Published online Jul 27, 2024. doi: 10.4240/wjgs.v16.i7.2308
Revised: May 23, 2024
Accepted: June 12, 2024
Published online: July 27, 2024
Processing time: 133 Days and 7.7 Hours
Surgical intervention involving the pancreas can lead to impaired glucose tole
To investigate the effects of different extents of pancreatic resection on endocrine function in S. murinus.
Eight-week-old male S. murinus shrews were randomly divided into three experimental groups according to different pancreatic resection ranges as follows: ventral pancreatectomy (VPx) group; partial pancreatectomy (PPx) group; subtotal pancreatectomy (SPx) group; and a sham-operated group. Postprandial serum insulin, glucagon-like peptide-1 (GLP-1), pancreatic polypeptide (PP), and somatostatin (SST) levels, as well as food intake, weight, blood glucose, and glucose tolerance were regularly measured for each animal.
S. murinus treated with PPx and SPx suffered from varying degrees of impaired glucose tolerance, but only a small proportion of the SPx group developed diabetes. Only S. murinus in the SPx group showed a significant decrease in food intake accompanied by severe weight loss, as well as a significant increase in postprandial serum GLP-1 levels. Postprandial serum PP levels decreased in both the VPx and PPx groups, but not in the SPx group. Postprandial serum SST levels decreased in both VPx and PPx groups, but the decrease was marginal.
Severe weight loss after pancreatectomy may be related to loss of appetite caused by compensatory elevation of GLP-1. PP and GLP-1 may play a role in resisting blood glucose imbalance.
Core Tip: Surgical intervention involving the pancreas can lead to impaired glucose tolerance and other types of endocrine dysfunction. The scope of pancreatectomy and whether it includes the ventral pancreas are the key factors in development of postoperative diabetes. Here, we investigated the impacts of three different pancreatic resection ranges (all containing ventral pancreas resection) on endocrine function in Suncus murinus and found that severe weight loss after pancreatectomy may be related to loss of appetite caused by compensatory elevation of glucagon-like peptide-1 (GLP-1) and that pancreatic poly
- Citation: Li RJ, Yang T, Zeng YH, Natsuyama Y, Ren K, Li J, Nagakawa Y, Yi SQ. Impacts of different pancreatic resection ranges on endocrine function in Suncus murinus. World J Gastrointest Surg 2024; 16(7): 2308-2318
- URL: https://www.wjgnet.com/1948-9366/full/v16/i7/2308.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v16.i7.2308
The pancreas is an important exocrine and endocrine organ, and there are complex interactions between the endocrine and exocrine functions of the pancreas and gut hormones. Surgical interventions involving the pancreas can lead to impaired glucose tolerance and other endocrine dysfunction. Gall et al[1] reported 49 partial pancreaticoduodenectomy and 68 total pancreaticoduodenectomy procedures performed for chronic pancreatitis. In the partial pancreatectomy group, 16% of patients had diabetes before surgery. This increased to 22% thereafter. Miyata et al[2] found no significant difference in fasting blood glucose levels compared to the normal control group in their study of insulin secretion after partial pancreaticoduodenectomy in 10 patients; however, in the resection group, hyperglycemia persisted for 3 h after oral glucose administration. Izbicki et al[3] studied the endocrine function of 30 patients who underwent pylorus-preserving pancreaticoduodenectomy and found that their glucose tolerance further deteriorated, requiring insulin to control blood glucose levels; 3 patients with normal preoperative glucose metabolism developed impaired glucose tolerance after surgery. Eddes et al[4] also reported that, in patients after pancreatic surgery that included pancreatic head resection, basal and stimulated pancreatic polypeptide (PP) secretions were significantly reduced. Therefore, the effect of pancreatectomy on glucose tolerance and other endocrine functions cannot be ignored.
Further research shows that preservation of the ventral pancreas is key to preventing postoperative diabetes after pancreatectomy. Büchler et al[5] indicated that preservation of the duodenum (or the PP and the ventral pancreas) was the main reason for improving postoperative glucose tolerance in their study of endocrine function after pylorus-preserving partial pancreaticoduodenectomy or duodenum-preserving pancreatic head resection. Central pancreatectomy is a parenchyma-sparing surgical procedure that enables the removal of benign or low-grade malignant lesions from the neck and proximal body of the pancreas. A systematic review indicated that central pancreatectomy is associated with a lower risk of endocrine insufficiency in comparison to distal pancreatectomy[6].
The liver is crucial for glucose homeostasis, and its impact on glucose metabolism largely depends on insulin, glucagon, and PP, which are all circulating hormones secreted by the pancreas[7]. Alpha cells (producing glucagon) and PP cells (producing PP) are respectively distributed in the tail and head of the pancreas, while β cells (producing glu
Glucagon-like peptide-1 (GLP-1) stimulates insulin secretion and reduces food intake. GLP-1 promotes insulin se
The pancreas secretes somatostatin (SST), which can inhibit pancreatic exocrine function. SST and its receptors are widely distributed in the digestive system. Enteric endocrine cells, D cells in the stomach, and δ cells in the pancreas secrete SST[19,20]. The digestive and absorption functions of the gastrointestinal (GI) system can be suppressed by SST and its receptors[21,22]. The use of perioperative SST or its analogs, which suppress pancreatic exocrine secretion, may contribute to reducing the incidence of postoperative complications in pancreatic surgery. Although many surgeons have accepted this viewpoint, it remains controversial[23].
In this study, we hypothesized that pancreatectomy involving the ventral pancreas could provide a stable model of impaired glucose tolerance/diabetes and endocrine dysfunction after surgery in S. murinus. The animals were randomly divided into three experimental groups and a control group according to different pancreatic resection ranges. Post
Male house musk shrews [S. murinus (n = 27; age of 8 wk; body weight of 80-105 g)] were used. S. murinus were maintained in a closed breeding colony (JIc: KAT-c, at our laboratory), which was kept in an experimental animal facility at a room temperature (RT) of 25-28 °C, under a 12-h light and dark cycle, with ad libitum access to trout chow and water[10]. The food pellets consisted of 45.0% protein, 4.0% fat, 3.0% fiber, 15.0% ash, and 26.2% complex carbohydrates (Oriental Yeast Co. Ltd. Bioindustry Division, Chiba, Japan). The metabolizable energy content was 357 kcal/100 g.
All animal experiments were approved by the Institutional Animal Care and Use Committee of the Tokyo Metro
All surgeries were performed under isoflurane anesthesia to alleviate pain, and all efforts were made to minimize suffering. Animals were monitored continuously after surgery until they were able to maintain sternal recumbency, as required by the Institutional Animal Care and Use Committee policy surgical guidelines. Subsequently, they were monitored twice daily for 3-5 d to ensure general health. The specific criteria used to monitor animal health included hunched posture, piloerection, abnormal feeding, drinking, and ambulation. If there were adverse signs that persisted for 24 h postoperatively or a pronounced sharp decrease in body weight (> 25%), the animals were euthanized using excessive anesthesia.
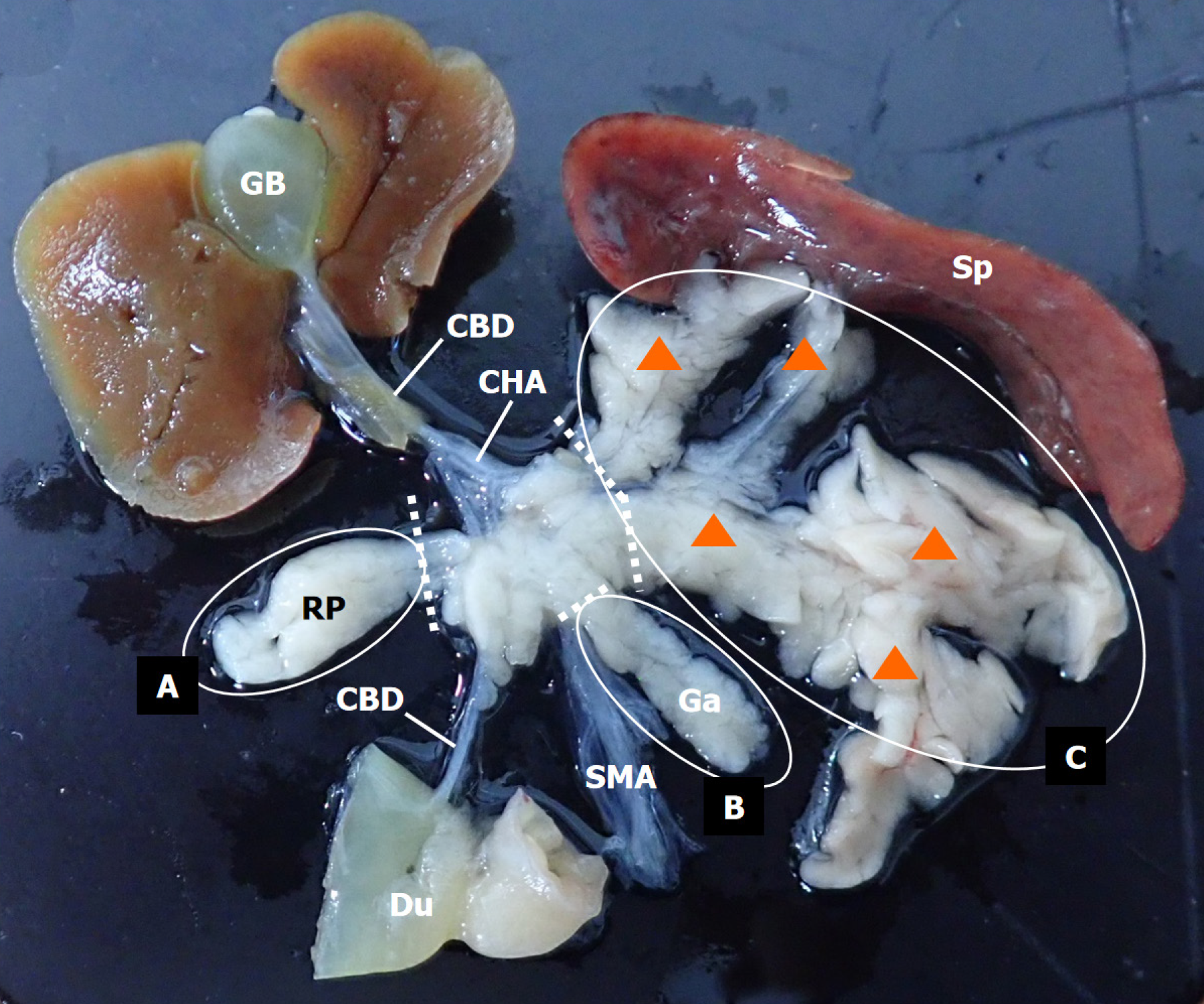
Animals were randomly sham-operated (Sham group, Sham, n = 6) or subjected to pancreatectomy (Px) (Experimental groups). The animals were divided into three groups: ventral pancreatectomy (VPx, n = 7), partial pancreatectomy (PPx, n = 8), and subtotal pancreatectomy (SPx, n = 6). For VPx, PPx, and SPx, different ranges of pancreatic resection were performed, as shown in Figure 1, corresponding to the resection of the right pancreas (VPx, Figure 1, area A, approximately 10% of the total pancreatic volume), the right pancreas and splenic lobe (area C, approximately 65%-75% of the total pancreatic volume) of the left pancreas (PPx, Figure 1, combined resection of areas A + C, approximately 75%-85% of the total pancreatic volume), right pancreas and gastric lobe (area B, approximately 5%-10% of the total pancreatic volume), and splenic lobes of the left pancreas (SPx, Figure 1, combined resection of areas A + B + C, approximately 85%-90% of the total pancreatic volume). The sham-operated animals were handled similarly, but their pancreas was not removed.
Experimental data were collected for each animal (Figure 2). Blood was collected through the submandibular vein 8, 10, 15, 17, 22, and 26 d after the operation, and body weight was measured on postoperative days 5, 11, 18, and 25. Intraperitoneal glucose tolerance test (IPGTT) and blood glucose testing were performed once and twice per week, respectively, after surgery. The average amount of food consumed for 7 consecutive days after surgery was taken as the first week’s food intake. The food intake for the second, third, and fourth weeks was calculated in sequence. Bromodeoxyuridine (BrdU) at a dose of 100 mg/kg body weight per day was injected intraperitoneally 3 d before sacrifice. All experimental animals were sacrificed 4 wk after surgery.
Blood glucose was measured twice a week at approximately 9:00 am on the tail snips using a portable glucometer (190725; TERUMO, Tokyo, Japan). IPGTT was performed when S. murinus were injected intraperitoneally with 1 g dextrose/kg body weight after overnight fasting (water not prohibited). Blood glucose concentrations were measured at various time points (0, 10, 30, 60, and 120 min), and blood was collected 30 min after intraperitoneal injection for insulin detection. The area under the curve was used to reflect the overall blood glucose level during IPGTT.
Immediately after euthanizing the animals, systemic perfusion was performed using 4% paraformaldehyde. Pancreatic tissue specimens were then collected, immersed, and fixed in 4% PFA at 4 °C overnight. The specimens were then washed thoroughly for 4-5 h under running tap water, dehydrated, and routinely embedded in paraffin. Sections (5 µm) were cut and placed on gelatin-coated glass slides. Immunohistochemical procedures for double fluorescence staining were performed as previously described[24]. Briefly, the tissue sections were deparaffinized with xylene and rehydrated in a graded ethanol series. The sections were then treated for 15 min in a methanol solution containing 0.3% (v/v) hydrogen peroxide. Rinsing was performed in 0.01 M PBS. After this, the sections were blocked with protein block (X0909; Dako, Nowy Sącz, Poland) for 1 h and incubated with 2 M hydrochloric acid for 45 min at RT. Next, the sections were incubated overnight at 4 °C with guinea pig anti-insulin antibody (ready to use) (IR00261-2J; Dako) in a humidified chamber, and then incubated with mouse anti-BrdU antibody (B35128; Invitrogen, ThermoFisher Scientific, Waltham, MA, United States) diluted to 1:50 in 0.01 M PBS in the same way. Thereafter, two types of corresponding secondary antibodies, Alexa Fluor 488-conjugated goat anti-guinea pig IgG (A11073; Invitrogen) and donkey anti-mouse IgG TRITC (sc-2300; Santa Cruz Biotechnology, Dallas, TX, United States), were diluted 1:100 in 0.01 M PBS and then incubated for 1 h at RT with the slides. Sections were then coverslipped with Fluoromount (K-024; Diagnostic Biosystems, Pleasanton, CA, United States) and viewed under a fluorescence microscope (Axio Imager M1; Zeiss, Oberkochen, Germany).
S. murinus were fasted (water not restricted) for 12 h, and blood was collected 30 min after feeding. After blood collection, samples were allowed to stand at RT for approximately 2 h to coagulate, then centrifuged at 3000 rpm for 10 min, and the serum was stored at -20 °C until the next experiment. These serum samples were used to measure insulin using the Insulin Eliza kit (10-1113-01; Mercodia AB, Uppsala, Sweden), PP using the Pancreatic Polypeptide ELISA kit (EK-054-02; Phoenix Pharmaceuticals, Mannheim, Germany), SST using the Somatostatin ELISA kit (NBP2-80269; Novus Biologicals, Centennial, CO, United States), and GLP-1 using a GLP-1 ELISA kit (10-1278-01; Mercodia AB). For the detailed steps, refer to the corresponding product protocols.
Data are expressed as the mean ± standard deviation. To determine statistical significance, a Mann–Whitney test was used for comparisons between two groups. Second-order smoothing was used to describe the trend of value variation over time. Data visualization was conducted using GraphPad Prism Version 9.0.0 (La Jolla, CA, United States). Differences were considered statistically significant at P < 0.05.
S. murinus in the SPx group experienced a temporary increase in blood glucose levels in the first 2 wk after surgery and then recovered to the same level as the Sham group (Figure 3A and Supplementary Table 1). Only one (1/6) animal maintained a sustained and stable hyperglycemic state, with an average postoperative blood glucose level of 151 mg/dL after surgery. S. murinus in both the PPx and SPx groups showed varying degrees of impaired glucose tolerance after surgery, which remained stable over time (Figure 3B and Supplementary Table 2).
Insulin release at 30 min after the IPGTT in the PPx and SPx groups was significantly reduced and relatively stable over time in comparison to the Sham group (Figure 4 and Supplementary Table 3). S. murinus subjected to PPx and SPx both suffered from varying degrees of stable impaired glucose tolerance due to insufficient insulin secretion, but only a small proportion of S. murinus in the SPx group developed diabetes.
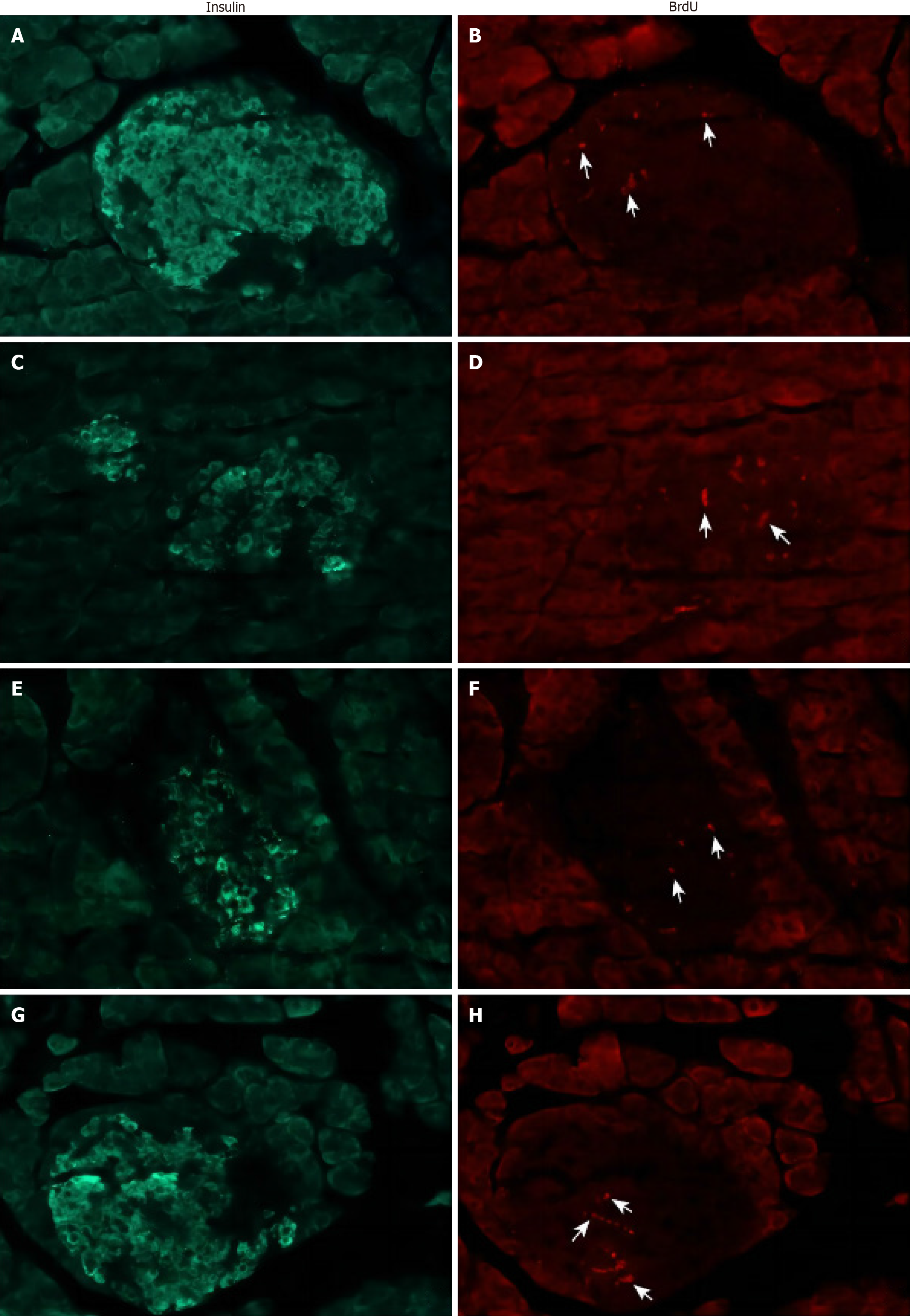
At the end of the experiment, no abnormally active proliferation of pancreatic endocrine cells was observed in any of the three surgical groups compared with the Sham group (Figure 5). Thus, it does not support the explanation of β-cell proliferation as a compensatory process for glucose homeostasis after Px in S. murinus.
All animals that underwent Px showed varying degrees of weight loss after surgery, with the SPx group exhibiting the most severe weight loss (Figure 6A and Supplementary Table 4). Only S. murinus in the SPx group showed a significant decrease in food intake post-surgery (Figure 6B and Supplementary Table 5). The decrease in food intake may be an essential reason for the severe weight loss in the SPx group.
Serum insulin levels at 30 min after feeding in both the PPx and SPx groups decreased significantly and remained relatively stable over time (Figure 7A and B, Supplementary Table 6). A 75%-85% Px can lead to insufficient postprandial insulin secretion in S. murinus. Serum GLP-1 levels at 30 min after feeding in the SPx group significantly increased and remained relatively stable over time (Figure 7C and D, Supplementary Table 7). SPx can lead to compensatory elevation in postprandial GLP-1 levels in S. murinus. The serum PP levels at 30 min after feeding in both the VPx and PPx groups decreased and remained relatively stable over time (Figure 7E and F, Supplementary Table 8), whereas there was no significant change in the SPx group. Partial Px, including that of the ventral pancreas, can cause a decrease in PP levels in the postprandial blood of S. murinus. However, when the scope of pancreatic resection expands further, it triggers compensatory mechanisms and rebounds to near-normal levels. Serum SST levels at 30 min after feeding in both the VPx and PPx groups decreased and remained relatively stable over time (Figure 7G and H, Supplementary Table 9), but the decrease was marginal.
In the present study, we divided the experimental animals into three Px groups (VPx, PPx, and SPx) and one sham-operated group. We regularly measured postprandial serum insulin, GLP-1, PP, and SST levels, as well as food intake, body weight, blood glucose, and glucose tolerance in all groups. We found that both S. murinus treated with PPx and SPx suffered from varying degrees of stable impaired glucose tolerance due to insufficient insulin secretion; however, only a small proportion of S. murinus in the SPx group had diabetes. Only S. murinus in the SPx group showed a significant decrease in food intake accompanied by severe weight loss. Only the SPx group showed a significant increase in serum GLP-1 levels 30 min after feeding. Postprandial serum PP levels decreased in both the VPx and PPx groups, but not in the SPx group.
We found that PPx (75%-85% Px) can lead to insufficient postprandial insulin release in S. murinus. Although insulin release in the SPx group decreased markedly (SPx vs Sham, 5.4 vs 30.4), only a small proportion of SPx S. murinus developed stable diabetes. This may be related to individual differences in the animals and subtle differences in the resection range. Kaufmann and Rodriguez[25] studied the incidence and development process of diabetes 12 mos after subtotal pancreatectomy in five strains of rats and found that only a few animals developed diabetes at the end of the experiment, and the severity of diabetes in different strains was distinct. Several animal experimental studies[26,27] have shown that partial pancreatic resection can lead to compensatory regeneration of pancreatic β cells. Although this study observed an increase in endocrine cells in the pancreatic islets of S. murinus after Px, the number of cells was not significantly different from that in the control group. Therefore, we believe that compensatory regeneration of β-cells is not the main reason S. murinus resists blood glucose imbalance after Px.
In addition, we observed that serum GLP-1 levels 30 min after feeding in the SPx group were significantly higher than those in the control group. The study by Cabou et al[28] showed that systemic glucose utilization and femoral artery blood flow in hyperinsulinemic-hyperglycemic mice were reduced by continuous intracerebral infusion of GLP-1 rece
We also found that the serum PP levels in the VPx and PPx groups significantly decreased 30 min after feeding. However, in the SPx group, it rebounded to normal levels in a compensatory manner, with no significant difference from the Sham group. Andersen et al[31] reported that the inhibition of hepatic glucose production significantly increased when PP was administered with insulin in dogs with chronic pancreatitis-induced PP deficiency. Seymour et al[32] studied male individuals who underwent Px after abdominal trauma, and found that patients with insufficient postoperative PP responses had severely impaired hepatic insulin responses. A few years later, in a study of a rat model of chronic pancreatitis[33], PP deficiency was associated with decreased availability of hepatic insulin receptors, which was reversible after PP administration. Therefore, we believe that the compensatory rebound of PP may contribute to the maintenance of blood glucose homeostasis in the SPx group by increasing liver sensitivity to insulin.
This study showed that S. murinus in the SPx group experienced severe weight loss accompanied by a decrease in food intake and an increase in postprandial serum GLP-1 levels. Punjabi et al[34] suggested that a regular chow meal transiently increased plasma active GLP-1 levels in the hepatic portal vein but not in the vena cava. However, an unphysiologically large postprandial release of GLP-1, for example, after gastric bypass surgery[35], may reach the circumventricular organs, and dipeptidyl peptidase 4 (DPP-4)-resistant GLP-1 receptor agonists clearly have central actions. Punjabi[34] also reported that intrameal hepatic portal vein GLP-1 infusion specifically reduced ongoing meal size by almost 40%. Peripherally administered GLP-1 receptor agonists reach the brainstem nuclei and hypothalamus, acting on the GLP-1 receptors of neurons in the arcuate nucleus that express proopiomelanocortin/cocaine and amphetamine-regulated transcript; the neurons are then depolarized by GLP-1 receptor stimulation and inhibit appetite producing NPY/appetite related peptide neurons, leading to decreased appetite and delayed eating[36,37], which are associated with reduced appetite and delayed initiation of meals. These studies indicate that abnormal increases in cir
This study showed that the serum PP levels 30 min after feeding in both the VPx and PPx groups decreased, whereas there was no significant change in the SPx group. Our previous study[9] showed that PP-producing cells were extremely abundant in the right lobe of the pancreas of S. murinus, whereas they were absent in the left lobe. Thus, we believe that the distribution characteristics of PP cells were the main reason for the decrease in PP in the VPx and PPx groups. Moreover, we previously reported[24] that PP family (peptide Y) immunoreactive cells of the GI tract in S. murinus were predominantly distributed in the rectum. This may be the main site of compensatory increase in PP secretion in S. murinus that underwent a SPx. However, Dammann et al[38] reported that PP was undetectable in 8 patients who underwent total Px. This implies the absence of a significant number of normally functioning PP cells at extrapancreatic sites in humans. Therefore, it should be noted that there may be differences in compensatory ability for PP secretion between humans and animals.
We also observed a decrease in postprandial serum SST levels in the VPx and PPx groups, but the decrease was minimal (VPx and PPx vs Sham, 14.1 and 15.6 vs 16.9). We have not found any studies suggesting a correlation between Px and changes in postprandial serum SST levels. In this study, we were not sure whether a slight decrease in SST after pancreatic surgery would affect the physiological function of S. murinus.
In summary, the maintenance of glucose homeostasis in S. murinus after Px is the result of the combined action of multiple hormones, among which PP and GLP-1 may play a non-negligible role, and severe weight loss may be related to poor appetite caused by the compensatory elevation of GLP-1.
This study suggests that severe weight loss after Px, in addition to easy-to-understand factors such as large surgical trauma and a sharp decrease in digestive enzymes, may be related to loss of appetite caused by compensatory elevation of GLP-1, and that PP and GLP-1 may play a non-negligible role in resisting blood glucose imbalance after Px. Our results provide a new direction for the treatment and prevention of diabetes after Px. It is suggested that this animal model can be used for research related to impaired glucose tolerance or diabetes, weight loss, and endocrine dysfunction after Px.
| 1. | Gall FP, Mühe E, Gebhardt C. Results of partial and total pancreaticoduodenectomy in 117 patients with chronic pancreatitis. World J Surg. 1981;5:269-275. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 83] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 2. | Miyata M, Takao T, Uozumi T, Okamoto E, Manabe H. Insulin secretion after pancreatoduodenectomy. Ann Surg. 1974;179:494-498. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 20] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 3. | Izbicki JR, Bloechle C, Broering DC, Knoefel WT, Kuechler T, Broelsch CE. Extended drainage versus resection in surgery for chronic pancreatitis: a prospective randomized trial comparing the longitudinal pancreaticojejunostomy combined with local pancreatic head excision with the pylorus-preserving pancreatoduodenectomy. Ann Surg. 1998;228:771-779. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 255] [Cited by in F6Publishing: 226] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 4. | Eddes EH, Verkijk M, Gielkens HA, Biemond I, Bemelman W, Lamers CB, Masclee AA. Pancreatic polypeptide secretion in patients with chronic pancreatitis and after pancreatic surgery. Int J Pancreatol. 2001;29:173-180. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 5. | Büchler MW, Friess H, Müller MW, Wheatley AM, Beger HG. Randomized trial of duodenum-preserving pancreatic head resection versus pylorus-preserving Whipple in chronic pancreatitis. Am J Surg. 1995;169:65-9; discussion 69. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 311] [Cited by in F6Publishing: 250] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 6. | Iacono C, Verlato G, Ruzzenente A, Campagnaro T, Bacchelli C, Valdegamberi A, Bortolasi L, Guglielmi A. Systematic review of central pancreatectomy and meta-analysis of central versus distal pancreatectomy. Br J Surg. 2013;100:873-885. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 108] [Cited by in F6Publishing: 128] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 7. | Andersen DK, Brunicardi FC. Pancreatic anatomy and physiology. In: Greenfield LJ. Surgery: Scientific Principles and Practice. 2nd. Philadelphia: Lippincott-Raven, 1997: 857-874. [Cited in This Article: ] |
| 8. | Orci L. Macro- and micro-domains in the endocrine pancreas. Diabetes. 1982;31:538-565. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 206] [Cited by in F6Publishing: 240] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 9. | Yi SQ, Akita K, Ohta T, Shimokawa T, Tanaka A, Ru F, Nakatani T, Isomura G, Tanaka S. Cellular localization of endocrine cells in the adult pancreas of the house musk shrew, Suncus murinus: a comparative immunocytochemical study. Gen Comp Endocrinol. 2004;136:162-170. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Yi SQ, Shimokawa T, Akita K, Ohta T, Kayahara M, Miwa K, Tanaka S. Anatomical study of the pancreas in the house musk shrew (Suncus murinus), with special reference to the blood supply and innervation. Anat Rec A Discov Mol Cell Evol Biol. 2003;273:630-635. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 11. | Pacini G, Ahrén B. Glucagon-like peptide-1 and glucose-dependent insulinotropic peptide: effects alone and in combination on insulin secretion and glucose disappearance in mice. Physiol Rep. 2017;5. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 12. | Mojsov S, Weir GC, Habener JF. Insulinotropin: glucagon-like peptide I (7-37) co-encoded in the glucagon gene is a potent stimulator of insulin release in the perfused rat pancreas. J Clin Invest. 1987;79:616-619. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 575] [Cited by in F6Publishing: 578] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 13. | Holst JJ, Orskov C, Nielsen OV, Schwartz TW. Truncated glucagon-like peptide I, an insulin-releasing hormone from the distal gut. FEBS Lett. 1987;211:169-174. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 409] [Cited by in F6Publishing: 407] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 14. | Weir GC, Mojsov S, Hendrick GK, Habener JF. Glucagonlike peptide I (7-37) actions on endocrine pancreas. Diabetes. 1989;38:338-342. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 128] [Cited by in F6Publishing: 140] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 15. | Ding WG, Renström E, Rorsman P, Buschard K, Gromada J. Glucagon-like peptide I and glucose-dependent insulinotropic polypeptide stimulate Ca2+-induced secretion in rat alpha-cells by a protein kinase A-mediated mechanism. Diabetes. 1997;46:792-800. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 73] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 16. | Turton MD, O'Shea D, Gunn I, Beak SA, Edwards CM, Meeran K, Choi SJ, Taylor GM, Heath MM, Lambert PD, Wilding JP, Smith DM, Ghatei MA, Herbert J, Bloom SR. A role for glucagon-like peptide-1 in the central regulation of feeding. Nature. 1996;379:69-72. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1354] [Cited by in F6Publishing: 1316] [Article Influence: 47.0] [Reference Citation Analysis (0)] |
| 17. | Orskov C, Poulsen SS, Møller M, Holst JJ. Glucagon-like peptide I receptors in the subfornical organ and the area postrema are accessible to circulating glucagon-like peptide I. Diabetes. 1996;45:832-835. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 173] [Cited by in F6Publishing: 185] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 18. | Flint A, Raben A, Astrup A, Holst JJ. Glucagon-like peptide 1 promotes satiety and suppresses energy intake in humans. J Clin Invest. 1998;101:515-520. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 951] [Cited by in F6Publishing: 956] [Article Influence: 36.8] [Reference Citation Analysis (0)] |
| 19. | Morisset J. Somatostatin: One of the Rare Multifunctional Inhibitors of Mammalian Species. Pancreas. 2017;46:8-18. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 20. | Rorsman P, Huising MO. The somatostatin-secreting pancreatic δ-cell in health and disease. Nat Rev Endocrinol. 2018;14:404-414. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 165] [Cited by in F6Publishing: 146] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 21. | Schubert ML, Rehfeld JF. Gastric Peptides-Gastrin and Somatostatin. Compr Physiol. 2019;10:197-228. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 22. | Engevik AC, Kaji I, Goldenring JR. The Physiology of the Gastric Parietal Cell. Physiol Rev. 2020;100:573-602. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 121] [Cited by in F6Publishing: 101] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 23. | Ramos-De la Medina A, Sarr MG. Somatostatin analogues in the prevention of pancreas-related complications after pancreatic resection. J Hepatobiliary Pancreat Surg. 2006;13:190-193. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 24. | Yi SQ, Li J, Yamaguchi T, Hori K, Hayashi K, Itoh M. Immunolocalization of the PP family and its receptors in the gastrointestinal tract of house musk shrew, Suncus murinus. Neuro Endocrinol Lett. 2011;32:212-219. [PubMed] [Cited in This Article: ] |
| 25. | Kaufmann F, Rodriguez RR. Subtotal pancreatectomy in five different rat strains: incidence and course of development of diabetes. Diabetologia. 1984;27:38-43. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 15] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 26. | Li W, Zhang H, Nie A, Ni Q, Li F, Ning G, Li X, Gu Y, Wang Q. mTORC1 pathway mediates beta cell compensatory proliferation in 60 % partial-pancreatectomy mice. Endocrine. 2016;53:117-128. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 27. | Hayashi KY, Tamaki H, Handa K, Takahashi T, Kakita A, Yamashina S. Differentiation and proliferation of endocrine cells in the regenerating rat pancreas after 90% pancreatectomy. Arch Histol Cytol. 2003;66:163-174. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 50] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 28. | Cabou C, Campistron G, Marsollier N, Leloup C, Cruciani-Guglielmacci C, Pénicaud L, Drucker DJ, Magnan C, Burcelin R. Brain glucagon-like peptide-1 regulates arterial blood flow, heart rate, and insulin sensitivity. Diabetes. 2008;57:2577-2587. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 97] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 29. | Cabou C, Vachoux C, Campistron G, Drucker DJ, Burcelin R. Brain GLP-1 signaling regulates femoral artery blood flow and insulin sensitivity through hypothalamic PKC-δ. Diabetes. 2011;60:2245-2256. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 30. | Parlevliet ET, de Leeuw van Weenen JE, Romijn JA, Pijl H. GLP-1 treatment reduces endogenous insulin resistance via activation of central GLP-1 receptors in mice fed a high-fat diet. Am J Physiol Endocrinol Metab. 2010;299:E318-E324. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 61] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 31. | Andersen DK. The Role of Pancreatic Polypeptide in Glucose Metabolism. Gastrointest Endocrinol. 1990;. [DOI] [Cited in This Article: ] |
| 32. | Seymour NE, Brunicardi FC, Chaiken RL, Lebovitz HE, Chance RE, Gingerich RL, Elahi D, Andersen DK. Reversal of abnormal glucose production after pancreatic resection by pancreatic polypeptide administration in man. Surgery. 1988;104:119-129. [PubMed] [Cited in This Article: ] |
| 33. | Seymour NE, Volpert AR, Lee EL, Andersen DK, Hernandez C. Alterations in hepatocyte insulin binding in chronic pancreatitis: effects of pancreatic polypeptide. Am J Surg. 1995;169:105-9; discussion 110. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 34] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 34. | Punjabi M, Arnold M, Rüttimann E, Graber M, Geary N, Pacheco-López G, Langhans W. Circulating glucagon-like peptide-1 (GLP-1) inhibits eating in male rats by acting in the hindbrain and without inducing avoidance. Endocrinology. 2014;155:1690-1699. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 35. | Korner J, Bessler M, Inabnet W, Taveras C, Holst JJ. Exaggerated glucagon-like peptide-1 and blunted glucose-dependent insulinotropic peptide secretion are associated with Roux-en-Y gastric bypass but not adjustable gastric banding. Surg Obes Relat Dis. 2007;3:597-601. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 277] [Cited by in F6Publishing: 274] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 36. | Secher A, Jelsing J, Baquero AF, Hecksher-Sørensen J, Cowley MA, Dalbøge LS, Hansen G, Grove KL, Pyke C, Raun K, Schäffer L, Tang-Christensen M, Verma S, Witgen BM, Vrang N, Bjerre Knudsen L. The arcuate nucleus mediates GLP-1 receptor agonist liraglutide-dependent weight loss. J Clin Invest. 2014;124:4473-4488. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 458] [Cited by in F6Publishing: 569] [Article Influence: 56.9] [Reference Citation Analysis (0)] |
| 37. | Gabery S, Salinas CG, Paulsen SJ, Ahnfelt-Rønne J, Alanentalo T, Baquero AF, Buckley ST, Farkas E, Fekete C, Frederiksen KS, Helms HCC, Jeppesen JF, John LM, Pyke C, Nøhr J, Lu TT, Polex-Wolf J, Prevot V, Raun K, Simonsen L, Sun G, Szilvásy-Szabó A, Willenbrock H, Secher A, Knudsen LB, Hogendorf WFJ. Semaglutide lowers body weight in rodents via distributed neural pathways. JCI Insight. 2020;5. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 119] [Cited by in F6Publishing: 255] [Article Influence: 63.8] [Reference Citation Analysis (0)] |
| 38. | Dammann HG, Besterman HS, Bloom SR, Schreiber HW. Gut-hormone profile in totally pancreatectomised patients. Gut. 1981;22:103-107. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 22] [Article Influence: 0.5] [Reference Citation Analysis (0)] |