Abstract
Pbx2 is one of four mammalian genes that encode closely related TALE homeodomain proteins, which serve as DNA binding partners for a subset of Hox transcription factors. The expression and contributions of Pbx2 to mammalian development remain undefined, in contrast to the essential roles recently established for family members Pbx1 and Pbx3. Here we report that Pbx2 is widely expressed during embryonic development, particularly in neural and epithelial tissues during late gestation. Despite wide Pbx2 expression, mice homozygous mutant for Pbx2 are born at the expected Mendelian frequencies and exhibit no detectable abnormalities in development and organogenesis or reduction of long-term survival. The lack of an apparent phenotype in Pbx2−/− mice likely reflects functional redundancy, since the Pbx2 protein is present at considerably lower levels than comparable isoforms of Pbx1 and/or Pbx3 in embryonic tissues. In postnatal bone marrow and thymus, however, Pbx2 is the predominant high-molecular-weight (MW)-isoform Pbx protein detectable by immunoblotting. Nevertheless, the absence of Pbx2 has no measurable effect on steady-state hematopoiesis or immune function in adult mice, suggesting possible compensation by low-MW-isoform Pbx proteins present in these tissues. We conclude that the roles of Pbx2 in murine embryonic development, organogenesis, hematopoiesis, immune responses, and long-term survival are not essential.
Pbx genes code for a family of highly conserved homeodomain proteins of the TALE (three-amino acid loop extension) class that participate in multiprotein complexes to regulate developmental gene expression (20). Pbx1 was originally identified in human pre-B acute lymphoblastic leukemias as a result of its disruption by t(1;19) chromosomal translocations (13, 23). The highly related Pbx2, Pbx3, and Pbx4 genes were subsequently identified on the basis of sequence conservation with Pbx1 (22, 40). Pbx orthologs in Caenorhabditis elegans (Ceh-20), Drosophila (Exd), and zebra fish (lazarus) (5, 28, 29, 39) have also been characterized. In Drosophila, Exd acts as a cofactor to direct homeotic selector proteins to target genes (44), thus conferring higher specificity to Hox DNA binding in developmental programs (6, 27, 30). In zebra fish, lazarus has been shown to globally mediate Hox gene function, while orchestrating the corresponding segmentation of the hindbrain and pharyngeal arches (28, 41).
Biochemical studies have demonstrated that Pbx proteins interact with a subset of Hox proteins to enhance their DNA binding affinities and specificities (6-9, 16, 19, 24-26, 37, 38). Pbx proteins also heterodimerize with the Meis/Prep subfamily of TALE-class homeodomain proteins (4, 8, 14) to form trimeric complexes with Hox proteins on appropriate DNA sites (3, 4, 12, 33) that regulate developmental gene expression. Pbx proteins are highly similar to each other and share extensive sequence identity within and flanking their DNA binding homeodomains. Additional isoforms of mammalian Pbx proteins arise from differential splicing of Pbx transcripts to yield high-molecular-weight (MW) (Pbx1a, Pbx2, Pbx3a, and Pbx4) and low-MW (Pbx1b and Pbx3b) forms of the respective proteins (22, 40). Although the DNA binding properties of Pbx proteins appear similar in vitro, the transcriptional effector properties of various isoforms can be distinguished on the basis of differential recruitment of transcriptional cofactors (2).
In contrast to their well-characterized roles as transcription factors, the differential contributions of Pbx proteins during mammalian development and organogenesis have not been fully determined. To assess their functional roles in vivo and their possible overlapping and unique contributions, we have generated and characterized mice deficient for each of the Pbx1, -2, and -3 proteins. Our previous studies demonstrated that Pbx1 and Pbx3 have unique, essential functions required for embryonic development and postnatal survival, respectively. Pbx1-deficient embryos die at gestational day 15 or 16 with severe hypoplasia or aplasia of multiple organs (15, 32, 34, 36), as well as homeotic transformation (36) and hematopoietic abnormalities (11). Mice deficient for Pbx3 develop to term but die within a few hours of birth due to central respiratory failure (J. Rhee, A. Arata, L. Selleri, Y. Jacobs, S. Arata, H. Onimaru, and M. Cleary, submitted for publication). In this study, we demonstrated that, despite widespread embryonic expression, Pbx2 is not an essential gene, whose loss does not affect normal development, organogenesis, fertility, hematopoiesis, or immune function, likely due to redundancy with the related Pbx family members.
MATERIALS AND METHODS
Targeted disruption of the Pbx2 gene and generation of Pbx2 knockout mice.
The Pbx2 gene was mutated by deletion of a 316-bp SacI-XbaI genomic fragment comprising 248 bp covering the entire Pbx2 exon 3, the largest 5′ exon containing a nonunit number of codons, and 68 bp of Pbx2 intron 3, to the XbaI site. In place of the removed 316-bp SacI-XbaI Pbx2 genomic fragment, a 2-kb PGK-neo cassette (from the pNT vector) (36) was inserted into the unique SacI site of exon 3. A 7.9-kb segment of genomic DNA spanning the disrupted Pbx2 exon 3 was then cloned into the targeting vector containing the herpes simplex virus thymidine kinase cassette (see Fig. 2). The targeting construct was linearized by NotI digestion and then electroporated into embryonic stem (ES) cell line GS (purchased from Genome Systems) and line W9.5 (18). Following positive/negative selection in G418 and ganciclovir (1), homologous recombinant clones were identified by Southern blot analysis using three different enzyme and probe combinations (5′ and 3′ external probes and a neo-specific internal probe). Of 182 informative clones, 18 yielded restriction digest patterns diagnostic for homologous recombination. Euploid clones were microinjected into C57BL/6J host blastocysts. Chimeric male mice from two independently derived ES clones (W9.5 # 68 and GS # 45) passed the targeted Pbx2 allele through the germ line. Phenotypes were analyzed in mice derived from the third or fourth backcross generation on a C57BL/6 background and on a fully inbred 129/SvTer background. All observations and results obtained were identical in mice derived from either of the two independently targeted clones (W9.5 # 68 and GS # 45). Genotype analysis was performed on DNA extracted from tail biopsies of adult mice or from yolk sacs of embryonal conceptuses (gestational days 9 to 17 [E9-17]) dissected free of maternal tissues. Following digestion with EcoRI, DNAs were subjected to Southern blot analysis using the Pbx2 5′ external probe.
FIG. 2.
Targeted inactivation of Pbx2 and Southern, Northern, and Western analyses of wt and mutant mice. (A) Schematic representation of the mouse Pbx2 genomic locus, targeting vector, and mutated allele following homologous recombination. Approximately 16 kb of the Pbx2 locus flanking exon 3 (black box) are depicted along with mapped restriction sites. The targeting construct carries a PGK-neo cassette (shaded box) inserted into the unique SacI site of Pbx2 exon 3, and the herpes simplex virus thymidine kinase gene (HSV-TK) (white box). The transcriptional orientations of the Pbx2 arms of homology are opposite to that of the PGK-neo cassette. The 5′ and 3′ external probes used for Southern blot analyses are shown as solid black boxes below the mutated allele. Restriction enzyme sites: E, EcoRI; Nhe, NheI; Sal, SalI; Xba, XbaI; Xho, XhoI. An EcoRI site (italicized) was introduced to facilitate diagnostic analysis. (B) Southern blot analysis of Pbx2 alleles. DNA from mouse tissues was analyzed with the probe and enzyme indicated beneath the panel. wt (5.5 kb) and mutant (6.5 kb) Pbx2 alleles are indicated to the right of the panel. (C and D) Western blot analysis of Pbx2 expression. Protein extracts were subjected to Western blot analysis using a monoclonal antibody specific for all Pbx long isoforms (α-PbxL) and a Pbx2-specific polyclonal antibody raised against a peptide mapping at the amino terminus of Pbx2 (α-Pbx2 in panel D). Smaller bands, of less than 50 kDa, are present in both wt and Pbx2−/− livers and represent nonspecific cross-reactivity by this antibody (D). Genotypes determined by Southern blotting are listed at the top. The left lane of panel C contains in vitro-translated Pbx2. (E) Northern blot analysis of Pbx2 expression. Poly(A)+ RNAs from E17 embryos were hybridized with a probe specific for the Pbx2 3′ UTR. (Left panel) No Pbx2 transcripts are present in Pbx2−/− embryos, in contrast to the 3.2-kb wt transcripts (as previously reported [22]) detected in Pbx2+/+ embryos. (Right panel) Methylene blue staining indicates equal amounts of 28S and 18S rRNAs in each lane.
Northern blot analysis.
Total RNA was isolated from E17 embryos using an RNeasy kit (Qiagen, Valencia, Calif.). Poly(A)+ RNA was purified from total RNA with the Polyadenylated RNA kit (Ambion, Austin, Tex.). After transfer and before hybridization, the membrane was stained with a 0.04% methylene blue solution for 5 min to visualize the quality of RNA transfer and to precisely quantify the amounts of rRNAs transferred to each lane. After the membrane was destained in 0.2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate)-1% sodium dodecyl sulfate (SDS), Northern blot analysis was conducted following standard protocols (22). The hybridization probe consisted of a murine Pbx2 cDNA containing approximately 650 bp of 3′ UTR (NCBI accession numbers MMAF20198 and AC AF020198).
Western blot analysis.
Embryonic tissues (E17), thymocytes, or adult bone marrow cells were lysed in 2× SDS sample buffer following homogenization using established procedures (12). Proteins were subjected to SDS-polyacrylamide gel electrophoresis and immobilized on nitrocellulose filters following electrophoretic transfer. Filters were probed with a murine monoclonal antibody that recognizes all three Pbx high-MW isoforms (α-PbxL) or monoclonal antibodies specific for Pbx1a or Pbx3a. A Pbx2 affinity-purified rabbit polyclonal antibody raised against a peptide mapping at the amino terminus of Pbx2 was also used (Santa Cruz Biotechnology, Inc.). Immune complexes were detected by using a horseradish peroxidase-conjugated secondary antibody and an enhanced luminescence system (ECL; Amersham Pharmacia Biotech, Inc., Piscataway, N.J.).
Histology.
For histological analysis, embryos were fixed in formalin and embedded in paraffin for sectioning using standard procedures (35). Sections of 5 μm thickness were stained with hematoxylin and eosin, mounted in distrene plasticizer xylene, and photographed.
Skeletal preparations.
Differential staining of cartilage and bone in whole mouse embryos (E16) and newborn mice was visualized with alcian blue and alizarin red (10, 21)
Whole-mount and section in situ hybridizations.
Whole-mount in situ hybridizations were performed on embryos at E9.5 and E10.5 as previously described (43). In situ hybridizations were performed on frozen sections, generated from wild-type (wt) embryos at E13.5 and E15.5, as previously described (43). Single-stranded sense and antisense riboprobes specific for the Pbx2 cDNA were generated and hybridized to frozen sections containing all developing organs in order to establish Pbx2 expression patterns in later stages of murine development. The Pbx2 hybridization probe was composed of approximately 650 bp of 3′ UTR as described above. Probes were labeled with digoxigenin, using standard procedures (42). Images were obtained using an Olympus BX41 compound light microscope and digital camera or an Olympus SZX12 dissection microscope.
Real-time quantitative RT-PCR.
For reverse transcription-PCR (RT-PCR) total RNA was isolated from E17.5 tissues by using Trizol reagent (Gibco BRL) and treated with DNase Treatment and Removal Reagent (DNA-free; Ambion). RNA was reverse transcribed by using the High Capacity cDNA Archive kit (Applied Biosystems, Foster City, Calif.), using random primers for initiating cDNA synthesis. TaqMan real-time quantitative PCR assays were performed on a Perkin-Elmer ABI PRISM 7700 sequence detection system, using SDS version 1.9 software (Applied Biosystems). Specific primers and fluorogenic TaqMan probes were custom designed using the Assay by Design Service (Applied Biosystems). Primers, probes, and PCR conditions are available upon request.
Hematologic studies.
Blood (50 to 100 μl) from the tail vein was collected in an EDTA-coated tube, and automated blood counts were performed using a Coulter cytometer.
Immunological studies.
Freshly isolated cells from bone marrow and thymus were stained for four-color analysis, and the fluorescence was analyzed by using a dual-laser FACS Vantage system (Becton Dickinson Immunocytometry Systems, Mountain View, Calif.) with a four-decade logarithmic amplifier. Dead cells were detected by staining with propidium iodide (1 μg/ml) and gated out electronically. Residual erythrocytes were also gated out electronically. All antibodies were purchased from BD Pharmingen (San Diego, Calif.). Specificities of antibodies were as follows: phycoerythrin-conjugated RM4-5 (anti-CD4), biotinylated 53-6.7 (anti-CD8a), fluorescein isothiocyanate-conjugated S7 (anti-CD43), and allophycocyanin-conjugated RA3-6B2 (anti-B220).
Mice were immunized by intraperitoneal injection of 100 μg of 2,4-dinitrophenol (DNP) conjugated to keyhole limpet hemocyanin (DNP-KLH) with complete Freund's adjuvant, as described previously (31). The titers of anti-DNP antibodies were measured by enzyme-linked immunosorbent assay using DNP-conjugated bovine albumin (DNP-albumin) as the capture antigen, alkaline phosphatase-conjugated rabbit anti-mouse immunoglobulin G (IgG) or IgM as the secondary antibody, and Sigma Fast p-nitrophenyl phosphate as the substrate for alkaline phosphatase. Absorbances (405 nm) of pre- and postimmune sera were compared to monitor immune responses against the hapten.
RESULTS AND DISCUSSION
Pbx2 is widely expressed during murine development.
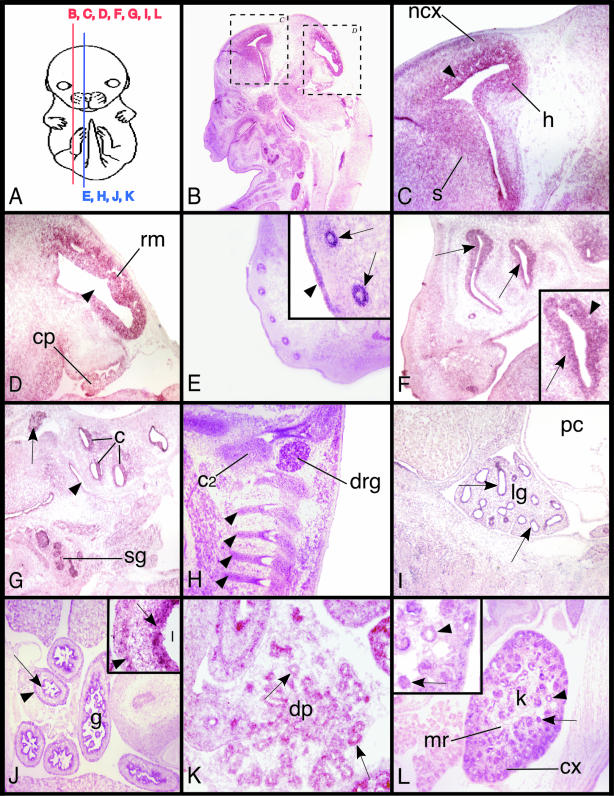
Whole-mount in situ hybridization experiments at E9.5 and 10.5 of murine gestation showed widespread expression of Pbx2 throughout the mouse embryo (data not shown). In situ hybridizations on frozen mouse embryonic sections at later days of gestation (E13.5 and 15.5) showed more restricted expression of Pbx2, which was nevertheless present in numerous distinct structures (Fig. 1). Pbx2 expression was prominent in several neural structures at E15.5, including the telencephalon (particularly in the neocortex, including the ventricular and subventricular zones), the hippocampus, and the developing striatum and globus pallidus (Fig. 1B and C). Pbx2 was also expressed in the mesencephalon, particularly the roof of the midbrain, the choroid plexus (Fig. 1B and D), the cranial ganglia (the trigeminal ganglion is depicted in Fig. 1F and G), and cervical dorsal root ganglia. High-level Pbx2 expression was observed in the epidermal layer of the developing skin and the vibrissae; in the latter it was limited to the root sheath and was not observed in the surrounding mesenchyme (Fig. 1E).
FIG. 1.
Pbx2 expression during mouse development. (A) In situ hybridizations were performed on frozen sagittal sections of E15.5 embryos (unless noted otherwise) as shown. (B to D) Pbx2 is expressed in the telencephalon, specifically in the neocortex (ncx), including the ventricular and subventricular zones, the hippocampus (h), and the developing striatum (s) and globus pallidus. The black arrowhead in panel C indicates the lateral ventricle. (D) In the mesenchephalon, Pbx2 expression is particularly prominent in the roof of the midbrain (rm) and in the choroid plexus (cp). The black arrowhead indicates the mesencephalic vesicle. (E) Pbx2 expression in the epidermal layer of the developing skin (as denoted by the black arrowhead in the inset) and in the developing vibrissae, where expression is limited to the root sheath (arrows) but is not evident in the surrounding mesenchyme. (F) Pbx2 is highly expressed in the olfactory epithelium, as indicated by arrows. The arrowhead indicates the nasal cavity (inset). (G) Pbx2 is expressed in the epithelium lining the cochlear ducts (c), in the trigeminal ganglion (arrow), in the epithelium of the tubotympanic recess (black arrowhead), and in the submandibular glands (sg). (H) Pbx2 expression is marked in the second cervical dorsal root ganglion (drg) and in the chondrocytes of the body of the second cervical vertebra (C2). Black arrowheads depict spinal nerves and their roots. (I) Pbx2 expression at E13.5 is predominant in the respiratory epithelium lining the segmental bronchi (arrows) of the lung (lg). Little to no expression of Pbx2 is detected in the mesenchyme of the lung or in the mesothelial lining of the pericardial cavity (pc). (J) Pbx2 is markedly expressed in distinct areas of the gut (g), specifically in the epithelium (arrow) covering the lumina (l, inset) and in the muscularis propria (black arrowhead). (K) Pbx2 is expressed in the developing dorsal pancreas (dp), specifically in the epithelium of the pancreatic acini (arrows) and ducts. (L) Pbx2 is expressed in the cortical (cx) and medullary (mr) kidney (k), in particular in the epithelium lining the collecting ducts (black arrowheads) and in the glomeruli (arrows). Little to no expression of Pbx2 is detected in the nephrogenic mesenchyme. Image B was obtained using a dissection microscope and digital camera, images E to J and L were photographed using a light microscope at a power of ×4. Image K and insets E, F, and L are at a magnification of ×10, while the inset of image J is at a magnification of ×40. All images were digitally processed.
At E15.5, expression of Pbx2 was also particularly prominent in numerous epithelial structures, including those lining the nasal cavity, cochlear ducts, and tubotympanic recess and in the submandibular glands (Fig. 1G). Respiratory epithelium lining the segmental bronchi of the lung also contained Pbx2, whereas little to no expression was detected in the pulmonary mesenchyme or mesothelial lining of the pericardial cavity (Fig. 1I). Although not typically expressed in mesenchymal tissues, Pbx2 was detected in vertebral chondrocytes (C2 is illustrated in Fig. 1H). Pbx2 was expressed in distinct areas of the gut, including the epithelium and muscularis propria (Fig. 1J) and the developing dorsal pancreas, specifically in the epithelium of the acini and ducts (Fig. 1K). Pbx2 was also markedly expressed in the cortical and medullary kidney, specifically in the epithelium lining the collecting ducts and in the glomeruli (Fig. 1L), whereas little to no expression of Pbx2 was detected in the nephrogenic mesenchyme. Heart tissue was notably devoid of Pbx2 expression. These in situ hybridization data are consistent with results of previous Northern blot and RT-PCR studies that detected Pbx2 RNA in most embryonic and adult tissues (22, 40).
In sum, in early mouse development Pbx2 expression is widespread, comparable to the ubiquitous Pbx2 expression during the first hours of zebra fish development (41). Later in mouse development, Pbx2 expression becomes more restricted and mostly preponderant in epithelial and neural tissues, comparable to expression patterns of the Drosophila homolog Exd (29). Conversely, Pbx2 is expressed at very low levels or is undetectable in the mesenchymal components of most tissues and organ systems, in contrast to Pbx1, which is highly expressed in mesenchymal and mesodermal cells (35).
Pbx2 is not essential for fetal and postnatal survival.
Mice with targeted disruption of Pbx2 were generated to determine whether the wide expression of Pbx2 during murine embryogenesis reflected a major role for its encoded protein in development. A null allele of the endogenous mouse Pbx2 gene was created by deletion of the entire third exon and insertion of a neomycin resistance gene through homologous recombination in ES cells (Fig. 2A). Exon 3 was chosen for targeting since: (i) it is the largest Pbx2 5′ exon (other than exon 1), whose disruption allows a truncation of Pbx2 immediately downstream of the initiating methionine to minimize possible expression of a truncated protein with dominant-negative properties; (ii) it lacks a unit number of codons, thereby minimizing the generation of a protein product with an in-frame deletion retaining partial or complete function, due to splicing around the targeted exon; and (iii) it codes for a portion of Pbx2 that is highly conserved in humans, mice, and flies. Exon 1 was not targeted since no information was available regarding possible alternative initiation sites downstream of exon 1, within the first intron. Overall, the above-described strategy proved successful, as a complete knockout of Pbx2 was obtained (Fig. 2).
The expected wt and mutated Pbx2 alleles were observed by Southern blot analysis of DNA extracted from targeted ES cell lines (results not shown) and mouse tissues (Fig. 2B). Western blot analysis of postnatal thymocytes and bone marrow cells showed that homozygous mutant mice did not produce Pbx2 protein, the only high-MW-isoform Pbx protein detectable in these tissues (Fig. 2C). Furthermore, Western blot analysis of fetal livers at E16.5 with a Pbx2-specific antibody raised against a peptide mapping at the amino terminus of Pbx2 clearly demonstrated that homozygous mutant mice did not produce full-length (50 kDa) or truncated forms of Pbx2 (Fig. 2D). Northern blot analysis was performed to exclude the possibility that a mutant Pbx2 protein, not detectable by Western blot analysis, might be produced from an altered transcript encoded by the targeted Pbx2 gene. This analysis demonstrated the absence of either full-length or truncated Pbx2 transcripts in Pbx2−/− embryos (Fig. 2E). Pbx2−/− mice were viable, with binomial proportion analysis showing no deviations from a 2:1 ratio of heterozygote to wt or heterozygote to homozygous mutant (data not shown). The sizes and body weights of Pbx2−/− mice were indistinguishable from those of their wt littermates (18.68 ± 1.25 g and 18.35 ± 1.34 g, respectively), as was their longevity and fertility (data not shown). The normal survival of Pbx2−/− mice suggested that Pbx2 is not solely or primarily responsible for any processes that are critical for development or survival.
Pbx2 is not essential for organogenesis.
Although lack of Pbx2 was compatible with life, this did not rule out possible subtle effects on organ structure. Major internal organs of Pbx2−/− newborn mice and their wt littermates were examined grossly and by histology. No structural or morphological abnormalities of internal organs were observed in Pbx2−/− mice (lung, liver, spleen, and kidney of wt and Pbx2−/− mice are shown in Fig. 3). Furthermore, cartilage/skeletal preparations of Pbx2−/− embryos and newborn mice showed neither gross nor subtle defects in cartilaginous and skeletal development (data not shown). These data demonstrate that lack of Pbx2 expression has no detectable effect on the morphogenesis of organs in which it is normally expressed.
FIG. 3.
Histological analysis of major organs of wt and Pbx2−/− newborn mice. Formalin-fixed, paraffin-embedded tissue sections of various organs (indicated to the left) of adult wt and Pbx2−/− littermates were stained with hematoxylin and eosin and photographed using a light microscope. Magnifications: lung, liver, and kidney, ×10; spleen, ×4; insets, ×20. All images were digitally processed.
Pbx proteins are redundantly present in major organs during late embryogenesis.
The absence of detectable phenotypes in Pbx2−/− mice raised the possibility that other Pbx family proteins may compensate for the lack of Pbx2 function. This was assessed by determining the relative levels of Pbx2 proteins compared to the levels of the other highly related Pbx proteins. Immunoblotting experiments were performed with a monoclonal antibody (α-PbxL) that recognizes a Pbx2 epitope shared with the high-MW isoforms of Pbx1 and Pbx3 (Pbx1a and Pbx3a, respectively) but not present in the low-MW isoforms Pbx1b or Pbx3b (Pbx2 has no low-MW isoform). In most tissues examined from embryos at E17.5, there were no differences in the abundance of the high-MW-isoform Pbx proteins in Pbx2−/− embryos as compared to wt embryos (Fig. 4A). A modest decrease was observed in fetal thymus, brain, and liver (Fig. 4A). The general lack of (or minimal) decrease in Pbx proteins detected with this antibody in tissues of Pbx2−/− embryos suggested that Pbx2 is not the preponderant Pbx protein expressed in these tissues during embryogenesis. Furthermore, tissues and organs such as the developing brain, where Pbx2 is highly expressed, as demonstrated by in situ hybridization (Fig. 1), were not affected either morphologically or functionally by its loss. Quantitative real-time RT-PCR showed that transcripts for other high-MW Pbx isoforms (Pbx1a and Pbx3a) were expressed in developing brain and other tissues (Fig. 4B and C), but there was no evidence of compensatory increases in their levels in Pbx2−/− compared to wt embryos. Taken together, these observations suggested that normal organ development and survival of Pbx2−/− mice is likely to result from functional compensation by other Pbx proteins. Interestingly, it has been reported that in zebra fish the Pbx genes are functionally equivalent in that each one, when overexpressed, can efficiently rescue the lazarus/Pbx4 mutant phenotype (41).
FIG. 4.
Western blot and quantitative RT-PCR analysis of Pbx expression. (A) Protein extracts prepared from various tissues (indicated at the top) of E17.5 embryos were analyzed by Western blotting using a monoclonal antibody specific for all Pbx long-form proteins (α-PbxL). (B and C) Quantitative RT-PCR analysis of Pbx1a and Pbx3a expression. Total RNA was isolated from the indicated tissues of E17.5 wt (black bars) and Pbx2−/− (grey bars) embryos. Bars (means ± standard deviations) indicate numbers of transcripts normalized against 105 β-actin transcripts.
Pbx2 in not essential for normal hematopoiesis.
In contrast to fetal tissues, Pbx2 was observed to be the predominant high-MW-isoform Pbx protein expressed in postnatal bone marrow (Fig. 2C). This raised the possibility that Pbx2 may contribute to adult hematopoiesis. To determine whether the absence of Pbx2 had any noticeable effect on adult hematopoiesis, peripheral blood cell counts were determined at various ages of Pbx2−/− and wt littermates. No statistically significant differences between Pbx2−/− and wt mice were observed in hemoglobin content, erythrocyte indices, or leukocyte or platelet counts (Table 1 and data not shown). These data suggest that, despite the fact that Pbx2 is the predominant Pbx protein (high-MW isoform) expressed in adult bone marrow, its absence produces no measurable effect on the production of mature blood cells from immature progenitors. This is consistent with the finding that a large cohort of Pbx2−/− mice achieved an age of 18 to 24 months without evidence of hematologic disorders, including malignancy (data not shown).
TABLE 1.
Hematological parameters of Pbx2-deficient mice
| Age and genotype of mice and reference | Hemoglobin (mg/dl) | Platelet counts (103/ml) | ANCa (103/ml) | ALCb (103/ml) |
|---|---|---|---|---|
| 9-11 mo | ||||
| Pbx2+/+ | 13.6 ± 1.9 | 1,167 ± 239 | 817 ± 469 | 7,266 ± 3,697 |
| Pbx2−/− | 14.2 ± 0.6 | 1,283 ± 305 | 915 ± 698 | 5,558 ± 2,305 |
| 14-16 mo | ||||
| Pbx2+/+ | 14.3 ± 0.3 | 1,342 ± 654 | 1,035 ± 798 | 8,986 ± 1,48 |
| Pbx2−/− | 14.6 ± 1.2 | 1,292 ± 572 | 855 ± 352 | 9,650 ± 1,805 |
| Normal range | 13.7-16.4 | 675-1,338 | 825-2,604 | 3,685-7,812 |
ANC, absolute neutrophil count.
ALC, absolute lymphocyte count.
Pbx2 is not essential for lymphoid cell production or function.
Pbx2 is also the predominant Pbx protein (high-MW isoform) expressed in the postnatal thymus (Fig. 2C). To investigate whether T-cell maturation might be affected by the absence of Pbx2, the relative proportions of lymphocyte subsets in wt versus Pbx2−/− littermates were quantified by immunofluorescent staining and flow cytometry (fluorescence-activated cell sorter). Using standard B- and T-cell surface markers, no differences were detected in the absolute or relative numbers of B and T cells (Table 1 and Fig. 5). Moreover, when Pbx2−/− mice were challenged with DNP conjugated to KLH they mounted humoral anti-DNP responses that were indistinguishable from those of wt littermates. These studies suggest that Pbx2 is not essential for normal lymphopoiesis and that lack of Pbx2 in the thymus does not detectably alter T-cell maturation. Since no overt autoimmunity manifestations (i.e., skin changes, weight loss) were observed in Pbx2−/− mice, it seems likely that Pbx2 is also not required for tolerance induction.
FIG. 5.
Immunological studies of Pbx2−/− mice. Fluorescence-activated cell sorter analyses of bone marrow cells (A) and thymocytes (B) of wt and Pbx2−/− littermates demonstrated no detectable differences in lymphoid cell subsets. (C) IgG levels were comparable in the sera of wt and Pbx2−/− littermates before and after immunization with the hapten DNP conjugated to KLH.
Functional redundancy in the Pbx protein family.
Pbx2 null mice are viable and healthy with normal organ structure and hematopoietic and immune function, despite widespread Pbx2 expression during embryogenesis. The lack of an obvious phenotype contrasts with the severe defects exhibited by mice deficient for Pbx1 or Pbx3. Pbx1 null embryos do not survive past E16.5 and exhibit severe developmental defects in nearly every organ system (11, 15, 32, 34, 36) and homeotic transformation (36). Conversely, mice lacking Pbx3 survive to term but die within hours of birth due to an inadequate respiratory drive (Rhee et al., submitted). These phenotypes likely reflect a disruption of multiple developmental pathways subordinate to Hox and/or other homeodomain transcription factors that require a Pbx binding partner (17, 44). The lack of any obvious phenotype in the absence of Pbx2 indicates that its function as a DNA binding partner for Hox and/or other transcription factors is not essential.
Two models for mammalian Pbx protein function can be envisioned for conceptualizing the role of multiple Pbx isoforms and the divergent phenotypes induced by their absence. In a quantitative model, the various Pbx protein isoforms may be largely redundant, but with a requirement to maintain a critical threshold of Pbx protein concentration, regardless of isotype, necessary for normal development. This scenario would be comparable to Pbx function in zebra fish, where the Pbx proteins are functionally equivalent (41) and is also supported by preliminary results indicating that Pbx1−/−Pbx2+/− compound mutant embryos die in utero at E13.5, earlier than Pbx1−/− embryos, which succumb at E15/16 (T. Capellini and L. Selleri, unpublished observations). Since our immunoblot analyses indicated that Pbx2 does not seem to be the preponderant Pbx protein in most tissues during embryonic development, its absence would not substantially reduce the total Pbx protein levels below the necessary threshold, thus accounting for the lack of measurable phenotypes. Alternatively, in a qualitative model, various Pbx isoforms (high versus low MW) may display different functional properties, consistent with in vitro biochemical studies of their divergent transcriptional effector properties (2). However, our analyses indicate that Pbx2 null mice lack detectable phenotypes attributable to deficiencies in tissues (thymus and adult bone marrow) that express Pbx2 as the only high-MW Pbx isoform protein. Since these tissues also express low-MW Pbx isoforms, such as Pbx1b, they may be able to functionally replace Pbx2. The alternative possibility that Pbx protein function is simply not required for postnatal hematopoietic and immune function has not been conclusively ruled out since Pbx1- and Pbx3-deficient mice die in utero or in the immediate postnatal period, respectively (Rhee et al., submitted; 36). Our results are most consistent with a quantitative model of mammalian Pbx protein function, but elucidation of the potential interrelationships and overlapping functions of Pbx family proteins in different developmental pathways will require detailed characterization of various organ systems in compound null mice.
Acknowledgments
We thank Joseph Lipsick and Uta Francke for many helpful discussions; Michael Depew, Inma Cobos, and Stewart Anderson for comments on the manuscript; and Cita Nicolas, Maria Ambrus, and Lawryn Kasper for expert technical assistance.
These studies were supported by grants to M.L.C. from the National Institutes of Health (CA42971, CA70704, and CA90735) and to L.S. from the March of Dimes and Birth Defects Foundation (6-FY03-071) and the National Institutes of Health (HD043997-01 A1).
REFERENCES
- 1.Abbondanzo, S. J., I. Gadi, and C. L. Stewart. 1993. Derivation of embryonic stem cell lines. Methods Enzymol. 225:803-823. [DOI] [PubMed] [Google Scholar]
- 2.Asahara, H., S. Dutta, H. Y. Kao, R. M. Evans, and M. Montminy. 1999. Pbx-Hox heterodimers recruit coactivator-corepressor complexes in an isoform-specific manner. Mol. Cell. Biol. 19:8219-8225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berthelsen, J., C. Kilstrup-Nielsen, F. Blasi, F. Mavilio, and V. Zappavigna. 1999. The subcellular localization of PBX1 and EXD proteins depends on nuclear import and export signals and is modulated by association with PREP1 and HTH. Genes Dev. 13:946-953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berthelsen, J., V. Zappavigna, E. Ferretti, F. Mavilio, and F. Blasi. 1998. The novel homeoprotein Prep1 modulates Pbx-Hox protein cooperativity. EMBO J. 17:1434-1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burglin, T. R., and G. Ruvkun. 1992. New motif in PBX genes. Nat. Genet. 1:319-320. [DOI] [PubMed] [Google Scholar]
- 6.Chan, S. K., L. Jaffe, M. Capovilla, J. Botas, and R. S. Mann. 1994. The DNA binding specificity of Ultrabithorax is modulated by cooperative interactions with extradenticle, another homeoprotein. Cell 78:603-615. [DOI] [PubMed] [Google Scholar]
- 7.Chang, C. P., L. Brocchieri, W. F. Shen, C. Largman, and M. L. Cleary. 1996. Pbx modulation of Hox homeodomain amino-terminal arms establishes different DNA-binding specificities across the Hox locus. Mol. Cell. Biol. 16:1734-1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang, C. P., Y. Jacobs, T. Nakamura, N. A. Jenkins, N. G. Copeland, and M. L. Cleary. 1997. Meis proteins are major in vivo DNA binding partners for wild-type but not chimeric Pbx proteins. Mol. Cell. Biol. 17:5679-5687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang, C. P., W. F. Shen, S. Rozenfeld, H. J. Lawrence, C. Largman, and M. L. Cleary. 1995. Pbx proteins display hexapeptide-dependent cooperative DNA binding with a subset of Hox proteins. Genes Dev. 9:663-674. [DOI] [PubMed] [Google Scholar]
- 10.Depew, M. J., J. K. Liu, J. E. Long, R. Presley, J. J. Meneses, R. A. Pedersen, and J. L. Rubenstein. 1999. Dlx5 regulates regional development of the branchial arches and sensory capsules. Development 126:3831-3846. [DOI] [PubMed] [Google Scholar]
- 11.DiMartino, J. F., L. Selleri, D. Traver, M. T. Firpo, J. Rhee, R. Warnke, S. O'Gorman, I. L. Weissman, and M. L. Cleary. 2001. The Hox cofactor and proto-oncogene Pbx1 is required for maintenance of definitive hematopoiesis in the fetal liver. Blood 98:618-626. [DOI] [PubMed] [Google Scholar]
- 12.Jacobs, Y., C. A. Schnabel, and M. L. Cleary. 1999. Trimeric association of Hox and TALE homeodomain proteins mediates Hoxb2 hindbrain enhancer activity. Mol. Cell. Biol. 19:5134-5142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kamps, M. P., C. Murre, X. H. Sun, and D. Baltimore. 1990. A new homeobox gene contributes the DNA binding domain of the t(1;19) translocation protein in pre-B ALL. Cell 60:547-555. [DOI] [PubMed] [Google Scholar]
- 14.Kilstrup-Nielsen, C., M. Alessio, and V. Zappavigna. 2003. PBX1 nuclear export is regulated independently of PBX-MEINOX interaction by PKA phosphorylation of the PBC-B domain. EMBO J. 22:89-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim, S. K., L. Selleri, J. S. Lee, A. Y. Zhang, X. Gu, Y. Jacobs, and M. L. Cleary. 2002. Pbx1 inactivation disrupts pancreas development and in Ipf1-deficient mice promotes diabetes mellitus. Nat. Genet. 30:430-435. [DOI] [PubMed] [Google Scholar]
- 16.Knoepfler, P. S., and M. P. Kamps. 1995. The pentapeptide motif of Hox proteins is required for cooperative DNA binding with Pbx1, physically contacts Pbx1, and enhances DNA binding by Pbx1. Mol. Cell. Biol. 15:5811-5819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krumlauf, R. 1994. Hox genes in vertebrate development. Cell 78:191-201. [DOI] [PubMed] [Google Scholar]
- 18.Lau, M. M., C. E. Stewart, Z. Liu, H. Bhatt, P. Rotwein, and C. L. Stewart. 1994. Loss of the imprinted IGF2/cation-independent mannose 6-phosphate receptor results in fetal overgrowth and perinatal lethality. Genes Dev. 8:2953-2963. [DOI] [PubMed] [Google Scholar]
- 19.Lu, Q., P. S. Knoepfler, J. Scheele, D. D. Wright, and M. P. Kamps. 1995. Both Pbx1 and E2A-Pbx1 bind the DNA motif ATCAATCAA cooperatively with the products of multiple murine Hox genes, some of which are themselves oncogenes. Mol. Cell. Biol. 15:3786-3795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mann, R. S., and M. Affolter. 1998. Hox proteins meet more partners. Curr. Opin. Genet. Dev. 8:423-429. [DOI] [PubMed] [Google Scholar]
- 21.McLeod, M. J. 1980. Differential staining of cartilage and bone in whole mouse fetuses by alcian blue and alizarin red S. Teratology 22:299-301. [DOI] [PubMed] [Google Scholar]
- 22.Monica, K., N. Galili, J. Nourse, D. Saltman, and M. L. Cleary. 1991. PBX2 and PBX3, new homeobox genes with extensive homology to the human proto-oncogene PBX1. Mol. Cell. Biol. 11:6149-6157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nourse, J., J. D. Mellentin, N. Galili, J. Wilkinson, E. Stanbridge, S. D. Smith, and M. L. Cleary. 1990. Chromosomal translocation t(1;19) results in synthesis of a homeobox fusion mRNA that codes for a potential chimeric transcription factor. Cell 60:535-545. [DOI] [PubMed] [Google Scholar]
- 24.Peers, B., S. Sharma, T. Johnson, M. Kamps, and M. Montminy. 1995. The pancreatic islet factor STF-1 binds cooperatively with Pbx to a regulatory element in the somatostatin promoter: importance of the FPWMK motif and of the homeodomain. Mol. Cell. Biol. 15:7091-7097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peltenburg, L. T., and C. Murre. 1996. Engrailed and Hox homeodomain proteins contain a related Pbx interaction motif that recognizes a common structure present in Pbx. EMBO J. 15:3385-3393. [PMC free article] [PubMed] [Google Scholar]
- 26.Phelan, M. L., I. Rambaldi, and M. S. Featherstone. 1995. Cooperative interactions between HOX and PBX proteins mediated by a conserved peptide motif. Mol. Cell. Biol. 15:3989-3997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Popperl, H., M. Bienz, M. Studer, S. K. Chan, S. Aparicio, S. Brenner, R. S. Mann, and R. Krumlauf. 1995. Segmental expression of Hoxb-1 is controlled by a highly conserved autoregulatory loop dependent upon exd/pbx. Cell 81:1031-1042. [DOI] [PubMed] [Google Scholar]
- 28.Popperl, H., H. Rikhof, H. Chang, P. Haffter, C. B. Kimmel, and C. B. Moens. 2000. Lazarus is a novel pbx gene that globally mediates hox gene function in zebrafish. Mol. Cell 6:255-267. [DOI] [PubMed] [Google Scholar]
- 29.Rauskolb, C., M. Peifer, and E. Wieschaus. 1993. extradenticle, a regulator of homeotic gene activity, is a homolog of the homeobox-containing human proto-oncogene pbx1. Cell 74:1101-1112. [DOI] [PubMed] [Google Scholar]
- 30.Rauskolb, C., and E. Wieschaus. 1994. Coordinate regulation of downstream genes by extradenticle and the homeotic selector proteins. EMBO J. 13:3561-3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sato, S., D. A. Steeber, and T. F. Tedder. 1995. The CD19 signal transduction molecule is a response regulator of B-lymphocyte differentiation. Proc. Natl. Acad. Sci. USA 92:11558-11562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schnabel, C. A., R. E. Godin, and M. L. Cleary. 2003. Pbx1 regulates nephrogenesis and ureteric branching in the developing kidney. Dev. Biol. 254:262-276. [DOI] [PubMed] [Google Scholar]
- 33.Schnabel, C. A., Y. Jacobs, and M. L. Cleary. 2000. HoxA9-mediated immortalization of myeloid progenitors requires functional interactions with TALE cofactors Pbx and Meis. Oncogene 19:608-616. [DOI] [PubMed] [Google Scholar]
- 34.Schnabel, C. A., L. Selleri, and M. L. Cleary. 2003. Pbx1 is essential for adrenal development and urogenital differentiation. Genesis 37:123-130. [DOI] [PubMed] [Google Scholar]
- 35.Schnabel, C. A., L. Selleri, Y. Jacobs, R. Warnke, and M. L. Cleary. 2001. Expression of Pbx1b during mammalian organogenesis. Mech. Dev. 100:131-135. [DOI] [PubMed] [Google Scholar]
- 36.Selleri, L., M. J. Depew, Y. Jacobs, S. K. Chanda, K. Y. Tsang, K. S. Cheah, J. L. Rubenstein, S. O'Gorman, and M. L. Cleary. 2001. Requirement for Pbx1 in skeletal patterning and programming chondrocyte proliferation and differentiation. Development 128:3543-3557. [DOI] [PubMed] [Google Scholar]
- 37.Shen, W. F., C. P. Chang, S. Rozenfeld, G. Sauvageau, R. K. Humphries, M. Lu, H. J. Lawrence, M. L. Cleary, and C. Largman. 1996. Hox homeodomain proteins exhibit selective complex stabilities with Pbx and DNA. Nucleic Acids Res. 24:898-906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van Dijk, M. A., and C. Murre. 1994. extradenticle raises the DNA binding specificity of homeotic selector gene products. Cell 78:617-624. [DOI] [PubMed] [Google Scholar]
- 39.Vlachakis, N., D. R. Ellstrom, and C. G. Sagerstrom. 2000. A novel pbx family member expressed during early zebrafish embryogenesis forms trimeric complexes with Meis3 and Hoxb1b. Dev. Dyn. 217:109-119. [DOI] [PubMed] [Google Scholar]
- 40.Wagner, K., A. Mincheva, B. Korn, P. Lichter, and H. Popperl. 2001. Pbx4, a new Pbx family member on mouse chromosome 8, is expressed during spermatogenesis. Mech. Dev. 103:127-131. [DOI] [PubMed] [Google Scholar]
- 41.Waskiewicz, A. J., H. A. Rikhof, and C. B. Moens. 2002. Eliminating zebrafish pbx proteins reveals a hindbrain ground state. Dev. Cell 3:723-733. [DOI] [PubMed] [Google Scholar]
- 42.Wilkinson, D. G. 1995. RNA detection using non-radioactive in situ hybridization. Curr. Opin. Biotechnol. 6:20-23. [DOI] [PubMed] [Google Scholar]
- 43.Wilkinson, D. G., and M. A. Nieto. 1993. Detection of messenger RNA by in situ hybridization to tissue sections and whole mounts. Methods Enzymol. 225:361-373. [DOI] [PubMed] [Google Scholar]
- 44.Wilson, D. S., and C. Desplan. 1995. Homeodomain proteins. Cooperating to be different. Curr. Biol. 5:32-34. [DOI] [PubMed] [Google Scholar]