Abstract
PURPOSE
The mammalian target of rapamycin (mTOR) kinase acts downstream of PI3K/Akt to regulate cellular growth, metabolism and cytoskeleton. Since approximately 60% of sporadic colorectal cancers (CRCs) exhibit high levels of activated Akt, we determined whether downstream mTOR signaling pathway components are overexpressed and activated in CRCs.
EXPERIMENTAL DESIGN
HCT116, KM20, Caco-2 and SW480 human CRC cells were utilized to determine the effects of pharmacological (using rapamycin) or genetic (using RNAi) blockade of mTOR signaling on cell proliferation, apoptosis, cell cycle progression and subcutaneous growth in vivo
RESULTS
We show that the mTOR complex proteins, mTOR, Raptor and Rictor, are overexpressed in CRC. Treatment with rapamycin significantly decreased proliferation of some CRC cell lines (rapamycin-sensitive), while other cell lines were resistant to its effects (rapamycin-resistant). Transient siRNA-mediated knockdown of the mTORC2 protein, Rictor, significantly decreased proliferation of both rapamycin-sensitive and rapamycin-resistant CRC cells. Stable shRNA-mediated knockdown of both mTORC1 and mTORC2 decreased proliferation, increased apoptosis and attenuated cell cycle progression in rapamycin-sensitive CRCs. Moreover, stable knockdown of both mTORC1 and mTORC2 decreased proliferation and attenuated cell cycle progression, while only mTORC2 knockdown increased apoptosis in rapamycin-resistant CRCs. Finally, knockdown of both mTORC1 and mTORC2 inhibited growth of rapamycin-sensitive and -resistant CRCs in vivo when implanted as tumor xenografts.
CONCLUSIONS
Targeted inhibition of the mTORC2 protein, Rictor, leads to growth inhibition and induces apoptosis in both rapamycin-sensitive and rapamycin-resistant CRCs, suggesting that selective targeting of mTORC2 may represent a novel therapeutic strategy for treatment of CRC.
Keywords: mTOR, Raptor, Rictor, Akt, rapamycin, colorectal cancer
INTRODUCTION
Activation of phosphatidylinositol 3-kinase (PI3K) by activating mutations in the PIK3CA gene (encoding the p110α catalytic subunit of PI3K) or loss of the tumor suppressor PTEN (encoding a lipid and protein phosphatase) is associated with growth and progression of a number of cancers, including colorectal cancer (CRC) (1, 2). PI3K effects on tumor growth and progression are mediated by two key downstream effectors, Akt and mTOR (3, 4). Treatment with wortmannin or LY294002, general PI3K chemical inhibitors, increased apoptosis and inhibited growth of CRC cells (5, 6); however, use is limited due to toxicity in vivo (7). We have previously shown that targeted inhibition of upstream PI3K/Akt pathway components decreases growth, increases apoptosis, increases sensitivity to chemotherapy and decreases metastatic capability of CRCs (8–12). Selective inhibition of downstream proteins that are directly involved in survival and proliferation may allow more targeted therapy with fewer toxicities.
mTOR, a serine/threonine kinase that regulates cell growth and metabolism (4), exists in two, distinct functional complexes: mTORC1 and mTORC2. mTORC1 consists of mTOR, mLST8, PRAS40 and Raptor, while mTORC2 consists of mTOR, mLST8, Rictor, Sin1 and PROTOR (4). mTORC1 mediates phosphorylation and activation of the eukaryotic translation initiation factor 4E (eIF4E) binding protein (4E-BP1) and the p70S6 ribosomal kinase (S6K), which together control protein synthesis (13). mTORC2 plays a role in cell growth, in addition to regulating cell cycle-dependent changes in the actin cytoskeleton (4, 14).
The bacterially derived drug, rapamycin, complexes with the FK506 binding protein (FKBP) 12 and the drug-receptor complex potently inhibits mTOR activity (4). mTORC1 is sensitive to rapamycin treatment; mTORC2 is thought to be rapamycin-insensitive, but prolonged treatment inhibits its assembly in certain cells (4, 15). Despite the seemingly clear rationale for use of an mTOR inhibitor in cancers addicted to PI3K/Akt signaling, rapamycin and its analogues have only shown limited benefit in clinical trials. Inhibition of mTORC1 by rapamycin leads to activation of a negative feedback loop via S6K and insulin-like growth factor-1 receptor (IGF-1R), which results in feedback activation of Akt (4, 16). This paradoxical Akt activation presents a problem as it promotes cell survival and resistance to the therapeutic benefits of mTORC1 inhibition (17).
mTORC2 has been implicated as the major hydrophobic kinase to phosphorylate the Ser473 residue of Akt, thus placing mTOR both upstream and downstream of Akt (4, 18). Since Akt activation is widespread in CRC, there is a rationale for inhibition of mTORC2 to prevent pAktSer473 phosphorylation. In addition, the finding that mTORC2 is not essential in Drosophila but becomes essential for phenotypes reliant on elevated PI3K activity (4, 19), further supports the possibility that mTORC2 inhibition might have therapeutic potential, particularly in malignant states dependent on elevated Akt signaling. Here, we show mTOR, Raptor and Rictor are overexpressed in CRCs. Inhibition of mTORC1 and mTORC2 had pronounced effects on CRC proliferation and growth. Targeted inhibition of the mTORC2 protein, Rictor, proved to be effective in growth inhibition and inducing apoptosis in both rapamycin-sensitive and rapamycin-resistant CRCs, suggesting that selective targeting of mTORC2 may represent a novel therapeutic strategy for treatment of CRC.
MATERIALS AND METHODS
Immunohistochemistry
Tissue microarrays containing normal and cancer tissues, A203 (VI), were purchased from ISUABXIS through Accurate Chemical & Scientific Corporation (Westbury, NY). Each array consisted of tissue derived from 45 patients: 90 tumor cores, 8 normal cores. Immunohistochemistry (IHC) was performed as described previously (10). For negative controls, primary antibody was omitted from the above protocol. Scoring was performed blindly by a pathologist according to a semi-quantitative seven-tier system developed by Allred et al. (20). This system assesses the percentage of positive cells (none = 0; <10% = 1; 10% to 50%, = 2; >50% = 3) and intensity of staining (none = 0; weak = 1; intermediate = 2; and strong = 3). The intensity and percentage scores are added to give a final immunoreactivity score ranging from 0 to 6. mTOR, Raptor and Rictor antibodies used for IHC in Fig. 1A were purchased from Bethyl Laboratories (Montgomery, TX), while pAktSer473 antibody was purchased from Cell Signaling (Danvers, MA). mTOR, Raptor and Rictor antibodies used in Supplemental Fig. 1 were purchased from Abcam (Cambridge, MA).
Figure 1. Expression of mTORC1 and mTORC2 in CRC tissues and cell lines.
A and B. Immunohistochemical analysis of mTOR, Raptor, Rictor, and pAkt in representative colorectal adenocarcinomas and adjacent normal mucosa (tissue microarray, 10X magnification; n=45 cases; 90 tumor cores, 8 non-neoplastic cores). C. Expression and activation of mTOR signaling pathway components in HCT116, KM20, SW480 and Caco-2 CRC cells treated with 20nM rapamycin for 24 h.
Cell Lines, Plasmid Transfections and Lentiviral Transductions
The human colon cancer cell lines HCT116, KM20, SW480 and Caco-2 were used in these studies. HCT116, SW480 and Caco-2 cells were obtained from American Type Culture Collection (Manassas, VA); KM20 cells were kindly provided by Dr. Isaiah J. Fidler (MD Anderson Cancer Center, Houston, TX). HCT116 cells were cultured in McCoy’s 5A medium supplemented with 10% fetal bovine serum (FBS; Hyclone, Logan, UT). SW480 cells were cultured in DMEM supplemented with 10% FBS. Caco-2 cells were cultured in MEM medium supplemented with 10% FBS. KM20 cells were cultured in MEM medium supplemented with 10% FBS, 1% sodium pyruvate, 1% non-essential amino acids and 2% MEM essential vitamins. All tissue culture media were purchased from Invitrogen (Carlsbad, CA). Non-targeting control (NTC) and SMARTPool Raptor and Rictor siRNA were purchased from Dharmacon (Lafayette, CO).
For generation of stable knockdown HCT116 and SW480 cells, shRNAs for human mTOR, Raptor and Rictor genes were constructed in pLKO.1-puro vector and purchased from Addgene (Cambridge, MA). A plasmid carrying a non-targeting sequence was used to create the control cells. For virus packaging, the control or mTOR/Raptor/Rictor shRNA constructs were co-transfected with Mission lentiviral packing mix (Sigma-Aldrich) into HEK 293T cells using FuGene 6. The virus-containing medium was collected, filtered and overlaid onto the parental cells in the presence of polybrene (10µg/mL) for 24 h. The infected cells were then selected with puromycin (2.5µg/mL).
Cell proliferation and Apoptosis analyses
Equal numbers of cells were seeded onto 24-well plates at a density of 1 × 104 cells per well in the appropriate culture medium with supplements. For proliferation assays, cells were treated with varying doses of rapamycin for 24–96 h. Cells were trypsinized and counted using a cell counter (Beckman-Coulter, Fullerton, CA) or Cell Proliferation ELISA (Roche, Indianapolis, IN). For apoptosis assays, cells were serum starved for 48 h and apoptosis was measured using the Cell Death Detection ELISAplus (Roche, Indianapolis, IN) as detailed in the manufacturer’s instructions.
Cell Cycle analysis
Cells (1×104) were trypsinized, washed with PBS and fixed in 70% methanol. Fixed cells were then washed with PBS, incubated with 100µg/ml RNAase for 30 min at 37°C, stained with propidium iodide (50µg/ml). Cells were subjected to flow cytometry analysis using Becton Dickinson FACScanto (Franklin Lakes, NJ). The percentages of cells in different cell cycle phases were analyzed using ModFit LT software (Verity Software House).
Western Blot analysis
Western blot analysis was performed as described previously (10). The following antibodies were from Cell Signaling (Danvers, MA): pAktSer473, pAktThr308, total Akt, β-Actin, p-mTORSer2448, Tubulin, p-p70S6KThr389, pS6Ser235/236. Antibodies for mTOR, Raptor and Rictor were obtained from Bethyl Labs (Montgomery, TX).
In vivo studies
The stable knockdown HCT116 and SW480 CRC cells were collected in 50µL of sterile PBS and inoculated subcutaneously into 6 week old male athymic nude mice at 2 × 106 cells per injection site (n=5 for each group). The tumor size was measured every 3–5 d with a vernier caliper, and the tumor volume was defined as (longest diameter) × (shortest diameter)2 / 2. At the end of the experiment on day 25 post-injection, mice were sacrificed and tumors removed, weighed and then extracted for protein analysis. All animal procedures were performed in the nude mouse facility using protocols approved by the UTMB Animal Care and Use Committee.
Statistical Analysis
Association between IHC score and stage (Fig. 1B) was assessed using Fisher’s exact test. Effects of: (1) rapamycin dose on cell proliferation (Fig. 2); (2) siRNA treatment on cell proliferation (Fig. 3); (3) shRNA treatment on cell proliferation (Fig. 4B and Fig. 5B); (4) shRNA treatment on tumor volume (transformed to cubic root) and tumor weight (Fig. 6) were analyzed using one-way analysis of variance. Effects of combinations of shRNA treatment and serum (Fig. 4C and Fig. 5C) were analyzed using analysis of variance for a two-factor experiment. All tests were assessed at the 0.05 level of significance (experiment-wise). Multiple comparisons were conducted using Fisher’s least significant difference procedure with Bonferroni adjustment for the number of comparisons. Statistical computations were carried out using SAS 9.1® (21).
Figure 2. HCT116 and KM20 cells are rapamycin-sensitive whereas Caco-2 and SW480 cells are rapamycin-resistant.
Assessment of cell proliferation by counting cell numbers directly (right) after rapamycin treatment for 48h in A. HCT116; B. KM20; C. Caco-2; D. SW480 cells (* p<0.05 vs. control). Western blot analysis (left) demonstrating expression patterns of pAktSer473 and p-p70S6KThr389 after rapamycin treatment.
Figure 3. Rictor siRNA decreases the proliferation of rapamycin-sensitive and rapamycin-resistant CRC cells.
Assessment of cell proliferation by counting cell numbers directly (middle) or MTS cell proliferation assay (right) in A. HCT116; B. KM20; C. Caco-2; D. SW480 cells transfected with Raptor, Rictor or NTC siRNA and assessed by western blotting (left) at 72h after transfection, (* p<0.05 vs. NTC siRNA).
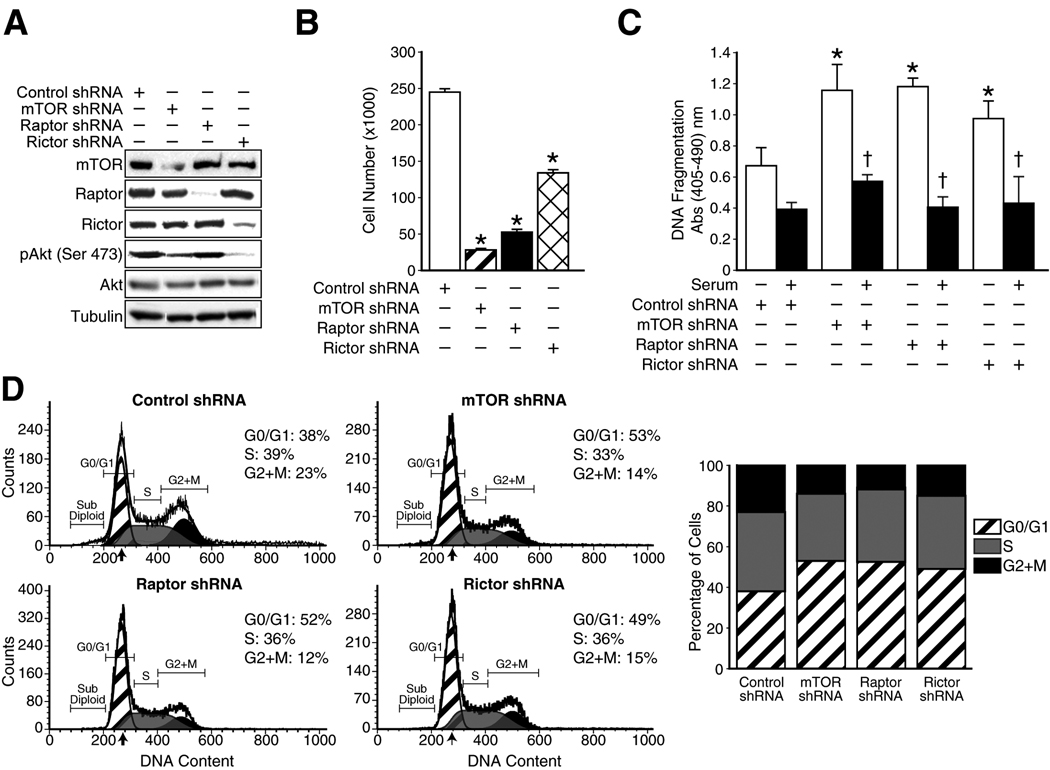
Figure 4. Functional effects of shRNA-mediated stable inhibition of mTORC1 and mTORC2 in rapamycin-sensitive HCT116 CRC cells.
A. Assessment of specific protein knockdown using Western blot analysis; B. Cell proliferation analysis by counting cell numbers directly (* p<0.05 vs. control shRNA); C. Apoptosis assessment using an ELISA detecting nucleosomes in the cytoplasm (* p<0.05 vs. control shRNA; † p<0.05 vs. -serum), D. Cell cycle progression analysis using flow cytometric analysis.
Figure 5. Functional effects of shRNA-mediated stable inhibition of mTORC1 and mTORC2 in rapamycin-resistant SW480 CRC cells.
A. Assessment of specific protein knockdown using Western blot analysis; B. Cell proliferation analysis by counting cell numbers directly (* p<0.05 vs. control shRNA); C. Apoptosis assessment using an ELISA detecting nucleosomes in the cytoplasm (* p<0.05 vs. control shRNA; † p<0.05 vs. -serum), D. Cell cycle progression analysis using flow cytometric analysis.
Figure 6. Inhibition of mTORC1 and mTORC2 reduces the tumorigenic potential of rapamycin-sensitive and rapamycin-resistant CRC cells in vivo.
Athymic nude mice were inoculated subcutaneously with A. HCT116 shNTC, HCT116 shmTOR, HCT116 shRaptor and HCT116 shRictor cells B. SW480 shNTC, SW480 shmTOR, SW480 shRaptor and SW480 shRictor cells. The size of the tumors was measured after 25 days. Five mice were used in each group, and the cells were inoculated at one site in each mouse. Bar graphs showing tumor volume (left) and tumor weight (middle) are shown along with representative mice with tumors from each group (right) (* p<0.05 vs. control shRNA).
RESULTS
mTORC1 and mTORC2 are overexpressed in CRC tissues and cells
To determine whether mTORC1 and mTORC2 proteins are overexpressed in CRCs, we examined CRCs and adjacent normal colonic tissue for expression of mTOR, Raptor and Rictor. Each sample was assigned an IHC immunoreactivity score ranging from 0–6. Representative patient samples for each protein are shown in Fig. 1A along with data analysis in Fig. 1B. The IHC score for tumor tissue was significantly higher than normal tissue for mTOR, Raptor and Rictor (p<0.0001). mTOR exhibits membranous and cytoplasmic staining; Raptor displays mixed cytoplasmic and nuclear staining of approximately equal intensity and Rictor exhibits granular cytoplasmic staining. These findings were confirmed using another set of antibodies (Supplemental Fig. 1); we found no differences in the localization and intensity of staining between the two sets of antibodies used for analysis. Furthermore, since mTORC2 has been implicated as the major kinase to phosphorylate the Ser473 residue of Akt, we also examined the expression of pAktSer473 in the same set of CRC tissues. The IHC score of tumor tissue was found to be significantly higher than normal tissue for pAktSer473 (p=0.002). Interestingly, expression of Rictor was found to correlate with elevated pAktSer473 expression; all patient samples that stained positive for pAktSer473 (regardless of staining intensity) exhibited elevated Rictor expression (IHC score=5 or 6). To further confirm our findings, we examined the expression levels of various mTOR complex components and their downstream effectors in a panel of CRC cell lines representing a spectrum of aberrations in this signaling pathway. Lysates were prepared from four human CRC cell lines (HCT116, KM20, SW480 and Caco-2) and probed with various antibodies using western blot analysis. As shown in Fig. 1C, we found that all cell lines expressed the mTORC1 components, mTOR and Raptor, with highest levels detected in HCT116 and KM20 cells. Moreover, all four cell lines expressed the mTORC2 protein, Rictor. Furthermore, all cell lines exhibit a decrease in levels of p-p70S6KThr389 upon rapamycin treatment (Fig. 1C and Supplemental Fig. 2). Taken together with the IHC results, these findings suggest that mTORC1 and mTORC2 proteins are overexpressed in CRCs. Furthermore, the mTORC2 kinase may contribute to the elevated pAktSer473 levels commonly seen in CRCs.
CRCs show differential sensitivity to rapamycin treatment
Many cancers demonstrate variable sensitivity to rapamycin treatment (4). The effects of mTOR inhibition on CRC growth are not well defined. Therefore, we determined the sensitivity or resistance of the four human CRC cell lines to rapamycin treatment. Cells were treated with increasing doses of rapamycin over a 48 h time period and cell proliferation quantitated. We found differential sensitivity to the effects of rapamycin with significant inhibition of proliferation noted in HCT116 and KM20 cells (rapamycin sensitive) as shown in Fig. 2A and 2B respectively. Rapamycin treatment of both sensitive cell lines for 48 h resulted in a dose-dependent decrease in pAktSer473 levels. In contrast, increasing doses of rapamycin had no significant effect on Caco-2 or SW480 cell proliferation (rapamycin resistant) as shown in Fig. 2C & 2D respectively. Interestingly, rapamycin treatment of both resistant cell lines for 48 h resulted in a dose-dependent increase in pAktSer473 levels. In summary, HCT116 and KM20 cells were sensitive to the anti-proliferative effects of rapamycin and exhibited a decrease in pAktSer473 levels with rapamycin treatment, while SW480 and Caco-2 cells were resistant to the anti-proliferative effects of rapamycin and exhibited an increase in pAktSer473 levels with rapamycin treatment.
Transient Rictor knockdown inhibits proliferation of both rapamycin-sensitive and rapamycin-resistant CRCs
We were interested in determining whether selective inhibition of either mTOR complex could inhibit proliferation of CRC cells. We first examined the rapamycin-sensitive cell lines, HCT116 and KM20, to determine the effects of Raptor or Rictor knockdown using siRNA (Fig. 3A & 3B). Specific knockdown of Raptor and Rictor by their corresponding siRNA was confirmed by western blot analysis. Transfection with Raptor siRNA significantly decreased KM20 cell proliferation, but not HCT116, compared to NTC siRNA. In contrast, transfection with Rictor siRNA significantly inhibited cell proliferation in both cell lines. The levels of pAktSer473 were dramatically reduced with Rictor knockdown, but not with Raptor knockdown. Next, we looked at rapamycin-resistant CRCs, Caco-2 and SW480, to determine the effects of Raptor or Rictor knockdown using specific siRNA (Fig. 3C & 3D). Transfection with Raptor siRNA had no effect on proliferation of either cell line. Surprisingly, transfection with Rictor siRNA significantly inhibited cell proliferation in both rapamycin-resistant cell lines. Levels of pAktSer473 were dramatically reduced with Rictor knockdown, but not with Raptor knockdown. These findings suggest that knockdown of Rictor can significantly inhibit the proliferation of both rapamycin-sensitive and rapamycin-resistant CRC cells.
Functional effects of stable inhibition of mTORC1 and mTORC2 in rapamycin-sensitive CRCs
To further confirm whether targeted inhibition of mTOR signaling affects the oncogenic properties of rapamycin-sensitive CRCs, we generated HCT116 cells with stable shRNA-mediated knockdown of mTOR, Raptor or Rictor protein expression. Cells expressing shRNA targeting mTOR, Raptor or Rictor had significantly reduced levels (>90% reduction) of each of the targeted proteins (Fig. 4A). Cells expressing shRNA targeting mTOR or Rictor had significantly reduced levels of pAktSer473, while levels of pAktSer473 in cells expressing shRNA targeting Raptor remained unaffected compared to control cells. Next, we determined whether knockdown of mTOR, Raptor and Rictor affected proliferation of these cells. As shown in Fig. 4B, HCT116 cells with knockdown of mTOR, Raptor or Rictor proliferate at significantly slower rates as compared to control cells. The decreased cell proliferation produced by knockdown of these proteins may be due to decreased cell cycle progression and/or increased induction of apoptosis.
We then determined whether knockdown of mTOR, Raptor and Rictor increased apoptosis in these cell lines. As shown in Fig. 4C, HCT116 cells expressing shRNA targeting mTOR, Raptor or Rictor demonstrated significantly increased rates of apoptosis compared to control cells. Furthermore, knockdown cells were sensitized to serum starvation-induced apoptosis compared to control cells. We also assessed the effect of knockdown of these mTOR complex proteins on cell cycle progression. As shown in Fig. 4D, the percentage of cells in the S- and G2/M-phases decreased markedly and the percentage of cells in the G0/G1 phase increased markedly in the population of cells with reduced mTOR, Raptor or Rictor compared with control cells. Taken together, these results suggest that inhibition of mTORC1 and mTORC2 proteins inhibits cell proliferation by inducing apoptosis as well as G0/G1 growth arrest in rapamycin-sensitive HCT116 cells.
Functional effects of stable inhibition of mTORC1 and mTORC2 in rapamycin-resistant CRCs
In order to determine the effects of inhibiting mTOR signaling on oncogenic properties of rapamycin-resistant CRCs, we generated SW480 cells with stable shRNA-mediated knockdown of mTOR, Raptor or Rictor protein expression. Cells expressing shRNA targeting mTOR, Raptor or Rictor had significantly reduced levels (>90% reduction) of each of the targeted proteins (Fig. 5A). Cells expressing shRNA targeting Rictor had significantly reduced levels of pAktSer473, while levels of pAktSer473 in cells expressing shRNA targeting mTOR and Raptor were increased in comparison to control cells. Next, we determined whether knockdown of mTOR, Raptor and Rictor affected the proliferation of these cells. As shown in Fig. 5B, SW480 cells with knockdown of mTOR, Raptor or Rictor proliferate at significantly slower rates as compared to control cells.
We then determined whether knockdown of mTOR, Raptor and Rictor increased apoptosis in these cells. Surprisingly, SW480 cells expressing shRNA targeting mTOR or Raptor had significantly reduced rates of apoptosis, while cells with stable Rictor knockdown demonstrated increased apoptosis compared to control cells (Fig. 5C). Furthermore, Rictor knockdown sensitized cells to serum starvation-induced apoptosis compared to control cells. We also assessed the effect of knockdown of these mTOR complex proteins on cell cycle progression. As shown in Fig. 5D, the percentage of cells in the S-phase decreased markedly and the percentage of cells in the G0/G1 phase increased markedly in the population of cells with reduced mTOR and Raptor compared with control cells. However, cells with reduced levels of Rictor did not undergo G0/G1 arrest; instead, the percentage of cells in S-phase was slightly decreased while the percentage of cells in the G2/M-phases was slightly increased. Taken together, these results suggest that inhibition of mTOR and Raptor components inhibits cell proliferation mainly by G0/G1 growth arrest, while inhibition of Rictor inhibits cell proliferation mainly by inducing apoptosis in rapamycin-resistant SW480 cells.
Stable knockdown of mTORC1 and mTORC2 inhibits xenograft tumor growth
To examine whether reduced mTOR, Raptor and Rictor expression in rapamycin-sensitive and rapamycin-resistant CRC cells affects growth in vivo, we injected highly tumorigenic, rapamycin-sensitive HCT116 cells and moderately tumorigenic, rapamycin-resistant SW480 cells with stable knockdown of each of the mTOR complex proteins subcutaneously into athymic nude mice and monitored tumor growth over a period of 25 days. Tumors derived from control HCT116 cells formed tumors with sizes of approximately 9500 mm3 within 25 days (Fig. 6A). In contrast, knockdown of mTOR, Raptor or Rictor significantly reduced tumor growth over the same time period; smaller tumors with sizes ranging from 750–1250 mm3 were detected at sacrifice (day 25). Lysates from HCT116-derived tumors expressing shRNA targeting mTOR or Rictor had significantly reduced levels of pAktSer473, while levels of pAktSer473 in lysates expressing shRNA targeting Raptor remained unaffected compared to the control (Supplemental Fig. 3A). Moreover, tumors derived from control SW480 cells formed tumors with sizes of approximately 690 mm3 within 25 days (Fig. 6B). In contrast, knockdown of mTOR, Raptor or Rictor significantly reduced tumor growth over the same period; smaller tumors with sizes ranging from 10–135 mm3 were detected at sacrifice (day 25). Lysates from SW480-derived tumors expressing shRNA targeting Rictor had significantly reduced levels of pAktSer473, while levels of pAktSer473 in lysates expressing shRNA targeting mTOR and Raptor were increased in comparison to the control (Supplemental Fig. 3B). Taken together, our results demonstrate that knockdown of mTOR, Raptor and Rictor inhibits growth of rapamycin-sensitive and -resistant CRC xenografts in nude mice.
DISCUSSION
In this study, we determined the role of mTORC1 and mTORC2 on CRC growth. First, we show that mTOR, Raptor and Rictor are overexpressed in CRC specimens compared to normal colonic tissue. Furthermore, Rictor overexpression correlates with elevated pAktSer473 levels. Second, we found that transient inhibition of Rictor, an essential component of mTORC2, significantly decreased proliferation of both rapamycin-sensitive and -resistant CRCs. Third, we found that stable inhibition of mTORC2 inhibited proliferation and induced apoptosis in both rapamycin-sensitive and -resistant CRCs. Finally, we showed that in vivo growth of CRC xenografts was significantly reduced with targeted inhibition of both mTORC1 and mTORC2.
Cellular levels of phosphorylated Akt are suspected to be important determinants of rapamycin sensitivity of cancer cells (22, 23). Current dogma suggests that cancers addicted to elevated Akt signaling are dependent upon downstream activation of mTORC1 to drive tumorigenesis (4). However, despite the seemingly clear rationale for the use of rapamycin in these cancers, clinical trials with rapamycin analogues have been, for the most part, disappointing (4). Rapamycin is a universal inhibitor of mTORC1-dependent S6K phosphorylation, but the existence of the negative feedback loop from S6K to Akt via IGF-1R presents a therapeutic problem as loss of feedback inhibition of Akt results in paradoxical Akt activation, which can promote cell survival and chemoresistance (4, 16, 17).
Consistent with these findings, our results demonstrate that rapamycin-resistant CRCs demonstrate a dose-dependent increase in pAktSer473 upon rapamycin treatment, while rapamycin-sensitive CRCs demonstrate a dose-dependent decrease in pAktSer473. Moreover, both rapamycin-sensitive and rapamycin-resistant CRCs demonstrate a decrease in p-p70S6KThr389 upon rapamycin treatment, but only the rapamycin-sensitive CRCs exhibit a significant decrease in proliferation upon rapamycin treatment. These results are consistent with the findings in other cancers in which rapamycin treatment demonstrates variable sensitivity; induction of pAktSer473 by negative feedback is often noted in rapamycin-resistant cancers (24). There are at least three possible explanations to this unexpected pattern of change in pAktSer473 levels. First, intrinsic differences in upstream signaling between rapamycin–sensitive and -resistant CRC cells may exist, which results in sustained feedback Akt activation by the negative feedback loop thus leading to rapamycin-resistance. Alternatively, there may be differential effects of rapamycin treatment on levels of the recently described PHLPP phosphatases, which regulate pAktSer473 and are lost or reduced in approximately 80% of CRC samples (25–27). Finally, it has been shown previously that long-term rapamycin treatment can lead to disassembly of mTORC2 in certain cells (15). It is interesting to speculate that rapamycin may be causing dissociation of mTORC2 only in the rapamycin-sensitive cell lines (HCT116 and KM20) but not in rapamycin-resistant cells (SW480 and Caco-2). Thus, changes in AktSer473 phosphorylation by mTORC2 may account for the differences in pAktSer473 levels noted in rapamycin-sensitive CRCs.
Targeting mTORC2 as an anti-cancer therapy is attractive for several reasons. First, almost 60% of CRCs demonstrate elevated Akt levels (2). In cultured cells and in the developing embryo, mTORC2 is a critical AktSer473 kinase (4, 18). Our results suggest that mTORC2 is the primary kinase that phosphorylates and activates AktSer473 in CRCs. We further show that targeted inhibition of Rictor can circumvent the paradoxical Akt activation noted with rapamycin treatment since there is no direct inhibition of mTORC1. Both transient and stable inhibition of the mTORC2 protein, Rictor, resulted in significantly reduced pAktSer473 levels in rapamycin-sensitive and -resistant CRCs. Second, an mTORC2 inhibitor may not be toxic since mTORC2 activity is dispensable in normal epithelium or cultured MEFs; rather, mTORC2 is required under conditions of elevated PI3K activity, which occurs in cancer (4, 28). This suggests the possibility that inhibition of mTORC2 could be more deleterious to cancer cells than to normal cells. Third, we found that transient inhibition of mTORC2, but not mTORC1, significantly reduced proliferation of all CRC cell lines tested, while stable inhibition of both mTORC1 and mTORC2 significantly reduced proliferation of the CRC cell lines and also reduced tumor growth in vivo. We hypothesize that CRC cells are more sensitive to inhibition of mTORC2 compared to mTORC1, such that even short-term loss of Rictor expression can inhibit proliferation of CRCs significantly. Finally, our results demonstrate that stable inhibition of Rictor, but not mTOR and Raptor, leads to significant induction of apoptosis in both rapamycin-sensitive and -resistant CRCs. This is important from a therapeutic standpoint as most successful therapeutic targets have both anti-proliferative and cytotoxic effects on cancer cells.
Recent studies suggest that an mTORC2-specific inhibitor may be a promising therapeutic agent for certain cancers driven by mutations promoting Akt signaling, such as activating mutations of PI3K or loss of PTEN. A recent study found that glioma cell lines and tissues exhibit Rictor overexpression, which results in elevated mTORC2 activity and promotes anchorage-independent growth, cellular motility and in vivo growth (29). Another study showed that prostate cancers lacking PTEN require mTORC2 to form tumors when injected into nude mice (28). In addition, the development of prostate cancer caused by Pten deletion in prostate epithelium required mTORC2, while mTORC2 activity is not essential for maintaining the integrity of normal prostate epithelium. Another recent study found that muscle-specific deletion of the gene encoding Raptor caused muscular dystrophy, whereas deletion of Rictor only had minor consequences (30).
Our studies on the effects of stable inhibition of mTORC1 and mTORC2 suggest that both complexes play a role in the proliferation and tumorigenesis of CRCs. Based on these results, it is tempting to speculate that the new generation of mTOR kinase inhibitors targeting the mTOR ATP-binding pocket, such as Torin1, PP242 and PP30, will hold greater therapeutic potential in the treatment of CRC than rapamycin analogues, since they will inhibit a wider spectrum of functions downstream of both mTOR complexes (31–33). Moreover, there may be rationale for the use of dual PI3K/mTOR inhibitors, such as PI-103 and NVP-BEZ235, in order to avoid feedback activation of PI3K/Akt signaling, especially in rapamycin-resistant CRCs that display a marked increase in pAktSer473 levels after rapamycin treatment.
In conclusion, our data supports a role for elevated mTORC1 and mTORC2 activity in CRC proliferation, apoptosis, cell cycle progression and tumorigenesis in vivo. mTOR and its interaction partners, Raptor (mTORC1) and Rictor (mTORC2), were noted to be overexpressed in CRCs. Inhibition of mTORC1 and mTORC2 had pronounced effects on CRC growth and proliferation. Targeted inhibition of the mTORC2 component, Rictor, effectively inhibited growth and induced apoptosis in both rapamycin-sensitive and rapamycin-resistant CRCs suggesting that selective targeting of mTORC2 may represent a novel therapeutic strategy for treatment of CRC.
STATEMENT OF TRANSLATIONAL RELEVANCE
Colorectal cancer (CRC) is the second leading cause of cancer deaths in the USA. Since approximately 60% of sporadic CRCs exhibit high levels of activated Akt, we determined whether downstream mTOR pathway components are overexpressed and activated in CRCs. Here, we show that the mTOR complex proteins, mTOR, Raptor and Rictor, are overexpressed in CRC tissues. We define two sub-populations of CRC cells, rapamycin-sensitive and rapamycin-resistant, exhibiting differential patterns of feedback induction of pAktSer473. We further show that targeted inhibition of the mTORC2 protein, Rictor, leads to growth inhibition and induces apoptosis in both rapamycin-sensitive and rapamycin-resistant CRCs, suggesting that selective targeting of mTORC2 may represent a novel therapeutic strategy for treatment of CRC. These findings have important ramifications for the treatment of patients with CRC, since one can envision mTOR kinase inhibitors and/or dual PI3K/mTOR inhibitors as one part of the treatment regimen for CRC patients.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank Mingjun Liao and Jun Song for technical assistance, Mark Griffin for assistance with Flow Cytometry, Karen Martin for manuscript preparation and Tatsuo Uchida for help with statistical analysis
This work was supported by grants RO1CA104748 and RO1DK48498 (BME) and K01CA10209 (TG) from the National Institutes of Health and a Sealy Center for Cancer Cell Biology Fellowship (PG).
REFERENCES
- 1.Philp AJ, Campbell IG, Leet C, et al. The phosphatidylinositol 3'-kinase p85alpha gene is an oncogene in human ovarian and colon tumors. Cancer Res. 2001;61:7426–7429. [PubMed] [Google Scholar]
- 2.Roy HK, Olusola BF, Clemens DL, et al. AKT proto-oncogene overexpression is an early event during sporadic colon carcinogenesis. Carcinogenesis. 2002;23:201–205. doi: 10.1093/carcin/23.1.201. [DOI] [PubMed] [Google Scholar]
- 3.Vivanco I, Sawyers CL. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2:489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- 4.Guertin DA, Sabatini DM. Defining the role of mTOR in cancer. Cancer Cell. 2007;12:9–22. doi: 10.1016/j.ccr.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 5.Khaleghpour K, Li Y, Banville D, Yu Z, Shen SH. Involvement of the PI 3-kinase signaling pathway in progression of colon adenocarcinoma. Carcinogenesis. 2004;25:241–248. doi: 10.1093/carcin/bgg195. [DOI] [PubMed] [Google Scholar]
- 6.Semba S, Itoh N, Ito M, Harada M, Yamakawa M. The in vitro and in vivo effects of 2-(4-morpholinyl)-8-phenyl-chromone (LY294002), a specific inhibitor of phosphatidylinositol 3'-kinase, in human colon cancer cells. Clin Cancer Res. 2002;8:1957–1963. [PubMed] [Google Scholar]
- 7.Ihle NT, Powis G. Take your PIK: phosphatidylinositol 3-kinase inhibitors race through the clinic and toward cancer therapy. Mol Cancer Ther. 2009;8:1–9. doi: 10.1158/1535-7163.MCT-08-0801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rychahou PG, Jackson LN, Silva SR, Rajaraman S, Evers BM. Targeted molecular therapy of the PI3K pathway: therapeutic significance of PI3K subunit targeting in colorectal carcinoma. Ann Surg. 2006;243:833–842. doi: 10.1097/01.sla.0000220040.66012.a9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rychahou PG, Murillo CA, Evers BM. Targeted RNA interference of PI3K pathway components sensitizes colon cancer cells to TNF-related apoptosis-inducing ligand (TRAIL) Surgery. 2005;138:391–397. doi: 10.1016/j.surg.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 10.Rychahou PG, Kang J, Gulhati P, et al. Akt2 overexpression plays a critical role in the establishment of colorectal cancer metastasis. Proc Natl Acad Sci U S A. 2008;105:20315–20320. doi: 10.1073/pnas.0810715105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sheng H, Shao J, Townsend CM, Jr, Evers BM. Phosphatidylinositol 3-kinase mediates proliferative signals in intestinal epithelial cells. Gut. 2003;52:1472–1478. doi: 10.1136/gut.52.10.1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Q, Li N, Wang X, Kim MM, Evers BM. Augmentation of sodium butyrate-induced apoptosis by phosphatidylinositol 3'-kinase inhibition in the KM20 human colon cancer cell line. Clin Cancer Res. 2002;8:1940–1947. [PubMed] [Google Scholar]
- 13.Ma XM, Blenis J. Molecular mechanisms of mTOR-mediated translational control. Nat Rev Mol Cell Biol. 2009;10:307–318. doi: 10.1038/nrm2672. [DOI] [PubMed] [Google Scholar]
- 14.Jacinto E, Loewith R, Schmidt A, et al. Mammalian TOR complex 2 controls the actin cytoskeleton and is rapamycin insensitive. Nat Cell Biol. 2004;6:1122–1128. doi: 10.1038/ncb1183. [DOI] [PubMed] [Google Scholar]
- 15.Sarbassov DD, Ali SM, Sengupta S, et al. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol Cell. 2006;22:159–168. doi: 10.1016/j.molcel.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 16.Wan X, Harkavy B, Shen N, Grohar P, Helman LJ. Rapamycin induces feedback activation of Akt signaling through an IGF-1R-dependent mechanism. Oncogene. 2007;26:1932–1940. doi: 10.1038/sj.onc.1209990. [DOI] [PubMed] [Google Scholar]
- 17.Manning BD. Balancing Akt with S6K: implications for both metabolic diseases and tumorigenesis. J Cell Biol. 2004;167:399–403. doi: 10.1083/jcb.200408161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the Rictor-mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 19.Hietakangas V, Cohen SM. Re-evaluating AKT regulation: role of TOR complex 2 in tissue growth. Genes Dev. 2007;21:632–637. doi: 10.1101/gad.416307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Allred DC, Clark GM, Elledge R, et al. Association of p53 protein expression with tumor cell proliferation rate and clinical outcome in node-negative breast cancer. J Natl Cancer Inst. 1993;85:200–206. doi: 10.1093/jnci/85.3.200. [DOI] [PubMed] [Google Scholar]
- 21.SAS/STAT 91 User's Guide. Cary, NC: SAS Institute Inc.; 2004. [Google Scholar]
- 22.Noh WC, Mondesire WH, Peng JY, et al. Determinants of rapamycin sensitivity in breast cancer cells. Clin Cancer Res. 2004;10:1013–1023. doi: 10.1158/1078-0432.ccr-03-0043. [DOI] [PubMed] [Google Scholar]
- 23.Zhou X, Tan M, Stone HV, et al. Activation of the Akt/mammalian target of rapamycin/4E-BP1 pathway by ErbB2 overexpression predicts tumor progression in breast cancers. Clin Cancer Res. 2004;10:6779–6788. doi: 10.1158/1078-0432.CCR-04-0112. [DOI] [PubMed] [Google Scholar]
- 24.Wang X, Yue P, Kim YA, Fu H, Khuri FR, Sun SY. Enhancing mammalian target of rapamycin (mTOR)-targeted cancer therapy by preventing mTOR/raptor inhibition-initiated, mTOR/Rictor-independent Akt activation. Cancer Res. 2008;68:7409–7418. doi: 10.1158/0008-5472.CAN-08-1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brognard J, Sierecki E, Gao T, Newton AC. PHLPP and a second isoform, PHLPP2, differentially attenuate the amplitude of Akt signaling by regulating distinct Akt isoforms. Mol Cell. 2007;25:917–931. doi: 10.1016/j.molcel.2007.02.017. [DOI] [PubMed] [Google Scholar]
- 26.Gao T, Furnari F, Newton AC. PHLPP: a phosphatase that directly dephosphorylates Akt, promotes apoptosis, and suppresses tumor growth. Mol Cell. 2005;18:13–24. doi: 10.1016/j.molcel.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 27.Liu J, Weiss HL, Rychahou P, Jackson LN, Evers BM, Gao T. Loss of PHLPP expression in colon cancer: role in proliferation and tumorigenesis. Oncogene. 2009;28:994–1004. doi: 10.1038/onc.2008.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guertin DA, Stevens DM, Saitoh M, et al. mTOR complex 2 is required for the development of prostate cancer induced by Pten loss in mice. Cancer Cell. 2009;15:148–159. doi: 10.1016/j.ccr.2008.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Masri J, Bernath A, Martin J, et al. mTORC2 activity is elevated in gliomas and promotes growth and cell motility via overexpression of Rictor. Cancer Res. 2007;67:11712–11720. doi: 10.1158/0008-5472.CAN-07-2223. [DOI] [PubMed] [Google Scholar]
- 30.Bentzinger CF, Romanino K, Cloetta D, et al. Skeletal muscle-specific ablation of raptor, but not of Rictor, causes metabolic changes and results in muscle dystrophy. Cell Metab. 2008;8:411–424. doi: 10.1016/j.cmet.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 31.Feldman ME, Apsel B, Uotila A, et al. Active-site inhibitors of mTOR target rapamycin-resistant outputs of mTORC1 and mTORC2. PLoS Biol. 2009;7:e38. doi: 10.1371/journal.pbio.1000038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guertin DA, Sabatini DM. The pharmacology of mTOR inhibition. Sci Signal. 2009;2:24. doi: 10.1126/scisignal.267pe24. [DOI] [PubMed] [Google Scholar]
- 33.Thoreen CC, Kang SA, Chang JW, et al. An ATP-competitive mammalian target of rapamycin inhibitor reveals rapamycin-resistant functions of mTORC1. J Biol Chem. 2009;284:8023–8032. doi: 10.1074/jbc.M900301200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.