Abstract
Inflammation in the tumor stroma greatly influences tumor development. In the present study, we investigated the roles of fibroblast growth factor (FGF)-2-induced chronic inflammation in the development of 4T1 murine mammary tumors. Administration of FGF-2 into the tumor inoculation site during the initial phase of tumor growth enhanced tumor growth and pulmonary metastasis as well as microvessel density in tumor tissues in normal but not in nude mice. Infiltration of T lymphocytes and macrophages, recruitment of pericytes/vascular mural cells in neovascular walls, and the expression levels of cyclooxygenase (COX)-2 and vascular endothelial growth factor A (VEGFA) were also enhanced in the FGF-2-activated host stroma of normal mice. In addition, FGF-2-induced tumor growth and metastasis was abrogated by administration of either an immunosuppressant, FK506, or a COX-2 inhibitor. FGF-2 enhanced prostaglandin E2 secretion in cultured T lymphocytes. In addition, VEGFA secretion was increased in a co-culture of T lymphocytes and fibroblasts in vitro. These results indicate that the massive infiltration of T lymphocytes into FGF-2-activated host stroma during the initial phase of tumor growth enhances neovascular stability by regulating endogenous COX-2 and VEGFA levels because both compounds are known to play important roles in marked 4T1 mammary tumor development via FGF-2-induced inflammatory reactions.
The host stroma microenvironment surrounding tumor tissue is recognized to influence tumor proliferation, invasion, metastasis, and angiogenesis. Recent studies show that inflammatory cells infiltrating into the host stroma support tumor angiogenesis and directly promote tumor malignancy by producing angiogenic and inflammatory factors.1,2 Clinical findings also showed that local and chronic inflammation in several types of cancer increase the risk of cancer malignancy.2 In breast carcinomas, inflammatory cells infiltrating into the host stroma surrounding the tumor are considered to be involved in a poor prognosis.3
Tumor-associated macrophages and tumor-infiltrated lymphocytes are major components of the inflammatory cells that are present in the host stroma surrounding breast cancers,3 and are considered to support the tumor-induced angiogenesis/lymphogenesis by producing prostaglandin E2 (PGE2), interleukin (IL)-1β, fibroblast growth factor (FGF)-2, and vascular endothelial growth factor (VEGF).1,2,4 Luo and colleagues5 reported that decreasing the tumor-associated macrophages in the host stroma resulted in reduced tumor angiogenesis, tumor growth, and metastasis in a murine mammary tumor model. Macrophages expressing cyclooxygenase-2 (COX-2) are essential for the formation of IL-1β-induced angiogenesis in mouse cornea assays.6 Tumor-infiltrated lymphocytes also support the tumor angiogenesis by producing angiogenic factors including FGF-2 and VEGF.7,8 Furthermore, deficiency of various subsets of T lymphocytes impairs arteriogenesis by decreasing macrophage and VEGF production in limb ischemia models.9,10,11
Among these factors associated with inflammatory-induced angiogenesis, FGF-2 has been reported to have synergistic potential for the recruitment of inflammatory cells in response to inflammatory cytokines.12 The activation of primary T lymphocytes13 and Jurkat T cells14 through FGF receptor 1 directly exerts cellular proliferation and IL-2 production in the presence of anti-CD3. Accordingly, FGF-2 is considered to be one of the key modulators for inducing chronic inflammation in the host stroma. Several overexpression models in tumor cells demonstrate that the FGF-2-induced tumor growth is dependent on the high neovascularization in tumor tissue.15,16 However, the wide varieties of FGF-2 functions on the tumor progression, especially via host stroma, were not fully understood. Therefore, we developed a direct injection model to evaluate the roles of FGF-2 in the tumor development. This model is beneficial to modulating host stroma by controlling the FGF injection. In this model, we have reported that FGF-2-induced tumor growth and metastasis are dependent on the marked development of angiogenesis accompanied by chronic inflammation in the melanoma stroma.17 However, the detailed mechanisms of FGF-2-induced chronic inflammation in the host stroma on tumor growth, metastasis, and angiogenesis remain unknown.
In the present study, we use 4T1 metastatic mammary tumor cells in this model and show new evidence that infiltrating T lymphocytes in the FGF-2-activated host stroma surrounding the tumor mass enhanced the recruitment of tumor-associated macrophages and pericytes/vascular mural cells (VMCs) to the neovascular wall. This may lead to an acceleration in FGF-2-induced tumor growth and metastasis of murine mammary tumors by up-regulation of endogenous COX-2 and VEGFA.
Materials and Methods
Antibodies and Reagents
Recombinant human FGF-2 was obtained from Scios Inc. (Mountain View, CA). A phosho-Akt (Ser 473; no. 9271) and a phospho-p38 (Thr180/Tyr182; no. 9125) rabbit monoclonal antibody were purchased from Cell Signaling Technology (Beverly, MA). Rabbit polyclonal antibodies against FGF receptor 1 (C-15) and VEGF (A-20), goat polyclonal antibodies against Akt (C-20), p38 (N-20), PECAM-1 (M-20), and CD3ε (M-20) were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). The mouse monoclonal antibody against actin and smooth muscle (Ab-1, α-SMA) and the rabbit polyclonal antibody against rat COX-2 were obtained from Lab Vision (Fremont, CA). Rat monoclonal antibody against mouse F4/80 (A3-1) was obtained from AbD Serotec (Oxford, UK). NS-398 (selective COX-2 inhibitor) was obtained from Cayman Chemical (Ann Arbor, MI). Tacrolimus (FK506, immunosuppresser agent) was obtained from Astellas Pharma (Tokyo, Japan). For the activation of primary T lymphocytes in the culture, mouse IL-2 and hamster monoclonal antibody against mouse CD3ε (145-2C11) were obtained from R&D Systems (Minneapolis, MN).
Animals
Female BALB/c normal and nude mice (6 or 7 weeks of age) were purchased from CREA Japan (Tokyo, Japan). Mice were provided with a commercial diet (CL-2, CREA Japan) and tap water ad libitum, kept in an animal room maintained specific pathogen-free at 20 to 26°C and 50 to 70% humidity, and housed with wood shavings in plastic cages. All experimental procedures were in accordance with the Guide for Care and Use of Experimental Animals of the University of Toyama.
Cell Culture
4T1 mouse mammary tumor cells (4T1 cells)18 with highly metastatic potentials were obtained from American Type Culture Collection (Manassas, VA), cultured in Dulbecco’s modified Eagle’s medium (DMEM) (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (FBS), 2 mmol/L of l-glutamine, 50 mmol/L of HEPES, 100 U/ml of penicillin, and 100 μg/ml of streptomycin. For serum starvation, cells were cultured in medium supplemented with 10% FBS for 24 hours and subsequently maintained in medium supplemented with 0.5% FBS. Peripheral blood mononuclear cells from BALB/c mice (7 weeks of age) were collected and separated with Lymphosepar II (Immuno-Biological Laboratories, Gunma, Japan) according to the manufacturer’s directions. To culture activating T lymphocytes, the peripheral blood mononuclear cells were cultured in flasks coated with anti-mouse CD3ε antibody and the RPMI 1640 (Invitrogen) medium was supplemented with 2 ng/ml of mouse IL-2, 10% FBS, 2-mercaptoethanol, 2 mmol/L of l-glutamine, 50 mmol/L of HEPES, 100 U/ml of penicillin, and 100 μg/ml of streptomycin. Each cell was kept at 37°C in 5% CO2/95% air.
Immunoblotting
The cells were lysed with sampling buffer containing 62.5 mmol/L Tris-HCl (pH 6.8 at 25°C), 2% w/v sodium dodecyl sulfate, 10% glycerol, 50 mmol/L dithiothreitol, and 0.01% w/v bromophenol. The cell lysates were resolved with sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membranes (Immobilon-P; Millipore, Billerica, MA). The membranes were incubated in blocking buffer (Block Ace; Dainippon Sumitomo Pharma, Osaka, Japan) for 2 hours at room temperature and subsequently reacted with the primary antibodies for 1 hour. The primary antibodies were detected using horseradish peroxidase-conjugated anti-rabbit or anti-goat antibodies. Target proteins were imaged with an enhanced chemiluminescence system (GE Health Care Bio-Sciences, Piscataway, NJ).
RNA Extraction, Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR), and Quantitative Real-Time RT-PCR
Total RNA was extracted from cultured cells and tissue samples using the RNeasy mini and fibrous tissue mini kits (Qiagen, Hilden, Germany), respectively, according to the manufacturer’s directions. First-strand cDNA was synthesized with random primers and SuperScript II reverse transcriptase (Invitrogen). The RT reaction was performed at 42°C for 50 minutes, then at 70°C for 15 minutes. Real-time PCR was conducted using SYBR Green I (SYBR Premix Ex Taq; Takara Bio, Shiga, Japan) and an Applied Biosystems (Foster, CA) 7300 real-time PCR system, for one cycle of 10 seconds at 95°C, and subsequently 40 cycles of 5 seconds at 95°C, and 32 seconds at 62°C according to the manufacturer’s instructions. The level of mRNA expression was determined with a standard curve and normalized to the mRNA level of GAPDH. The primers of mouse FGF-2, VEGFA, VEGFC, COX-2, tumor necrosis factor-α, IL-1β, and GAPDH were obtained from Takara Bio. The sequences that were optimized for real-time PCR are shown in Table 1.
Table 1.
Primer Sequences and Quantitative Real-Time RT-PCR
| Species | Gene | Primer sequence |
|---|---|---|
| FGF-2 | ||
| Forward | 5′-GGACGGCTGCTGGCTTCTAA-3′ | |
| Reverse | 5′-CCAGTTCGTTTCAGTGCCACATAC-3′ | |
| VEGFA | ||
| Forward | 5′-GTGCACTGGACCCTGGCTTTA-3′ | |
| Reverse | 5′-GGTCTCAATCGGACGGCAGTA-3′ | |
| VEGFC | ||
| Forward | 5′-AGGCAGCTAACAAGACATGTCCAAC-3′ | |
| Reverse | 5′-GGGTCCACAGACATCATGGAATC-3′ | |
| Mouse | COX-2 | |
| Forward | 5′-GTGTGCGACATACTCAAGCAGGA-3′ | |
| Reverse | 5′-TGAAGTGGTAACCGCTCAGGTG-3′ | |
| TNF-α | ||
| Forward | 5′-AAGCCTGTAGCCCACGTCGTA-3′ | |
| Reverse | 5′-GGCACCACTAGTTGGTTGTCTTTG-3′ | |
| IL-1β | ||
| Forward | 5′-TCCAGGATGAGGACATGAGCAC-3′ | |
| Reverse | 5′-GAACGTCACACACCAGCAGGTTA-3′ | |
| GAPDH | ||
| Forward | 5′-AAATGGTGAAGGTCGGTGTG-3′ | |
| Reverse | 5′-TGAAGGGGTCGTTGATGG-3′ |
The Orthotopic Inoculation and Spontaneous Metastasis of Mammary Tumor Cells
4T1 cells (0.7 × 106 cells) were inoculated into the right inguinal mammary fat pad of the BALB/c normal and nude mice. The major and minor axes of the tumor mass were measured, using a digital gauge (500-401 type; Mitsutoyo, Kanagawa, Japan) under anesthesia with ether to calculate the tumor volume using the following formulas: (large diameter × short diameter2/2).19 Tumor volumes were measured on days 4, 8, 12, 16, and 20 after the inoculation. The primary tumor was surgically removed on day 21 under anesthesia with diethyl ether. The mice were autopsied on day 30 and the number of metastasis colonies in the lung was counted.
The Effect of FGF-2 on Cellular Proliferation and Intracellular Signaling of 4T1 Cells in Vitro
4T1 cells were seeded in a 6-cm dish and cultured for 24 hours. After starvation, FGF-2 (10 ng/ml) and heparin (10 μg/ml; Sigma-Aldrich, St. Louis, MO) were added to the medium. The cellular lysates were collected and the activation of intracellular signaling was evaluated with immunoblotting. 4T1 cells were seeded in a 3.5-cm dish and cultured in the DMEM for 24 hours. After starvation, FGF-2 (10 and 100 ng/ml) and heparin (10 μg/ml) were added to the medium and cultured for 24 hours. The cells were stained with trypan blue and the number of living cells was counted with a hemocytometer.
The Effect of FGF-2 on the Tumor Growth, Spontaneous Metastasis, and mRNA Expression of Tumor Stroma in Normal and Nude Mice
To investigate the role of FGF-2-activated host stroma on the initial and autonomous growth phases of mammary tumor, 4T1 cells were inoculated into the mammary fat pads of BALB/c normal mice, then FGF-2 (40 μg/mouse) was administered once daily into the tumor inoculation site from days 1 to 2 (initial growth phase) or from days 9 to 10 (autonomous growth phase) after the inoculation. The vehicle was also administrated in the site of control mice using the same procedure. The initial and autonomous phase was defined by development of mature vasculature-oriented tumor tissue in the host stroma. We previously reported that the administration of this dose of FGF-2 into the tumor inoculation site induced intensive leukocyte and fibroblast infiltration into the tumor stroma,17 and exerted the maximum response for the tumor growth and metastasis in the preliminary studies. We next examined the role of T-lymphocyte infiltration into the host stroma on FGF-2-induced tumor growth and metastasis. 4T1 cells were inoculated into the mammary fat pads of BALB/c nude mice. FGF-2 administration, the evaluations of tumor volumes until day 20, and the pulmonary metastases on day 30 were performed using the same protocol as the normal mice study.
To investigate the mRNA expression of the host stroma in the normal and nude mice, FGF-2 was administered into the inoculation site from days 1 to 2 after the tumor inoculation. The mice were autopsied on days 3 or 5 and the host stroma was collected within 3 to 5 mm from surrounding tumor tissue. Total RNA was extracted and the mRNA levels of FGF-2, VEGFA, VEGFC, COX-2, tumor necrosis factor-α, and IL-1β were measured with real-time RT-PCR.
Immunostaining of Tumor Tissue and Host Stroma in Normal and Nude Mice Administered with FGF-2
FGF-2 was administered into the inoculation site from days 1 to 2 in normal and nude mice. Then, the mice were autopsied on days 3, 5, and 7 after inoculation, and the host stroma including the tumor tissue was collected. After the preparation of paraffin-embedded samples (4 μm thick) of the inoculation sites fixed in 10% formalin solution, the sections were routinely stained with hematoxylin and eosin (H&E). Immunostained samples of the tissues were reacted with the primary antibodies against the target proteins for 1 hour at room temperature. The primary antibodies were detected with the secondary antibodies anti-rabbit, anti-rat, and anti-goat Fab′ fragment combined amino acid polymers with peroxidase according to the manufacturer’s instructions (Histofine simple stain kit; Nichirei Co., Tokyo, Japan), and imaged with 3,3′-diaminobenzidine (Dako Cytomation, Kyoto, Japan). The vascular endothelial cells, pericytes/VMCs, macrophages, and T lymphocytes were detected with primary antibodies against mouse PECAM-1 (1:50), α-SMA (1:100), F4/80 (1:1000), and CD3ε (1:100), respectively. To detect the cells that produced mouse VEGFA and COX-2, we used antibodies against mouse VEGFA (1:100) and COX-2 (1:100), respectively. The host stroma surrounding the tumor tissue was observed with a microscope at ×200 or ×400 power. The density of PECAM-1-positive microvessels (MVD) in the tumor tissue was analyzed as described by Weidner20 with some modifications. The number of PECAM-1 and/or α-SMA-positive microvessels, CD3ε-positive T lymphocytes, and F4/80-positive macrophages was counted in the host stroma. The measured area in the tumor tissue and the host stroma was analyzed with Adobe Photoshop (San Jose, CA) and Image J (National Institutes of Health, Bethesda, MD). The number of cells per area (mm2) was calculated.
The Effects of FK506 on FGF-2-Induced Tumor Growth Metastasis and mRNA Expression in the Host Stroma
To investigate the direct effect of FK506 on the proliferation of 4T1 cells in vitro, 4T1 cells were seeded in a 3.5-cm dish and cultured in DMEM for 24 hours, FK506 (1 and 10 μmol/L) was added to the medium supplemented with 10% FBS, and living cells were counted after 24 hours using the same method as described previously. We also evaluated the direct effect of FK506 on the proliferation of 4T1 cells in an in vivo study. 4T1 cells were inoculated into the mammary fat pad of BALB/c normal and nude mice. FK506 (3 mg/kg)21 was intraperitoneally administered once daily from days 1 to 2 before the tumor inoculation and from days 1 to 5 after. The vehicle was also administered intraperitoneally in the control mice using the same procedure. The tumor volumes were measured until day 20.
To suppress FGF-2-induced T-lymphocyte activation into the host stroma at the initial phase of tumor growth, FK506 (3 mg/kg)21 was intraperitoneally administered once daily using the same protocol described in the previous section when FGF-2 was administered once a daily into the tumor inoculation site from days 1 to 2. The vehicle for FGF-2 and FK506 was also administered to the mice using the same procedure. The tumor volume until day 20 and the pulmonary metastasis on day 30 were evaluated using the same protocol as described previously. On day 5, the host stroma was collected and the mRNA levels of COX-2 and VEGFA were measured using real-time RT-PCR.
The Effects of COX-2 Inhibitor (NS-398) on FGF-2 Induced the Tumor Growth, Pulmonary Metastasis, and VEGFA mRNA Expression in Host Stroma
To investigate the direct effect of NS-398 on the proliferation of 4T1 cells in vitro, 4T1 cells were seeded in a 3.5-cm dish and cultured in DMEM for 24 hours, NS-398 (2 and 20 μmol/L) was added to the medium supplemented with 10% FBS, and living cells were counted after 24 hours using the same method as described previously. To suppress high COX-2 expression in the FGF-2-activated host stroma at the initial phase of tumor growth, a selective COX-2 inhibitor, NS-398 (20 mg/kg),22,23 was intraperitoneally administered once daily from days 1 to 9 after the tumor inoculation whereas FGF-2 was administered once daily into the tumor inoculation site from the days 1 to 2. The vehicle for FGF-2 and NS-398 was also administered to the mice using the same procedure. The tumor volume until day 20 and pulmonary metastasis on day 30 were evaluated. On day 5, the host stroma was collected and the mRNA level of VEGFA was measured by real-time RT-PCR. The mice administered NS-398 was not observed the gastrointestinal injury and peritonitis, which exerts COX-1 inhibition (data not shown).
The Effect of FGF-2 on PGE2 Production in T Lymphocytes and VEGFA Production in Co-Cultured T Lymphocytes and Dermal Fibroblasts
The peripheral blood T lymphocytes (1 × 105 cells/dish) were seeded in a 12-well dish with RPMI 1640 medium supplemented with 1% FBS, and then FGF-2 (10 ng/ml) and heparin (10 μg/ml) were added to the medium, and incubated for 24 hours. The concentration of PGE2 in the medium was measured with an enzyme immunoassay (Cayman Chemical) according to the manufacturer’s instructions.
The primary dermal fibroblast cells (1 × 105 cell/dish) were seeded in a 12-well dish, cultured for 24 hours, and removed from the medium. The peripheral blood T lymphocytes (1 × 105 cells/dish) were suspended in RPMI 1640 medium supplemented with 1% FBS. Cultured fibroblasts were added to the dish and co-cultured for 24 hours. The media were collected and the T lymphocytes were removed by centrifugation. The concentration of mouse VEGFA in the medium was measured with an enzyme-linked immunoassay (Quantikine, R&D Systems) in accordance with the manufacturer’s instructions.
Statistical Analysis
The statistical differences in each parameter were analyzed with the Dunnett-type multiple comparison test in the studies using both normal and nude mice or the Tukey-type multiple comparison test in the studies of administered FK506 and COX-2 inhibitor. P values of less than 0.05 were considered statistically significant.
Results
T-Lymphocyte Deficiency Suppresses FGF-2-Induced Mammary Tumor Growth and Metastasis
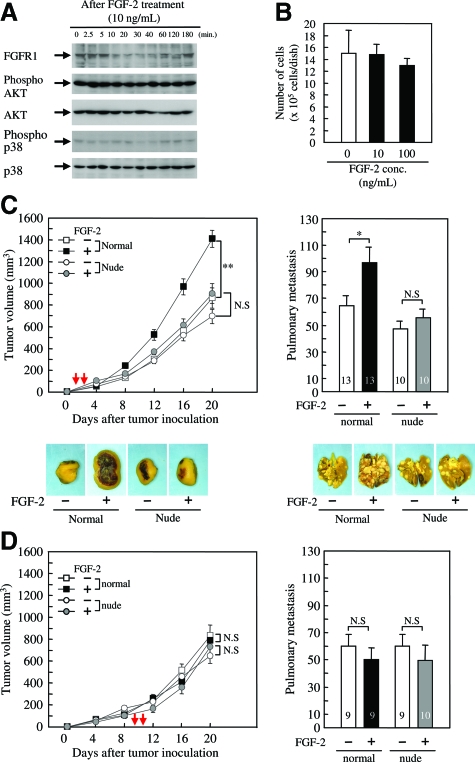
To investigate the direct action of FGF-2, we examined the effect of FGF-2 on the intracellular signaling and cell proliferation in vitro. 4T1 cells expressed FGF receptor 1 (Figure 1A); however, FGF-2 did not activate AKT or p38 (Figure 1A). AKT and p38 were successively phosphorylated regardless of FGF-2 addition (Figure 1A). Cell proliferation under serum starvation was also not affected by FGF-2 (Figure 1B). Therefore, exogenous FGF-2 did not directly activate 4T1 cells.
Figure 1.
FGF-2 indirectly enhances 4T1 mammary tumor growth and spontaneous metastasis in normal mice but not in nude mice. 4T1 cells were cultured under the serum-starved conditions for 24 hours and then treated with FGF-2. A: Whole cell lysates were collected and immunoblotted with antibodies against FGF receptor 1, phospho-Akt, Akt, phospho-p38, and p38. B: The number of living 4T1 cells was counted at 24 hours after the FGF-2 treatment. Each value represents the mean ± SEM for five samples. Normal and nude female mice (6 weeks old) were inoculated with 4T1 cells into the mammary fat pad, and then FGF-2 was administered once daily at the inoculation site from days 1 to 2 (C) and from days 9 to 10 (D) after the inoculation. Tumor volumes until day 20 and the number of pulmonary metastases on day 30 were evaluated. The timing of FGF-2 administration is represented with a red arrow in each graph of tumor growth. A similar result was obtained in an independent experiment. The number of mice examined is shown in each column. Each value represents the mean ± SEM, and significant differences from each control group are shown as *P < 0.05; **P < 0.01; and N.S, not significant.
The effect of FGF-2 on tumor growth and metastasis was examined by directly injecting it into the tumor inoculation site during the growth phase of the tumors. FGF-2 significantly enhanced tumor growth (Figure 1C) and pulmonary metastasis (Figure 1D) when injected during the initial phase of tumor growth (from days 1 to 2). Surprisingly, FGF-2 did not affect tumor growth and metastasis during the autonomous phase (from days 9 to 10). These results suggest that FGF-2 indirectly enhanced tumor growth and metastasis by activating the host stroma, which exerts itself during the initial growth phase rather than the autonomous phase. Interestingly, the significant increases in tumor growth and pulmonary metastasis were not observed when FGF-2 activated host stroma of nude mice during the initial or autonomous phase (Figure 1, C and D). The tumor volumes in nude mice decreased by ∼20% compared with those in the normal mice (Figure 1, C and D).
FGF-2 Induces T Lymphocyte and Macrophage Infiltration into the Host Stroma
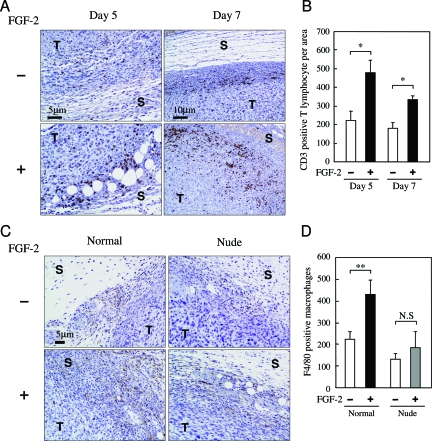
The deficiency of T lymphocytes influenced FGF-2-induced tumor growth and metastasis. Therefore, we investigated the numbers of T lymphocytes in the host stroma when FGF-2 was administered into the tumor inoculation site. The number of T lymphocytes was significantly increased in the FGF-2-activated host stroma on days 5 and 7 (Figure 2, A and B). We confirmed the deficiency of T lymphocytes in the FGF-2-activated host stroma of nude mice (data not shown). This result demonstrated that FGF-2 induced T lymphocyte infiltration into the activated host stroma.
Figure 2.
FGF-2 induces intensive T-lymphocyte and macrophage infiltration into the host stroma of normal mice. Normal and nude mice were inoculated with 4T1 cells into the mammary fat pad, and then FGF-2 was administered from days 1 to 2 after inoculation using the same procedure described in Figure 1. A: Representative photographs show the immunostaining of CD3-positive T lymphocytes in the host stroma on days 5 and 7. B: The number of T lymphocytes was counted in the host stroma on days 5 and 7. C: Representative photographs show the immunostaining of F4/80-positive macrophages in the host stroma on day 7. D: The number of macrophages was counted in the host stroma on day 7. Five mice were examined in each group. Each value represents the mean number of cells per area (mm2) ± SEM. Significant differences from each control group are shown as *P < 0.05; **P < 0.01; and N.S, not significant. T and S in the photographs represent the tumor tissue and host stroma, respectively.
It has been shown that macrophages are one of the key components in T-lymphocyte-associated atherogenesis.10 We next examined the infiltration of macrophages into the FGF-2-activated host stroma of normal and nude mice. On day 7, the number of infiltrating macrophages was significantly increased in the FGF-2-activated host stroma of normal mice but not the nude mice (Figure 2, C and D), suggesting that the deficiency of T lymphocytes suppressed the macrophage infiltration into the FGF-2-activating host stroma.
T-Lymphocyte Deficiency Suppresses FGF-2-Induced MVD in Tumor Tissue and Pericyte/VMC Recruitment in Host Stoma
MVD was examined in the tumor tissue on days 5 and 7 when FGF-2 was administered into the tumor inoculation site. A significant increase in the number of microvessels was observed on day 7 in the normal mice but not in nude mice (Figure 3, A and B). These results demonstrated that the deficiency of T lymphocytes suppressed the FGF-2-induced high MVD in the tumor tissue.
Figure 3.
FGF-2 increases microvessel formation in tumor tissue and the host stroma of normal mice but not nude mice. Normal and nude mice were inoculated with 4T1 cells, and then FGF-2 was administered at the inoculation site from days 1 to 2. A: Representative photographs show the immunostaining of the PECAM-1-positive microvessels developed in the tumor tissue on day 7. B: MVD in the tumor tissue was evaluated on day 7. Each value represents the mean number of cells per area (mm2) ± SEM. Significant differences from each control group are shown as **P < 0.01. C: Representative photographs show the PECAM-1 (e--h) and α-SMA-positive pericyte/VMC (i--l) in the host stroma surrounding the tumor on day 7. The square drawn with the orange lines in the photograph of H&E staining (a--d) show the PECAM-1 and α-SMA photograph area in the host stroma. T and S in the photographs represent the tumor tissue and host stroma, respectively.
Although the number of microvessels was comparable between normal and nude mice on day 5 of FGF-2-activated host stroma (data not shown), FGF-2 significantly increased the number of PECAM-1-positive microvessels in normal mice but not nude mice on day 7 (Table 2 and Figure 3C, e–h). This result indicates that the potency of neovascular formation induced by administering FGF-2 was suppressed during T-lymphocyte deficiency. FGF-2-activated host stroma developed a higher density of the various migrating cells compared with that of nude mice (Figure 3C, a–d). Thus, to elucidate the difference in neovascular potency, we next examined VMC recruitment by new microvessels in the host stroma by immunostaining with α-SMA. Although the number of microvessel-recruited pericytes/VMCs was significantly increased in the FGF-2-activated host stroma of the normal mice on day 7 (Table 2; Figure 3C, i and j), FGF-2 did not enhance the VMC recruitment in the nude mice (Table 2; Figure 3, k and l). The percentage of pericyte/VMC-positive microvessels in the host stroma was also raised in normal mice but not in nude mice (Table 2). These results suggest that the number of PECAM-1-positive microvessels in the FGF-2-activated host stroma was closely associated with VMC recruitment via T-lymphocyte infiltration.
Table 2.
PECAM-1- and α-SMA-Positive Microvessel Formation in the FGF-2-Activated Host Stroma
| Day after inoculation | Mouse | Group | Microvessel number
|
α-SMA/PECAM-1 | |
|---|---|---|---|---|---|
| PECAM-1 | α-SMA | (%) | |||
| Day 7 | Normal | Control | 114 ± 11]* | 59 ± 9]† | 42 ± 5]† |
| FGF-2 | 253 ± 18 | 207 ± 9 | 83 ± 2 | ||
| Nude | Control | 131 ± 26]n.s. | 75 ± 17]n.s. | 59 ± 11]n.s. | |
| FGF-2 | 168 ± 47 | 107 ± 35 | 60 ± 4 | ||
Number of PECAM-1- and α-SMA-positive microvessels per area (mm2) was counted with microscopy. The value represents the mean of five mice ± SEM. Significant differences were observed between control and FGF-2 groups in normal and nude mice at
P < 0.05 and
P < 0.01. N.S: not significant.
Deficiency of T Lymphocytes Suppresses FGF-2-Induced COX-2 Expression in the Host Stroma
COX-2 is one of the key regulators in VMC recruitment in the neovascular wall.24 Therefore, we identified the types of COX-2-positive cells in the host stroma of normal and nude mice after the FGF-2 administration. On day 3, COX-2 was mainly detected in leukocytes and fibroblasts (Figure 4A, a–h). The density of COX-2-positive leukocytes and fibroblasts was higher in the FGF-2-activated host stroma of normal mice (Figure 4Af) than in those of nude mice (Figure 4Ah). COX-2 mRNA was significantly increased in the FGF-2-activated host stroma but not in those of nude mice (Figure 4B); however, IL-1β and tumor necrosis factor-α mRNA were not increased in the stroma (data not shown). On day 5, more of the various cells were found in the FGF-2-activated host stroma of normal mice than in the stroma of control mice (Figure 4A, i–l). COX-2 was mainly detected in vascular endothelium, leukocytes, and fibroblasts in both normal and nude mice. A higher density of COX-2-positive T lymphocytes and fibroblasts was observed in the FGF-2-activated host stroma of normal mice compared with those of nude mice (Figure 4A, j and l; and Supplemental Figure 1, A and B, see http://ajp.amjpathol.org). Therefore, these results demonstrated that the massive migration/infiltration of fibroblasts and leukocytes, including T lymphocytes, is closely associated with high COX-2 production in FGF-2-activated host stroma.
Figure 4.
FGF-2-induced COX-2 expression in the host stroma is different between normal and nude mice. Normal and nude female mice were inoculated with 4T1 cells, and then FGF-2 was treated from days 1 to 2. A: The photographs show the immunostaining of COX-2 in the host stroma on days 3 and 5. The square drawn with the orange lines in the photograph of H&E staining on day 3 shows the photograph area of the host stroma-stained COX-2. The T and S represent the tumor tissue and host stroma, respectively. B: The level of COX-2 mRNA in the host stroma was measured on day 3. Each value represents the mean ± SEM for five samples. Significant differences between groups are shown as *P < 0.05 and N.S; not significant.
Deficiency of T Lymphocytes Suppresses FGF-2-Induced VEGFA Expression in the Host Stroma
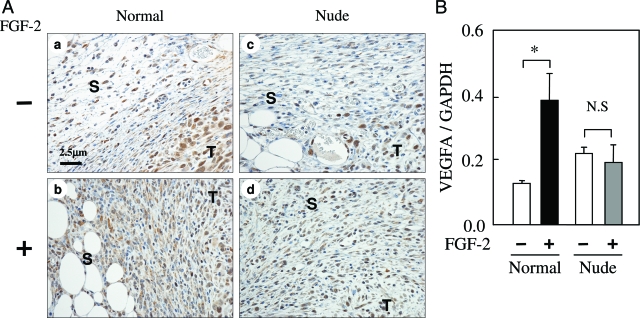
We examined the density and type of VEGFA-positive cells in the FGF-2-activated host stroma of normal and nude mice. On day 5, VEGFA was mainly detected in the vascular endothelium, leukocytes, and fibroblasts in the host stroma of normal and nude ice. A higher density of VEGFA-positive fibroblasts and T lymphocytes was observed in the FGF-2-activated host stroma of normal mice compared with those of nude mice (Figure 5A; and Supplementary Figure 1, see http://ajp.amjpathol.org). The level of VEGFA mRNA in the host stroma was evaluated in normal and nude mice. VEGFA mRNA was significantly increased in the FGF-2-activated host stroma of normal mice but not nude mice (Figure 5B). In contrast, FGF-2 did not induce mRNA of angiogenic and/or lymphogenic factors such as FGF-2 and VEGFC in the host stroma of normal and nude mice (data not shown). Therefore, these results demonstrate that the massive migration/infiltration of fibroblasts and T lymphocytes is closely associated with high VEGFA production in the FGF-2-activated host stroma.
Figure 5.
FGF-2-induced VEGFA expression in the host stroma is different between normal and nude mice. Normal and nude female mice were inoculated with 4T1 cells, and then FGF-2 was administered from days 1 to 2. A: The photographs show the immunostaining of VEGFA in the host stroma on day 5. The T and S represent the tumor tissue and host stroma, respectively. B: The VEGFA mRNA levels in the host stroma were measured on day 5. Each value represents the mean ± SEM for five samples. Significant differences between groups are shown as *P < 0.05 and N.S; not significant. T and S in the photographs represent the tumor tissue and host stroma, respectively.
FK506 Inhibited FGF-2-Induced Tumor Growth, Metastasis, and COX-2 and VEGFA Expression in the Host Stroma
To rule out the possibility that FK506 directly or indirectly affects tumor growth, we examined the direct effect of FK506 on cellular proliferation in vitro and tumor growth in vivo. FK506 did not influence cellular proliferation after 24 hours (Figure 6A). After intraperitoneal FK506 administration in normal (Figure 6B) and nude mice (data not shown), FK506 did not directly affect tumor growth in vivo regardless of T-lymphocyte infiltration.
Figure 6.
FK506 inhibits FGF-2-induced tumor growth and COX-2 and VEGFA mRNA expression in the host stroma. A: The 4T1 cells were cultured in DMEM, then FK506 (1 and 10 μmol/L) was added to the medium, and the number of cells was counted after 24 hours. B: Mice were inoculated with 4T1 cells in the mammary fat pad. FGF-2 was administered at the inoculation site from days 1 to 2. Tumor volumes (B) until day 20 and the number of pulmonary metastases (C) on day 30 were evaluated. Ten mice were examined in each group. A similar result was obtained in an independent experiment. D: The relative expression level of COX-2 and VEGFA mRNA in the host stroma was measured on day 5 using real-time PCR. Five mice were examined in each group. Each value represents the mean ± SEM. Significant differences between groups are shown as *P < 0.05 and **P < 0.01.
We investigated whether inactivation of the T lymphocyte activation with FK506 influences FGF-2-induced tumor growth and metastasis during the initial phase of tumor growth. FK506 significantly decreased FGF-2-induced tumor growth (Figure 6B) and slightly decreased (P = 0.088) pulmonary metastasis (Figure 6C). We next examined the suppression of T-lymphocyte activation on COX-2 and VEGFA mRNA expression in the FGF-2-activated host stroma. FK506 significantly suppressed FGF-2-induced mRNA expression of COX-2 and VEGFA on day 5 (Figure 6D). These findings support the results from the nude mice that T-lymphocyte activation plays an important role in marked tumor growth, and COX-2 and VEGFA mRNA expression via FGF-2 activation of the host stroma.
NS-398 Inhibited the FGF-2-Induced Tumor Growth, Metastasis, and VEGFA Expression in the Tumor Stroma
Up-regulation of COX-2 synthesis in the FGF-2-activated host stroma is closely associated with marked tumor growth and metastasis. Therefore, we examined the role of COX-2 in FGF-2-induced tumor growth and metastasis by administering a selective COX-2 inhibitor NS-398. Proliferation of 4T1 cells was not directly affected by NS-398 in vitro (Figure 7A). The continuous administration of COX-2 inhibitor is known to exert the regression of tumor growth and metastasis.22,23,24,25 Therefore, we administered NS-398 for a short period in FGF-2-activated host stroma. Although intraperitoneal administration from days 1 to 9 did not significantly affect the tumor growth and metastasis, FGF-2-induced tumor growth and metastasis were significantly suppressed by NS-398 (Figure 7, B and C). To examine the role of FGF-2-induced COX-2 expression in the host stroma, we examined the effects of NS-398 on VEGFA expression on days 3 and 5. FGF-2-induced VEGFA mRNA expression was significantly suppressed on day 5 but not on day 3 (Figure 7D). These results indicate that high COX-2 activity in the FGF-2-activated host stroma is essential for marked tumor growth and metastasis and VEGFA expression.
Figure 7.
NS-398 inhibits FGF-2-induced tumor growth, pulmonary metastasis, and VEGFA mRNA expression in the host stroma. A: The 4T1 cells were cultured in DMEM, then NS-398 (2 and 20 μmol/L) was added to the medium, and the number of cells was counted after 24 hours. Mice were inoculated with 4T1 cells in the mammary fat pad. FGF-2 was administered at the inoculation site from days 1 to 2 after inoculation. NS-398 (20 mg/kg) was administered intraperitoneally from days 1 to 9. Tumor volume (B) until day 20 and the number of pulmonary metastases (C) on day 30 were evaluated. Fifteen mice were examined in each group. A similar result was obtained in an independent experiment. D: The relative level of VEGFA mRNA in the host stroma was measured on day 5 using real-time PCR. Five mice were examined in each group. Each value represents the mean ± SEM. Significant differences between groups are shown as **P < 0.01 and *P < 0.05.
Co-Cultured T Lymphocytes and Dermal Fibroblasts Induce PGE2 and VEGFA in Vitro
We examined PGE2 concentration in the culture medium of T lymphocytes after FGF-2 addition (Figure 8A). FGF-2 increased significantly PGE2 production from T lymphocytes. A significant increase in VEGFA production was also observed in the co-cultured T lymphocytes and fibroblasts (Figure 8B). On the contrary, T lymphocytes did not produce VEGFA in the medium. These results demonstrate that FGF-2 directly induces PGE2 production in T lymphocytes, and that the interaction of T lymphocytes with fibroblasts enhanced VEGFA production.
Figure 8.
The effects of FGF-2 on PGE2 and VEGFA production in co-culture of T lymphocytes and dermal fibroblasts. A: The T lymphocytes were cultured with the DMEM supplemented with 1% FBS, and then FGF-2 (20 ng/ml) was added to the medium. The concentration of PGE2 in the medium was measured with ELISA. B: The T lymphocytes and dermal fibroblasts were co-cultured with DMEM supplemented with 1% FBS. The concentration of VEGFA in the medium was measured with ELISA. Five dishes were examined in each group. Each value represents the mean ± SEM. Significant differences between groups are shown as *P < 0.05 and **P < 0.001.
Discussion
FGF-2 is well known as one of the key factors for tumor angiogenesis by activating vascular endothelial cells. In addition, FGF receptors, expressed in various cells, exert the multiple functions including angiogenesis.26 However, little is known about the multiple roles of FGF-2 in the tumor malignancy via host stroma activation. In the present study, we show new evidence that massive infiltration of T lymphocytes into the FGF-2-activated host stroma play a critical role in the progression of tumor growth and metastasis of 4T1 mammary tumors by enhancing the neovascular stability, which is regulated by endogenous COX-2 and VEGFA.
In the spontaneous metastasis model of mouse 4T1 mammary tumors, the presence of FGF-2-activated host stroma during the initial phase of tumor growth led to the induction of the tumor growth and pulmonary metastasis (Figure 1C). Moreover, FGF-2-induced tumor progression was not observed during T-lymphocyte deficiency in nude mice (Figure 1C) and during suppression of T-lymphocyte activation by FK506 (Figure 6, B and C). These results were supported by histological evidence that intensive T-lymphocyte infiltration was observed in the FGF-2-activated host stroma of normal mice (Figure 2, A and B). On the contrary, FGF-2 did not induce tumor growth and metastasis when injected during the autonomous growth phase (Figure 1D), suggesting that abundant blood flow from mature vasculatures provided a stromal microenvironment favorable for tumor growth. We previously reported that FGF-2 developed the intensive leukocyte infiltration into the melanoma stroma accompanying angiogenesis.17 FGF-2 has been reported to have potential to recruit leukocytes after the administration of rat dermal skin.12 Moreover, FGF induces the proliferation of T lymphocytes13 and Jurkat T cells14 with CD3ε antibody in vitro. Meij and colleagues27 reported that inducible myocardium injury in FGF-2 transgenic mice promoted the infiltration of FGF receptor-positive T lymphocytes to the injury site. Taken together, our findings indicate that the marked induction of mammary tumor growth and metastasis via host stroma activation by FGF-2 depends on the infiltration and/or activation of T lymphocytes during the initial phase of tumor growth when tumor cells escape from dormancy.
FGF-2 enhanced the formation of pericyte/VMC-positive microvessels in the host stroma of normal mice accompanying with the intensive infiltration of T lymphocytes and macrophages (Table 2, Figure 3C). The MVD in tumor tissues with FGF-2-activated host stroma was increased in normal mice but not nude mice (Figure 3, A and B). MVD in tumor tissue is recognized to correlate well with tumor malignancy.20 Pericytes/VMCs act to stabilize vessels28 and support the increase of blood perfusion.29 Accordingly, the pericyte/VMC formation in neovascular walls developed in host stroma is considered to sustain the increase of blood flow in the tumor tissue. The infiltration of T lymphocytes and macrophages into the FGF-2-activated host stroma regulates pericyte/VMC formation in neovascular walls to sustain vascular stability and keep the blood flow into tumor tissues, which enhances tumor progression by developing a high MVD. Because the migration potency of vascular endothelial cells in the FGF-2-activated host stroma is identical between normal and nude mice, the direct action of FGF2 on the vascular endothelial cells was not involved in the T-lymphocyte infiltration.
FGF-2 enhanced the expression of COX-2 in the stroma, which had intensive T-lymphocyte infiltration (Figure 4A). In addition, the expression of COX-2 mRNA in FGF-2-activated host stroma is suppressed during T-lymphocyte deficiency (Figure 4A) as well as by the immunosuppressant FK506 (Figure 6D). Although the COX-2 inhibitor did not directly affect the mammary tumor proliferation in vitro, FGF-2-induced tumor growth and metastasis was suppressed (Figure 7, B and C). The immunostaining of host FGF-2-activated stroma revealed that the high level of COX-2 expression was mainly detected in T lymphocytes in normal mice (Figure 4B). In addition, abundant fibroblasts expressing COX-2 were observed in the host stroma of normal mice as compared with those of nude mice (Figure 4B). This evidence shows that pericyte/VMC formation in the neovascular wall was reduced during T-lymphocyte deficiency, which accompanies lower COX-2 expression. Lee and colleagues25 reported that a COX-2 inhibitor suppressed pericyte/VMC recruitment in the neovascular endothelium consequently leading to tumor regression. COX-2 is a key enzyme for inflammation-induced angiogenesis.30 Therefore, the present results indicate that high expression of COX-2 in the FGF-2-activated host stroma is associated with the maintenance of pericyte/VMC formation in the neovascular wall as well as vascular endothelial cell proliferation. As shown in Figure 8A, FGF-2 increased the production of PGE2 from cultured T lymphocytes. In addition, the co-culture of abundant T lymphocytes and gingival fibroblasts (ratio, 10:1) has been reported to enhance PGE2 production.31 Yamamura and colleagues32 reported that co-culture of resting T lymphocytes and synovial fibroblasts resulted in increased PGE2 production in the presence of IL-17. These phenomena may be based on the interaction between T lymphocytes and immature mesenchymal cells, including fibroblasts, inducing COX-2 activity. Therefore, the present in vivo results indicate that the up-regulation of COX-2 by T lymphocytes and/or the interaction of T lymphocytes and fibroblasts in FGF-2-activated host stroma lead to pericyte/VMC formation in the neovascular wall to sustain vascular stability and successive supply of the vascular endothelial cells. Cytotoxic T-lymphocyte (CTL) infiltration is recognized to depend mainly on the rejection of tumor cells. Several studies demonstrated that the depletion of CD4+- or CD8+-positive T cells did not affect the tumor growth and metastasis of 4T1 cells even if mice had an up-regulated immune response.33,34,35 In addition, the number of CD4+ and CD8+ T cells in the peripheral blood during the 4T1 tumor development was not significantly different compared with intact mice.36 Recently, Sharma and colleagues37 showed that the high production of PGE2 from tumor cells increases the regulatory T cells (Tregs) suppressing the anti-tumor immune response. Chaput and colleagues38 showed that the depletion of CD25+ T cells in the tumor site induces the 4T1 tumor regression by increasing the number of CD8+ T cells, and that the massive infiltration of Tregs in tumor prevents the development of a successful helper response. Moreover, myeloid-derived suppresser cells inhibit immune surveillance of CD4+ and CD8+ T lymphocytes.39 PGE2 has been shown to induce directly Treg function in human CD4+ T cells40 or indirectly via myeloid-derived suppresser cells.41 FGF-2 failed to induce directly the CTL response. T lymphocytes infiltrating into the FGF-2-activated host stroma with high COX-2 activity may be mainly CD4+ and CD8+ T lymphocytes, including Tregs. In these T lymphocytes, Tregs and/or myeloid-derived suppresser cells contribute to escape from the anti-tumor response of CTL even in massive T-lymphocyte infiltration. Characterization of T-lymphocyte subsets supporting the neovascular satiability is essential to understand the FGF-2-induced activation of host stroma.
VEGFA is well known to have the potential to proliferate and survive in the vascular endothelium.42 VEGFA production was enhanced by the co-culture of T lymphocytes and dermal fibroblasts without FGF-2 activation. During T-lymphocyte deficiency (Figure 5A) and suppression of T-lymphocyte activation (Figure 6D), the expression of VEGFA mRNA was suppressed in the FGF-2-activated host stroma. Immunohistochemical analysis revealed that a high density of VEGFA-positive T lymphocytes and fibroblasts was observed in the FGF-2-activated host stroma of normal mice (Figure 5B). VEGFA produced by T lymphocytes increases the local angiogenic response in the tumor stroma.7,43 The co-culture of activating T lymphocytes and synovial fibroblasts enhanced VEGFA production compared with T lymphocytes alone.44 These results suggest that continual VEGFA production in the FGF-2-activated host stroma depends partly on the synergic mechanism between T lymphocytes and fibroblasts. The macrophages are also involved in the induction of angiogenesis by delivering VEGF to inflammatory sites.45 The intensive migration of macrophages into the FGF-2-activated host stroma (Figure 2, C and D) may be one of the key factors in inducing high VEGFA production. As shown in Figure 7D, a selective COX-2 inhibitor reduced the high expression of VEGFA in the FGF-2-activated host stroma. PGE2 promotes the production of VEGFA from the fibroblasts.46,47 In particular, Seno and colleagues48 shows that COX-2 up-regulates PGE2 in the tumor stroma that stimulates the EP2 receptor, followed by VEGFA induction. The high PGE2 production via T lymphocytes may contribute to VEGFA production from fibroblasts in the FGF-2-activated host stroma. VEGFA produced from T lymphocytes and macrophages is closely associated with the development of collateral vessels in the ischemic limb model using CD4, CD8 knockout9,11 and nude mice.10 FGF-2-induced tumor angiogenesis and tumor growth were inhibited by the blockage of endogenous VEGF.17,49 Taking account of this evidence, the intensive infiltration of T lymphocytes, interacting directly or indirectly with fibroblasts and macrophages, sustains the production of VEGFA in the FGF-2-activated host stroma. This high and continual production of VEGFA via these T-lymphocyte functions is also associated with the proliferation, migration, and survival of vascular endothelial cells in the FGF-2-activated host stroma.
In conclusion, these results indicate that massive T-lymphocyte infiltration into the FGF-2-acitivated inflammatory host stroma during the initial phase of tumor growth enhances stable neovascular formation-oriented tumor tissue by regulating endogenous COX-2 and VEGFA, which plays an important role in FGF-2-induced tumor growth and metastasis of 4T1 mammary tumors. Accordingly, the suppression of COX-2 activity is a reasonable therapeutic target for FGF-2-induced inflammation-associated breast cancer malignancy.
Supplementary Material
Acknowledgments
We thank Toshiyuki Shibanushi, Kazuhito Hashimoto, and Kenji Minowa for technical support.
Footnotes
Address reprint requests to Satoshi Tsunoda, Division of Pathogenic Biochemistry, Institute of Natural Medicine, University of Toyama, 2630 Sugitani, Toyama 930-0194, Japan. E-mail: [email protected].
Supported by the 21st Century Center of Excellence Program and the Ministry of Education, Culture, Sports, Science, and Technology, Japan (Cooperative Link of Unique Science and Technology for Economy Revitalization grant).
Supplemental material for this article can be found on http://ajp.amjpathol.org.
References
- Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow. Lancet. 2001;357:539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Baruch A. Inflammatory cells and cytokines and chemokines in breast cancer progression: reciprocal tumor-microenvironment interaction. Breast Cancer Res. 2002;5:31–36. doi: 10.1186/bcr554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelo LS, Kurzrock R. Vascular endothelial growth factor and its relationship to inflammatory mediators. Clin Cancer Res. 2007;15:2825–2830. doi: 10.1158/1078-0432.CCR-06-2416. [DOI] [PubMed] [Google Scholar]
- Luo Y, Zhou H, Krueger J, Kaplan C, Lee SH, Dolman C, Markowitz D, Wu W, Liu C, Reisfeld RA, Xiang R. Targeting tumor-associated macrophages as a novel strategy against breast cancer. J Clin Invest. 2006;116:2132–2141. doi: 10.1172/JCI27648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakao S, Kuwano T, Tsutsumi-Miyahara C, Ueda S, Kimura YN, Hamano S, Sonoda KH, Saijo Y, Nukiwa T, Strieter RM, Ishibashi T, Kuwano M, Ono M. Infiltration of COX-2-expressing macrophages is a prerequisite for IL-1 beta-induced neovascularization and tumor growth. J Clin Invest. 2005;115:2979–2991. doi: 10.1172/JCI23298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman MR, Schneck FX, Gagnon ML, Corless C, Soker S, Niknejad K, Peoples GE, Klagsbrun M. Peripheral blood T lymphocytes and lymphocytes infiltrating human cancers express vascular endothelial growth factor: a potential role for T cells in angiogenesis. Cancer Res. 1995;55:4140–4145. [PubMed] [Google Scholar]
- Peoples GE, Blotnick S, Takahashi K, Freeman MR, Klagsbrun M, Eberlein TJ. T lymphocytes that infiltrate tumors and atherosclerotic plaques produce heparin-binding epidermal growth factor-like growth factor and basic fibroblast growth factor: a potential pathologic role. Proc Natl Acad Sci USA. 1995;92:6547–6551. doi: 10.1073/pnas.92.14.6547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stabile E, Burnett MS, Watkins C, Kinnaird T, Bachis A, la Sala A, Miller JM, Shou M, Epstein SE, Fuchs S. Impaired arteriogenic response to acute hindlimb ischemia in CD4-knockout mice. Circulation. 2003;108:205–210. doi: 10.1161/01.CIR.0000079225.50817.71. [DOI] [PubMed] [Google Scholar]
- Couffinhal T, Silver M, Kearney M, Sullivan A, Witzenbichler B, Magner M, Annex B, Peters K, Isner JM. Impaired collateral vessel development associated with reduced expression of vascular endothelial growth factor in ApoE−/− mice. Circulation. 1999;99:3188–3198. doi: 10.1161/01.cir.99.24.3188. [DOI] [PubMed] [Google Scholar]
- Stabile E, Kinnaird T, la Sala A, Hanson SK, Watkins C, Campia U, Shou M, Zbinden S, Fuchs S, Komfeld H, Epstein SE, Burnett MS. CD8+ T lymphocytes regulate the arteriogenic response to ischemia by infiltrating the site of collateral vessels development and recruiting CD4+ mononuclear cells through the expression of interleulin-16. Circulation. 2006;113:118–124. doi: 10.1161/CIRCULATIONAHA.105.576702. [DOI] [PubMed] [Google Scholar]
- Zittermann SI, Issekutz AC. Basic fibroblast growth factor (bFGF, FGF-2) potentiates leukocyte recruitment to inflammation by enhancing endothelial adhesion molecule expression. Am J Pathol. 2006;168:835–846. doi: 10.2353/ajpath.2006.050479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao XM, Byrd VM, McKeehan WL, Reich MB, Miller GG, Thomas JW. Costimulation of human CD4+ T cells by fibroblast growth factor-1 (acidic fibroblast growth factor). J Immunol. 1995;155:3904–3911. [PubMed] [Google Scholar]
- Byrd VM, Ballard DW, Miller GG, Thomas JW. Fibroblast growth factor-1 (FGF-1) enhances IL-2 production and nuclear translocation of NF-kappaB in FGF receptor-bearing Jurkat T cells. J Immunol. 1999;162:5853–5859. [PubMed] [Google Scholar]
- Giavazzi R, Giuliani R, Coltrini D, Bani MR, Ferri C, Sennino B, Tosatti MP, Stoppacciaro A, Presta M. Modulation of tumor angiogenesis by conditional expression of fibroblast growth factor-2 affects early but not established tumors. Cancer Res. 2001;61:309–317. [PubMed] [Google Scholar]
- Nissen LJ, Cao R, Hedlund EM, Wang Z, Zhao X, Wetterskog D, Funa K, Bråkenhielm E, Cao Y. Angiogenic factors FGF2 and PDGF-BB synergistically promote murine tumor neovascularization and metastasis. J Clin Invest. 2007;117:2766–2777. doi: 10.1172/JCI32479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsunoda S, Nakamura T, Sakurai H, Saiki I. Fibroblast growth factor-2-induced host stroma reaction during initial tumor growth promotes progression of mouse melanoma via vascular endothelial growth factor A-dependent neovascularization. Cancer Sci. 2007;98:541–548. doi: 10.1111/j.1349-7006.2007.00432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aslakson CJ, Miller FR. Selective events in the metastatic process defined by analysis of the sequential dissemination of subpopulations of a mouse mammary tumor. Cancer Res. 1992;52:1399–1405. [PubMed] [Google Scholar]
- Price JE. Xenograft models in immunodeficient animals. Brooks SA, Schumacher U, editors. Totowa: Humana Press; 2001:pp 205–213. [Google Scholar]
- Weidner N. Current pathologic methods for measuring intratumoral microvessel density within breast carcinoma and other solid tumors. Breast Cancer Res Treat. 1995;36:169–180. doi: 10.1007/BF00666038. [DOI] [PubMed] [Google Scholar]
- Harada N, Shimada M, Okano S, Suehiro T, Soejima Y, Tomita Y, Maehara Y. IL-12 gene therapy is an effective therapeutic strategy for hepatocellular carcinoma in immunosuppressed mice. J Immunol. 2004;173:6635–6644. doi: 10.4049/jimmunol.173.11.6635. [DOI] [PubMed] [Google Scholar]
- Hiraga T, Myoui A, Choi ME, Yoshikawa H, Yoneda T. Stimulation of cyclooxygenase-2 expression by bone-derived transforming growth factor-beta enhances bone metastases in breast cancer. Cancer Res. 2006;66:2067–2073. doi: 10.1158/0008-5472.CAN-05-2012. [DOI] [PubMed] [Google Scholar]
- Ferrario A, Fisher AM, Rucker N, Gomer CJ. Celecoxib and NS-398 enhance photodynamic therapy by increasing in vitro apoptosis and decreasing in vivo inflammatory and angiogenic factors. Cancer Res. 2005;65:9473–9478. doi: 10.1158/0008-5472.CAN-05-1659. [DOI] [PubMed] [Google Scholar]
- Lee A, Frischer J, Serur A, Huang J, Bae JO, Kornfield ZN, Eljuga L, Shawber CJ, Feirt N, Mansukhani M, Stempak D, Baruchel S, Bender JG, Kandel JJ, Yamashiro DJ. Inhibition of cyclooxygenase-2 disrupts tumor vascular mural cell recruitment and survival signaling. Cancer Res. 2006;66:4378–4384. doi: 10.1158/0008-5472.CAN-05-3810. [DOI] [PubMed] [Google Scholar]
- Leahy KM, Ornberg RL, Wang Y, Zweifel BS, Koki AT, Masferrer JL. Cyclooxygenase-2 inhibition by celecoxib reduces proliferation and induces apoptosis in angiogenic endothelial cells in vivo. Cancer Res. 2002;62:625–631. [PubMed] [Google Scholar]
- Bikfalvi A, Klein S, Pintucci G, Rifkin DB. Biological roles of fibroblast growth factor-2. Endocr Rev. 1997;18:26–45. doi: 10.1210/edrv.18.1.0292. [DOI] [PubMed] [Google Scholar]
- Meij JT, Sheikh F, Jimenez SK, Nickerson PW, Kardami E, Cattini PA. Exacerbation of myocardial injury in transgenic mice overexpressing FGF-2 is T cell dependent. Am J Physiol. 2002;282:H547–H555. doi: 10.1152/ajpheart.01019.2000. [DOI] [PubMed] [Google Scholar]
- Huang J, Soffer SZ, Kim ES, McCrudden KW, Huang J, New T, Manley CA, Middlesworth W, O'Toole K, Yamashiro DJ, Kandel JJ. Vascular remodeling marks tumors that recur during chronic suppression of angiogenesis. Mol Cancer Res. 2004;2:36–42. [PubMed] [Google Scholar]
- Schildmeyer LA, Braun R, Taffet G, Debiasi M, Burns AE, Bradley A, Schwartz RJ. Impaired vascular contractility and blood pressure homeostasis in the smooth muscle alpha-actin null mouse. FASEB J. 2000;14:2213–2220. doi: 10.1096/fj.99-0927com. [DOI] [PubMed] [Google Scholar]
- Kuwano T, Nakao S, Yamamoto H, Tsuneyoshi M, Yamamoto T, Kuwano M, Ono M. Cyclooxygenase 2 is a key enzyme for inflammatory cytokine-induced angiogenesis. FASEB J. 2004;18:300–310. doi: 10.1096/fj.03-0473com. [DOI] [PubMed] [Google Scholar]
- Yucel-Lindberg T, Brunius G, Wondimu B, Andurén I, Modéer T. Enhanced cyclooxygenase-2 mRNA expression in human gingival fibroblasts induced by cell contact with human lymphocytes. Eur J Oral Sci. 2001;109:187–192. doi: 10.1034/j.1600-0722.2001.00999.x. [DOI] [PubMed] [Google Scholar]
- Yamamura Y, Gupta R, Morita Y, He X, Pai R, Endres J, Freiberg A, Chung K, Fox DA. Effector function of resting T cells: activation of synovial fibroblasts. J Immunol. 2001;166:2270–2275. doi: 10.4049/jimmunol.166.4.2270. [DOI] [PubMed] [Google Scholar]
- Pulaski BA, Clements VK, Pipeling MR, Ostrand-Rosenberg S. Immunotherapy with vaccines combining MHC class II/CD80+ tumor cells with interleukin-12 reduces established metastatic disease and stimulates immune effectors and monokine induced by interferon gamma. Cancer Immunol Immunother. 2000;49:34–45. doi: 10.1007/s002620050024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakhmilevich AL, Janssen K, Hao Z, Sondel PM, Yang NS. Interleukin-12 gene therapy of a weakly immunogenic mouse mammary carcinoma results in reduction of spontaneous lung metastases via a T-cell-independent mechanism. Cancer Gene Ther. 2000;7:826–838. doi: 10.1038/sj.cgt.7700176. [DOI] [PubMed] [Google Scholar]
- Huang X, Wong MK, Yi H, Watkins S, Laird AD, Wolf SF, Gorelik E. Combined therapy of local and metastatic 4T1 breast tumor in mice using SU6668, an inhibitor of angiogenic receptor tyrosine kinases, and the immunostimulator B7.2-IgG fusion protein. Cancer Res. 2002;62:5727–5735. [PubMed] [Google Scholar]
- DuPre' SA, Hunter KW., Jr Murine mammary carcinoma 4T1 induces a leukemoid reaction with splenomegaly: association with tumor-derived growth factors. Exp Mol Pathol. 2007;82:12–24. doi: 10.1016/j.yexmp.2006.06.007. [DOI] [PubMed] [Google Scholar]
- Sharma S, Yang SC, Zhu L, Reckamp K, Gardner B, Baratelli F, Huang M, Batra RK, Dubinett SM. Tumor cyclooxygenase-2/prostaglandin E2-dependent promotion of FOXP3 expression and CD4+ CD25+ T regulatory cell activities in lung cancer. Cancer Res. 2005;65:5211–5220. doi: 10.1158/0008-5472.CAN-05-0141. [DOI] [PubMed] [Google Scholar]
- Chaput N, Darrasse-Jèze G, Bergot AS, Cordier C, Ngo-Abdalla S, Klatzmann D, Azogui O. Regulatory T cells prevent CD8 T cell maturation by inhibiting CD4 Th cells at tumor sites. J Immunol. 2007;179:4969–4978. doi: 10.4049/jimmunol.179.8.4969. [DOI] [PubMed] [Google Scholar]
- Bunt S, Shnha P, Clements V, Leips J, Ostrand-Rosenbeg S. Inflammation induces myloid-drived suppressor cells that facilitate tumor progression. J Immunol. 2006;176:284–290. doi: 10.4049/jimmunol.176.1.284. [DOI] [PubMed] [Google Scholar]
- Baratelli F, Lin Y, Zhu L, Yang SC, Heuzé-Vourc'h N, Zeng G, Reckamp K, Dohadwala M, Sharma S, Dubinett SM. Prostaglandin E2 induces FOXP3 gene expression and T regulatory cell function in human CD4+ T cells. J Immunol. 2005;175:1483–1490. doi: 10.4049/jimmunol.175.3.1483. [DOI] [PubMed] [Google Scholar]
- Sinha P, Clements V, Fulton A, Ostrand-Rosenbeg S. Prostaglandin E2 promotes tumor progression by inducing myeloid-derived suppressor cells. Cancer Res. 2007;67:4507–4513. doi: 10.1158/0008-5472.CAN-06-4174. [DOI] [PubMed] [Google Scholar]
- Veikkola T, Karkkainen M, Claesson-Welsh L, Alitalo K. Regulation of angiogenesis via vascular endothelial growth factor receptors. Cancer Res. 2000;60:203–212. [PubMed] [Google Scholar]
- Mor F, Quintana FJ, Cohen IR. Angiogenesis-inflammation cross-talk: vascular endothelial growth factor is secreted by activated T cells and induces Th1 polarization. J Immunol. 2004;172:4618–4623. doi: 10.4049/jimmunol.172.7.4618. [DOI] [PubMed] [Google Scholar]
- Cho CS, Cho ML, Min SY, Kim WU, Min DJ, Lee SS, Park SH, Choe J, Kim HY. CD40 engagement on synovial fibroblast up-regulates production of vascular endothelial growth factor. J Immunol. 2000;164:5055–5061. doi: 10.4049/jimmunol.164.10.5055. [DOI] [PubMed] [Google Scholar]
- Cursiefen C, Chen L, Borges LP, Jackson D, Cao J, Radziejewski C, D'Amore PA, Dana MR, Wiegand SJ, Streilein JW. VEGF-A stimulates lymphangiogenesis and hemangiogenesis in inflammatory neovascularization via macrophage recruitment. J Clin Invest. 2004;113:1040–1050. doi: 10.1172/JCI20465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Av P, Crofford LJ, Wilder RL, Hla T. Induction of vascular endothelial growth factor expression in synovial fibroblasts by prostaglandin E and interleukin-1: a potential mechanism for inflammatory angiogenesis. FEBS Lett. 1995;372:83–87. doi: 10.1016/0014-5793(95)00956-a. [DOI] [PubMed] [Google Scholar]
- Amano H, Hayashi I, Endo H, Kitasato H, Yamashina S, Maruyama T, Kobayashi M, Satoh K, Narita M, Sugimoto Y, Murata T, Yoshimura H, Narumiya S, Majima M. Host prostaglandin E(2)-EP3 signaling regulates tumor-associated angiogenesis and tumor growth. J Exp Med. 2003;197:221–232. doi: 10.1084/jem.20021408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seno H, Oshima M, Ishikawa TO, Oshima H, Takaku K, Chiba T, Narumiya S, Taketo MM. Cyclooxygenase 2- and prostaglandin E(2) receptor EP(2)-dependent angiogenesis in Apc(Delta716) mouse intestinal polyps. Cancer Res. 2002;62:506–511. [PubMed] [Google Scholar]
- Giavazzi R, Sennino B, Coltrini D, Garofalo A, Dossi R, Ronca R, Tosatti MP, Presta M. Distinct role of fibroblast growth factor-2 and vascular endothelial growth factor on tumor growth and angiogenesis. Am J Pathol. 2003;162:1913–1926. doi: 10.1016/S0002-9440(10)64325-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.