Abstract
Background
Treatment of pancreatic cancer with pharmacological ascorbate (ascorbic acid, vitamin C) decreases tumor progression in pre-clinical models. A phase I clinical trial was performed to establish safety and tolerability of pharmacological ascorbate combined with gemcitabine in patients with biopsy-proven stage IV pancreatic adenocarcinoma.
Design
Nine subjects received twice weekly intravenous ascorbate (15–125 g) employing Simon’s Accelerated Titration design to achieve a targeted post-infusion plasma level of ≥ 350 mg/dL (≥20 mM). Subjects received concurrent gemcitabine. Disease burden, weight, performance status, hematologic and metabolic labs, time to progression and overall survival were monitored.
Results
Mean plasma ascorbate trough levels were significantly higher than baseline (1.46 ± 0.02 vs. 0.78 ± 0.09 mg/dL, i.e. 83 vs. 44μM, p < 0.001). Adverse events attributable to the drug combination were rare and included diarrhea (n = 4) and dry mouth (n = 6). Dose-limiting criteria were not met for this study. Mean survival of subjects completing at least 2 cycles (8 weeks) of therapy was 13 ± 2 months.
Conclusions
Data suggest pharmacologic ascorbate administered concurrently with gemcitabine is well-tolerated. Initial data from this small sampling suggest some efficacy. Further studies powered to determine efficacy should be conducted.
Keywords: Pancreatic neoplasm, Ascorbic acid, Clinical Trial, phase 1, Gemcitabine, Drug toxicity
Introduction
The incidence rate of pancreatic cancer is increasing, from 11.4 in 2000 to 12.0 per100,000 in 2008 (1). Of the 44,000 diagnosed with pancreatic cancer in 2011, just over 37,000 are expected to succumb to the disease that year—a mortality rate of over 80% (2). Despite being the fourth leading cause of cancer-related death in the United States, the National Cancer Institute reports progress in pancreatic cancer treatment has not made the same advances as treatments for other cancers (3). A significant therapeutic advance for pancreatic cancer was the initiation of the gemcitabine regimen as described by Burris and colleagues in 1997 (4). FOLFIRINOX, a multi-drug combination chemotherapy, was recently shown to improve overall survival in patients with metastatic pancreatic cancer when compared to gemcitabine alone, but with added toxicity (5). Contributions to foster therapeutic breakthroughs for this disease are greatly needed.
Ascorbate (ascorbic acid, vitamin C) can be both an antioxidant and pro-oxidant; in the presence of catalytic metals it typically exhibits pro-oxidant properties (6). Pharmacological ascorbate is the use of high-doses of ascorbate, administered IV, to achieve plasma levels of ascorbate on the order of 100–1000 times that of healthy nutritional levels. At these high levels, pharmacological ascorbate has been proposed as a pro-drug for the delivery of H2O2 to tumors (7). Pharmacological ascorbate, at doses achievable in humans, selectively kills pancreatic cancer cells via an H2O2-mediated mechanism, i.e. acting as a pro-oxidant (7–9). Additionally, preclinical data indicate adding pharmacological ascorbate to the standard gemcitabine regimen may improve outcomes (10).
Reported clinical outcomes regarding the efficacy of high-dose ascorbate as a therapeutic agent are conflicting. The absence of benefit from studies of oral ascorbate are predictable and expected (11–13). Considering the difference in route of administration the decision was made to conduct a phase I trial to determine the safety and tolerability of pharmacological ascorbate when administered concomitantly with gemcitabine in pancreatic cancer patients. Given the recent preclinical study indicating ascorbate has the potential to improve antitumor therapy, plasma levels of at least 20 mM were targeted (9). Primary objectives of this study were to characterize the toxicity profile associated with IV ascorbate and the dose effect on plasma ascorbate levels when given twice weekly with gemcitabine for patients with metastatic pancreatic cancer.
Patients and methods
Regulatory requirements
An investigational new drug application (IND) was filed and obtained from the Center for Drug Evaluation and Research (CDER) of the FDA. The phase I protocol was reviewed and approved by CDER. Approval was sought and obtained from The University of Iowa IRB-01 [Biomedical] and the trial was listed on clinicaltrials.gov [NCT01049880]. Good clinical practice consistent with ICH E8 was maintained and protocol compliance was monitored quarterly by the Data and Safety Monitoring Board (DSMB) through an active audit process of all subjects. Annual reports compliant with 21CFR§312.33 were filed as required with the FDA.
Patient population
Patients were required to have histologically or cytologically confirmed unresectable, metastatic, or recurrent pancreatic adenocarcinoma. Pharmacological ascorbate infusions may cause red blood cell hemolysis in those deficient in the glucose-6-phosphate dehydrogenase (G6PD) enzyme. Thus, those with low G6PD levels were excluded (normal range 7 – 20.5 U/g hemoglobin, ARUP laboratories, Salt Lake, UT). Other criteria included an ECOG performance status (PS) of 0, 1, or 2 with a life expectancy of at least 3 months or greater. Required initial laboratory values included a neutrophil count of ≥ 1,500/mm3, platelet count of ≥ 100,000/mm3, creatinine of ≤ 1.5 mg/dL or creatinine clearance ≥ 60 mL/min, total bilirubin ≤ 2 x upper limit of normal (ULN), transaminases ≤ 3 x ULN (< 5 x ULN if liver metastases were present), and a prothrombin time or international normalized ratio (INR) within normal institutional limits. Key exclusion criteria included active comorbidities such as end stage congestive heart failure, unstable angina, or a myocardial infarction within 6 months of enrollment. All patients were required to provide written informed consent. All enrollment eligibility was confirmed through a formal registration process utilizing an independent monitor provided by the Holden Comprehensive Cancer Center’s DSMB.
Treatment plan
The treatment schema is provided (Figure 1). Subjects received a test dose of 15 g ascorbate (Bioniche Pharma USA, Lake Forest, IL) in 250 mL 5% dextrose water (D5W) infused over 30 min. If tolerated, a second test dose was administered within a calendar week. The dose of ascorbate was then increased weekly until a plasma level of ≥ 350 mg/dL (>20 mM) was achieved (Supplemental information, Table 1). Once this level was attained, the ascorbate dose was adjusted as needed. Ascorbate infusions were given each week of the four week cycle. For the purposes of this phase I study, dose-limiting toxicities (DLT) were defined as a grade 3 or greater non-hematologic toxicity with attribution to the ascorbate. If a subject experienced a DLT, ascorbate treatment was terminated. In absence of DLT, subjects continued ascorbate treatment until progression (defined by RECIST).
Figure 1. Schematic of phase I clinical treatment plan.
Red blood cell hemolysis may occur in people deficient in the G-6-PD enzyme, so this is an exclusion criterion. If the results of the G-6-PD laboratory test are negative, a test dose of 15 g of ascorbic acid was infused over 30 min. If tolerated throughout the first week, the dose of ascorbate was increased weekly (i.e., every 2 infusions) until the plasma level reaches at least 350 mg/dL (20 mM). Ascorbate infusions were given each week of the four-week cycle.
Gemcitabine was administered following the method established by Burris and colleagues: IV infusion at a dose of 1000 mg/m2 over 30 minutes, with each cycle consisting of weekly gemcitabine infusions for 3 consecutive weeks with one week rest (4).
Blood samples were drawn pre- and post-infusion. Plasma was analyzed by a colorimetric/kinetic assay for ascorbate determination (14). Each sample was divided into at least 3 aliquots and analyzed separately. Blood samples were stored on ice in a dark refrigerator until prepared for assay. No ascorbate degradation was expected to occur during processing and storage conditions. CT imaging was obtained at baseline and after completing every two cycles in accordance with standard of care at the University of Iowa Hospitals and Clinics.
Response and toxicity criteria
Toxicity was graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events 3.0 (CTCAE). The RECIST guideline was used for evaluation of antitumor activity. Toxicity attribution to ascorbic acid was initially determined by treating physician, reviewed by the study PI (JJC) and medical monitor (DJB), and confirmed by the DSMB.
Sample analysis
Sample preparation for plasma and RBCs
Whole blood (NaHeparin 75 USP units, BD Vacutainer® green top blood collection tube, 4 mL) was collected from clinical trial participants. Samples were centrifuged at 500 g for 5 min, and then RBC-free plasma was collected for ascorbate and F2-isoprostane measurements. Most samples were analyzed immediately; some plasma samples were frozen at −80 °C and analyzed within a week.
For glutathione redox determinations, RBCs were collected, then washed twice with cold isotonic saline solution. After washing, an aliquot was taken for CBC analysis (Sysmex XE-2100™ Automated Hematology System). RBCs were lysed with 5% perchloric acid/100 μM diethylenetriaminepentaacetic acid (DETAPAC; Sigma-Aldrich Chemical Co, St Louis, MO); this precipitates the protein and preserves glutathione (GSH) and glutathione disulfide (GSSG). The sample was centrifuged to pellet the protein (4000 g, 5 min). The clean supernatant was stored at −80 °C or immediately analyzed using HPLC.
Measurement of Plasma Ascorbate
Ascorbate levels in patient plasma samples were estimated with a plate reader-based assay as described in Vislisel et al. 2007 (14) with minor modifications to accommodate the 1000-fold differences in ascorbate concentrations seen under nutritional (μM) as opposed to the post-IV ascorbate infusion levels (mM). Briefly, plasma samples were extracted with a buffer containing 90% methanol and 10% water with 250 μM DETAPAC (90:10, v/v), mixed, and incubated on ice for 10 min to precipitate the protein. The sample was then clarified by centrifugation, 10 min at 16 g with an Eppendorf model 5415D Microfuge.
Specifically for this study, we standardized the extraction procedure so that plasma samples from the enrollment screen and pre-infusion were diluted using 150 μL of the plasma and 600 μL (5x dilution) of the extraction buffer. In contrast, the post IV ascorbate samples were diluted at a ratio of 50 μL of the plasma to 450 μL (10x dilution) of the extraction buffer. The screen and pre-IV ascorbate plasma samples were further diluted another 3x (15x overall) into a buffer containing 72% methanol and 28% water with 250 μM DETAPAC (72:28, v/v). Post-intravenous ascorbate infusion plasma samples were diluted an additional 77.5- to 105-fold in this same buffer. Overall, the dilutions of the screen and pre-infusion samples were 5- and 15-fold (final) whereas the post-infusion samples were diluted from 775- to 1050-fold (final). This protocol ensured that all samples assayed had the same methanol content (72%): a critical factor for the success of this assay (14). The diluted plasma samples were then assayed immediately without further storage. All samples were placed as 100 μL aliquots in 96-well optical bottom black plates (Thermo Fisher Scientific, Rochester, NY). Authentic L-ascorbic acid (Macron Chemicals, Avantor Performance Materials, Center Valley, PA) standards in 72% methanol and 28% water with 250 uM DETAPAC (72:28, v/v) were included in the assay as 100 μL aliquots with stock solution concentrations ranging from 2.5 to 50 μM: again the 72% methanol content in the assay was maintained in the standards. The assay was initiated at room temperature by adding 100 μL of a 2.3 mM 4-hydroxy-2,2,6,6-tetramethylpiperidinyloxyl (Tempol; Sigma-Aldrich, St. Louis, MO) in 2 M sodium acetate dihydrate (Sigma-Aldrich) buffer previously adjusted to pH 5.5 with acetic acid. The samples were then incubated for 10 min in the dark, during which 2 equivalents of Tempol oxidized 1 ascorbic acid by 2 separate 1-electron oxidations to dehydroascorbic acid (DHA). After 10 min, 42 μL of 5.5 mM ortho-phenylenediamine (oPD) in 2 M sodium acetate buffer (pH 5.5) was added to the samples. Kinetic measurements were initiated and collected every 22 s with a TECAN SpectraFluor Plus plate reader (Tecan, Research Triangle Park, NC) using a 345 nm bandpass filter for excitation and a 425 nm bandpass filter for emission. The condensation product of the reaction of oPD with DHA is the fluorescent 3-(dihydroxyethyl)furo[3,4-b]quinoxaline-1-one; this product was monitored under these instrumental settings. Plasma sample concentrations were determined by comparison to the standard curve established from the rates of the DHA-oPD condensation product formation from the authentic ascorbate standards using linear portions of the progress curves: this linear portion occurs in the first 4 kinetic time points (<90 s) under the concentrations and conditions described here. Full kinetic curves were collected and examined to ensure no abnormalities.
Ascorbate Radical in Blood
Whole blood was drawn into 100 mm Hirschmann melting point determination tubes (Z61174, Sigma-Aldrich) by capillary action; breaking off the sealed end of the capillary tubes allowed 20–50 μL of the whole blood to flow freely into the tubes without air bubbles. The tubes were then sealed at one end with capillary tube sealant. The capillary tube, with the sealed end down, was placed into the bottom of a 250 mm × 3 mm (ID) thin walled quartz EPR tube (707-SQ-250M Wilmad –Lab Glass, Vineland, NJ). The EPR tube was then placed into a Bruker HS EPR cavity (Bruker, Billerica, MA) so that the blood sample was centered within the cavity without air bubbles filling the spectral acquisition volume and that any sample leakage and capillary sealant being outside the spectral acquisition volume. EPR spectra were collected with a Bruker EMX EPR spectrometer: room temperature; microwave power, 20 mW; frequency, 9.853 GHz; scanning 10 G with a sweep time of 20.972 s; receiver gain, 5.02 × 104; modulation frequency, 100 kHz; modulation amplitude, 0.70 G; signal channel time constant, 327.680 ms. All spectra were collected in the additive mode using 5 scans. Estimates of [Asc•−]ss in whole blood were made using 3-carboxy-PROXYL (3-CP)(Sigma-Aldrich) as a standard; the concentration of 3-CP in the standard was verified using ε234 = 2370 ± 50 M−1 cm−1 (15); signals were double integrated using the Bruker WIN-EPR software with corrections for differences in saturation behavior (16).
Measurement of Isoprostanes
Isoprostanes are a unique series of prostaglandin-like compounds formed in vivo via the nonenzymatic free radical-initiated peroxidation of arachidonic acid, a ubiquitous polyunsaturated fatty acid. The F2-isoprostanes have become the biomarker of choice for assessing endogenous oxidative stress because these molecules are chemically stable and have been detected in all biological fluids and tissues analyzed (17). Blood from subjects was collected before and after ascorbate infusion to assess parameters indicative of oxidative stress. To quantify F2-isoprostane levels, gas chromatography-mass spectrometry was utilized. This assay was chosen because it has been shown to be capable of detecting changes indicative of in vivo oxidative stress in plasma samples (17) and is verifiable (17–19).
Measurement of GSH and GSSG in RBCs with HPLC-BDD
To determine the status of the GSSG,2H+/2GSH couple in red blood cells we used HPLC with electrochemical detection following the protocol outlined by Park et al. (20). The method is based on an electrochemical detection (ECD) system using a boron-doped diamond (BDD) electrode (Model 5040, ESA Biosciences, Chelmsford, MA). The BDD electrode is an excellent detector for thiol and disulfide compounds as these analytes require a high electrode potential. Derivatization of the sample is not required allowing higher throughput. With the minimal sample processing required there is less opportunity for the sample to oxidize, which would lead to overestimation of GSSG (21). The results from the HPLC/BDD along with the information from the complete blood count, allow determination of the intracellular concentrations of GSH and GSSG. These concentrations in conjunction with the Nernst equation (pH 7.40) were used to determine the intracellular redox status of the couple in red blood cells, Ehc (22).
Study design and statistical analysis
This phase I study was designed as a single institution, prospective, open label study of safety and tolerability of ascorbate when administered concurrently with gemcitabine. Enrollment followed a two-stage design with influence from Simon’s accelerated titration design. Three subjects were accrued in the first stage of this study and completed at least 1 month of study treatment. If ≥ 2 subjects experienced a DLT, a stopping rule would be invoked and the study stopped. If <2 subjects experience a DLT, the study would progress to the second stage, where up to seven additional subjects would complete at least one month of therapy. If ≥ 3 dose limiting toxicities occur in the combination of stage 1 and 2 subjects, the study will be terminated and the treatment deemed unsafe. If less than 3 subjects experienced DLT, the treatment would be considered tolerable and investigated in a phase II study powered for efficacy.
Results
Patient population
A total of 15 patients were enrolled from April 2010 to November 2011, with 14 of these patients eligible. One patient was found to be ineligible due to a high G6PD. Two patients withdrew due to the travel required for treatment. One patient withdrew due to insurance denial.
Treatment delivery and protocol compliance
Of the 11 subjects treated with ascorbate, two discontinued protocol treatment the first month due to progressive disease necessitating palliative treatment. The characteristics of the subjects are listed in Table 1. The remaining nine completed at least one month protocol therapy, making them evaluable for statistical purposes. Quarterly audits identified no compliance deficiencies. Adverse events were reviewed monthly at investigator’s meetings and reviewed against protocol requirements for dose limiting toxicities. Individual ascorbate doses for maintenance of 350 mg/dL ranged from 50 g to 125 g per infusion. Ascorbate levels ranged from 20–25 mM in the first hour post-infusion; levels shown in pre-clinical studies to provide antitumor effects (9).
Table 1.
Patient characteristics
| Characteristic | Number of Patients |
|---|---|
| Sex | |
| Male | 6 |
| Female | 5 |
| Age (years) | |
| Median | 62 |
| Range | 50–69 |
| ECOG Score | |
| 0 | 3 |
| 1 | 7 |
| 2 | 1 |
| Smoking History | |
| Never | 3 |
| Former | 6 |
| Current | 2 |
| Clinical Stage | |
| IV | 11 |
| T Stage | |
| T3 | 8 |
| T4 | 3 |
| N Stage | |
| N1 | 11 |
| M Stage | |
| M1 | 11 |
| Histological Type | |
| Adenocarcinoma | 11 |
| CA 19-9 | |
| < 40 | 3 |
| > 40 | 8 |
Six of the nine treated subjects maintained or improved their performance status. Average treatment duration was 6 months (177 days, range 69–556 days) during which mean weight loss was 5..3 ± 1.6 kg while receiving study treatment (Supplemental Figure 1). No subjects were lost to follow-up. Of the nine patients who completed at least one month of protocol therapy, time to progression was 26 ± 7 weeks, while overall survival was 13 ± 2 months (Means ± SEM).
Safety and toxicity
No dose limiting toxicities or serious adverse events (as defined by 21CFR312.32) occurred. Toxicities were comparable with published trials of gemcitabine regimens (4). Table 2 provides the maximum grade for toxicities in the initial treatment cycle while Table 3 provides the same data for all treatment cycles. The increased toxicities with repeated doses were attributable to the gemcitabine treatment. In the nine subjects treated, few grade 3 and 4 toxicities were observed and none were attributed to ascorbate. Grade 3 laboratory toxicities were limited to elevated GGT (n = 2) and hypokalemia (n = 1), both likely related to the disease process. There were no grade 4 laboratory toxicities. Grade 3 and 4 hematologic toxicities included leukopenia (n = 1), lymphopenia (n = 1), neutropenia (n = 2), and thrombocytopenia (n = 1), consistent with the percentages reported for these toxicities with gemcitabine alone (4). Constitutional toxicities (Table 2) possibly attributable to ascorbate included nausea (n = 6) and diarrhea (n = 4). Thirst/dry mouth was definitively attributed to ascorbate (n = 4), a transient symptom alleviated within the same day as infusion.
Table 2.
Summary of Maximum Grade for Laboratory and Constitutional Toxicities (Initial Treatment Cycle Only)
| Toxicity | Number of Subjects§ | ||||
|---|---|---|---|---|---|
|
| |||||
| Grade of Adverse Event† | |||||
| 0
|
1
|
2
|
3
|
4
|
|
| Metabolic | |||||
| Hypoalbuminemia | 6 | 0 | 3 | 0 | 0 |
| Alkaline phosphatase, elevated | 5 | 2 | 2 | 0 | 0 |
| Gamma-glutamyl transpeptidase, elevated | 1 | 0 | 1 | 1 | 0 |
| Aspartate aminotransferase, elevated | 5 | 2 | 2 | 0 | 0 |
| Alanine aminotransferase, elevated | 1 | 6 | 2 | 0 | 0 |
| Hyperbilirubinemia | 7 | 2 | 0 | 0 | 0 |
| Creatinine, elevated | 9 | 0 | 0 | 0 | 0 |
| Hypocalcemia | 6 | 2 | 1 | 0 | 0 |
| Hypercalcemia | 9 | 0 | 0 | 0 | 0 |
| Hypokalemia | 4 | 5 | 0 | 0 | 0 |
| Hyperkalemia | 7 | 2 | 0 | 0 | 0 |
| Hyponatremia | 6 | 3 | 0 | 0 | 0 |
| Hypernatremia | 9 | 0 | 0 | 0 | 0 |
| Hematologic | |||||
| Anemia | 0 | 6 | 3 | 0 | 0 |
| Leukopenia | 2 | 4 | 2 | 1 | 0 |
| Lymphopenia | 6 | 0 | 2 | 0 | 0 |
| Neutropenia | 1 | 2 | 2 | 2 | 0 |
| Thrombocytopenia | 1 | 7 | 0 | 1 | 0 |
| Constitutional | |||||
| Nausea/vomiting | 4 | 5 | 0 | 0 | 0 |
| Diarrhea | 8 | 1 | 0 | 0 | 0 |
| Constipation | 9 | 0 | 0 | 0 | 0 |
| State of consciousness | 9 | 0 | 0 | 0 | 0 |
| Pain | 7 | 2 | 0 | 0 | 0 |
| Fever | 7 | 2 | 0 | 0 | 0 |
| Cutaneous | 7 | 2 | 0 | 0 | 0 |
| Oral | 8 | 1 | 0 | 0 | 0 |
| Hemorrhage | 9 | 0 | 0 | 0 | 0 |
| Infection | 8 | 1 | 0 | 0 | 0 |
| Pulmonary | 9 | 0 | 0 | 0 | 0 |
| Hair | 9 | 0 | 0 | 0 | 0 |
| Peripheral neurotoxicity | 9 | 0 | 0 | 0 | 0 |
| Proteinuria | 9 | 0 | 0 | 0 | 0 |
| Cardiac rhythm | 9 | 0 | 0 | 0 | 0 |
| Allergic | 9 | 0 | 0 | 0 | 0 |
| Hematuria | 9 | 0 | 0 | 0 | 0 |
graded using Common Terminology Criteria for Adverse Events v.3
represents number of subjects (of total N=9) experiencing adverse event during initial treatment cycle with ascorbate + gemcitabine
Table 3.
Summary of Maximum Grade for Laboratory and Constitutional Toxicities (Aggregate for All Treatment Cycles)
| Toxicity | Number of Subjects§ | ||||
|---|---|---|---|---|---|
|
| |||||
| Grade of Adverse Event† | |||||
| 0
|
1
|
2
|
3
|
4
|
|
| Metabolic | |||||
| Hypoalbuminemia | 6 | 0 | 3 | 0 | 0 |
| Alkaline phosphatase, elevated | 0 | 6 | 3 | 0 | 0 |
| Gamma-glutamyl transpeptidase, elevated | 4 | 2 | 1 | 2 | 0 |
| Aspartate aminotransferase, elevated | 2 | 4 | 3 | 0 | 0 |
| Alanine aminotransferase, elevated | 0 | 4 | 5 | 0 | 0 |
| Hyperbilirubinemia | 7 | 2 | 0 | 0 | 0 |
| Creatinine, elevated | 8 | 1 | 0 | 0 | 0 |
| Hypocalcemia | 4 | 4 | 1 | 0 | 0 |
| Hypercalcemia | 8 | 1 | 0 | 0 | 0 |
| Hypokalemia | 3 | 5 | 0 | 1 | 0 |
| Hyperkalemia | 6 | 3 | 0 | 0 | 0 |
| Hyponatremia | 4 | 5 | 0 | 0 | 0 |
| Hypernatremia | 9 | 0 | 0 | 0 | 0 |
| Hematologic | |||||
| Anemia | 0 | 3 | 6 | 0 | 0 |
| Leukopenia | 2 | 3 | 3 | 1 | 0 |
| Lymphopenia | 4 | 0 | 3 | 1 | 1 |
| Neutropenia | 1 | 1 | 3 | 2 | 2 |
| Thrombocytopenia | 1 | 7 | 0 | 1 | 0 |
| Constitutional | |||||
| Nausea/vomiting | 3 | 4 | 2 | 0 | 0 |
| Diarrhea | 5 | 2 | 1 | 1 | 0 |
| Constipation | 9 | 0 | 0 | 0 | 0 |
| State of consciousness | 9 | 0 | 0 | 0 | 0 |
| Pain | 2 | 1 | 5 | 1 | 0 |
| Fever | 7 | 2 | 0 | 0 | 0 |
| Cutaneous | 7 | 2 | 0 | 0 | 0 |
| Oral | 9 | 0 | 0 | 0 | 0 |
| Hemorrhage | 9 | 0 | 0 | 0 | 0 |
| Infection | 4 | 1 | 4 | 0 | 0 |
| Pulmonary | 8 | 0 | 1 | 0 | 0 |
| Hair | 9 | 0 | 0 | 0 | 0 |
| Peripheral neurotoxicity | 9 | 0 | 0 | 0 | 0 |
| Proteinuria | 9 | 0 | 0 | 0 | 0 |
| Cardiac rhythm | 9 | 0 | 0 | 0 | 0 |
| Allergic | 9 | 0 | 0 | 0 | 0 |
| Hematuria | 9 | 0 | 0 | 0 | 0 |
graded using Common Terminology Criteria for Adverse Events v.3
represents number of subjects (of total N=9) experiencing adverse event during entire course of treatment with ascorbate + gemcitabine
Pharmacology
In all subjects treated, ascorbate levels were measured before and immediately after infusions. Figure 2A demonstrates peak plasma ascorbate levels achieved with increasing doses for all subjects. Pre- and post-infusion ascorbate levels in a typical patient in the dose-escalation scheme are depicted in Figure 2B. For this subject, a 75 g dose yielded peak plasma levels ranging between 320–630 mg/dL. Interestingly, once targeted levels were achieved, ascorbate mean trough levels were significantly higher than baseline in all patients (1.46 ± 0.02 vs. 0.78 ± 0.09 mg/dL; i.e. 83 μM vs. 44 μM; p < 0.001) (Figure 2C). In addition to measuring plasma ascorbate levels, generation of ascorbate radical was measured via EPR in whole blood of both pre-and post-infusion samples to examine the degree of ascorbate oxidation occurring in the blood with pharmacological dosing. The level of ascorbate radical in pre-infusion samples at nutritional levels of ascorbate was below the limit of detection, Figure 2D. However, in post-infusion samples at pharmacological levels of ascorbate, the rate of ascorbate oxidation is greatly increased as seen by readily detectable ascorbate radical (23).
Figure 2. Ascorbate infusions achieve plasma millimolar levels.
Dose escalation of ascorbate infusions was administered to achieve a target level of 350 – 450 mg/dL (20 – 25 mM). Once subjects achieved these levels, the dose of ascorbate was not changed.
A. Peak ascorbate levels of 20 – 30 mM were reached with doses ranging from 0.75 – 1.75 g/kg.
B. Typical plasma levels of ascorbate achieved in a single patient over time. Peak ascorbate levels post-infusion were approximately 500-fold higher than both baseline and pre-infusion trough ascorbate levels.
C. Trough ascorbate levels after steady dosing regimen were significantly higher than baseline screening ascorbate levels.
D. Ascorbate radical is observed in whole blood only with high levels of ascorbate. Ascorbate radical is below the limit of detection (<10 nM under these experimental conditions) in pre-infusion samples of whole blood that have typical nutritional levels of ascorbate, here 60 – 80 μM. Ascorbate radical (100 – 150 nM) is easily detectable in post-infusion samples that have very high levels of ascorbate (19 – 23 mM). This presence of ascorbate radical indicates the ongoing oxidation of ascorbate in whole blood (23).
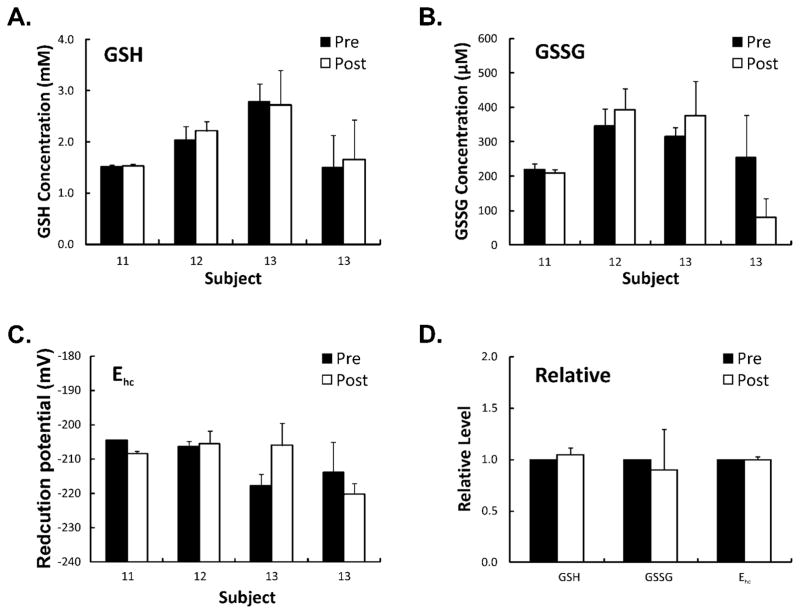
Our hypothesis is that ascorbate is a pro-drug for the delivery of H2O2 to pancreatic tumors to induce cytotoxicity (9). Lipids are readily attacked by oxidative cascades initiated by H2O2 resulting in the formation of a number of oxidation products (17). F2-isoprostanes have become the biomarker of choice for assessing endogenous oxidative stress; these molecules are chemically stable and have been detected in all biological fluids and tissues analyzed (17). Measurement of F2-isoprostanes levels before and after ascorbate infusions demonstrated a decrease in F2-isoprostane levels in all patients when compared to baseline levels (Figure 4). These data suggest that these high levels of ascorbate do not induce systemic oxidative stress. We hypothesize that the antioxidant properties of ascorbate play a role in the systemic decrease in the F2-isoprostanes levels, while the local pro-oxidant effects induce tumor cell killing. Similarly, glutathione and its half-cell reduction potential are other measurable markers indicative of the oxidation state of the redox buffer. In severe systemic oxidative stress, glutathione (GSH) may become depleted when the degree of oxidative stress overwhelms the capability of glutathione disulfide reductase (GR) and the glutathione redox cycle with cell death occurring when the cell becomes overly oxidized (24). As seen in Figure 5, the intracellular concentrations of glutathione (GSH) and glutathione disulfide (GSSG) in red blood cells pre- and post-infusion of ascorbate and the calculated half-cell reduction potentials (Ehc) demonstrate no significant change in red blood cell intracellular concentration of GSH or Ehc, suggesting although pharmacological ascorbate is a pro-oxidant toward tumor cells, there is no evidence of oxidative stress to red blood cells as seen by the stability of the intracellular redox buffer.
Figure 4. Pharmacological ascorbate does not increase markers of systemic oxidative damage.

Baseline and post-treatment F2-isoprostane levels in five patients receiving gemcitabine + ascorbate twice weekly to achieve peak plasma levels ≥ 20 mM (≥ 350 mg dL−1). In all patients tested, the F2-isoprostane level decreased after ascorbate infusions.
Figure 5. Pharmacological ascorbate does not alter the GSSG/2GSH redox buffer of RBCs.
Blood was collected from patients before and immediately after infusion and assayed for the intracellular concentrations of GSH and GSSG in the RBCs; this information was used to assess the intracellular redox status as manifest by Ehc. Plotted in panels A through C are the intracellular concentrations for samples from three individuals and the corresponding calculated half-cell reduction potential (Ehc); the error bar represents measurement variability from triplicate determinations. In panel D, the relative average levels for all analytes are presented. Pre-infusion concentrations for each individual are normalized to 1, then the corresponding relative values for the post sample are calculated. The error bars represents differences between patients.
Discussion
Our results compare to other trials of pharmacological ascorbate in advanced malignancies. In a phase I toxicity study, Hoffer and colleagues treated patients with advanced cancers or hematologic malignancies with ascorbate infused at doses of 0.4–1.5 g/kg three times weekly with 5 subjects receiving the highest dose (25). All tolerated the ascorbate infusions well. While two subjects had unexpected stable disease all eventually succumbed. The investigators concluded ascorbate may need to be combined with cytotoxic or other redox-active molecules to be an efficacious treatment.
Recently, Monti and colleagues conducted a trial of pharmacological ascorbate plus gemcitabine and erlotinib in patients with metastatic pancreatic cancer (26). A dose escalation design was used, with maximum ascorbate dose of 100 grams three times weekly but for only 8 weeks. Gemcitabine and erlotinib were administered per standard treatment regimens. Findings included fifteen non-serious adverse events and eight serious adverse in 14 patients. All of the adverse events were attributed to the chemotherapy regimen or progression of disease. Eighty-two percent of evaluable patients had weight loss of approximately 5.5 kg.
Adverse events in the present study attributable to ascorbate were rare and included diarrhea (n = 4) and dry mouth (n = 6) while subjects maintained performance status and lost minimal weight, only 5.3 ± 1.6 kg on average over 6 months of evaluation. Observed adverse events appear to be less severe when compared to the adverse events published in the literature for the gemcitabine regimen. While such a small sample size (n = 9) limits broader applicability, these results are encouraging and may warrant further investigation.
In contrast to the Monti study, we infused ascorbate doses based on the plasma levels of achieved after infusion was completed. Our goal was to achieve a targeted peak ascorbate level ≥350 mg/dL (≥20 mM), which was determined as antitumoral based on the findings of Du and colleagues (9). Maximum doses ranged from 50 g (n = 1), 75 g (n = 3), 100 g (n = 2) and 125 g (n = 3).
The Monti study had a short ascorbate treatment of only eight weeks to assess safety and tolerability. Even so, during that time, eight of the nine patients had a decrease in the size of the primary tumors. Monti et al. report an estimated mean progression free survival (PFS) of 12.7 weeks (89 days) and overall survival (OS) of 6 months (182 days) (26). In contrast, our study strategy was to continue treatment until progression per RECIST. Our mean PFS was 26 weeks and OS of 12 months (n = 9). While neither study was powered to determine therapeutic efficacy, results are striking when compared to those of Burris et al., who reported a PFS of 9 weeks and OS of approximately 6 months (4).
In a recent multi-site trial, patients treated with FOLFIRINOX increased survival by 4.3 months compared to gemcitabine (5). Median PFS was nearly double and the response rate nearly tripled with the FOLFIRINOX regimen. However, quality of life was significantly lower and serious grade 3 and 4 adverse events were significantly increased in the FOLFIRINOX group of patients.
Further studies with pharmacological ascorbate will be needed to demonstrate efficacy and confirm safety, but adverse events attributable to ascorbate were uncommon in our current study. Ascorbate infusions significantly increased the trough levels of ascorbate prior to intravenous administration. In addition, the half-cell reduction potential of the intracellular redox buffer of red blood cells remained stable. Furthermore, plasma levels of F2-isoprostanes were decreased in subjects post-infusion, suggesting ascorbate may be protective to normal tissue. This may explain the apparent decrease in adverse event frequency observed during this trial, the reduced weight loss throughout treatment, and maintained PS scores.
In summary, IV administration of ascorbate 50 to 125 g twice weekly produced plasma levels of at least 350 mg/dL (20 mM) with no significant adverse events or shift in a toxicity profile. The use of pharmacological ascorbate in combination with gemcitabine in patients with metastatic or unresectable pancreatic adenocarcinoma should be safe and well tolerated. A phase II clinical trial powered to determine efficacy of concomitant ascorbic acid combined with gemcitabine is warranted.
Supplementary Material
Figure 3. Response to therapy.

A. Baseline and post-treatment CT scans in a patient receiving ascorbate plus gemcitabine. The patient tolerated dose escalation of ascorbate, which stabilized at 75 g twice a week. Post-infusion analysis demonstrated plasma ascorbate concentrations of 22–27 mM (390 – 475 mg dL−1) at 1 h. The patient had a 9-fold decrease in the primary tumor size within 4 months of treatment.
B. Overall survival. Our phase I trial was designed to determine the effect of escalating doses of ascorbate when combined with gemcitabine in stage IV pancreatic cancer patients. The trial utilized a modified Burris regimen, administering gemcitabine for 3 weeks for each cycle of therapy along with ascorbate given twice weekly for every week. Historic median survival for gemcitabine-treated patients is 5.65 months (4). The mean survival is 12 months.
Acknowledgments
This work was supported by the National Institutes of Health [grant numbers GM42056, GM073929, P42ES013661, P30 CA086862, CA137230, CA148062], the Medical Research Service of the Department of Veterans Affairs, the Holden Comprehensive Cancer Center, and the Susan L. Bader Foundation of Hope. Dr. Mark Levine is supported by the Intramural Research Program NIDDK, NIH.
The authors thank the John (Jack) Widness lab and the Sysmex Corporation, Kobe, Japan for use of the XE-2100 and XT-2000 automated hematology analyzers. The authors also thank the Holden Comprehensive Cancer Center for its support for the clinical trial. TJvE gratefully acknowledges support from the Iowa Superfund Research Program (P42 ES013661) Training Core. The content is solely the responsibility of the authors and does not represent views of the National Institutes of Health. The University of Iowa ESR Facility provided invaluable support.
Footnotes
Disclosure
The authors have declared no conflicts of interest.
References
- 1.Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61(4):212–36. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62(1):10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 3.Winstead ER. Pancreatic Cancer Report Urges Changes in Clinical Trials. NCI Cancer Bulletin. 2009 [Google Scholar]
- 4.Burris HA, 3rd, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. Journal of Clinical Oncology: official journal of the American Society of Clinical Oncology. 1997;15(6):2403–13. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- 5.Conroy T, Desseigne F, Ychou M, Bouche O, Guimbaud R, Becouarn Y, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364(19):1817–25. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 6.Buettner GR, Jurkiewicz BA. Catalytic metals, ascorbate and free radicals: combinations to avoid. Radiat Res. 1996;145(5):532–41. [PubMed] [Google Scholar]
- 7.Chen Q, Espey MG, Krishna MC, Mitchell JB, Corpe CP, Buettner GR, et al. Pharmacologic ascorbic acid concentrations selectively kill cancer cells: action as a pro-drug to deliver hydrogen peroxide to tissues. Proc Natl Acad Sci U S A. 2005;102(38):13604–9. doi: 10.1073/pnas.0506390102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen Q, Espey MG, Sun AY, Lee JH, Krishna MC, Shacter E, et al. Ascorbate in pharmacologic concentrations selectively generates ascorbate radical and hydrogen peroxide in extracellular fluid in vivo. Proc Natl Acad Sci U S A. 2007;104(21):8749–54. doi: 10.1073/pnas.0702854104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Du J, Martin SM, Levine M, Wagner BA, Buettner GR, Wang SH, et al. Mechanisms of ascorbate-induced cytotoxicity in pancreatic cancer. Clin Cancer Res. 2010;16(2):509–20. doi: 10.1158/1078-0432.CCR-09-1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Espey MG, Chen P, Chalmers B, Drisko J, Sun AY, Levine M, et al. Pharmacologic ascorbate synergizes with gemcitabine in preclinical models of pancreatic cancer. Free Radic Biol Med. 2011;50(11):1610–9. doi: 10.1016/j.freeradbiomed.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levine M, Conry-Cantilena C, Wang Y, Welch RW, Washko PW, Dhariwal KR, et al. Vitamin C pharmacokinetics in healthy volunteers: evidence for a recommended dietary allowance. Proc Natl Acad Sci U S A. 1996;93(8):3704–9. doi: 10.1073/pnas.93.8.3704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Padayatty SJ, Sun H, Wang Y, Riordan HD, Hewitt SM, Katz A, et al. Vitamin C pharmacokinetics: implications for oral and intravenous use. Ann Intern Med. 2004;140(7):533–7. doi: 10.7326/0003-4819-140-7-200404060-00010. [DOI] [PubMed] [Google Scholar]
- 13.Levine M, Padayatty SJ, Espey MG. Vitamin C: a concentration-function approach yields pharmacology and therapeutic discoveries. Adv Nutr. 2011;2(2):78–88. doi: 10.3945/an.110.000109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vislisel JM, Schafer FQ, Buettner GR. A simple and sensitive assay for ascorbate using a plate reader. Anal Biochem. 2007;365(1):31–9. doi: 10.1016/j.ab.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Venkataraman S, Martin SM, Schafer FQ, Buettner GR. Detailed methods for the quantification of nitric oxide in aqueous solutions using either an oxygen monitor or EPR. Free Radic Biol Med. 2000;29(6):580–5. doi: 10.1016/s0891-5849(00)00404-4. [DOI] [PubMed] [Google Scholar]
- 16.Buettner GR, Kiminyo KP. Optimal EPR detection of weak nitroxide spin adduct and ascorbyl free radical signals. J Biochem Biophys Methods. 1992;24(1–2):147–51. doi: 10.1016/0165-022x(92)90054-e. [DOI] [PubMed] [Google Scholar]
- 17.Morrow JD, Hill KE, Burk RF, Nammour TM, Badr KF, Roberts LJ., 2nd A series of prostaglandin F2-like compounds are produced in vivo in humans by a non-cyclooxygenase, free radical-catalyzed mechanism. Proc Natl Acad Sci U S A. 1990;87(23):9383–7. doi: 10.1073/pnas.87.23.9383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kadiiska MB, Gladen BC, Baird DD, Germolec D, Graham LB, Parker CE, et al. Biomarkers of oxidative stress study II: are oxidation products of lipids, proteins, and DNA markers of CCl4 poisoning? Free Radic Biol Med. 2005;38(6):698–710. doi: 10.1016/j.freeradbiomed.2004.09.017. [DOI] [PubMed] [Google Scholar]
- 19.Kadiiska MB, Gladen BC, Baird DD, Graham LB, Parker CE, Ames BN, et al. Biomarkers of oxidative stress study III. Effects of the nonsteroidal anti-inflammatory agents indomethacin and meclofenamic acid on measurements of oxidative products of lipids in CCl4 poisoning. Free Radic Biol Med. 2005;38(6):711–8. doi: 10.1016/j.freeradbiomed.2004.10.024. [DOI] [PubMed] [Google Scholar]
- 20.Park HJ, Mah E, Bruno RS. Validation of high-performance liquid chromatography-boron-doped diamond detection for assessing hepatic glutathione redox status. Anal Biochem. 2010;407(2):151–9. doi: 10.1016/j.ab.2010.08.012. [DOI] [PubMed] [Google Scholar]
- 21.Jones DP, Liang Y. Measuring the poise of thiol/disulfide couples in vivo. Free Radic Biol Med. 2009;47(10):1329–38. doi: 10.1016/j.freeradbiomed.2009.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schafer FQ, Buettner GR. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic Biol Med. 2001;30(11):1191–212. doi: 10.1016/s0891-5849(01)00480-4. [DOI] [PubMed] [Google Scholar]
- 23.Buettner GR, Jurkiewicz BA. Ascorbate free radical as a marker of oxidative stress: an EPR study. Free Radic Biol Med. 1993;14(1):49–55. doi: 10.1016/0891-5849(93)90508-r. [DOI] [PubMed] [Google Scholar]
- 24.Buettner GR, Wagner BA, Rodgers VG. Quantitative Redox Biology: An Approach to Understand the Role of Reactive Species in Defining the Cellular Redox Environment. Cell Biochem Biophys. 2011 doi: 10.1007/s12013-011-9320-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoffer LJ, Levine M, Assouline S, Melnychuk D, Padayatty SJ, Rosadiuk K, et al. Phase I clinical trial of i.v. ascorbic acid in advanced malignancy. Ann Oncol. 2008;19(11):1969–74. doi: 10.1093/annonc/mdn377. [DOI] [PubMed] [Google Scholar]
- 26.Monti DA, Mitchell E, Bazzan AJ, Littman S, Zabrecky G, Yeo CJ, et al. Phase I evaluation of intravenous ascorbic acid in combination with gemcitabine and erlotinib in patients with metastatic pancreatic cancer. PLoS One. 2012;7(1):e29794. doi: 10.1371/journal.pone.0029794. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.