Following Immunization in combined associated/indeterminate applicants by vaccine type.
Figure 3.
Diagnoses of Adverse Events Following Immunization in combined associated/indeterminate applicants by vaccine type.
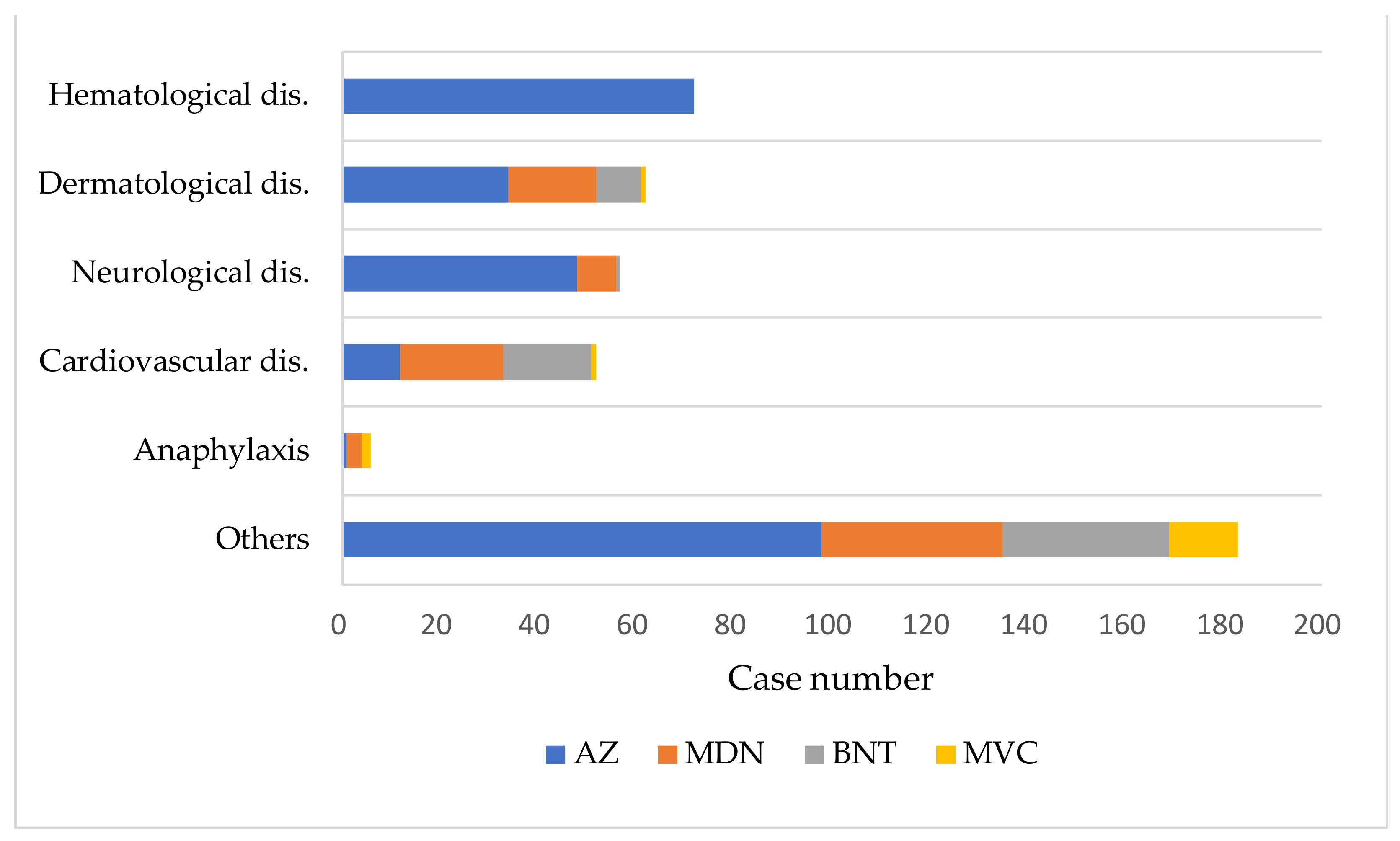
Table 1.
Comparison between associated/indeterminate and unassociated groups.
| Associated/ Indeterminate n = 426 |
Unassociated n = 2515 |
||
|---|---|---|---|
| Case Number (%) | Case Number (%) | p | |
| Female | 238 (55.9) | 1156 (46.0) | <0.05 |
| Age (years) | 40.7 ± 21.8 | 58.2 ± 22.5 | <0.05 |
| Preexisting chronic disease* | 209 (66.1) | 1477 (78.3) | <0.05 |
| Symptom onset time interval (days) | 4.5 ± 7.2 | 5.4 ± 11.3 | 0.129 |
| Death | 11 (2.6) | 324 (12.9) | <0.05 |
| Vaccine | |||
| AZ | 259 (60.8) | 1331 (52.9) | <0.05 |
| MDN | 82 (19.2) | 700 (27.8) | <0.05 |
| BNT | 65 (15.3) | 361 (14.4) | 0.603 |
| MVC | 20 (4.7) | 123 (4.9) | 0.711 |
* Among 2202 cases with complete medical records logged in Vaccine Injury Compensation Program data.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Copyright: This open access article is published under a Creative Commons CC BY 4.0 license, which permit the free download, distribution, and reuse, provided that the author and preprint are cited in any reuse.
supplementary.pdf (155.89KB )
Submitted:
02 May 2024
Posted:
07 May 2024
You are already at the latest version
Alerts
A peer-reviewed article of this preprint also exists.
supplementary.pdf (155.89KB )
This version is not peer-reviewed
Preprints on COVID-19 and SARS-CoV-2
Submitted:
02 May 2024
Posted:
07 May 2024
You are already at the latest version
Alerts
Abstract
The potential adverse effects of coronavirus disease 2019 (COVID-19) vaccinations raise public concerns. Data from Taiwan’s Vaccine Injury Compensation Program (VICP) can provide valuable insights. This study analyzed preliminary application data for COVID-19 vaccine compensation in Taiwan’s VICP, focusing on applicants receiving vaccines between March 2021 and June 2022. Among the 2941 adverse events, 113 cases (3.8%) were deemed causally associated with vaccination, 313 (10.6%) were indeterminate, and 2515 (85.5%) had no causal association. Nearly half (47.6%) of applicants were over 60 years old, and 76.6% had a history of preexisting chronic dis-eases. Among the 426 vaccine-associated or indeterminate cases, the most common causes were hematological diseases and thrombosis. There were 920 mortality cases reported, and 97.4% were unassociated with vaccination. Only five deaths were judged to be associated with the COVID-19 vaccination, all involving the adenovirus vector vaccine and thrombosis with thrombocytopenia syndrome. In conclusion, most compensation applications were not causally linked to vaccination. Compared to other countries, the number of applications in Taiwan’s VICP is relatively high. These findings may indicate a need to adjust the application requirements for compensation in Taiwan’s program.
Keywords:
Subject: Public Health and Healthcare - Primary Health Care
1. Introduction
The coronavirus disease 2019 (COVID-19) is a global epidemic characterized by severe outbreaks, and vaccination represents the most effective strategy to mitigate and ultimately end the pandemic. A variety of COVID-19 vaccines have received emergency use authorization to combat the infection and achieve widespread immunity across the entire population. Among these vaccines, one adenovirus vector COVID-19 vaccine, ChAdOx1 nCoV-19 (Oxford/AstraZeneca, AZ), two mRNA vaccines, mRNA-1273 (Moderna, MDN) and BNT162b2 (Pfizer/BioNTech, BNT), and one protein-based vaccine, MVC-COV1901 (Medigen, MVC), all have received authorization for emergency use in Taiwan since February 2021 [1]. AZ was the first vaccine available in Taiwan, starting in March 2021 [1]. Subsequently, the MDN vaccine was introduced in June 2021, followed by the MVC vaccine in August 2021, and the BNT vaccine in September 2021 [2]. Billions of doses of COVID-19 vaccines have been administered worldwide in the fight against severe acute respiratory syndrome coronavirus 2. While many have lauded the efficiency of these newly developed vaccines, a considerable number of individuals remain skeptical. This skepticism has been exacerbated by a surge of reports on vaccine-related complications [3,4]. Vaccine refusal could be attributable to distrust in health authorities, low confidence in vaccines, misconceptions, and political beliefs [5]. In Taiwan, applications for the Vaccine Injury Compensation Program (VICP) are free and easy to access, leading to a relatively high number of applications compared to other countries. The study aimed to provide clinicians with an overview of the preliminary data regarding the demographics, clinical manifestations, and symptoms of COVID-19 vaccine Adverse Events Following Immunization (AEFI) reported to Taiwan’s VICP.
2. Materials and Methods
We have compiled data on VICP judgments from 2021 to June 2023 for COVID-19 vaccination compensation claims. For each patient, the following data were collected: vaccination types, vaccination dosage, age, gender, preexisting chronic diseases, symptoms experienced, onset timing of symptoms, final diagnoses of symptoms after vaccination, prognosis, and VICP’s decision on the application. Preexisting chronic diseases were categorized into cardiovascular diseases (such as hypertension, coronary artery diseases, cardiac arrhythmia, congenital cardiac malformation, etc.), endocrine/metabolic diseases (such as diabetes mellitus, hyperlipidemia, thyroid disorders, etc.), urinary system diseases (such as renal failure on dialysis, hydronephrosis, renal stones, renal tumors, etc.), neurological diseases (such as stroke, cerebral hemorrhage, epilepsy, dementia, Parkinsonism, psychiatric disorders, etc.), and others (such as hematological disorders, gastrointestinal and hepatobiliary disorders, chronic respiratory tract diseases, ophthalmic disorders, ear, nose, and throat disorders, dermatological disorders, orthopedic disorders, gynecological diseases, etc.). The compensation claim diagnoses were classified into categories, such as cardiovascular diseases, neurological diseases, infectious diseases, hematological diseases, dermatological diseases, anaphylaxis, and others. Apart from anaphylaxis, the final diagnoses of symptoms following vaccination applications were grouped into five main categories: hematological, dermatological, neurological, cardiovascular diseases, and others. This study was approved by the Mackay Hospital Institutional Review Board (21MMHIS408e), and all participant’s informed consent are waived following the Helsinki Declaration.
The VICP committee determined the association between adverse events and COVID-19 vaccines by reviewing patients’ medical charts and vaccination histories. Compensation was awarded based on the established association and the severity of the health damage incurred. Based on VICP’s judgments, the association between adverse events and the vaccines was categorized into three groups: causally associated, indeterminate, and unassociated. Cases deemed associated or indeterminate required special attention, and they were grouped together for further analysis compared to the unassociated cases. For fatal cases, additional analyses were conducted, including the examination of autopsy results. Statistical analysis was performed using the Statistical Package for the Social Sciences (Version 26.0, SPSS, Inc., Chicago, Illinois, USA). The chi-squared test was used, as appropriate, to assess differences between categorical variables. A p-value of less than 0.05 was considered statistically significant.
3. Results
3.1. Demographics of the Applicants
From 2021 to June 2023, a total of 2941 cases inoculated with COVID-19 vaccines between March 2021 and June 2022 applied for the VICP. The distribution of cases by vaccine type was as follows: 1590 (54.1%) after AZ vaccine, 782 (26.6%) after MDN vaccine, 426 (14.5%) after BNT vaccine, and 143 (4.9%) after MVC vaccine (Figure S1). Among the applicants, 1547 (52.6%) were male, and 1394 (47.4%) were female. The most common age group ranged from 40 to 79 years old, with nearly half (47.6%) of cases being over 60 years old (Figure 1). Out of the total cases, 2202 individuals (74.8%) had complete previous three-year medical records available in the VICP data. Preexisting chronic diseases were identified in more than three-fourths (76.6%) of these individuals. Cardiovascular diseases were the most prevalent preexisting condition, affecting 51.3% of individuals, followed by endocrine/metabolic diseases (40.2%), neurological diseases (30.0%), and urinary system diseases (11.9%). Comorbidities were common, with only 23.3% of cases having a single preexisting disease, while the majority had multiple preexisting chronic conditions.
3.2. Adverse Events Following Vaccination
Approximately one-third (31.6%) of AEFI occurred within one day after the administration of the COVID-19 vaccine, with 42.9% occurring between one day and one week, 23.5% between two and six weeks, and 2.1% more than six weeks postvaccination (Figure S2). Figure 2 summarizes the diagnoses for compensation claims and the associated vaccine types. The most common claimed problems were related to the cardiovascular system, accounting for 24.7% (758 cases) of reported issues. This category included 379 cases (12.3%) of arrhythmia or heart failure, 286 cases (9.3%) of coronary artery disease or myocardial infarction, 55 cases (1.8%) of myocarditis or pericarditis, and 38 cases (1.2%) of aortic dissection. Manifestations related to the nervous system amounted to 691 cases (22.5%), including 477 cases (15.5%) of stroke, cerebral hemorrhage, or hypoxic brain lesions; 34 cases (1.1%) of Guillain–Barre syndrome (GBS); 12 cases (0.4%) of encephalitis; and 168 cases (5.5%) of peripheral nervous system disorders, such as facial palsy, hearing loss, or dizziness. Infection-related manifestations amounted to 389 cases (12.7%), including 260 cases (8.5%) of pneumonia or sepsis and 129 cases (4.2%) of local cellulitis. Thrombocytopenia and thrombosis were reported in 210 cases (6.8%), urticaria and other skin allergic reactions in 92 cases (3%), and anaphylaxis in six cases (0.2%).
3.3. Vaccine Types and Associations
Of the reported AEFI, 113 cases (3.8%) were classified as causally associated with vaccination, 313 cases (10.6%) were indeterminate, and 2515 cases (85.5%) were deemed unassociated. Table 1 summarizes the demographic and clinical characteristics between the combined associated/indeterminate cases and unassociated cases. The combined associated/indeterminate group had more female and younger patients with fewer preexisting chronic diseases and lower mortality rates, but no significant differences in vaccine-symptom onset intervals. On the contrary, the unassociated group had higher MDN vaccination rates and lower AZ vaccination rates.
Among the 426 associated/indeterminate cases, the AZ vaccine accounted for 259 cases, with 91.9% (238 cases) following the first dose and 8.1% (21 cases) following the second dose. However, the MDN vaccine accounted for 82 cases, with 61.0% (50 cases) following the first dose, 35.4% (29 cases) following the second dose, and 3.6% (three cases) following the third dose. Furthermore, the BNT vaccine accounted only for 65 cases, with 81.5% (53 cases) following the first dose, 17% (11 cases) following the second dose, and 1.5% (one case) following the third dose. Finally, the MVC vaccine accounted for 20 cases, with 70.0% (14 cases) following the first dose and 30.0% (six cases) following the second dose.
Among the associated/indeterminate group, hematological diseases were the most common diagnoses (75 cases, 17.2%), followed by dermatological diseases (62 cases, 14.3%), neurological diseases (57 cases, 13.1%), and cardiovascular diseases (52 cases, 12%) (Figure 3, Figures S3 and S4). All cases of hematological diseases were inoculated with AZ vaccine, with 94.7% occurring after the first dose. Twenty-five cases of thrombosis with thrombocytopenia syndrome (TTS) were diagnosed, five of which resulted in death. The time intervals between vaccine administration and symptom onset were between one and 15 days in these five patients. Most dermatological cases (58, 93.5%) were diagnosed as urticaria. GBS was reported in 43, with 36 occurring after the AZ vaccination and seven after the MDN vaccination. Similarly, 35 cases of myocarditis/pericarditis were identified, with 15 occurring after the BNT vaccination, 12 after the MDN vaccination, and eight after the AZ vaccination. Anaphylaxis occurred in six cases, including one case after AZ vaccination, three cases after MDN vaccination, and two cases after MVC vaccination.
3.4. Fatal Cases
There were 920 fatal cases, including 357 (38.8%) over 80 years old, 367 (39.9%) between 60–79 years, 149 (16.2%) between 40–59 years, 39 (4.2%) between 21–39 years, and eight (0.9%) less than 20 years (Figure S5). Cardiovascular diseases were the leading cause of death (473 cases, 49.7%), followed by infection diseases (203 cases, 21.3%), neurological diseases (107 cases, 11.2%), and hematological diseases (26 cases, 2.7%) (Figure S6). Most of the fatal cases (896 cases, 97.4%) were unassociated with vaccination, while five cases (0.5%, four females and one male) were judged as causally associated with vaccination. All five cases occurred after the first dose of the AZ vaccine and were diagnosed with TTS. There were 19 cases (2.1%) judged as having an indeterminate association with vaccination, including 15 cases of cardiovascular diseases (heart failure [n = 9], myocarditis [n = 5], and acute myocardial infarction [n = 1]), three cases of hematological diseases (subdural hemorrhage due to thrombocytopenia [n = 2] and idiopathic thrombocytopenia purpura [n = 1]), and one case of anaphylactic shock.
3.5. Autopsy Cases
Autopsies were performed on 198 fatal cases, with none linked to vaccination. Among these, 162 cases (81.8%) had preexisting diseases, and 95 cases (47.9%) had new, unrelated conditions identified. Cardiovascular diseases were the most common newly identified condition (38.9%), followed by neurological diseases (15.8%), pulmonary diseases (12.6%), renal disease (1.1%), and others (14.7%). In 16 autopsy cases (16.8%), deaths were classified as unnatural due to chocking, trauma, intoxication, and other causes.
4. Discussion
Vaccination is a cornerstone in controlling the spread of severe acute respiratory syndrome coronavirus 2 and saving lives. However, like any medication, AEFI can occur. To compensate individuals who experience serious AEFI potentially linked to COVID-19 vaccines, many countries have established VICPs. Due to the rapid development of the pandemic, COVID-19 vaccines were swiftly authorized for emergency use, further raising doubts about their safety [5]. Taiwan’s VICP stands out for its unique features, offering free and easy application processes and providing subsidies for autopsies in suspected vaccine-related deaths. These factors contribute to a high volume of applications, potentially overwhelming the review process. To quickly gain insight into the association between AEFI and COVID-19 vaccines, we analyzed preliminary data from Taiwan’s VICP.
The number of vaccine compensation applicants is likely correlated with the number of vaccine doses administered. This study primarily focused on cases involving early recipients, resulting in a significantly higher proportion of applications for first doses compared to subsequent doses. The AZ vaccine was the first COVID-19 vaccine authorized in Taiwan [1]. A higher number of vaccine compensation applicants in this study does not necessarily equate to a higher risk of adverse events. People are more likely to attribute health problems to vaccination if symptoms appear soon after inoculation. In this study, nearly one-third of AEFI occurred within one day of vaccination, and almost two-thirds occurred within one week.
Elderly individuals with preexisting health conditions are more susceptible to experiencing AEFI. The presence of comorbidities further complicates the assessment of causality. Patients with conditions such as hypertension, poorly controlled diabetes, or end-stage renal disease on dialysis are more prone to sudden health deterioration, constituting a significant proportion of applicants. In Taiwan, the National Health Insurance System provides access to medical records for the past three years, which the VICP committee uses to review vaccines’ medical histories. However, limitations in recordkeeping may result in underreporting of preexisting conditions.
Acute symptoms from cardiovascular or neurological conditions can deteriorate rapidly and are more likely to be misattributed to vaccination, leading to a higher rate of compensation claims. Cardiovascular diseases account for approximately one-quarter and neurological conditions for over one-fifth of the applications in this study. Infectious diseases, logically unrelated to vaccination, still constitute one-eighth of reports, possibly due to postvaccination fever raising suspicion among applicants. Beyond the higher number of people initially receiving the AZ vaccine, another factor contributing to a higher rate of compensation claims from AZ vaccine recipients is likely the public awareness of possible thrombosis events after AZ vaccination, which was heavily publicized during the program’s rollout.
Standards for assessing the association between COVID-19 vaccines and AEFI vary globally [6,7]. Our findings revealed that the majority of AEFI (85.5%) were not attributed to the vaccine, suggesting a relatively high safety profile. Many cases were deemed indeterminate due to symptom timing, clinical presentation, or insufficient supporting data. Since more population-based studies on the relationship between vaccination and AEFI have been published, these judgments may be revised.
A higher proportion of females were observed in the associated or indeterminate group, which may be linked to AZ vaccines with hematological complications. Older individuals with underlying health conditions are more likely to have non-vaccine-related reasons for AEFI, resulting in most cases being unassociated. In this study, thrombosis events are predominantly associated with the AZ vaccine, consistent with previous research [8,9]. AEFI complaints were more common after the first dose of the AZ vaccine, aligning with existing literature [10]. Despite reports of higher AEFI rates after the second dose of mRNA vaccines [2], our study found more AEFI after the first dose of mRNA vaccines. This discrepancy may be due to our focus on the initial data from the COVID-19 vaccine compensation cases. Nonetheless, even within this limited timeframe, a trend toward a higher rate of AEFI after the second dose of the MDN vaccine compared to the AZ vaccine was observed in this study.
The most documented reactions associated with COVID-19 vaccines include thrombosis/TTS [11,12], myocarditis/pericarditis [13,14], GBS [14,15], and anaphylaxis [16]. TTS, a rare but serious adverse event linked mostly to the AZ vaccine, is characterized by low platelet counts and blood clots in large blood vessels [5]. Previous studies suggest two main mechanisms: the action of anti-platelet factor 4 antibodies and direct interaction between the adenovirus vector and platelets [17,18]. In our study, myocarditis and pericarditis are more commonly associated with BNT and MDN vaccines, consistent with the higher proportion of cardiovascular complications, including myocarditis and pericarditis, reported with mRNA vaccines [3,19,20]. The suspected mechanisms for COVID-19 mRNA vaccine-induced myocarditis involve hyperimmunity, potentially due to mRNA immune reactivity, the production of antibodies to SARS-CoV-2 spike glycoproteins cross-reacting with myocardial contractile proteins, and hormonal differences [21,22]. Neurological disorders such as GBS or encephalitis can be challenging to link to the vaccine [23]. Diagnosis often relies on the timing of onset and the exclusion of other diagnoses, leading to some cases being categorized as indeterminate [24]. Similarly, urticaria and certain skin allergic reactions can fall into this category [25]. Anaphylaxis is diagnosed based on clinical presentation and occurs rarely with COVID-19 vaccines, typically without lasting effects [26].
The vast majority of death cases reviewed are not linked to the COVID-19 vaccination. Older individuals with preexisting health conditions have a higher mortality rate, and most deaths are unrelated to vaccination, often attributed to the exacerbation of the preexisting illnesses. Autopsies have confirmed these findings and sometimes uncovered preexisting health problems. Occasionally, autopsies may reveal unnatural causes of death. Taiwan’s VICP offers funeral subsidies to promote autopsies, a unique feature of the program.
Our study has several limitations. The VICP is a passive reporting system, which may result in underreporting. In Taiwan, the government encourages people to report adverse events following the COVID-19 vaccination through a free and convenient reporting system. However, retrospective medical records may have missing data, affecting the accuracy of determining the association between the vaccine and adverse events. We have only analyzed approximately one-third of the total applicants, so our findings are preliminary and may not represent the full scope of the situation. As new data on COVID-19 vaccines continue to emerge, decisions made by the VICP may change based on updated evidence from published literature.
5. Conclusions
This is a preliminary report, and more data and time are required to investigate additional cases before drawing a definitive conclusion. Based on the current information from Taiwan’s VICP, severe adverse reactions associated with COVID-19 vaccines are rare. Therefore, the COVID-19 vaccination can be considered safe. The VICP is currently overwhelmed by a large volume of applications, which cannot be resolved in a short time frame. It may be advisable to consider implementing more stringent regulations for compensation applications, especially for cases that are clearly unrelated.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org, Figure S1: Types and doses of COVID-19 vaccines applied for compensation; Figure S2: Time interval from vaccine administration to symptom onset and their association with the vaccination; Figure S3: Diagnoses of problems and vaccine types in associated cases; Figure S4: Diagnoses of problems and vaccine types in indeterminate cases; Figure S5: Age distribution of fatal cases; Figure S6: Diagnoses of problems in fatal cases.
Author Contributions
Conceptualization, N.C.C.; methodology, N.C.C. and Y.A.L.; software, Y.A.L.; validation, H.F.Y., C.H. and C.Y.L.; formal analysis, N.C.C and Y.A.L.; resources, N.C.C, H.F.Y. and C.H.; writing—Y.A.L. and N.C.C.; visualization, Y.A.L and N.C.C.; supervision, N.C.C.; project administration, N.C.C. All authors have read and agreed to the published version of the manuscript.”
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board of MacKay Memorial Hospital (protocol code 21MMHIS408e, approved on March 17, 2022)
Informed Consent Statement
When the applicant submits an application for VICP, they have already consented to the relevant medical records being used within the scope of the application. Our data are anonymized. Thus, patient consent was waived.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Acknowledgments
We thank the members of the Taiwan Vaccine Injury Compensation Program committee for their evaluation of the association between the COVID-19 vaccine and possible adverse reactions.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Food and Drug Administration, M.o.H.a.W. TFDA granted emergency use authorization (EUA) for four COVID-19 vaccines in Taiwan. 2021. Available online: https://www.mohw.gov.tw/cp-4745-64023-2.html (accessed on 30 March 2024).
- Su, W.J.; Arnold Chan, K.; Chuang, J.H.; Wang, T.A.; Chen, S.F.; Chang, Y.C.; Chen, M.Y.; Chang, C.C.; Yang, C.H. Acute reactions after a homologous primary COVID-19 vaccination series: Analysis of Taiwan V-Watch data. Vaccine 2023, 41, 2853–2859. [Google Scholar] [CrossRef] [PubMed]
- Goddard, K.; Lewis, N.; Fireman, B.; Weintraub, E.; Shimabukuro, T.; Zerbo, O.; Boyce, T.G.; Oster, M.E.; Hanson, K.E.; Donahue, J.G.; et al. Risk of myocarditis and pericarditis following BNT162b2 and mRNA-1273 COVID-19 vaccination. Vaccine 2022, 40, 5153–5159. [Google Scholar] [CrossRef] [PubMed]
- Rosenblum, H.G.; Gee, J.; Liu, R.; Marquez, P.L.; Zhang, B.; Strid, P.; Abara, W.E.; McNeil, M.M.; Myers, T.R.; Hause, A.M.; et al. Safety of mRNA vaccines administered during the initial 6 months of the US COVID-19 vaccination programme: An observational study of reports to the Vaccine Adverse Event Reporting System and v-safe. Lancet Infect Dis 2022, 22, 802–812. [Google Scholar] [CrossRef] [PubMed]
- Lun, P.; Ning, K.; Wang, Y.; Ma, T.S.W.; Flores, F.P.; Xiao, X.; Subramaniam, M.; Abdin, E.; Tian, L.; Tsang, T.K.; et al. COVID-19 Vaccination Willingness and Reasons for Vaccine Refusal. JAMA Netw Open 2023, 6, e2337909. [Google Scholar] [CrossRef] [PubMed]
- Schönborn, L.; Pavord, S.; Chen, V.M.Y.; Pai, M.; Gwarzo, D.H.; Buttery, J.; Munoz, F.M.; Tran, H.; Greinacher, A.; Law, B. Thrombosis with thrombocytopenia syndrome (TTS) and vaccine-induced immune thrombocytopenia and thrombosis (VITT): Brighton Collaboration case definitions and guidelines for data collection, analysis, and presentation of immunisation safety data. Vaccine 2024, 42, 1799–1811. [Google Scholar] [CrossRef] [PubMed]
- Sexson Tejtel, S.K.; Munoz, F.M.; Al-Ammouri, I.; Savorgnan, F.; Guggilla, R.K.; Khuri-Bulos, N.; Phillips, L.; Engler, R.J.M. Myocarditis and pericarditis: Case definition and guidelines for data collection, analysis, and presentation of immunization safety data. Vaccine 2022, 40, 1499–1511. [Google Scholar] [CrossRef] [PubMed]
- Bayas, A.; Menacher, M.; Christ, M.; Behrens, L.; Rank, A.; Naumann, M. Bilateral superior ophthalmic vein thrombosis, ischaemic stroke, and immune thrombocytopenia after ChAdOx1 nCoV-19 vaccination. Lancet 2021, 397, e11. [Google Scholar] [CrossRef] [PubMed]
- Schultz, N.H.; Sørvoll, I.H.; Michelsen, A.E.; Munthe, L.A.; Lund-Johansen, F.; Ahlen, M.T.; Wiedmann, M.; Aamodt, A.H.; Skattør, T.H.; Tjønnfjord, G.E.; et al. Thrombosis and Thrombocytopenia after ChAdOx1 nCoV-19 Vaccination. N Engl J Med 2021, 384, 2124–2130. [Google Scholar] [CrossRef] [PubMed]
- Eterafi, M.; Fouladi, N.; Golizadeh, M.; Shaker, H.; Matin, S.; Safarzadeh, E. Reported side-effects following Oxford/AstraZeneca COVID-19 vaccine in the north-west province, Iran: A cross-sectional study. PLoS ONE 2024, 19, e0296669. [Google Scholar] [CrossRef] [PubMed]
- Marcucci, R.; Marietta, M. Vaccine-induced thrombotic thrombocytopenia: The elusive link between thrombosis and adenovirus-based SARS-CoV-2 vaccines. Intern Emerg Med 2021, 16, 1113–1119. [Google Scholar] [CrossRef] [PubMed]
- Günther, A.; Brämer, D.; Pletz, M.W.; Kamradt, T.; Baumgart, S.; Mayer, T.E.; Baier, M.; Autsch, A.; Mawrin, C.; Schönborn, L.; et al. Complicated Long Term Vaccine Induced Thrombotic Immune Thrombocytopenia-A Case Report. Vaccines (Basel) 2021, 9. [Google Scholar] [CrossRef] [PubMed]
- Weintraub, E.S.; Oster, M.E.; Klein, N.P. Myocarditis or Pericarditis Following mRNA COVID-19 Vaccination. JAMA Network Open 2022, 5, e2218512. [Google Scholar] [CrossRef]
- Faksova, K.; Walsh, D.; Jiang, Y.; Griffin, J.; Phillips, A.; Gentile, A.; Kwong, J.C.; Macartney, K.; Naus, M.; Grange, Z.; et al. COVID-19 vaccines and adverse events of special interest: A multinational Global Vaccine Data Network (GVDN) cohort study of 99 million vaccinated individuals. Vaccine 2024. [Google Scholar] [CrossRef]
- Abara, W.E.; Gee, J.; Marquez, P.; Woo, J.; Myers, T.R.; DeSantis, A.; Baumblatt, J.A.G.; Woo, E.J.; Thompson, D.; Nair, N.; et al. Reports of Guillain-Barré Syndrome After COVID-19 Vaccination in the United States. JAMA Netw Open 2023, 6, e2253845. [Google Scholar] [CrossRef] [PubMed]
- Shimabukuro, T.T.; Cole, M.; Su, J.R. Reports of Anaphylaxis After Receipt of mRNA COVID-19 Vaccines in the US-December 14, 2020-January 18, 2021. Jama 2021, 325, 1101–1102. [Google Scholar] [CrossRef] [PubMed]
- Rzymski, P.; Perek, B.; Flisiak, R. Thrombotic Thrombocytopenia after COVID-19 Vaccination: In Search of the Underlying Mechanism. Vaccines (Basel) 2021, 9. [Google Scholar] [CrossRef] [PubMed]
- Stone, D.; Liu, Y.; Shayakhmetov, D.; Li, Z.Y.; Ni, S.; Lieber, A. Adenovirus-platelet interaction in blood causes virus sequestration to the reticuloendothelial system of the liver. J Virol 2007, 81, 4866–4871. [Google Scholar] [CrossRef] [PubMed]
- Oster, M.E.; Shay, D.K.; Su, J.R.; Gee, J.; Creech, C.B.; Broder, K.R.; Edwards, K.; Soslow, J.H.; Dendy, J.M.; Schlaudecker, E.; et al. Myocarditis Cases Reported After mRNA-Based COVID-19 Vaccination in the US From December 2020 to August 2021. JAMA 2022, 327, 331–340. [Google Scholar] [CrossRef]
- Alami, A.; Krewski, D.; Farhat, N.; Mattison, D.; Wilson, K.; Gravel, C.A.; Farrell, P.J.; Crispo, J.A.G.; Haddad, N.; Perez-Lloret, S.; et al. Risk of myocarditis and pericarditis in mRNA COVID-19-vaccinated and unvaccinated populations: A systematic review and meta-analysis. BMJ Open 2023, 13, e065687. [Google Scholar] [CrossRef]
- Vojdani, A.; Kharrazian, D. Potential antigenic cross-reactivity between SARS-CoV-2 and human tissue with a possible link to an increase in autoimmune diseases. Clin Immunol 2020, 217, 108480. [Google Scholar] [CrossRef]
- Heymans, S.; Eriksson, U.; Lehtonen, J.; Cooper, L.T., Jr. The Quest for New Approaches in Myocarditis and Inflammatory Cardiomyopathy. J Am Coll Cardiol 2016, 68, 2348–2364. [Google Scholar] [CrossRef]
- Woo, E.J.; Mba-Jonas, A.; Dimova, R.B.; Alimchandani, M.; Zinderman, C.E.; Nair, N. Association of Receipt of the Ad26.COV2.S COVID-19 Vaccine With Presumptive Guillain-Barré Syndrome, February-July 2021. Jama 2021, 326, 1606–1613. [Google Scholar] [CrossRef]
- Garg, R.K.; Paliwal, V.K. Spectrum of neurological complications following COVID-19 vaccination. Neurol Sci 2022, 43, 3–40. [Google Scholar] [CrossRef] [PubMed]
- Magen, E.; Yakov, A.; Green, I.; Israel, A.; Vinker, S.; Merzon, E. Chronic spontaneous urticaria after BNT162b2 mRNA (Pfizer-BioNTech) vaccination against SARS-CoV-2. Allergy Asthma Proc 2022, 43, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Jaggers, J.; Wolfson, A.R. mRNA COVID-19 Vaccine Anaphylaxis: Epidemiology, Risk Factors, and Evaluation. Curr Allergy Asthma Rep 2023, 23, 195–200. [Google Scholar] [CrossRef] [PubMed]
Figure 1.
Age distribution of applicants and their association with COVID-19 vaccines.

Figure 2.
Diagnoses associated with compensation claims by vaccine type.

Figure 3.
Diagnoses of Adverse Events Following Immunization in combined associated/indeterminate applicants by vaccine type.
Figure 3.
Diagnoses of Adverse Events Following Immunization in combined associated/indeterminate applicants by vaccine type.

Table 1.
Comparison between associated/indeterminate and unassociated groups.
| Associated/ Indeterminate n = 426 |
Unassociated n = 2515 |
||
|---|---|---|---|
| Case Number (%) | Case Number (%) | p | |
| Female | 238 (55.9) | 1156 (46.0) | <0.05 |
| Age (years) | 40.7 ± 21.8 | 58.2 ± 22.5 | <0.05 |
| Preexisting chronic disease* | 209 (66.1) | 1477 (78.3) | <0.05 |
| Symptom onset time interval (days) | 4.5 ± 7.2 | 5.4 ± 11.3 | 0.129 |
| Death | 11 (2.6) | 324 (12.9) | <0.05 |
| Vaccine | |||
| AZ | 259 (60.8) | 1331 (52.9) | <0.05 |
| MDN | 82 (19.2) | 700 (27.8) | <0.05 |
| BNT | 65 (15.3) | 361 (14.4) | 0.603 |
| MVC | 20 (4.7) | 123 (4.9) | 0.711 |
* Among 2202 cases with complete medical records logged in Vaccine Injury Compensation Program data.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Copyright: This open access article is published under a Creative Commons CC BY 4.0 license, which permit the free download, distribution, and reuse, provided that the author and preprint are cited in any reuse.
Incidence and Nature of Adverse Events Following COVID-19 Second Boosters: Insights from Taiwan's Universal Vaccination Strategy
Ching-Hao Lin
et al.
,
2024
Risk of Blood Clots After COVID-19 Vaccination and Infection: A Risk-Benefit Analysis
Huong NQ Tran
et al.
,
2024
Who should be Prioritized for COVID-19 Vaccination in China? A Descriptive Study
Juan Yang
et al.
,
2020
MDPI Initiatives
Important Links
© 2024 MDPI (Basel, Switzerland) unless otherwise stated









