Abstract
Marchalina hellenica is a sap sucking scale insect endemic to the Aegean basin and it has been introduced to several regions in Greece and Turkey to increase pine honey production. It is also considered as a pest since heavy infestation may leave the host trees vulnerable to secondary pests. An understanding of its natural predators would facilitate planning biocontrol programs. Although there are several studies reporting the predators of M. hellenica in its native range, there is no study identifying those in its introduced range. We aimed to determine predators of M. hellenica in Burdur, one of its introduced sites in Turkey. We carried out sampling through regular visits in an M. hellenica-infested locality nearby Burdur Lake. Through field and laboratory observations, we identified 19 species predating upon M. hellenica. Comparing predators reported in previous studies in its native range and those we found in the present study showed that 12 of the species that we found are new reports for the species predating upon M. hellenica. The highest number of predator individuals belonged to the monophagous Neoleucopis kartliana. Myrrha octodecimguttata, Chilocorus bipustulatus and Harmonia quadripunctata were also the most frequently observed predators.
1 Introduction
The giant pine scale, Marchalina hellenica (Gennadius 1883; Hemiptera: Marchalinidae), is an economically important species for two contrasting reasons. First, it is the most significant of several honeydew-producing insects in Greece and Turkey; pine honey production relies mainly on M. hellenica honeydew in both the countries [1,2]. For this reason, it has been intentionally introduced at many natural pine forests in these countries and its population has increased locally [3,4,5]. Second, it is a pine pest as it feeds on pine sap and can cause increment loss, desiccation, branch dieback, increasing crown transparency and tree decline [5,6,7,8,9]. Furthermore, Petrakis et al. [9] showed that the giant pine scale can cause decrease in diversity of pine-forest-related insects. Therefore, its population size must be in a balance to provide the highest possible honey yield and the lowest possible damage to the pine ecosystem. However, this is not always possible due to climate warming and human-mediated introduction of the species, and it can turn to be a pest which may necessitate control measures to be taken. Being endemic to Greece and Western Turkey, M. hellenica has been introduced to the Italian island of Ischia and Australia, where it is accepted as a dangerous exotic species [10,11]. It has also been reported in Armenia, Georgia and Krasnodar and Sochi in Russia [12,13], although taxonomic status of the Caucasian population is controversial [14].
Predators and parasitoids play a significant role in suppressing the population size of forest pest insects [15]. Exotic species usually benefit from the enemy-free advantage of the new ecosystems during the early stages of the invasion [16]. This advantage can subside as the host-specific parasitoids and predators follow the invader or the generalist parasitoids and predators start to feed on the invader. Generalist parasitoids and predators can use “host/prey switching” strategy; thus, they can forage on the most abundant prey, the invader in the case of an invasion, in a habitat (e.g., ref. [17]).
The giant pine scale occurs almost in the entire western coasts of Turkey [4,18,19]. It has also been introduced into several regions in the country by beekeepers (Figure A1). Burdur basin, where the present study was undertaken, is one of those regions. Although the first report of M. hellenica in the region was in 2004 [20], the local beekeepers introduced the giant pine scale-infested branches into the region in the early 1990s from Muğla province ca. 200 km west of the study site (M. Bilgiç/Burdur Association of Beekeepers, personal communication). The species has been well established in this isolated basin during the last 30 years. However, it has not been investigated which predatory species prey upon M. hellenica during this period in its recent range. This information may be important for biological control against M. hellenica. Studies on the predators of the giant pine scale revealed 28 predaceous species (two of which being ectoparasitic) so far in Georgia, Greece and Turkey ([13,14,19] Süreyya and Hovasse 1931 cited in ref. [21,22,23,24]) with 3, 7 and 22 species, respectively (Table A1). Although the striking difference in the number of described predatory species among the three countries could be related to the number of studies conducted in each country, the natural history of M. hellenica or a combination of these factors, it is not in our scope to resolve this issue. However, our preliminary observations suggested that the list of predacious species from Turkey seems to be even longer. Accordingly, we asked the following questions in the present study: (1) What are the arthropod predator species in Burdur basin where M. hellenica has been an introduced species? (2) How different is the predator composition in the introduced range of M. hellenica from those in its natural range?
2 Materials and methods
The study area, Burdur Urban Forest (Southwestern Turkey), is located at an average altitude of 910 m (a.s.l., ±30 m; 37°41′30″N, 30°11′54″E) and at the northwestern aspect facing the lake (Figure 1). It is an isolated area of ca. 100 ha, consisting of mainly Pinus brutia, but also of P. nigra and P. pinea. Approximately 300 beehives have been placed in the area for pine honey production. Giant pine scale introductions were carried out intensely in the early 1990s in the region. The Brutian pine in the area was heavily infested not only by M. hellenica but also by bark beetles (mainly Mediterranean pine beetle, Orthotomicus erosus and pine shoot beetle, Tomicus destruens).
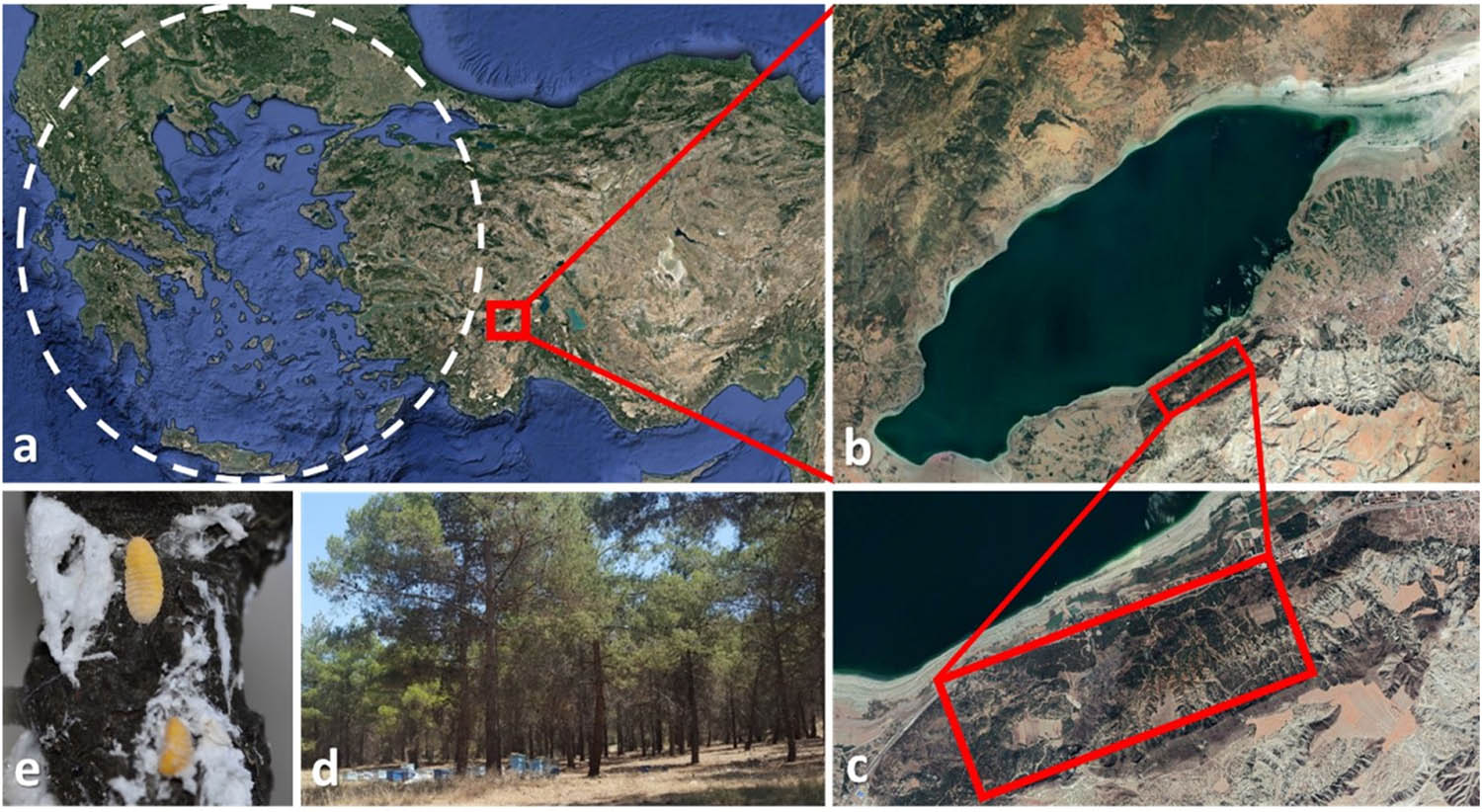
Study site. Dashed circle depicts the approximate natural distribution of Marchalina hellenica (a), Burdur Lake (b) and the study site near the lake (c), beehives in Pinus brutia stand in the study site (d) and M. hellenica adults on the stem bark (e).
We identified one site of heavy infestation around Burdur Lake in Burdur basin through visual inspection and visited this site (ca. 5 ha, 37°42′0.81″N–30°12′52.32″E; 37°41′49.78″N–30°12′27.65″E; 37°41′36.83″N–30°11′52.99″E; 37°41′26.94″N–30°11′44.08″E; 37°41′26.22″N–30°11′29.60″E) once in October 2017, 13 times between April and October 2018 and 5 times between March and May 2019 (a total of 19 visits). We always conducted the field visits between 11:00 am and 02:00 pm and during this period, we visited 30 trees which we randomly selected in each visit. We collected adult insects from the infested branches by using an entomological beating sheet and a mouth aspirator. We took advantage of the trap-logs placed in the stand in 2019 by the local department of forestry as a part of the management practices applied against O. erosus. We recorded and collected the insects preying on M. hellenica on the trap-logs. During the field observations, we also found pupae on the cottony secretions of M. hellenica. In order to identify them, we reared these pupae in plastic cages (680 mm × 465 mm × 360 mm, 80 lt) with a ventilated lid in the laboratory and recorded the adult emergences. Then we offered M. hellenica nymphs and adults to the adults emerging from the pupae collected from the field to observe predation in the laboratory. Although this was not a setting designed for a choice experiment, it provided us a chance to evaluate possible predation by the species whose immature stages were found in M. hellenica-related structures. Finally, we evaluated the collected species according to the relevant literature, and we discarded those which have not been shown to feed on Coccoidea. Thus, we provided evidence for predation through direct observation in the field and the laboratory, and literature records.
The criteria we used to determine predation on M. hellenica were as follows: (1) If the potential predator was seen feeding directly on M. hellenica (but not on its honeydew) in the field, then we identified the species as a predator. If not, we did not collect it. (2) If it was seen feeding directly on M. hellenica in the laboratory, then we identified it as a predator. If not, we discarded it. (3) If the predation could not be detected through field and laboratory observations but was previously reported in the literature, then we identified the considered species as a predator.
Taxonomical identification of the specimens confirmed by specialists is acknowledged at the end of the article. Specimens were stored at the Entomological Museum of Isparta University of Applied Sciences, Faculty of Forestry.
3 Results
We found 29 insect species during the study, but we eliminated ten species that do not normally prey on scale insects including the giant pine scale. As a result of field and laboratory observations, we identified 19 species (295 individuals) as predators of M. hellenica. Among them, one belonged to Acari, ten to Coleoptera, one to Diptera, five to Hemiptera and two to Neuroptera (Table 1, Figure 2). Detailed descriptions of the identified species are given below. Among all the predator species, the one with the highest number of individuals, both in the field and laboratory observations, was Neoleucopis kartliana (116 individuals), which was followed by Myrrha octodecimguttata (29), Chilocorus bipustulatus (26), Anthocoris nemorum (24) and Temnostethus reduvinus (21) (Table 1). The most frequently detected species was M. octodecimguttata which was recorded as 68.4% of all field visits. Other frequently detected species were C. bipustulatus (57.9%), Harmonia quadripunctata (47.4%), A. nemorum (42.1%) and T. reduvinus (42.1%) (Table 1). The highest number of predator species (7) was recorded on 22 April 2018 and 9 September 2018; whereas the highest number of predator individuals (55) was recorded on 18 March 2019, which was followed by the record on 12 April 2019 (36) and 12 May 2019 (32) (Figure 3). The lowest number of predator species (1) was recorded on 6 April 2018, 27 April 2019 and 19 May 2019; whereas the lowest number of predator individuals (1) was recorded on 6 April 2018 and 19 May 2019 (Figure 3).
Predators of Marchalina hellenica found in Burdur, Turkey
| No | Order | Family | Species | Number of individuals | Individual detection rate (%)* | Species detection rate (%)** |
|---|---|---|---|---|---|---|
| 1 | Acari | Anystidae | Anystis baccarum ‡ | 5 | 1.7 | 5.3 |
| 2 | Diptera | Chamaemyiidae | Neoleucopis kartliana | 116 | 39.3 | 21.1 |
| 3 | Coleoptera | Coccinellidae | Chilocorus bipustulatus ‡ | 26 | 8.8 | 57.9 |
| 4 | Harmonia quadripunctata | 16 | 5.4 | 47.4 | ||
| 5 | Myrrha octodecimguttata ‡ | 29 | 9.8 | 68.4 | ||
| 6 | Nephus quadrimaculatus +‡ | 5 | 1.7 | 21.1 | ||
| 7 | Novius cruentatus +‡ | 16 | 5.4 | 36.8 | ||
| 8 | Rodolia cardinalis ‡ | 1 | 0.3 | 5.3 | ||
| 9 | Scymnus pallipediformis +‡ | 1 | 0.3 | 5.3 | ||
| 10 | Scymnus subvillosus | 2 | 0.7 | 5.3 | ||
| 11 | Scymnus syriacus +‡ | 1 | 0.3 | 5.3 | ||
| 12 | Stethorus gilvifrons +‡ | 9 | 3.1 | 15.8 | ||
| 13 | Hemiptera | Anthocoridae | Anthocoris nemoralis +‡ | 1 | 0.3 | 5.3 |
| 14 | Anthocoris nemorum +‡ | 24 | 8.1 | 42.1 | ||
| 15 | Orius majusculus +‡ | 1 | 0.3 | 5.3 | ||
| 16 | Temnostethus reduvinus +‡ | 21 | 7.1 | 42.1 | ||
| 17 | Reduviidae | Nagusta goedelii +‡ | 1 | 0.3 | 5.3 | |
| 18 | Neuroptera | Chrysopidae | Chrysoperla carnea +‡ | 1 | 0.3 | 5.3 |
| 19 | Raphidioptera | Raphidiidae | Raphidia ambigua + | 19 | 6.4 | 5.3 |
*Percentage of detection of the individuals among all other species.
**Percentage of detection of the species in all field visits.
+New record as a predator of M. hellenica.
‡New record for Burdur region.
![Figure 2
Predators of Marchalina hellenica identified in Burdur, Turkey: (a) Anystis baccarum, (b) Neoleucopis kartliana, (c) Chilocorus bipustulatus [88], (d) Harmonia quadripunctata, (e) Myrrha octodecimguttata, (f) Nephus quadrimaculatus, (g) Novius cruentatus, (h) Rodolia cardinalis, (i) Scymnus pallipediformis, (j) S. subvillosus, (k) S. syriacus, (l) Stethorus gilvifrons, (m) Anthocoris nemoralis, (n) A. nemorum, (o) Temnostethus reduvinus, (p) Orius majusculus, (q) Nagusta goedelii, (r) Chrysoperla carnea and (s) Raphidia ambigua.](/https/www.degruyter.com/document/doi/10.1515/biol-2021-0066/asset/graphic/j_biol-2021-0066_fig_002.jpg)
Predators of Marchalina hellenica identified in Burdur, Turkey: (a) Anystis baccarum, (b) Neoleucopis kartliana, (c) Chilocorus bipustulatus [88], (d) Harmonia quadripunctata, (e) Myrrha octodecimguttata, (f) Nephus quadrimaculatus, (g) Novius cruentatus, (h) Rodolia cardinalis, (i) Scymnus pallipediformis, (j) S. subvillosus, (k) S. syriacus, (l) Stethorus gilvifrons, (m) Anthocoris nemoralis, (n) A. nemorum, (o) Temnostethus reduvinus, (p) Orius majusculus, (q) Nagusta goedelii, (r) Chrysoperla carnea and (s) Raphidia ambigua.
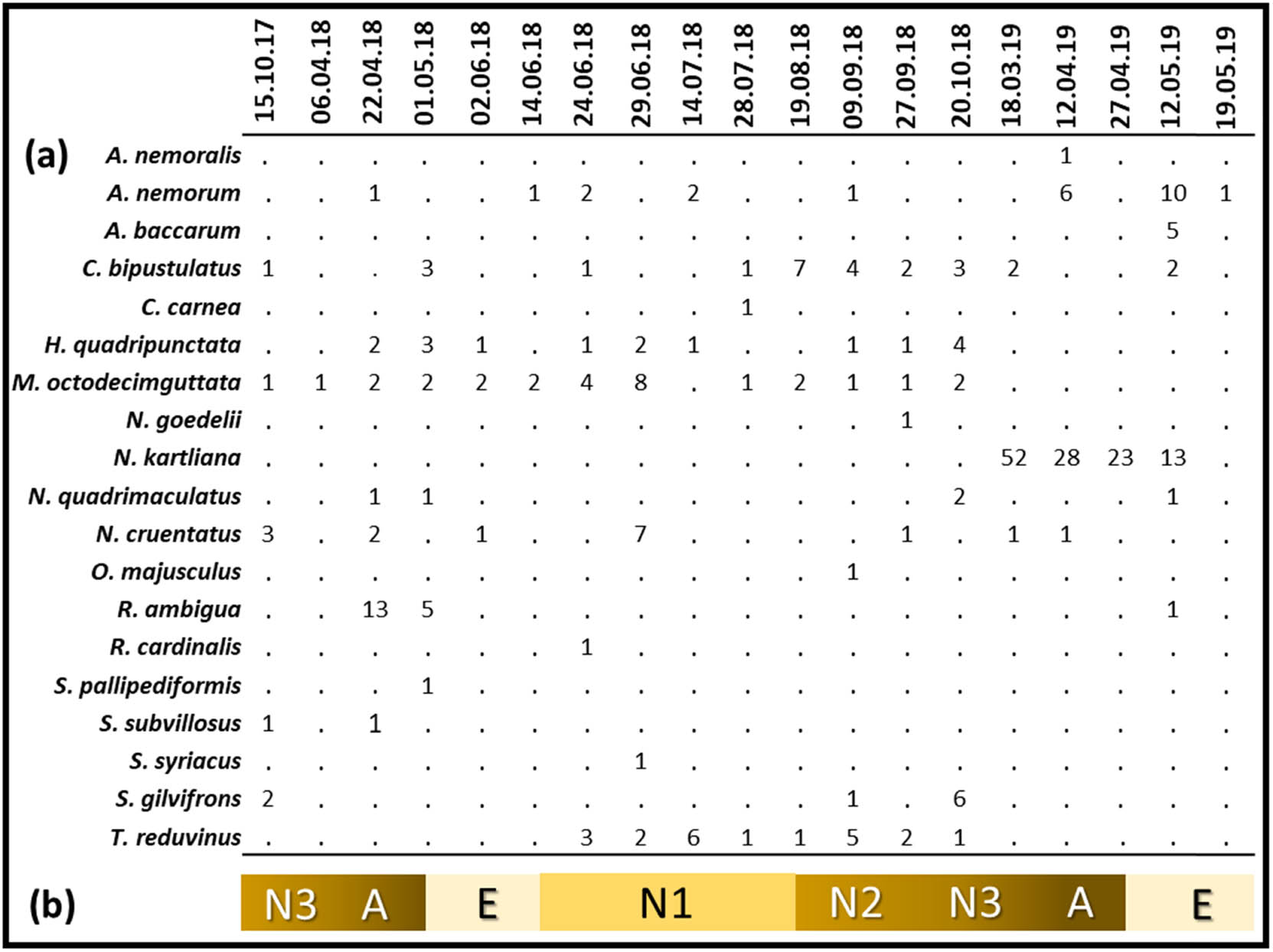
(a) Number of predator individuals per species per field visit. (b) Seasonal development of M. hellenica in the region (A: Adult, E: Egg and N: Nymph).
ACARI: ANYSTIDAE
Anystis baccarum (Linnaeus 1758)
Material examined: 12.05.2019 (5 individuals)
Polyphagous predator is known to feed on M. hellenica [19]. In Turkey, it occurs in Denizli and Manisa [25], İzmir [26] and Muğla [19]. This is the first report from Burdur.
COLOPTERA: COCCINELLIDAE
Chilocorus bipustulatus (Linnaeus 1758)
Material examined: 15.10.2017 (1), 01.05.2018 (3), 24.06.2018 (1), 28.07.2018 (1), 19.08.2018 (7), 09.09.2018 (4), 27.09.2018 (2), 20.10.2018 (3), 18.03,2019 (2) and 12.05.2019 (2) (26 individuals)
It is known to feed on Coccoidea species [27,28]. In Turkey, it occurs in Aydın, Artvin Denizli, İzmir, Muğla [28], Kahramanmaraş [29], Ankara [30], Antalya [31,32] and Çanakkale [33]. This is the first report from Burdur.
Harmonia quadripunctata (Pontoppidan 1763)
Material examined: 22.04.2018 (2), 01.05.2018 (3), 02.06.2018 (1), 24.06.2018 (1), 29.06.2018 (2), 14.07.2018 (1), 09.09.2018 (1), 27.09.2018 (1) and 20.10.2018 (4) (16 individuals)
It is known to feed on Torosaspis cedricola (Balachowsky & Alkan) [34] and M. hellenica [24]. In Turkey, it occurs in Adana, Ankara, Afyonkarahisar, Bursa, Denizli, Isparta, [28], Kahramanmaraş [29], Artvin [35], Antalya [31,32], Çanakkale [33], Bartın [36] and Burdur [37].
Myrrha ( Myrrha ) octodecimguttata (Linnaeus 1758)
Material examined: 15.10.2017 (1), 06.04.2018 (1), 22.04.2018 (2), 01.05.2018 (2), 02.06.2018 (2), 14.06.2018 (2), 24.06.2018 (4), 29.06.2018 (8), 28.07.2018 (1), 19.08.2018 (2), 09.09.2018 (1), 27.09.2018 (1) and 20.10.2018 (2) (29 individuals)
It is known to feed on M. hellenica [19], Matsucoccus pini (Green) (Hemiptera: Matsucoccidae) [38], Carulaspis juniperi (Bouché 1851) and Leucaspis lowi Colvée 1882 (Hemiptera: Diaspididae) [39]. In Turkey, it occurs in Adana, Ankara, Afyonkarahisar, Bursa, Denizli, Isparta, Rize, [28], Kahramanmaraş [29], Balıkesir, Çanakkale, İzmir and Muğla [19]. This is the first report from Burdur.
Nephus (Nephus) quadrimaculatus (Herbst 1783)
Material examined: 22.04.2018 (1), 01.05.2018 (1), 20.10.2018 (2) and 12.05.2019 (1) (5 individuals)
It is known to feed on Palaeococcus fuscipennis (Hemiptera: Monophlebidae) (Burmeister 1835) [40] and Coccus pseudomagnoliarum (Kuwana 1914) [41,47,84]. This is the first report showing that it predates upon M. hellenica. In Turkey, it occurs in Antalya [32] and Diyarbakır [42]. This is the first report from Burdur.
Novius cruentatus (Mulsant 1850)
Material examined: 15.10.2017 (3), 22.04.2018 (2), 02.06.2018 (1), 29.06.2018 (7), 27.09.2018 (1), 18.03.2019 (1) and 12.04.2019 (1) (16 individuals)
It is known to feed on Palaeococcus fuscipennis [43]. This is the first report showing that it predates upon M. hellenica. Löbl and Smetana [83] reported the species in Turkey without any locality detail. This is the first report from Burdur.
Rodolia cardinalis (Mulsant 1850)
Material examined: 24.06.2018 (1 individual)
It is known to feed on M. hellenica [19,78] and P. fuscipennis [40,43]. In Turkey, it occurs in Mediterranean region [28], Aydın, İzmir, Muğla [19] and Antalya [32]. This is the first report from Burdur.
Scymnus pallipediformis Günther 1958
Material examined: 01.05.2018 (1 individual)
It is a polyphagous species feeding on aphids and scale insects [29,42,51]. This is the first report showing that it predates upon M. hellenica. In Turkey, it occurs in Mediterranean region [28], Adıyaman, Diyarbakır, Şanlıurfa [42], Ağrı [44], Antalya [31,32], Çanakkale [33], Kahramanmaraş [29], İzmir [45] and Yalova [46]. This is the first report from Burdur.
Scymnus ( Pullus ) subvillosus (Goeze 1777)
Material examined: 15.10.2018 (1) and 22.04.2018 (1) (2 individuals)
It is a polyphagous species feeding on aphids and scale insects [29,42,51]. It is known to feed on M. hellenica [19], Coccus pseudomagnoliarum [47] and Planococcus citri (Risso) (Hemiptera: Pseudococcidae) [48]. In Turkey, it occurs in Adıyaman, Diyarbakır [42], Antalya [32], Aydın, Balıkesir, İzmir, Muğla [19], Burdur [49], Çanakkale [33], Kahramanmaraş [29] and Yalova [46].
Scymnus syriacus (Marsuel 1868)
Material examined: 29.06.2018 (1 individual)
It is known to feed on Aphididae [42,50], Cicadellidae, Coccidae, Diaspididae and Psyllidae [51,52]. This is the first report showing that it predates upon M. hellenica. In Turkey, it occurs in Adana, Hatay, Mersin [28], Adıyaman, Şanlıurfa [51], Antalya [32], Diyarbakır [42] and Kahramanmaraş [29]. This is the first report from Burdur.
Stethorus gilvifrons (Mulsant 1850)
Material examined: 15.10.2017 (2), 09.09.2018 (1) and 20.10.2018 (6) (9 individuals)
It is known to feed on aphids and scale insects [29,31,51] and Tetranychidae [28]. Anapulvinaria pistaciae (Bodenheimer 1926), Eulecanium rugulosum (Arch. 1937; Coccidae), Pistaciaspis pistaciae Arch. and Suturaspis pistaciae (Lindinger 1906) (Diaspididae) are reported to be its prey species [51]. This is the first report showing that it predates upon M. hellenica. In Turkey, it occurs in Mediterranean region and Adıyaman, Diyarbakır, Şanlıurfa [42], Antalya [31,32], Çanakkale [33], Isparta [53], İzmir [28] and Kahramanmaraş [29]. This is the first report from Burdur.
DIPTERA: CHAMAEMYIIDAE
Neoleucopis kartliana (Tanasijtshuk 1986)
Material examined: 18.03.2019 (52), 12.04.2019 (28), 27.04.2019 (23) and 12.05.2019 (13) (116 individuals). It feeds only on Marchalina [19,54]. In Turkey, it occurs in Antalya, Aydın, Balıkesir, Burdur, Bursa, Çanakkale, Denizli, Edirne, İstanbul, İzmir, Manisa and Muğla [19].
HEMIPTERA: ANTHOCORIDAE
Anthocoris nemoralis (Fabricius 1794)
Material examined: 12.04.2019 (1 individual)
It is a polyphagous species feeding on aphids, mites, psyllids and lepidopteran eggs and young larva [55,56,57]. This is the first report showing that it predates upon M. hellenica. In Turkey, it occurs in Antalya, Erzincan, Erzurum, İzmir, Manisa and Mersin [58,59]. This is the first report from Burdur.
Anthocoris nemorum (Linnaeus 1761)
Material examined: 22.04.2017 (1), 14.06.2018 (1), 24.06.2018 (2), 14.07.2018 (2), 09.09.2018 (1), 12.04.2019 (6), 12.05.2019 (10) and 19.05.2019 (1) (24 individuals)
It is a polyphagous species reported to feed on Cacopsylla pyri (L.) (Hemiptera: Psyllidae) and Brevicoryne brassicae (L.) (Hemiptera: Aphididae) [56,85,86]. This is the first report showing that it predates upon M. hellenica. In Turkey, it occurs in Antalya and Erzurum [59], Artvin, Bolu and Trabzon [60]. This is the first report from Burdur.
Orius ( Heterorius ) majusculus (Reuter 1879)
Material examined: 09.09.2018 (1 individual)
It is a polyphagous species [61] reported to feed on aphids, thrips and whiteflies [62,63,64]. This is the first report showing that it predates upon M. hellenica. In Turkey, it occurs in Eastern Black Sea and Eastern and Central Anatolia regions [60]. This is the first report from Burdur.
Temnostethus ( Ectemnus ) reduvinus (Herrich-Schäffer 1850)
Material examined: 24.06.2018 (3), 29.06.2018 (2), 14.07.2018 (6), 28.07.2018 (1), 19.08.2018 (1), 09.09.2018 (5), 27.09.2018 (2) and 20.10.2018 (1) (21 individuals)
It is known to feed on Lepidosaphes ulmi (L.) (Diaspididae) and Agonoscena pistaciae Burck. & Laut. (Psyllidae) [65,66,67]. This is the first report showing that it predates upon M. hellenica. In Turkey, it occurs in Adıyaman, Siirt [67], Antalya, Artvin, Erzincan, Erzurum and Kars [59]. This is the first report from Burdur.
HEMIPTERA: REDUVIIDAE
Nagusta goedelii (Kolenati 1857)
Material examined: 27.09.2018 (1 individual)
It is a polyphagous species reported to feed on aphids, psyllids and pseudococcids [32,68,69,70]. This is the first report showing that it predates upon M. hellenica. In Turkey, it has a wide distribution [71,72,73]. This is the first report from Burdur.
NEUROPTERA: CHRYSOPIDAE
Chrysoperla carnea (Stephens 1836)
Material examined: 28.07.2018 (1 individual)
It is a polyphagous species reported to feed on aphids, scale insects, lepidopter eggs and larvae, psyllids, chrysomelid larvae, thrips and acars [74]. This is the first report showing that it predates upon M. hellenica. Ülgentürk et al. [19] reported another species of the genus (C. lucasina) as a predator of M. hellenica in Turkey. It occurs widely in Turkey [75,76]. This is the first report from Burdur.
RAPHIDIOPTERA: RAPHIDIIDAE
Raphidia ambigua Aspöck&Aspöck 1964
Material examined: 22.04.2018 (13), 01.05.2018 (5) and 12.05.2019 (1) (19 individuals)
It feeds on Coccoidea and Raphidiidae [77]. This is the first report showing that it predates upon M. hellenica. Argyriou et al. [78] reported another species of the genus (R. notata F.) as a predator of M. hellenica in Greece. In Turkey, it occurs in Afyonkarahisar, Ankara, Antalya, Balıkesir, Bilecik, Bitlis, Burdur, Çorum, Denizli, Diyarbakır, Elazığ, Isparta, İzmir, Kırşehir, Konya, Mersin, Muğla and Muş [79].
4 Discussion
In the present study, we identified 19 species foraging on M. hellenica in an isolated P. brutia stand, where M. hellenica is an introduced species. We report 12 of these species as the predators of M. hellenica for the first time (A. nemoralis, A. nemorum, C. carnea, N. quadrimaculatus, N. cruentatus, N. goedelii, O. majusculus, R. ambigua, S. pallipediformis, S. syriacus, S. gilvifrons and T. reduvinus), whereas 12 of the identified species were the first records for the Burdur region (A. nemoralis, A. nemorum, A. baccarum, C. bipustulatus, M. octodecimguttata, N. quadrimaculatus, N. cruentatus, R. cardinalis, S. syriacus, S. pallipediformis, S. gilvifrons and T. reduvinus). Among the 19 species we found, 7 species were also reported from the native range of M. hellenica (Table A1 in Appendix). The number of M. hellenica predator species in all its distribution reached 40 after the present study. The updated predator list is provided in Table A1 (Appendix). On the other hand, our low sample size along with suboptimal assumption approach we adopted to identify predation on M. hellenica necessitate further studies focusing directly on the predatory behavior of the species found in this study in order to confirm their predatory status. We additionally provided eight new occurrence records for M. hellenica out of its native range in Turkey (Figure A1).
The western end of the Taurus Mountain chain is a significant geographical barrier separating the coastal Aegean and Mediterranean regions from the Burdur basin. Furthermore, the forest cover is not continuous into the inner regions which must be a major barrier for the spread of a weak disperser such as the giant pine scale (40 m/year) (Nicolopoulos 1965 cited in ref. [80]). Thus, human-aided introduction seems to be the only way for M. hellenica to spread the appropriate spots in the inner regions. The local beekeepers have been introducing M. hellenica to several areas in Burdur since 1990s but some of these efforts failed probably due to inappropriate ecological conditions for M. hellenica. However, the humid climate of the study site owing to the nearby lake seems to have facilitated its establishment. We found 12 new species predating upon M. hellenica in its introduced range and we could not find in the study site the other 12 species that had been reported as M. hellenica predators from its native range. The two ranges (native and introduced) had seven species in common (Table A1). Although some of the predators (particularly N. kartliana) might be accidentally introduced into the region during the introduction of M. hellenica, most of them were cosmopolitan species occurring also in M. hellenica-free ecosystems. Therefore, we believe that most of the predator species we identified have started foraging on M. hellenica in the past 30 years in the study site, although they most probably occur in the natural range of M. hellenica. These results also suggest that native predator community can rather quickly include the giant pine scale into the menu in scale’s introduced range. This is not surprising as prey switching is a strategy that predators use in the process of accepting a new prey species [17]. Although identifying the predation strategy of M. hellenica predators was out of the scope of the present study and its sampling design, we hypothesize that prey switching strategy might be used by most of the predators we identified. As a supporting observation for this hypothesis, the abundance of the Aphididae and Coccoidae species, which are the main preys of the predatory species we identified in the present study, was significantly low in the study region during all the field visits. Therefore, the most abundant prey species in the region which was always available to the predators was M. hellenica. This hypothesis should be tested by further studies.
The highest number of individuals we found belonged to N. kartliana. This result was in accordance with that of Ülgentürk et al. [19] who demonstrated that N. kartliana was the most common predator of M. hellenica in the entire range of the giant pine scale in Turkey. This is not surprising as N. kartliana is a monophagous predator [43]. On the other hand, it was found only in March, April and May 2019, but not in 2018 probably due to the sampling bias. Its pupae were available only in the cottony secretions of M. hellenica which were more abundant in 2019 because of trap-logs established for O. erosus management program. We found N. kartliana pupae in March and larvae by April. If the species were in the adult stage in late May (e.g., Gaimari et al. [54] found adults in June in Greece), it might escape from the sampling. Although revealing the biology of N. kartliana was out of our scope in this study, the abundance of it and its monophagous nature suggests that it is the best candidate for biological control against the giant pine scale as suggested by Avtzis et al. [14]. Considering observed high frequencies of M. octodecimguttata (68.4% of all field visits), C. bipustulatus (57.9%) and H. quadripunctata (47.4%), and absence or scarcity of other possible prey species in the study site, the hypothesis of these Coccinellids being significant suppressors of the giant pine scale populations seems reliable. However, this hypothesis remains for testing through future studies.
5 Conclusion
The giant pine scale is an important component of the biodiversity in its native range as it supports several insect species by its honeydew secretion. On the other hand, it can be a pest in habitats where the host trees are weak due to several reasons such as poor soil quality and climatic change. In such habitats, the presence of M. hellenica can favor outbreaks of secondary pests such as bark beetles which can eventually lead to death of the host trees [3,80]. Indeed, during the present study, we noted several P. brutia deaths due to the Mediterranean pine beetle particularly on the hosts heavily infested with the giant pine scale. Therefore, introducing M. hellenica out of its native range in favor of apiculture can lead to serious pest problems which may ultimately threaten not only the biodiversity but also the sustainability of pine honey production. Considering the serious impact of the climate change predicted for the eastern Mediterranean [81], control of M. hellenica can be expected to be one of the major issues in the near future in the pine forests of the region and in other introduction spots such as Australia. This makes research on M. hellenica predators and parasitoids, particularly those from Anatolia where the giant pine scale most probably originated from ref. [80,82], a priority. In the future studies related to biological control of populations of the giant pine scale, a special importance should be given to N. kartliana as it seems to be a host specific predator of the pest [14]. Additionally, since all the predatory species feeding upon M. hellenica described so far have been diurnal species, research on the nocturnal predatory species may also contribute to a list of giant pine scale predators.
Acknowledgements
The authors thank Asst. Prof. Dr Derya Şenal for Coccinellidae, Prof. Dr Ahmet Dursun and Prof. Dr Meral Fent for Hemiptera, Prof. Dr Savaş Canbulat for Neuroptera and Raphidioptera identifications. The authors thank anonymous reviewers for their comments and suggestions on the earlier version of the article.
-
Funding information: The study was financially supported by theTurkish Beekeeping Association.
-
Conflict of interest: The authors state no conflict of interest.
-
Data availability statement: The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Appendix
Predators of Marchalina hellenica identified so far in Georgia, Greece and Turkey (○: in the natural range of M. hellenica in TR, ●: in the introduced range of M. hellenica in TR)
| Order: family | Species | Country | Reference |
|---|---|---|---|
| Acarina: Trombidiidae | Allothrombium pulvinum ○ | Turkey | Ülgentürk et al. 2013 [19] |
| Allothrombium triticium ○ | Turkey | Ülgentürk et al. 2013 [19] | |
| Acarina: Anystidae | Anystis baccarum ○● | Turkey | Ülgentürk et al. 2013 [19]; Present study |
| Neuroptera: Chrysopidae | Chrysopa pallens (Rambur) | Greece | Argyriou et al. 1976 [78] |
| Chrysopa (=Dichochrysa) flavifrons | Greece | Argyriou et al. 1976 [78]; Nicolopoulos 1965 [80] | |
| Chrysoperla carnea ● | Turkey | Present study | |
| Chrysoperla lucasina ○ | Turkey | Ülgentürk et al. 2013 [19] | |
| Dichochrysa genei ○ | Turkey | Ülgentürk et al. 2013 [19] | |
| Dichochrysa prasina ○ | Turkey | Ülgentürk et al. 2013 [19] | |
| Pseudomallada flavifrons | Greece | Nicolopoulos 1965 [80] | |
| Neuroptera: Hemerobiidae | Wesmaelius subnebulosus ○ | Turkey | Ülgentürk et al. 2013 [19] |
| Raphidioptera: Raphidiidae | Raphidia ambigua ● | Turkey | Present study |
| Raphidia (=Phaeostigma) notata | Armenia, Georgia | Hadzibejli 1969 [89] | |
| Greece | Nicolopoulos 1965 [80]; Gaimari et al. 2007 [54] | ||
| Diptera: Chameamyiidae | Leucopis sp. ○ | Turkey | Bodenheimer 1953; Selmi 1983 [24] |
| Leucopis obscura | Greece | Nicolopoulos 1965 [80] | |
| Neoleucopis kartliana ○● | Turkey | Ülgentürk et al. 2013 [19]; Present study | |
| Georgia, Greece | Gaimari et al. 2007 [54] | ||
| Hemiptera: Anthocoridae | Anthocoris nemoralis ● | Turkey | Present study |
| Anthocoris nemorum ● | Turkey | Present study | |
| Brachysteles parvicornis ○ | Turkey | Selmi 1983 [24] | |
| Cardiastethus nazarenus ○ | Turkey | Ülgentürk et al. 2013 [19] | |
| Ectemnus sp. ○ | Turkey | Selmi 1983 [24] | |
| Elatophilus pachycnemis ○ | Turkey | Ülgentürk et al. 2013 [19] | |
| Orius majusculus ● | Turkey | Present study | |
| Temnostethus reduvinus ● | Turkey | Present study | |
| Hemiptera: Reduviidae | Nagusta goedelii ● | Turkey | Present study |
| Coleoptera: Coccinellidae | Chilocorus bipustulatus ○● | Turkey | Giray 1970 [23]; Selmi 1983 [24]; Present study |
| Coccinella septempunctata ○ | Turkey | Selmi 1983 [24] | |
| Exochomus sp. | Georgia | Hadzibejli 1969 | |
| Exochomus quadripustulatus ○ | Turkey | Selmi 1983 [24] | |
| Harmonia quadripunctata ○● | Turkey | Selmi 1983 [24]; Present study | |
| Hippodamia variegata | Greece | Nicolopoulos 1965 [80] | |
| Myrrha octodecimguttata ○● | Turkey | Ülgentürk et al. 2013 [19]; Present study | |
| Nephus quadrimaculatus ● | Turkey | Present study | |
| Novius cruentatus ● | Turkey | Present study | |
| Rodolia cardinalis ○● | Turkey | Ülgentürk et al. 2013 [19]; Present study | |
| Scymnus pallipediformis ● | Turkey | Present study | |
| Scymnus subvillosus ○● | Turkey | Ülgentürk et al. 2013 [19]; Present study | |
| Scymnus syriacus ● | Turkey | Present study | |
| Stethorus gilvifrons ● | Turkey | Present study | |
| Coleoptera: Cantharidae | Dasytes flavipes | Turkey | Bodenheimer 1953 [22] |
References
[1] Hatjina F, Bouga M. Portrait of Marchalina hellenica Gennadius (Hemiptera: Margarodidae), the main producing Insect of pine honeydew-biology, genetic variability, and honey production. Uludag Bee J. 2009;9:162–7.Search in Google Scholar
[2] Ülgentürk S, Özdemir I, Kozar F, Kaydan M, Dostbil Ö, Sarıbaşak H, et al. Honeydew producing insect species in forest areas in Western Turkey. Turk Bul Entomol. 2013a;3:125–33.Search in Google Scholar
[3] Williams N. Buzzing off. Cur Biol. 2007;17:R526.10.1016/j.cub.2007.06.050Search in Google Scholar
[4] Avcı M. Marchalina hellenica: morfolojisi, biyolojisi, ekolojisi, arıcılık için önemi, kızılçamlarda beslenmesi Muğla kızılçam ormanlarında arıcılık ormancılık ilişkileri. In: Avcı M, Korkmaz M, editors. Basralı Ormanların Geleceği. Muğla Kızılçam Ormanlarında Arıcılık Ormancılık İlişkileri. Muğla, Turkey: Esin Yayıncılık; 2016.Search in Google Scholar
[5] Mendel Z, Branco M, Battisti A. Invasive sap-sucker insects in the Mediterranean Basin. In: Paine TD, François L, editors. Insects and diseases of mediterranean forest systems. Cham: Springer; 2016. p. 261–91.10.1007/978-3-319-24744-1_10Search in Google Scholar
[6] Çanakçioğlu H, Mol T. Forest entomology harmful and useful insects. İstanbul, Turkey: İstanbul University Faculty of Forestry; 1998. p. 451.Search in Google Scholar
[7] Yeşil A, Gürkan B, Saraçoğlu O, Zengin H. Effect of the pest Marchalina hellenica Gennadius (Homoptera, Margarodidae) on the growth parameters of Pinus brutia Ten in Mugla Region (Turkey). Pol J Ecol. 2005;53:451–8.Search in Google Scholar
[8] Gallis AT. Evaluation of the damage by insect Marchalina hellenica (Genn.) in Eastern Attica, Greece: conclusions for sustainable management of forests ecosystems. Proceedings of the 10th International Conference on Environmental Science and Technology; 2007. p. 191–6.Search in Google Scholar
[9] Petrakis PV, Spanos K, Feest A. Insect biodiversity reduction of pinewoods in southern Greece caused by the pine scale (Marchalina hellenica). For Syst. 2011;20:27–41.10.5424/fs/2011201-8924Search in Google Scholar
[10] Fimiani P, Solino G. An exotic insect dangerous to the native plants of the island of Ischia. Inf Agrar. 1994;50:65–8.Search in Google Scholar
[11] Nahrung HF, Loch AD, Matsuki M. Invasive insects in Mediterranean forest systems: Australia. In: Paine TD, François L, editors. Insects and diseases of mediterranean forest systems. Cham: Springer; 2016. p. 475–98.10.1007/978-3-319-24744-1_17Search in Google Scholar
[12] Gavrilov IA, Kuznetsova VG. New data on the scale insect (Homoptera: Coccinea) fauna of European Russia, their taxonomy and cytogenetics. Proceedings of 10th International Symposium on Scale Insect Studies. 408, 2004.Search in Google Scholar
[13] García Morales M, Denno BD, Miller DR, Miller GL, Ben-Dov Y, et al. ScaleNet: a literature-based model of scale insect biology and systematics. Database. 2016;bav118:1–5. 10.1093/database/bav118. http://scalenet.infoSearch in Google Scholar PubMed PubMed Central
[14] Avtzis DN, Lubanga UK, Lefoe GK, Kwong RM, Eleftheriadou N, Andreadi A, et al. Prospects for classical biological control of Marchalina hellenica in Australia. BioCont. 2020;65:413–23. 10.1007/s10526-020-10012-3.Search in Google Scholar
[15] Watt AD, Stork NE, Hunter MD. Forests and insects. London: Chapman and Hall; 1997.Search in Google Scholar
[16] Jeffries MJ, Lawton JH. Enemy free space and the structure of ecological communities. Biol J Linn Soc. 1984;23:269–86.10.1111/j.1095-8312.1984.tb00145.xSearch in Google Scholar
[17] Jaworski CC, Bompard A, Genies L, Amiens-Desneux E, Desneux N. Preference and prey switching in a generalist predator attacking local and invasive alien pests. PLoS One. 2013;8:e82231.10.1371/journal.pone.0082231Search in Google Scholar PubMed PubMed Central
[18] Gürkan B. Çam pamuklu koşnili Marchalina hellenica (Gennadius)’nın biyo-ekolojisi ve populasyon dinamiği (Ph.D. thesis). Ankara: Hacettepe University; 1989.Search in Google Scholar
[19] Ülgentürk S, Szentkirályi F, Uygun N, Fent M, Gaimari SD, Civelek H, et al. Predators of Marchalina hellenica (Hemiptera: Marchalinidae) on pine forests in Turkey. Phytopar. 2013;41:529–37.10.1007/s12600-013-0313-1Search in Google Scholar
[20] Ülgentürk S, Çanakçioğlu H, Kaygin AT. Scale insects of the conifer trees in Turkey and their zoogeographical distribution. J Pest Sci. 2004;77:99–104.10.1007/s10340-003-0035-0Search in Google Scholar
[21] Ülgentürk S, Balci Ş. New records of Chamaemyiidae and Cryptochaetidae (Diptera) on Scale Insects (Hemiptera: Coccomorpha) in Turkey. Turk J Biol Cont. 2019;10:127–32.Search in Google Scholar
[22] Bodenheimer FS. The Coccoidea of Turkey III. Rev Fac Sci Unin Ist (Ser B). 1953;18:91–164.Search in Google Scholar
[23] Giray H. Harmful and useful species of Coccinellidae (Coleoptera) from Aegian Region with on their localities, collecting dates and hosts. Yearb Fac Agri. 1970;1:35–52.Search in Google Scholar
[24] Selmi E. Marchalina hellenica (Gennadius) (Coccoidea, Homoptera)‘nın Marmara Bölgesindeki biyolojisi ve doğal düşmanları. J Fac For Ist Uni. 1983;33:93–103.Search in Google Scholar
[25] Göven MA, Çobanoğlu S, Güven B. Predatory mite fauna in Aegean vineyards. Plant Prot Bul. 2009;49:1–10.Search in Google Scholar
[26] Kılıç T, Çobanoğlu S, Yoldaş Z, Madanlar N. Mite (Acari) species determined in fresh onion fields in Izmir province. Turk J Entomol. 2012;36:401–11.Search in Google Scholar
[27] Giorgi JA, Vandenberg NJ, McHugh JV, Forrester JA, Ślipiński SA, Miller KB, et al. The evolution of food preferences in Coccinellidae. Biol Cont. 2009;51:215–31.10.1016/j.biocontrol.2009.05.019Search in Google Scholar
[28] Uygun N. Taxonomic research on the Coccinellidae (Coleoptera) fauna of Turkey. Adana, Turkey: Çukurova University, Faculty of Agriculture, Publications; 1981. p. 15.Search in Google Scholar
[29] Aslan MM, Uygun N. The Aphidophagus Coccinellid (Coleoptera: Coccinellidae) Species in Kahramanmaraş, Turkey. Turk J Zool. 2005;29:1–8.Search in Google Scholar
[30] Ülgentürk S. Parasitoids and predators of Coccidae (Homoptera: Coccoidae) species on ornamental plants in Ankara, Turkey. Acta Phytop Entomol Hun. 2001;36:369–75.10.1556/APhyt.36.2001.3-4.18Search in Google Scholar
[31] Bali B. Coccinellidae species and their distribution in Antalya province (M.Sc. thesis). Van, Turkey: Van Yüzüncü Yıl University; 2011.Search in Google Scholar
[32] Başar M, Yaşar B. Parasitoid and predator species in olive plantations in Antalya Province, Turkey. Turk J Biol Cont. 2018;9:82–101.Search in Google Scholar
[33] Baştuğ G, Kasap İ. Faunistic studies on Coccinellidae (Coleoptera) family in the province of Çanakkale. Turk J Biol Cont. 2015;6:41–50.Search in Google Scholar
[34] Dostbil Ö, Ülgentürk S. Bio-ecology of cedar scale insect Torosaspis cedricola (Balachowsky & Alkan) (Hemiptera Diaspididae) in Ankara, Turkey. Redia. 2016;99:163.10.19263/Redia-99.16.21Search in Google Scholar
[35] Portakaldalı M, Satar S. Research on Coccinellidae (Coleoptera) fauna in Artvin and Rize province. Plant Prot Bul. 2010;50:89–99.Search in Google Scholar
[36] Toper Kaygın A, Sobutay Kaptan U. Coccinellidae (Insecta: Coleoptera) species of Bartın province. J Bartın Fac For. 2017;19:227–36.Search in Google Scholar
[37] Oğuzoğlu Ş, Avcı M. Distribution, biology, morphology, and damage of Cinara cedri Mimeur, 1936 (Hemiptera: Aphididae) in the Isparta Regional Forest Directorate. Forestist. 2019;69:1–10.10.26650/forestist.2019.346284Search in Google Scholar
[38] Siewniak M. On the morphology and bionomy of the pine scale Matsucoccus pini (Green) (Hom., Coccoidea: Margarodidae). Z Angew Entomol. 1976;81:337–62.10.1111/j.1439-0418.1976.tb04247.xSearch in Google Scholar
[39] Kuznetsov NN. Four little known species of scales (Homoptera, Diaspididoidea) damaging conifers. Zool Zh. 1970;49:1644–55.Search in Google Scholar
[40] Eizaguirre M, Arenas N, Lumbierres B, Pons X. Daños de Palaeococcus fuscipennis Burm (Homoptera: Margarodidae) en pinos y cipreses de los parques de Lleida. Bol Sanidad Veget Plag. 2002;28:199–205.Search in Google Scholar
[41] Güncan A, Yoldaş Z, Koçlu T. Conservation and classical biological control of Satsuma citrus pests in western Turkey. IOBC-WPRS Bul. 2013;95:99–108.Search in Google Scholar
[42] Gözüaçık C, Yiğit A, Uygun N. Coccinellidae (Coleoptera) species in different habitats at Southeastern Anatolia Region of Turkey. Turk J Biol Cont. 2012;3:69–88.Search in Google Scholar
[43] Mendel Z, Assael F, Zeidan S, Zehavi A. Classical biological control of Palaeococcus fuscipennis (Burmeister) (Homoptera: Margarodidae) in Israel. Biol Cont. 1998;12:151–7.10.1006/bcon.1998.0621Search in Google Scholar
[44] Kaydan MB, Atlihan R, Uygun N, Şenal D. Coccinellid (Coleoptera: Coccinellidae) species feeding on coccoids (Hemiptera: Coccoidea) in Van Lake basin, Turkey. Turk J Biol Cont. 2012;3:37–46.Search in Google Scholar
[45] Keskin N. Fauna of Coccinellidae (Coleoptera: Insecta) in recreation areas of Bornova district (İzmir) in Turkey (M.Sc. thesis). Konya: Selçuk University; 2012.Search in Google Scholar
[46] Buğday H, Şenal D, Atlıhan R. Coccinellidae (Coleoptera) species and their distribution in different habitats of Yalova Province in Turkey. Turk J Biol Cont. 2015;6(127):138.Search in Google Scholar
[47] Öncüer C. Investigation on the identification, distribution, and efficiency of the natural enemies of some Coccoidae (Homoptera) which infect the fruit trees in Izmir Province. Ege Univ Fac Agri. 1977;336:122–9.Search in Google Scholar
[48] Elekçioğlu NZ, Senal D. Pest and natural enemy fauna in organic citrus production in the eastern Mediterranean region of Turkey. Int J Nat Eng Sci. 2007;1:29–34.Search in Google Scholar
[49] Kaya M. The determination of laydbird species (Col.: Coccinellidae) on fruit trees in Isparta provincial and districts (M.Sc. thesis). Isparta: Süleyman Demirel University; 2009.Search in Google Scholar
[50] Allawi TF. Biological and ecological studies on Scymnus syriacus and Scymnus levaillanti (Coleoptera: Coccinellidae). Eur J Entomol. 2006;103:501–3.10.14411/eje.2006.065Search in Google Scholar
[51] Bolu H, Özgen İ, Bayram A, Çınar M. Coccinelidae species, distribution areas and their preys in pistachio, almond and cherry orchards in southeastern and eastern Anatolia regions. J Harran Univ Fac Agri. 2007;11:39–47.Search in Google Scholar
[52] Abd-Rabou S, Ahmed N. Seasonal incidence of scale insects, whiteflies and psyllids (Hemiptera) of olive and their natural enemies in Egypt. Egypt Acad J Biol Sci A Entomol. 2011;4:59–74.10.21608/eajbsa.2011.15172Search in Google Scholar
[53] Kaya Başar M, Yaşar B. Determination of ladybird species (Coleoptera: Coccinellidae) on fruit trees in Isparta, Turkey. Turk J Entomol. 2011;35:519–34.Search in Google Scholar
[54] Gaimari SD, Milonas P, Souliotis C. Notes on the taxonomy, biology, and distribution of Neoleucopis kartliana (Diptera: Chamaemyiidae). Fol Heyrov. 2007;15:7–16.Search in Google Scholar
[55] Er H. Ankara ilinde armut ağaçlarında zararlı Cacopsylla pyri (L.) (Homoptera: Psyllidae) ile doğal düşmanlarının yoğunluklarının saptanması üzerinde araştırmalar (M.Sc. thesis). Ankara: Ankara University; 1996.Search in Google Scholar
[56] Sigsgaard L. Habitat and prey preferences of the two predatory bugs Anthocoris nemorum (L.) and A. nemoralis (Fabricius) (Anthocoridae: Hemiptera-Heteroptera). Biol Cont. 2010;53:46–54.10.1016/j.biocontrol.2009.11.005Search in Google Scholar
[57] Karaca I, Kayahan A, Şimşek B, Çelikpençe Y. First record of Glycaspis brimblecombei Moore (Hemiptera: Aphalaridae), in Turkey. Phytopar. 2015;43:171–5.10.1007/s12600-015-0457-2Search in Google Scholar
[58] Tezcan S, Önder F. Faunistical studies in ecological cherry orchards in İzmir and Manisa provinces of Turkey: an evaluation on the species of Heteroptera. Anadolu J Aegean Agri Res Inst. 2003;13:124–31.Search in Google Scholar
[59] Yıldırım E, Yazıcı G, Kul R, Moulet P. Contribution to the knowledge of the Anthocoridae, Lyctocoridae, Nabidae, Reduviidae and Tingidae (Hemiptera, Heteroptera) fauna of Turkey. J Entomol Res Soc. 2013;15:53–66.Search in Google Scholar
[60] Önder F, Karsavuran Y, Tezcan S, Fent M. Türkiye Heteroptera (Insecta) Kataloğu. İzmir, Turkey: Meta Basım Matbaacılık; 2006.Search in Google Scholar
[61] Pumariño L, Alomar O. The role of omnivory in the conservation of predators: Orius majusculus (Heteroptera: Anthocoridae) on sweet alyssum. Biol Cont. 2012;62:24–8.10.1016/j.biocontrol.2012.03.007Search in Google Scholar
[62] Arno J, Roig J, Riudavets J. Evaluation of Orius majusculus and O. laevigatus as predators of Bemisa tabaci and estimation of their prey preference. Biol Cont. 2008;44:1–6.10.1016/j.biocontrol.2007.10.009Search in Google Scholar
[63] Messelink GJ, Bloemhard CJB, Sabelis MW, Janssen A. Biological control of aphids in the presence of thrips and their enemies. BioControl. 2013;58:45–55.10.1007/s10526-012-9462-2Search in Google Scholar
[64] Ünal Bahşi Ş, Tunç İ. Optimization of Orius majusculus release: photoperiodic sensitivity at different temperatures and storage of diapausing adults. Turk J Agri For. 2014;38:935–41.10.3906/tar-1403-144Search in Google Scholar
[65] Çiftçi K, Türkyılmaz N, Kumaş F, Özkan A. Antalya ili elma bahçelerindeki önemli zararlılar ile doğal düşmanlarının tespiti üzerinde ön çalışmalar. Plant Prot Bul. 1985;25:49–61.Search in Google Scholar
[66] Aydoğdu S, Toros S. Investigations on bio-ecology of Lepidosaphes ulmi L. (Homoptera: Diaspididae) and its relation with natural enemies in Erzincan and neighbouring province. Plant Prot Bul. 1987;27:147–78.Search in Google Scholar
[67] Bolu H, Kornoşor S, Altın M. Indicating the population development of nymph parazitoid and Agonoscena pistaciae Burckhardt and Lauterer (Homoptera; Psyllidae), predator Heteroptera species and their spread areas at the Southeastern Anatolia Region pistachio (Pistacia vera L.) areas. Proceedings of the 4th Turkish National Congress of Biological Control. 4, 1999. p. 26–9Search in Google Scholar
[68] Sahayaraj K. Reduviids and their merits in biological control. In: Saharayaj K, editor. Basic and applied aspects of biopesticides. New Delhi: Springer; 2014. p. 195–21410.1007/978-81-322-1877-7_10Search in Google Scholar
[69] Baghi R, Maurel JP. Première observation de la punaise prédatrice Nagusta goedelii (Kolenati, 1857) en région Occitanie (Hemiptera, Reduviidae). Carnets Nat. 2017;4:1–4.Search in Google Scholar
[70] Kitherian S, Kumar V, Banu N, Avery PB, Radhika A, McKenzie CL, et al. Predation potential of Rhynocoris marginatus (Hemiptera: Reduviidae) against three mealybug species of agricultural importance. Appl Entomol Zool. 2018;53:475–82.10.1007/s13355-018-0576-6Search in Google Scholar
[71] Yıldırım E, Moulet P, Külekçi G, Bulak Y. Contribution to the knowledge of Reduviidae (Hemiptera) fauna of Turkey. Linz biol Beit. 2010;42:825–31.Search in Google Scholar
[72] Dursun A. A study on the Nabidae and Reduviidae (Hemiptera: Heteroptera) of the Kelkit Valley and Amasya. Turkey Acta Entomol Serb. 2011;16:35–43.Search in Google Scholar
[73] Dursun A, Salur A. Presence of Sphedanolestes sanguineus (Fabricius, 1794) in Turkey, followed by an annotated checklist of Reduviidae (Hemiptera: Heteroptera). Turk J Zool. 2013;37:610–20.10.3906/zoo-1203-17Search in Google Scholar
[74] Kaya U, Öncüer C. Investigations on the effect of two different food to the biology of Chrysoperla carnea (Steph.) (Neuroptera: Chrysopidae) reared in laboratory. Turk J Entomol. 1988;12:151–9.Search in Google Scholar
[75] Canbulat S. Güney Batı Anadolu Raphidiopter’leri ve Neuropter’leri (Insecta, Neuropterida) (Ph.D. thesis). Ankara: Gazi University; 2003.Search in Google Scholar
[76] Arı İ, Aktaş M, Kiyak S. Notes on the Chrysopidae (Neuroptera) fauna of Ardahan, Iğdır and Kars provinces of Turkey. Turk J Zool. 2007;1976(31):201–8.Search in Google Scholar
[77] Miller GL, Oswald JD, Miller DR. Lacewings and scale insects: a review of predator/prey associations between the Neuropterida and Coccoidea (Insecta: Neuroptera, Raphidioptera, Hemiptera). Ann Entomol Soc Am. 2004;97:1103–25.10.1603/0013-8746(2004)097[1103:LASIAR]2.0.CO;2Search in Google Scholar
[78] Argyriou LC, Stavraki HG, Mourikis PA. A list of recorded entomophagous insects of Greece. Athens, Greece: Benaki Phytopathological Institute; 1976.Search in Google Scholar
[79] Canbulat S, Kıyak S. Contribution to the distribution of Raphidioptera (Raphidiidae, Inocellidae) from Southwest Anatolia. Acta Mus Regina. 2006;31:141–7.Search in Google Scholar
[80] Petrakis PV, Roussis V, Vagias C, Tsoukatou M. The interaction of pine scale with pines in Attica, Greece. Eur J For Res. 2010;129:1047–56.10.1007/s10342-010-0389-9Search in Google Scholar
[81] Önol B, Semazzi FHM. Regionalization of climate change simulations over the Eastern Mediterranean. J Clim. 2009;22:1944–61.10.1175/2008JCLI1807.1Search in Google Scholar
[82] Bouga M, Evangelou V, Lykoudis D, Cakmak I, Hatjina F. Genetic structure of Marchalina hellenica (Hemiptera: Margarodidae) populations from Turkey: preliminary mtDNA sequencing data. Biochem Genet. 2011;49:683–94.10.1007/s10528-011-9442-8Search in Google Scholar PubMed
[83] Löbl I, Smetana A. Catalogue of Palaearctic Coleoptera Volume 4: Elateroidea – Derodontoidea – Bostrichoidea – Lymexyloidea – Cleroidea – Cucujoidea. Stenstrup, Denmark: Apollo Books; 2007.10.1163/9789004260894Search in Google Scholar
[84] Öncüer C. The Coccus species (Homoptera: Coccidae) damaging Citrus groves in the Aegean region; studies on their morphological characters, distribution, and natural enemies. Plant Prot Bul. 1974;1:59.Search in Google Scholar
[85] Sigsgaard L, Esbjerg P, Philipsen H. Controlling pear psyllids by mass-releasing Anthocoris nemoralis and A. nemorum (Heteroptera: Anthocoridae). J Fru Ornam Plant Res. 2006;14:89–98.Search in Google Scholar
[86] Simonsen ML, Enkegaard A, Bang CN, Sigsgaard L. Temperature effect on the predation rate of Anthocoris nemorum (Het.: Anthocoridae) on cabbage aphids (Hom.: Aphididae). J Appl Entomol. 2009;133:198–200.10.1111/j.1439-0418.2008.01327.xSearch in Google Scholar
[87] DMAPS. d-maps.com. Accession date: 14-04-2021.Search in Google Scholar
[88] Oğuzoğu Ş, Avcı M. Natural enemies of Cinara cedri Mimeur 1936 (Hemiptera: Aphididae) in cedar forests in Isparta Regional Forest Directorate. Kastamonu Üni Orman Fak D. 2019;19(2):172–84.10.17475/kastorman.625698Search in Google Scholar
[89] Hadzibejli ZK. On Marchalina caucasica, sp. n. (Homoptera, Coccoidea) from the Caucasus. Ento Rev. 1969;48:391–8.Search in Google Scholar
© 2021 Şükran Oğuzoğlu et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.



![Figure A1
Natural (circles) and introduced (triangles) distribution of Marchalina hellenica in Turkey (data from ref. [ 4,18,19,24] and personal observations) along with the first records made in the present study (asterisks) (counter map from ref. [87]).](/https/www.degruyter.com/document/doi/10.1515/biol-2021-0066/asset/graphic/j_biol-2021-0066_fig_004.jpg)
