Abstract
The NCCN Guidelines for Kidney Cancer focus on the screening, diagnosis, staging, treatment, and management of renal cell carcinoma (RCC). Patients with relapsed or stage IV RCC typically undergo surgery and/or receive systemic therapy. Tumor histology and risk stratification of patients is important in therapy selection. The NCCN Guidelines for Kidney Cancer stratify treatment recommendations by histology; recommendations for first-line treatment of ccRCC are also stratified by risk group. To further guide management of advanced RCC, the NCCN Kidney Cancer Panel has categorized all systemic kidney cancer therapy regimens as “Preferred,” “Other Recommended Regimens,” or “Useful in Certain Circumstances.” This categorization provides guidance on treatment selection by considering the efficacy, safety, evidence, and other factors that play a role in treatment selection. These factors include pre-existing comorbidities, nature of the disease, and in some cases consideration of access to agents. This article summarizes surgical and systemic therapy recommendations for patients with relapsed or stage IV RCC.
Overview
An estimated 76,080 Americans will be diagnosed with cancers of the kidney and renal pelvis and 13,780 will die of the disease in the United States in 2021.1 Renal cell carcinoma (RCC) comprises approximately 3.8% of all new cancers, with a median age at diagnosis of 64 years.2 Approximately 85% of kidney tumors are RCC, and approximately 70% of these have a clear cell histology (ccRCC).3–5 Other less common cell types include papillary, chromophobe, translocation, and Bellini duct (collecting duct) tumors.6 Medullary renal carcinoma is a rare and aggressive RCC variant that almost exclusively arises in patients who are sicklecell trait positive.7 The histologic diagnosis of RCC is established after surgical removal of renal tumors or after biopsy.
Smoking, obesity, and hypertension are established risk factors for RCC development. Several hereditary types of RCC also exist, with von Hippel-Lindau (VHL) disease being the most common. VHL disease is caused by an autosomal-dominant constitutional mutation in the VHL gene that predisposes to ccRCC and other proliferative vascular lesions.8–11
Analysis of the SEER database indicates that RCC incidence has been rising on average 0.6% each year and death rates have been falling on average 0.7% each year from 2006 through 2015.2 The 5-year survival rate for localized RCC has increased from 88.4% (during 1992–1995) to 92.6% (during 2007–2013) and for advanced disease from 7.3% (during 1992–1995) to 11.7% (during 2007–2013).12 The most important prognostic determinants of 5-year survival are the tumor stage, grade, local extent of the tumor, presence of regional nodal metastases, and evidence of metastatic disease at presentation.13–22 RCC primarily metastasizes to the lung, bone, liver, lymph nodes, adrenal gland, and brain.9,23,24
The NCCN Guidelines for Kidney Cancer provide multidisciplinary recommendations for the clinical management of patients with ccRCC and nonclear cell RCC (nccRCC). These NCCN Guidelines are intended to assist with clinical decision-making, but they cannot incorporate all possible clinical variations and are not intended to replace good clinical judgment or individualization of treatments. Medical practitioners should note that unusual patient scenarios (presenting in <5% of patients) are not specifically discussed in these guidelines.
Management of Relapsed or Stage IV Disease
Prognostic Models for Metastatic Disease
Prognostic scoring systems have been developed to define risk groups of patients by combining independent prognostic factors for survival in patients with metastatic RCC.25,26
The first prognostic factor model to be widely applied is from Memorial Sloan Kettering Cancer Center (MSKCC). The model was derived from examining prognostic factors in patients (n=463) with metastatic RCC enrolled in clinical trials and treated with interferon.25 Prognostic factors for multivariable analysis included 5 variables: interval from diagnosis to treatment of less than 1 year; Karnofsky performance status less than 80%; serum lactate dehydrogenase greater than 1.5 times the upper limit of normal (ULN); corrected serum calcium greater than the ULN; and serum hemoglobin less than the lower limit of normal. Patients with none of these factors are considered low risk or with good prognosis, those with 1 or 2 factors present are considered intermediate risk, and patients with 3 or more of the factors are considered poor risk. The MSKCC criteria have been additionally validated by an independent group at the Cleveland Clinic.27
A prognostic model derived from a population of patients with metastatic RCC treated with vascular endothelial growth factor (VEGF)-targeted therapy has been developed and is known as the IMDC (International Metastatic RCC Database Consortium) or Heng’s model.26 This model was derived from a retrospective study of 645 patients with metastatic RCC treated with sunitinib, sorafenib, or bevacizumab plus interferon. Patients who received prior immunotherapy (ie, received their targeted therapy as second-line treatment) also were included in the analysis. The analysis identified 6 clinical parameters to stratify patients into favorable, intermediate, and poor prognosis groups. Four of the five adverse prognostic factors are those previously identified by MSKCC as independent predictors of short survival: hemoglobin less than the lower limit of normal, serum-corrected calcium greater than the ULN, Karnofsky performance score <80%, and time from initial diagnosis to initiation of therapy <1 year. Additional, independent, adverse prognostic factors validated in this model are absolute neutrophil count greater than ULN and platelets greater than ULN.26
Patients with none of the identified 6 adverse factors were in the favorable-risk category (n=133; 22.7%) in which a median overall survival (OS) was not reached and a 2-year OS was 75% (95% CI, 65%–82%). Patients with 1 or 2 adverse factors were in the intermediate-risk category (n=301; 51.4%) in which a median OS was 27 months and a 2-year OS was 53% (95% CI, 46%–59%). Finally, patients with 3 to 6 adverse factors were in the poor-risk category (n=152; 25.9%) in which a median OS was 8.8 months and a 2-year OS was 7% (95% CI, 2%–16%).26 This model was validated in an independent dataset.28
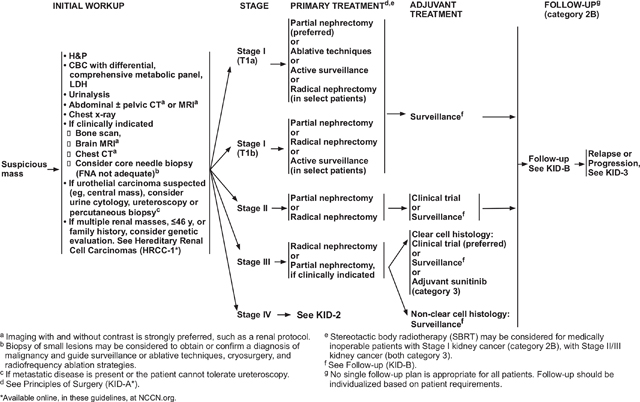
Surgical Options for Patients With Relapsed or Stage IV Disease
Patients with stage IV disease also may benefit from surgery. For example, lymph nodes suspicious for metastatic disease on CT may be hyperplastic and not involved with tumor; thus, the presence of minimal regional adenopathy does not preclude surgery.
Cytoreductive nephrectomy before systemic therapy is recommended in select patients with a potentially surgically resectable primary tumor mass. A retrospective analysis conducted in the cytokine era indicated that patients most likely to benefit from cytoreductive nephrectomy before systemic therapy were those with lung-only metastases, good prognostic features, and good performance status.29 Retrospective data from the IMDC suggested that cytoreductive nephrectomy continues to play a role in patients treated with VEGF-targeted agents.30 The efficacy of newer systemic therapies is challenging the standard in some patients with metastatic disease. Results from the CARMENA phase III trial of patients with metastatic RCC who were eligible for cytoreductive nephrectomy found that sunitinib alone was noninferior to sunitinib after nephrectomy.31 The median OS was 18.4 months in the sunitinib-alone group and 13.9 months in the sunitinib after nephrectomy group (hazard ratio [HR], 0.89; 95% CI, 0.71–1.10), which did not exceed the fixed noninferiority limit (1.20). However, many of the patients in this trial had poor-risk features, underscoring the importance of patient selection to obtain the greatest benefit from nephrectomy or targeted therapy.31,32 A posthoc analysis of the CARMENA trial reported that for patients with only one IMDC risk factor, OS was longer after nephrectomy (31.4 vs 25.2 months).33 At this point, no prospective data are available defining the role of cytoreductive nephrectomy in patients who subsequently receive checkpoint antibody therapy. Further study will better define the role of cytoreductive nephrectomy in the rapidly evolving treatment landscape for RCC.
Patients with metastatic disease who present with hematuria or other symptoms related to the primary tumor should be offered palliative nephrectomy if they are surgical candidates. In addition, the small subset of patients with potentially surgically resectable primary RCC and oligometastatic sites are candidates for nephrectomy and management of metastases by surgical metastasectomy or with ablative techniques for selected patients who are not candidates for metastasectomy. Candidates include patients who: (1) initially present with primary RCC and oligometastatic sites; or (2) develop oligometastases after a prolonged disease-free interval from nephrectomy. Oligometastatic sites that are amenable to this approach include the lung, bone, and brain. The primary tumor and the metastases may be resected during the same operation or at different times. Most patients who undergo targeted treatment of oligometastases experience recurrence, but long-term relapse-free survival has been reported in these patients.
In patients whose tumors are surgically unresectable, the NCCN Panel recommends performing tissue sampling to confirm diagnosis of RCC to determine histology and guide subsequent management. Systemic therapy is generally recommended after recurrence, cytoreductive nephrectomy in patients with multiple metastatic sites, or for patients with surgically unresectable tumors.
Patients who have undergone a nephrectomy and years later develop an oligometastatic recurrence also have the option of metastasectomy, stereotactic body radiation therapy,34–36 or ablative techniques, in addition to the first-line therapy options discussed in subsequent sections.
Systemic Therapy Options for Patients With Relapsed or Stage IV Disease
Targeted therapy utilizing tyrosine kinase inhibitors (TKIs), and/or anti-VEGF antibodies, has been widely used in first- and second-line treatments. Agents targeting the mammalian target of rapamycin (mTOR) are also used in highly selected settings. A number of targeted agents have been approved by the FDA for the treatment of advanced RCC in the first and/or subsequent lines of therapy. Immune checkpoint inhibitors provided a revolution in treatment options. Checkpoint antibodies alter the interaction between immune cells and antigen-presenting cells, including tumor cells. These agents can augment an antitumor immune response and have shown promise in a number of tumor indications.
Tumor histology and risk stratification of patients is important in therapy selection. The NCCN Guidelines for Kidney Cancer stratify treatment recommendations by histology. Recommendations for first-line treatment of ccRCC are also stratified by risk group.
NCCN Categories of Preference
To further guide management of advanced RCC, the NCCN Kidney Cancer Panel has categorized all systemic kidney cancer therapy regimens as “Preferred,” “Other Recommended Regimens,” or “Useful in Certain Circumstances.” This categorization provides guidance on treatment selection by considering the efficacy, safety, evidence, and other factors that play a role in treatment selection. These factors include pre-existing comorbidities, nature of the disease, and in some cases consideration of access to agents.
Data Tables According to Line of Treatment and RCC Histology
Due to the increasing number of NCCN-recommended systemic therapy options for metastatic RCC, the panel has organized efficacy data from key studies into tables according to RCC histology and line of treatment (when applicable) for category 1 and 2A, preferred and other recommended regimens. See the full version of the NCCN Guidelines for Kidney Cancer, including these data tables, on NCCN.org.
Information about drug mechanism of action, FDA approval, summaries of study conclusions and safety data, and Categories of Evidence and Categories of Preference for NCCN-recommended regimens is discussed subsequently, and is stratified by RCC histology, line of treatment (when applicable), and Category of Preference.
Systemic Therapy for Patients With ccRCC
ccRCC: First-Line, Preferred Regimens
Axitinib With Pembrolizumab (All Risk Groups)
Axitinib is a selective, second-generation TKI of VEGFRs, whereas pembrolizumab is a monoclonal antibody that selectively binds to programmed death-1 (PD-1; expressed on activated T cells) and blocks the interaction between PD-1 and its ligands, programmed death ligand 1 (PD-L1) and PD-L2 (both expressed on antigen-presenting cells). In April 2019, the FDA approved axitinib in combination with pembrolizumab for first-line treatment of patients with advanced RCC.37,38 Data from the randomized phase III KEYNOTE-426 trial, which included patients with favorable-, intermediate-, or poor-risk RCC, supported the combination therapy’s approval for this indication. Patients received either axitinib/pembrolizumab or sunitinib; those receiving the combination regimen had a significantly higher overall response rate (ORR) and longer progression-free survival (PFS) than those receiving sunitinib. Median OS was not reached for either group, but the HR favored axitinib/pembrolizumab.39 A subsequent exploratory analysis with a 31-month median follow-up period agreed with these data.40 Based on these data, the panel recommends first-line axitinib/pembrolizumab as a category 1, preferred option for patients with ccRCC across all risk groups.
Cabozantinib With Nivolumab (All Risk Groups)
Cabozantinib is a multitargeted TKI of VEGFRs, MET, and AXL, while nivolumab is an anti–PD-1 antibody. In January 2021, the FDA approved cabozantinib in combination with nivolumab for first-line treatment of patients with advanced RCC.41 Data from the randomized phase III CheckMate 9ER trial, which included patients with favorable-, intermediate-, or poor-risk RCC, supported the combination therapy’s approval for this indication. Patients received either cabozantinib/nivolumab or sunitinib; those receiving cabozantinib/nivolumab had significantly longer ORR and PFS than those receiving sunitinib. Median OS was not reached for either group, but the HR favored cabozantinib/nivolumab.40,42 Based on these data, the panel recommends first-line cabozantinib/nivolumab as a category 1, preferred option for patients with ccRCC across all risk groups.
Lenvatinib With Pembrolizumab (All Risk Groups)
Lenvatinib is a multitargeted TKI of VEGFR-1, -2, and -3; fibroblast growth factor receptor-1, -2, -3, and 4; platelet-derived growth factor receptor-α (PDGFR-α); c-KIT; and RET. Pembrolizumab’s mechanism of action was described previously. In August 2021, the FDA approved lenvatinib in combination with pembrolizumab for first-line treatment of patients with advanced RCC.43 Data from the randomized phase III CLEAR trial, which included patients with favorable-, intermediate-, or poor-risk RCC, supported the combination therapy’s approval for this indication. Patients received either lenvatinib/pembrolizumab, lenvatinib/everolimus, or sunitinib. Those receiving lenvatinib/pembrolizumab had significantly longer PFS and a higher ORR than those receiving sunitinib. Median OS was not reached for either group, but the HR for lenvatinib/pembrolizumab versus sunitinib favored the combination regimen. In contrast, OS was not significantly different between the lenvatinib/everolimus and sunitinib groups.44 Based on these data, the panel recommends first-line lenvatinib/pembrolizumab as a category 1, preferred treatment option for patients with ccRCC across all risk groups.
In contrast, the panel felt that the CLEAR data for lenvatinib/everolimus did not yet support the combination regimen’s inclusion in the NCCN Guidelines for Kidney Cancer.
Ipilimumab With Nivolumab (Poor-/Intermediate-Risk Groups)
Ipilimumab is a monoclonal antibody that selectively blocks the interaction between the negative regulator cytotoxic T-lymphocyte antigen 4 (CTLA-4; expressed early on activated T cells) and its ligands CD80/CD86 (expressed on antigen-presenting cells); nivolumab’s mechanism of action was described previously. In April 2018, the FDA approved ipilimumab in combination with nivolumab for first-line treatment of patients with poor-/intermediate-risk advanced RCC.45 Data from the randomized phase III CheckMate 214 trial, which supported the FDA approval, compared combination ipilimumab/nivolumab followed by nivolumab monotherapy with sunitinib monotherapy in patients with advanced RCC.46 The study’s coprimary endpoints were ORR, OS, and PFS in intermediate- and poor-risk patients only; exploratory analyses of data in favorable-risk patients were reported separately. In intermediate-/poor-risk patients, combination ipilimumab/nivolumab led to a higher ORR and complete response (CR) rate versus sunitinib monotherapy. Median PFS did not meet the prespecified threshold and was not statistically significant between the 2 treatment arms. Treatment-related adverse events occurred in 93% of patients in the ipilimumab/nivolumab group and 97% of patients in the sunitinib group; grade 3 or 4 events occurred in 46% and 63%, respectively. Adverse events led to treatment discontinuation in 22% and 12% of patients receiving ipilimumab/nivolumab and sunitinib, respectively. Treatment-related deaths occurred in 8 patients receiving the combination therapy and 4 patients receiving sunitinib. Thirty-five percent of patients who developed immune-mediated adverse events after ipilimumab/nivolumab treatment received high-dose steroids.46 Based on these data, the panel recommends first-line ipilimumab/nivolumab as a category 1, preferred treatment option for poor- and intermediate-risk patients with ccRCC.
Cabozantinib (Poor-/Intermediate-Risk Groups)
In the open-label, randomized phase II CABOSUN trial, patients with intermediate- or poor-risk advanced RCC received either cabozantinib or sunitinib. Those treated with cabozantinib showed a significantly increased median PFS and higher ORR compared with those treated with sunitinib.47 Based on these results, the panel recommends first-line cabozantinib as a category 2A, preferred treatment option for poor- and intermediate-risk patients with ccRCC.
ccRCC: First-Line, Other Recommended Regimens
Axitinib With Avelumab (All Risk Groups)
Avelumab is a monoclonal antibody that selectively binds to PD-L1; axitinib’s mechanism of action was described previously. In May 2019, the FDA approved axitinib/avelumab for first-line treatment of patients with advanced RCC. Data from the randomized phase III JAVELIN Renal 101 trial, which included patients with favorable-, intermediate-, or poor-risk RCC, supported the combination therapy’s approval for this indication.48,49 For both the overall population and PD-L1-positive patients, those receiving axitinib/avelumab had significantly longer PFS than those receiving sunitinib. This benefit was observed across all risk groups. For median OS, data were immature for all groups in both the primary48 and 13-month interim49 analyses. Based on these results, the panel added first-line axitinib/avelumab as a category 2A, other recommended regimen for patients with ccRCC across all risk groups.
Cabozantinib (Favorable-Risk Group)
Extrapolating on the CABOSUN data for poor-/intermediate-risk patients (discussed previously), the panel added first-line cabozantinib as a category 2B, other recommended regimen for favorable-risk patients with ccRCC.
Ipilimumab With Nivolumab (Favorable-Risk Group)
The CheckMate 214 trial included favorable-risk patients treated with ipilimumab/nivolumab or sunitinib. The 18-month OS in poor-/intermediate-risk patients favored ipilimumab/nivolumab over sunitinib, but an exploratory analysis of OS data from favorable-risk patients favored sunitinib over the combination regimen. ORR and median PFS were also lower in favorable-risk patients receiving ipilimumab/nivolumab than those receiving sunitinib. However, CR rates were higher in favorable-risk patients than in poor-/intermediate-risk patients, regardless of treatment regimen.46
Based on these data, the panel recommends combination first-line ipilimumab/nivolumab as a category 2A, other recommended regimen for patients with favorable-risk ccRCC. As mentioned previously, the FDA approval for ipilimumab/nivolumab is narrower, only including patients with intermediate- or poor-risk ccRCC.
Pazopanib (All Risk Groups)
Pazopanib is an oral multitargeted TKI/angiogenesis inhibitor of VEGFRs, PDGFR-α and -β, and stem cell factor receptor (c-KIT). The drug’s safety and efficacy were evaluated in an open-label phase III study. Patients with advanced ccRCC who received 0–1 prior treatments received either pazopanib or placebo. PFS was significantly longer and ORR was significantly higher with pazopanib versus placebo in the treatment-naïve subpopulation,50 but there was no difference in OS between the 2 groups.51 Notable grade 3 toxicity was hepatotoxicity, indicated by elevated levels of alanine (30%) and aspartate (21%) transaminase.50 Therefore, it is critical to monitor liver function before and during treatment with the drug.
Additionally, the COMPARZ noninferiority study of sunitinib versus pazopanib showed that these 2 drugs have similar safety and efficacy.52,53 Based on these data, the panel has listed first-line pazopanib as a category 2A, other recommended regimen for patients with ccRCC across all risk groups.
Sunitinib (All Risk Groups)
Sunitinib is a multikinase inhibitor targeting several receptor tyrosine kinases, including PDGFR-α and -β VEGFR-1, -2, and -3; c-KIT; FMS-like tyrosine kinase 3 (FLT3); colony-stimulating factor-1 receptor; and neurotrophic factor receptor (RET).54–57 The efficacy of first-line sunitinib was studied in a randomized phase III trial, in which patients with metastatic RCC received either sunitinib or IFN-α.54 Median PFS was longer in those receiving sunitinib across all risk groups. Updated results showed a strong trend toward OS advantage of sunitinib over IFN-α in the first-line setting.58 Based on these data, the panel includes first-line sunitinib as a category 2A, other recommended regimen for patients with ccRCC across all risk groups.
ccRCC: First-Line, Useful in Certain Circumstances Treatments
Active Surveillance for Select, Asymptomatic Patients With ccRCC
A subset of patients with advanced ccRCC show indolent progression of disease and could benefit from initial active surveillance because of the toxicity of systemic therapies. A phase II trial of patients with treatment-naïve, asymptomatic, metastatic RCC followed patients on active surveillance through radiographic assessment at defined intervals until a decision was made to initiate systemic therapy.59 Of the 48 patients included in the analysis, the median time of surveillance from registration to initiation of systemic therapy was 14.9 months. This study demonstrated that a subset of patients with advanced ccRCC can safely undergo active surveillance before starting systemic therapy. Therefore, the panel included active surveillance as a category 2A, useful in certain circumstances option for select, asymptomatic patients with favorable-risk ccRCC.
Axitinib (All Risk Groups)
As a second-line therapy for patients with ccRCC, axitinib treatment led to higher ORR and longer median PFS compared with sorafenib.60 In a randomized phase III trial, treatment-naïve patients received either axitinib or sorafenib; median PFS was not significantly longer in patients receiving axitinib versus sorafenib but had an acceptable toxicity profile.61 Based on these data, the panel has included first-line axitinib as a category 2B, useful in certain circumstances option for patients with ccRCC across all risk groups.
High-Dose IL-2 (All Risk Groups)
IL-2–based immunotherapy achieved long-lasting complete or partial remissions in a small subset of patients, but high-dose IL-2 is associated with substantial toxicity, and attempts to characterize tumor or patient factors for best response to this therapy have been unsuccessful.62–64 For highly selected patients with ccRCC, first-line high-dose IL-2 has been designated as useful in certain circumstances (category 2B designation for favorable-risk patients and category 3 for poor-/intermediate-risk patients).
Temsirolimus (Poor-/Intermediate-Risk Groups)
Temsirolimus is an inhibitor of the mTOR protein. The randomized, open-label phase III ARCC study enrolled previously untreated patients with advanced RCC who had 3 or more unfavorable prognostic factors.65 Patients received IFN-α alone, temsirolimus alone, or the combination of temsirolimus and IFN-α. Those who received temsirolimus alone showed improvement in OS and median PFS over those receiving IFN-α alone or combination therapy. Based on these data, the panel has included first-line temsirolimus as a category 3, useful in certain circumstances option for poor-/intermediate-risk patients with ccRCC.
ccRCC: Subsequent, Preferred Regimens
Cabozantinib
In the randomized phase III METEOR trial, patients with disease progression after previous TKI therapy receive cabozantinib or everolimus. Median PFS was significantly longer and ORR significantly higher in patients receiving cabozantinib versus everolimus.66 The final analysis of the METEOR trial showed a statistically significant increase in OS in the cabozantinib arm versus the everolimus arm.67,68
Additionally, a network meta-analysis comparing the relative effectiveness of subsequent treatment options for RCC found the probability of longer PFS during the analyzed 3 years to be higher with cabozantinib compared with everolimus, nivolumab, axitinib, sorafenib, and best supportive care.69 Based on these data, the Panel has included cabozantinib as a category 1, preferred subsequent therapy option for patients with ccRCC.
Lenvatinib With Everolimus
In May 2016, the FDA approved lenvatinib, a multitargeted kinase inhibitor, in combination with everolimus, an mTOR inhibitor, for treating advanced RCC after 1 prior antiangiogenic therapy.70,71 In a randomized phase II trial, patients with metastatic or unresectable, locally advanced ccRCC who had received prior antiangiogenic therapy received either combination lenvatinib/everolimus, single-agent lenvatinib, or single-agent everolimus. PFS and median OS were significantly longer in patients receiving lenvatinib/everolimus versus everolimus monotherapy.72,73 Based on these data, the Panel considers lenvatinib/everolimus a category 1, preferred subsequent therapy option for patients with ccRCC.
Nivolumab
In the randomized phase III CheckMate 025 trial, patients with advanced ccRCC, who were previously treated with one or more lines of therapy (excluding mTOR inhibitors) received either nivolumab or everolimus. Patients receiving nivolumab had significantly longer OS and significantly higher ORR than those receiving everolimus.74 An independent analysis was performed to determine the efficacy of nivolumab-based baseline factors such as number and location of metastases, risk group, number of prior therapies, and specific prior therapies (ie, sunitinib, pazopanib, IL-2); a consistent OS benefit and ORR were observed across all baseline factors.75 Based on these data, the panel has included nivolumab as a category 1, preferred subsequent therapy option for patients with ccRCC.
ccRCC: Subsequent, Other Recommended Regimens
Axitinib
The randomized phase III AXIS study compared second-line axitinib versus sorafenib. Median PFS was significantly longer and ORR significantly higher in patients receiving axitinib versus sorafenib.60 Updated AXIS results showed that while OS did not significantly differ between the 2 groups, patients receiving axitinib had a continued improvement in PFS.76 Based on these data, the panel included axitinib as a category 1, other recommended subsequent therapy option for patients with ccRCC.
Axitinib With Pembrolizumab
Upon axitinib/pembrolizumab’s FDA approval in a first-line setting,37,38 the panel discussed whether the combination therapy might be used in clinical practice as an off-label subsequent treatment option in patients with relapsed or stage IV ccRCC. Although they conceded that no published data were available to support the use of axitinib/pembrolizumab in a second-line setting, they thought that clinicians were likely to consider the combination as a treatment option in patients with advanced ccRCC whose disease progressed after first-line sunitinib therapy. Thus, the panel added axitinib/pembrolizumab as a category 2A, other recommended subsequent therapy option for patients with ccRCC.
Cabozantinib With Nivolumab
In 2020, Apolo et al77 published data from an ongoing phase I dose escalation trial (ClinicalTrials.gov identifier: NCT02496208) in which patients with metastatic urothelial carcinoma or other genitourinary tumors (including 3 patients with ccRCC) received combination cabozantinib/nivolumab with or without ipilimumab; data from patients with ccRCC were not reported separately. In 2021, a conference abstract78 reported a pooled analysis of the phase I dose-finding cohort and 7 subsequent expansion cohorts, which included 16 patients with metastatic RCC. In these patients, median OS was 38.6 months (95% CI, 19.4–not estimable [NE]). Based on these data, the panel considers cabozantinib/nivolumab a category 2A, other recommended subsequent therapy option for patients with ccRCC.
Ipilimumab With Nivolumab
The phase I CheckMate 016 trial included treatment-naïve patients and those who had received 1 to 4 or more prior treatment regimens. Only the ORR results were stratified by treatment status: ORR in the N3I1 and N1I3 was approximately 46% and 39%, respectively. OS and PFS data were not stratified by treatment line, but were similar.79 Based on these data, the panel considers ipilimumab/nivolumab a category 2A, other recommended subsequent therapy option for patients with ccRCC.
Lenvatinib With Pembrolizumab
The ongoing phase II KEYNOTE-146 trial included 3 groups of patients: treatment-naïve, those who had previously received at least one line of treatment that did not include anti–PD-1 or anti–PD-L1 immune checkpoint inhibitors, and those who had previously received at least I anti–PD-1 or anti–PD-L1 immune checkpoint inhibitor. Treatment-naïve patients had the highest ORR and the longest PFS; ORR and PFS were comparable in the ICI-naïve and ICI treatment-experienced groups. Median OS was only met in the ICI-naive group.80 Based on these data, the panel considers lenvatinib/pembrolizumab a category 2A, other recommended subsequent therapy option for patients with ccRCC.
Pazopanib
A phase III trial comparing pazopanib with placebo (detailed in the section, “ccRCC: First-line, Other Recommended Regimens”) also included patients who had received prior cytokine therapy. PFS was significantly longer with pazopanib versus placebo in the treatment-experienced subpopulation,50 but OS was similar between the 2 groups.51 Additionally, a prospective phase II trial evaluated second-line pazopanib in patients with advanced metastatic RCC previously treated with a targeted agent (ie, bevacizumab, sunitinib). Twenty-seven percent of patients had an objective response to pazopanib; 49% had stable disease (SD). Median PFS was 7.5 months, regardless of prior treatment regimen. Estimated OS rate at 24 months was 43%.81 Based on these data, the panel considers pazopanib a category 2A, other recommended subsequent therapy option for patients with ccRCC.
Sunitinib
Sunitinib also has shown substantial antitumor activity as a second-line therapy in patients with metastatic RCC who progressed on cytokine therapy.55,82 Studies investigating the sequential use of sunitinib and sorafenib are mostly retrospective. There are limited prospective data that suggest a lack of total cross-resistance between TKIs, either sorafenib followed by sunitinib failures or vice versa—an observation that is consistent with their differences in target specificities and slightly different toxicity spectra that sometimes permit tolerance of one agent over another.83–87 Sunitinib is considered a category 2A, other recommended subsequent therapy option for patients with ccRCC.
Tivozanib
In March 2021, the FDA approved tivozanib, a multitargeted TKI, for patients with relapsed or refractory advanced RCC who previously received 2 or more systemic therapies.88 Data from the randomized phase III TIVO-3 trial, which enrolled treatment-experienced patients with relapsed or refractory advanced ccRCC, supported the drug’s approval. Patients receiving tivozanib had significantly longer PFS than those receiving sorafenib; OS was similar between the 2 groups.89 Based on these data, the panel considers tivozanib as a category 2A, other recommended subsequent therapy option for patients with ccRCC.
Axitinib With Avelumab
Extrapolating on the first-line JAVELIN Renal 101 data for poor-/intermediate-risk patients (see “First-line, Other Recommended Regimens,” page 79), the panel added axitinib/avelumab as a category 3, other recommended subsequent therapy option for patients with ccRCC.
ccRCC: Subsequent, Useful In Certain Circumstances Regimens
Everolimus
Everolimus is an orally administered mTOR inhibitor. In the randomized phase III RECORD-1 trial, everolimus was compared with placebo for the treatment of metastatic RCC in patients whose disease had progressed on treatment with sunitinib or sorafenib. The median PFS was significantly longer for everolimus versus placebo, but OS was similar between the 2 groups.90,91 Everolimus is listed as a category 2A, useful in certain circumstances subsequent therapy option for patients with ccRCC.
Bevacizumab
Phase II trials have shown benefit of bevacizumab monotherapy after prior treatment with a cytokine.92 Bevacizumab is a category 2B, useful in certain circumstances subsequent therapy option for patients with ccRCC.
High-Dose IL-2 (For Selected Patients)
High-dose IL-2 is listed as a category 2B, useful in certain circumstances subsequent therapy option for selected patients with excellent performance status and normal organ function.
Sorafenib
Sorafenib tosylate is a small molecule that inhibits multiple isoforms of the intracellular serine/threonine kinase, RAF, and other receptor tyrosine kinases, including VEGFR-1, -2, and -3; PDGFR-β; FLT3; c-KIT; and RET.93–97 Efficacy of sorafenib was studied in the randomized phase III TARGET trial, which enrolled patients with ccRCC who progressed on a prior therapy (mostly cytokines). Sorafenib-treated patients had significantly longer OS and PFS than those receiving placebo.98,99 Sorafenib is listed as a category 3, useful in certain circumstances subsequent therapy option for patients with ccRCC.
Temsirolimus
The randomized phase III INTORSECT trial compared the efficacy of temsirolimus to sorafenib after first-line sunitinib as a treatment for patients with ccRCC or nccRCC. Although a significant OS advantage was seen for sorafenib, PFS was similar between the 2 groups.100 The panel considers temsirolimus a category 2B, useful in certain circumstances subsequent therapy option for patients with ccRCC.
Systemic Therapy for Patients With nccRCC
Clinical trials of targeted agents have predominantly focused on patients with ccRCC due to the high prevalence of ccRCC.101 Data from systematic reviews, meta-analyses, and phase II studies with targeted agents also show some activity in patients with nccRCC. Compared with responses in ccRCC, however, the response rates with these agents are significantly lower for nccRCC. Therefore, according to the panel, enrollment in clinical trials is the preferred strategy for patients with nccRCC.
nccRCC: Preferred Regimens
Cabozantinib
The randomized phase II SWOG 1500 trial compared the MET-targeted TKIs cabozantinib, crizotinib, and savolitinib with standard-of-care sunitinib in patients with advanced papillary RCC who had previously received up to 1 previous systemic therapy, excluding VEGF- and MET-targeted TKIs. Assignment to the crizotinib and savolitinib arms was halted due to results of a prespecified futility analysis.102 Patients receiving cabozantinib had significantly longer PFS and a higher ORR than those receiving sunitinib. Based on these data, the panel included cabozantinib as a category 2A, preferred option for patients with nccRCC.
Sunitinib
Two recent randomized phase II studies compared first-line sunitinib with first-line everolimus in patients with nccRCC. Although data from the ASPEN trial103 suggested that patients receiving sunitinib had significantly longer PFS than those receiving everolimus, data from the ESPN trial104 suggested that both OS and PFS were similar between the 2 groups.
Additionally, a meta-analysis of randomized clinical trials for patients with nccRCC found that TKI treatment reduced the risk of progression compared with mTOR inhibitors.105 The study found that sunitinib significantly reduced the risk of progression compared with everolimus in the first-line setting. However, no significant differences between TKIs and mTOR inhibitor treatment were found for OS and ORR. Based on these data, sunitinib is listed as a category 2A, preferred option for patients with nccRCC.
nccRCC: Other Recommended Regimens
Lenvatinib With Everolimus
Extrapolating on data from the phase III lenvatinib/everolimus trial in patients with ccRCC72 (see “ccRCC: Subsequent, Preferred Regimens,” page 81), the panel added the combination regimen as a category 2A, other recommended regimen for patients with nccRCC.
They also reviewed data106 from an ongoing singlearm phase II trial (ClinicalTrials.gov identifier: NCT02915783) enrolling patients with unresectable advanced or metastatic nccRCC who had not previously received prior systemic therapy; all patients in the trial received combination lenvatinib/everolimus. Authors reported that ORR was 26% (95% CI, 12–45). Eight patients in the trial experienced a partial response (PR; papillary, n=3; chromophobe, n=4; unclassified, n=1); no patients had a CR. The median duration of response was NE. Eighteen patients (58.1%) had SD, and the clinical benefit rate (CR + PR + durable SD [duration ≥23 weeks]) was 61% (95% CI, 42–78). The median PFS was 9.2 months (95% CI, 5.5–NE) and OS was 15.6 months (95% CI, 9.2–NE). While the panel conceded that the number of enrolled patients was small, they generally felt that lenvatinib/everolimus treatment led to improved patient outcomes across all nccRCC subtypes.
Nivolumab
A retrospective analysis evaluated the response to at least one dose of nivolumab in patients with metastatic nccRCC.107 This study evaluated 35 patients for response and found that 20% had a PR and 29% had SD, with a median follow-up of 8.5 months and median PFS of 3.5 months. A separate retrospective analysis found modest responses with PD-1/PD-L1 inhibitors in 43 patients also with metastatic nccRCC.108 An objective response was achieved in 8 patients (19%), including 4 patients (13%) who received PD-1/PD-L1 monotherapy. Based on these data, the Panel considers nivolumab a category 2A, other recommended regimen for patients with nccRCC.
Pembrolizumab
Cohort B of the phase II KEYNOTE-427 study assessed the efficacy and safety of pembrolizumab monotherapy in 165 patients with systemic therapy-naïve, newly diagnosed or recurrent stage IV nccRCC.109 The majority (about 72%) of patients had confirmed papillary RCC, about 13% had chromophobe RCC, and about 16% had unclassified RCC histology. ORR across all subtypes was approximately 27% (ORR by histology was 29% for papillary, 10% for chromophobe, and 31% for unclassified). Overall PFS and OS were 4.2 months and 28.9 months, respectively. Based on these data, the panel added pembrolizumab as a category 2A, other recommended regimen for patients with nccRCC.
nccRCC: Useful in Certain Circumstances Regimens
Axitinib
A phase II trial of axitinib in 40 patients with recurrent or metastatic nccRCC that failed treatment with temsirolimus found a median PFS of 7.4 months and ORR of 37.5%.110 The panel considers axitinib a category 2A, useful in certain circumstances option for patients with nccRCC.
Bevacizumab
A small phase II trial studied bevacizumab monotherapy in patients with papillary RCC. The PFS reported for each of these patients was 25, 15, 11, 10, and 6 months.111 The panel has included bevacizumab as a category 2A, useful in certain circumstances option for patients with nccRCC.
Bevacizumab With Erlotinib for Advanced Papillary RCC, including Hereditary Leiomyomatosis and RCC-Associated RCC
Hereditary leiomyomatosis and RCC (HLRCC) is a hereditary condition in which affected patients are at risk for development of skin and uterine leiomyomas, as well as an aggressive form of papillary kidney cancer.112 Bevacizumab in combination with either erlotinib or everolimus is currently being investigated for treatment of advanced papillary RCC, including HLRCC.
An abstract detailed the results of a phase II trial of patients with advanced papillary RCC (HLRCC-associated RCC; n=42 or sporadic papillary RCC; n=41) treated with bevacizumab plus erlotinib.113 All enrolled patients received 2 or fewer VEGFR TKIs; 27 (33%) had at least one prior treatment. Most patients had intermediate-risk disease. The ORR was 64% for those with HLRCC compared with 37% with sporadic papillary RCC. Median PFS was 21.1 months in the HLRCC group compared with 8.7 months in the sporadic papillary RCC group.113 Based on these data, the panel recommends bevacizumab plus erlotinib as a category 2A, useful in certain circumstances option for select patients with nccRCC and papillary histology, including HLRCC.
Bevacizumab With Everolimus
A phase II trial of 34 treatment-naïve patients with metastatic nccRCC studied the efficacy and safety of treatment with bevacizumab plus everolimus.114 Median PFS, OS, and ORR were 11.0 months, 18.5 months, and 29%, respectively. Patients with tumors that contained appreciable papillary or chromophobe elements showed significantly higher PFS and ORR than other histologies.115 Based on these data, the panel recommends bevacizumab plus everolimus as a category 2A, useful in certain circumstances option for patients with nccRCC.
Erlotinib
The efficacy of erlotinib, an oral epidermal growth factor receptor (EGFR) TKI, was studied in 52 patients with advanced papillary RCC.116 ORR was 11% (5 of 45 patients; 95% CI, 3%–24%), and the disease control rate (defined as SD for 6 weeks, or confirmed PR or CR using RECIST) was 64%. Median OS was 27 months.116 Based on these data, the panel has included erlotinib as a category 2A, useful in certain circumstances option for patients with nccRCC.
Everolimus
The efficacy and safety of everolimus in patients with metastatic nccRCC were evaluated in a subgroup of 75 patients enrolled in the REACT trial. ORR and rate of SD were similar between patients with ccRCC and nccRCC.117 In a phase II study of treatment-experienced patients with nccRCC,118 OS was 14 months and PFS was 5.2 months. According to data from the phase II RAPTOR trial,119 OS and PFS ranged from 24 to 28 months and PFS ranged from 5 to 8 months; patients with type 1 nccRCC had better responses than those with type 2 histology. Based on these data, the panel included everolimus as a category 2A, useful in certain circumstances option for patients with nccRCC.
Pazopanib
In a Korean phase II trial of pazopanib in 28 patients with locally advanced or metastatic nccRCC, 8 patients experienced a confirmed PR with an ORR of 28%.120 A retrospective analysis of an Italian multicenter cohort of nccRCC patients found treatment with pazopanib to be effective and safe.115 Based on these data, the panel considers pazopanib a category 2A, useful in certain circumstances option for patients with nccRCC. A clinical trial evaluating the efficacy of second-line pazopanib in patients with nccRCC is ongoing.121
Temsirolimus
A retrospective subset analysis of the global phase III ARCC trial demonstrated benefit of temsirolimus not only in ccRCC but also in nccRCC.65 122 In patients with nccRCC (predominantly papillary RCC), the median OS was 11.6 months with temsirolimus and 4.3 months with IFN-α. Randomized clinical trials in rarer subgroups of patients are often challenging. Consistent with the results of the ARCC trial, a case report of a patient with a diagnosis of metastatic chromophobe RCC that was refractory to treatment with sunitinib achieved durable clinical response lasting 20 months on treatment with temsirolimus.123 Temsirolimus is a useful in certain circumstances option for nccRCC; it has a category 1 designation for poor-risk patients and a category 2A designation for favorable-/intermediate-risk patients.
Additional Treatment Options for Rare Types of nccRCC
Among the nccRCC histologies, renal medullary carcinoma is extremely rare, comprising approximately 2% of all primary renal tumors in young people.124,125 Metastatic disease is seen at presentation in 67%–95% of patients.124–126 Chemotherapy remains the focus of treatment for this subtype, although the prognosis remains dismal.
Collecting-duct carcinoma is also a very rare type of nccRCC, often presenting at an advanced stage of disease. Up to 40% of patients have metastatic spread at initial presentation, and most patients die within 1 to 3 years of primary diagnosis.127–130 Collecting duct carcinoma shares biologic features with urothelial carcinoma. In a multicenter prospective study, 23 patients with no prior therapy were treated with a combination of gemcitabine and either cisplatin or carboplatin.131 The results showed a response rate of 26% and an OS of 10.5 months.131
The NCCN Kidney Cancer Panel notes that in patients with other nccRCC subtypes such as collecting duct or medullary subtypes, PRs to cytotoxic chemotherapy have been observed (gemcitabine in combination with carboplatin or cisplatin; or paclitaxel with carboplatin) as well as for other platinum-based chemotherapies currently used for urothelial carcinomas.126,132 Oral targeted therapies generally do not produce responses in patients with renal medullary carcinoma. Outside of clinical trials, platinum-based chemotherapy regimens should be the preferred therapy for renal medullary carcinoma.
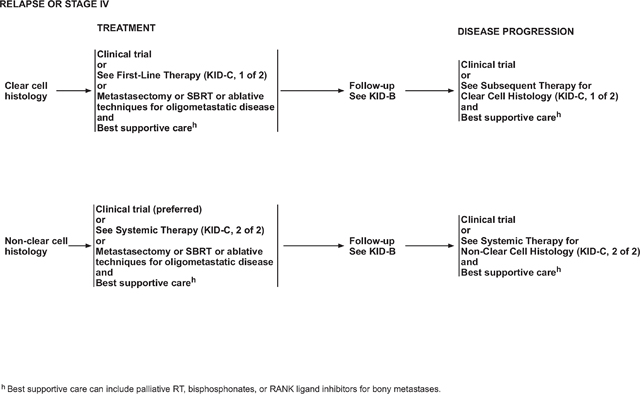
Follow-up Recommendations for Relapsed or Stage IV Disease and Surgically Unresectable Disease
The NCCN Panel recommends a history and physical examination every 6 to 16 weeks for patients receiving systemic therapy, or more frequently as clinically indicated. Other laboratory evaluations may be performed as per the requirements for the therapeutic agent being used.
Imaging tests such as CT or MRI should be performed before starting systemic treatment/observation; subsequent imaging may be performed every 6 to 16 weeks as per physician discretion and patient’s clinical status. Imaging interval frequency should be altered according to rate of disease change and sites of active disease. The panel recommends additional imaging such as CT or MRI of the head or spine, and bone scan at baseline and then as clinically indicated.
Supportive Care
Supportive care remains a mainstay of therapy for all patients with metastatic RCC (See NCCN Guidelines for Palliative Care on NCCN.org). This includes surgery for patients with oligometastatic disease in the brain whose disease is well-controlled extracranially. Stereotactic radiotherapy, if available, is an alternative to surgery for limited-volume brain metastasis, and whole brain irradiation is recommended for those patients with multiple brain metastases.133
Surgery also may be appropriate for selected patients with malignant spinal cord compression, or impending or actual fractures in weight-bearing bones, if the rest of the disease burden is limited or patients remain symptomatic. Also, radiation therapy along with bisphosphonates is considered for palliation, particularly for painful bone metastases. The frequency of clinic visits or radiographic and laboratory assessments depends on the individual needs of the patient.
Bone metastasis occurs in 30%–40% of patients with advanced RCC.134–136 Bone lesions in patients with RCC are typically osteolytic and cause considerable morbidity, leading to skeletal-related events, including bone pain with need for surgery or radiotherapy, hypercalcemia, pathologic fractures, and spinal cord compression. Two studies of patients with bone metastases showed an improvement in bone pain using different radiotherapy modalities.137,138
The role of bone-modifying agents such as bisphosphonates (eg, zoledronic acid) has been established in patients with various malignancies.139,140 The newer bone-modifying agent approved for use in patients with RCC that has metastasized to the bone is the RANK-L inhibitor, denosumab. A phase III randomized trial directly compared the development of SREs on either denosumab or zoledronic acid in patients with multiple myeloma or bone metastases with a solid tumor (excluding breast or prostate cancer). The study enrolled 1776 patients with bone metastases from a wide range of cancer types, including patients with RCC (6%) not previously treated with a bisphosphonate.141 Denosumab was reported to be noninferior to zoledronic acid in delaying time to first on-study SRE (HR, 0.84; 95% CI, 0.71–0.98; P=.0007).141
The panel recommends a bisphosphonate or a RANK ligand inhibitor for selected patients with bony metastases and creatinine clearance ≥30 mL/min. Daily supplemental calcium and vitamin D are strongly recommended. Treatment for palliation of symptoms, especially in patients with marginal performance status and evidence of metastatic disease, includes optimal pain management (See NCCN Guidelines for Adult Cancer Pain on NCCN.org).
Supplementary Material
NCCN CATEGORIES OF EVIDENCE AND CONSENSUS.
Category 1: Based upon high-level evidence, there is uniform NCCN consensus that the intervention is appropriate. Category 2A: Based upon lower-level evidence, there is uniform NCCN consensus that the intervention is appropriate.
Category 2B: Based upon lower-level evidence, there is NCCN consensus that the intervention is appropriate. Category 3: Based upon any level of evidence, there is major NCCN disagreement that the intervention is appropriate.
All recommendations are category 2A unless otherwise noted.
Clinical trials: NCCN believes that the best management of any patient with cancer is in a clinical trial. Participation in clinical trials is especially encouraged.






Footnotes
PLEASE NOTE
The NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) are a statement of evidence and consensus of the authors regarding their views of currently accepted approaches to treatment. Any clinician seeking to apply or consult the NCCN Guidelines is expected to use independent medical judgment in the context of individual clinical circumstances to determine any patient’s care or treatment. The National Comprehensive Cancer Network® (NCCN®) makes no representations or warranties of any kind regarding their content, use, or application and disclaims any responsibility for their application or use in any way.
The complete NCCN Guidelines for Kidney Cancer are not printed in this issue of JNCCN but can be accessed online at NCCN.org.
© National Comprehensive Cancer Network, Inc. 2022. All rights reserved. The NCCN Guidelines and the illustrations herein may not be reproduced in any form without the express written permission of NCCN.
Disclosures for the NCCN Kidney Cancer Panel
At the beginning of each NCCN Guidelines Panel meeting, panel members review all potential conflicts of interest. NCCN, in keeping with its commitment to public transparency, publishes these disclosures for panel members, staff, and NCCN itself.
Individual disclosures for the NCCN Kidney Cancer Panel members can be found on page 90. (The most recent version of these guidelines and accompanying disclosures are available at NCCN.org.)
The complete and most recent version of these guidelines is available free of charge at NCCN.org.
References
- 1.Siegel RL, Miller KD, Fuchs HE, et al. Cancer Statistics, 2021. CA Cancer J Clin 2021;71:7–33. [DOI] [PubMed] [Google Scholar]
- 2.Cancer Stat Facts SEER. Kidney and Renal Pelvis Cancer. Bethesda, MD: National Cancer Institute. Accessed June 28, 2019. Available at: http://seer.cancer.gov/statfacts/html/kidrp.html. [Google Scholar]
- 3.Moch H, Gasser T, Amin MB, et al. Prognostic utility of the recently recommended histologic classification and revised TNM staging system of renal cell carcinoma: a Swiss experience with 588 tumors. Cancer 2000;89:604–614. [PubMed] [Google Scholar]
- 4.Leibovich BC, Lohse CM, Crispen PL, et al. Histological subtype is an independent predictor of outcome for patients with renal cell carcinoma. J Urol 2010;183:1309–1315. [DOI] [PubMed] [Google Scholar]
- 5.Lipworth L, Morgans AK, Edwards TL, et al. Renal cell cancer histological subtype distribution differs by race and sex. BJU Int 2016;117:260–265. [DOI] [PubMed] [Google Scholar]
- 6.Pathology and genetics of tumours of the urinary system and male genital organs. In: World Health Organization Classification of Tumours. Lyon, France: IARC press;2004. [Google Scholar]
- 7.Msaouel P, Hong AL, Mullen EA, et al. Updated recommendations on the diagnosis, management, and clinical trial eligibility criteria for patients with renal medullary carcinoma. Clin Genitourin Cancer 2019;17:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choyke PL, Glenn GM, Walther MM, et al. Hereditary renal cancers. Radiology 2003;226:33–46. [DOI] [PubMed] [Google Scholar]
- 9.DeVita VT Jr., Lawrence TS, Rosenberg SA. DeVita, Hellman, and Rosenberg’s Cancer: Principles and Practice of Oncology, et al. Ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2008. [Google Scholar]
- 10.Schmidt LS, Linehan WM. Genetic predisposition to kidney cancer. Semin Oncol 2016;43:566–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DeVita VT Jr., Lawrence TS, Rosenberg SA. DeVita, Hellman, and Rosenberg’s Cancer: Principles and Practice of Oncology, 10th Ed. Philadelphia, PA: Wolters Kluwer Health; 2015. [Google Scholar]
- 12.Howlader N, Noone A, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2014, based on November 2016 SEER data submission, posted to the SEER web site, April 2017. National Cancer Institute, Bethesda, MD. 2017. Available at https://seer.cancer.gov/csr/1975_2014/ [Google Scholar]
- 13.Ficarra V, Schips L, Guillè F, et al. Multiinstitutional European validation of the 2002 TNM staging system in conventional and papillary localized renal cell carcinoma. Cancer 2005;104:968–974. [DOI] [PubMed] [Google Scholar]
- 14.Frank I, Blute ML, Leibovich BC, et al. Independent validation of the 2002 American Joint Committee on cancer primary tumor classification for renal cell carcinoma using a large, single institution cohort. J Urol 2005;173:1889–1892. [DOI] [PubMed] [Google Scholar]
- 15.Zisman A, Pantuck AJ, Chao D, et al. Reevaluation of the 1997 TNM classification for renal cell carcinoma: T1 and T2 cutoff point at 4.5 rather than 7 cm. better correlates with clinical outcome. J Urol 2001;166:54–58. [PubMed] [Google Scholar]
- 16.Klatte T, Patard JJ, Goel RH, et al. Prognostic impact of tumor size on pT2 renal cell carcinoma: an international multicenter experience. J Urol 2007;178:35–40., discussion 40. [DOI] [PubMed] [Google Scholar]
- 17.Lam JS, Klatte T, Patard JJ, et al. Prognostic relevance of tumour size in T3a renal cell carcinoma: a multicentre experience. Eur Urol 2007;52:155–162. [DOI] [PubMed] [Google Scholar]
- 18.Minervini A, Lilas L, Minervini R, et al. Prognostic value of nuclear grading in patients with intracapsular (pT1-pT2) renal cell carcinoma. Long-term analysis in 213 patients. Cancer 2002;94:2590–2595. [DOI] [PubMed] [Google Scholar]
- 19.Dall’Oglio MF, Antunes AA, Sarkis AS, et al. Microvascular tumour invasion in renal cell carcinoma: the most important prognostic factor. BJU Int 2007;100:552–555. [DOI] [PubMed] [Google Scholar]
- 20.Dall’Oglio MF, Ribeiro-Filho LA, Antunes AA, et al. Microvascular tumor invasion, tumor size and Fuhrman grade: a pathological triad for prognostic evaluation of renal cell carcinoma. J Urol 2007;178:425–428., discussion 428. [DOI] [PubMed] [Google Scholar]
- 21.Lam JS, Shvarts O, Said JW, et al. Clinicopathologic and molecular correlations of necrosis in the primary tumor of patients with renal cell carcinoma. Cancer 2005;103:2517–2525. [DOI] [PubMed] [Google Scholar]
- 22.Sengupta S, Lohse CM, Leibovich BC, et al. Histologic coagulative tumor necrosis as a prognostic indicator of renal cell carcinoma aggressiveness. Cancer 2005;104:511–520. [DOI] [PubMed] [Google Scholar]
- 23.Bianchi M, Sun M, Jeldres C, et al. Distribution of metastatic sites in renal cell carcinoma: a population-based analysis. Ann Oncol 2012;23:973–980. [DOI] [PubMed] [Google Scholar]
- 24.Meyer CP, Sun M, Karam JA, et al. Complications after metastasectomy for renal cell carcinoma: a population-based assessment. Eur Urol 2017;72:171–174. [DOI] [PubMed] [Google Scholar]
- 25.Motzer RJ, Bacik J, Murphy BA, et al. Interferon-alfa as a comparative treatment for clinical trials of new therapies against advanced renal cell carcinoma. J Clin Oncol 2002;20:289–296. [DOI] [PubMed] [Google Scholar]
- 26.Heng DY, Xie W, Regan MM, et al. Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor-targeted agents: results from a large, multicenter study. J Clin Oncol 2009;27:5794–5799. [DOI] [PubMed] [Google Scholar]
- 27.Mekhail TM, Abou-Jawde RM, Boumerhi G, et al. Validation and extension of the Memorial Sloan-Kettering prognostic factors model for survival in patients with previously untreated metastatic renal cell carcinoma. J Clin Oncol 2005;23:832–841. [DOI] [PubMed] [Google Scholar]
- 28.Heng DY, Xie W, Regan MM, et al. External validation and comparison with other models of the International Metastatic Renal-Cell Carcinoma Database Consortium prognostic model: a population-based study. Lancet Oncol 2013;14:141–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Culp SH, Tannir NM, Abel EJ, et al. Can we better select patients with metastatic renal cell carcinoma for cytoreductive nephrectomy? Cancer 2010;116:3378–3388. [DOI] [PubMed] [Google Scholar]
- 30.Choueiri TK, Xie W, Kollmannsberger C, et al. The impact of cytoreductive nephrectomy on survival of patients with metastatic renal cell carcinoma receiving vascular endothelial growth factor targeted therapy. J Urol 2011;185:60–66. [DOI] [PubMed] [Google Scholar]
- 31.Méjean A, Ravaud A, Thezenas S, et al. Sunitinib alone or after nephrectomy in metastatic renal-cell carcinoma. N Engl J Med 2018;379:417–427. [DOI] [PubMed] [Google Scholar]
- 32.Motzer RJ, Russo P. Cytoreductive nephrectomy - patient selection is key. N Engl J Med 2018;379:481–482. [DOI] [PubMed] [Google Scholar]
- 33.M_ejean A, Ravaud A, Thezenas S, et al. Sunitinib alone or after nephrectomy for patients with metastatic renal cell carcinoma: is there still a role for cytoreductive nephrectomy? Eur Urol 2021;80:417–424. [DOI] [PubMed] [Google Scholar]
- 34.Siva S, Ellis RJ, Ponsky L, et al. Consensus statement from the International Radiosurgery Oncology Consortium for Kidney for primary renal cell carcinoma. Future Oncol 2016;12:637–645. [DOI] [PubMed] [Google Scholar]
- 35.Siva S, Louie AV, Warner A, et al. Pooled analysis of stereotactic ablative radiotherapy for primary renal cell carcinoma: A report from the International Radiosurgery Oncology Consortium for Kidney (IROCK). Cancer 2018;124:934–942. [DOI] [PubMed] [Google Scholar]
- 36.Meyer E, Pasquier D, Bernadou G, et al. Stereotactic radiation therapy in the strategy of treatment of metastatic renal cell carcinoma: a study of the Getug group. Eur J Cancer 2018;98:38–47. [DOI] [PubMed] [Google Scholar]
- 37.Food and Drug Administration. FDA approves pembrolizumab plus axitinib for advanced renal cell carcinoma. Accessed December 6, 2021. Available at: https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-pembrolizumab-plus-axitinib-advanced-renal-cell-carcinoma
- 38.Pembrolizumab (KEYTRUDA) prescribing information. Accessed November 4, 2021. Available at: https://www.merck.com/product/usa/pi_circulars/k/keytruda/keytruda_pi.pdf
- 39.Rini BI, Plimack ER, Stus V, et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med 2019;380:1116–1127. [DOI] [PubMed] [Google Scholar]
- 40.Powles T, Plimack ER, Soulières D, et al. Pembrolizumab plus axitinib versus sunitinib monotherapy as first-line treatment of advanced renal cell carcinoma (KEYNOTE-426): extended follow-up from a randomised, open-label, phase 3 trial. Lancet Oncol 2020;21:1563–1573 [DOI] [PubMed] [Google Scholar]
- 41.FDA approves nivolumab plus cabozantinib for advanced renal cell carcinoma. Accessed November 4, 2021. Available at: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-nivolumab-plus-cabozantinib-advanced-renal-cell-carcinoma
- 42.Choueiri TK, Powles T, Burotto M, et al. Nivolumab plus cabozantinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med 2021;384:829–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.FDA approves lenvatinib plus pembrolizumab for advanced renal cell carcinoma. Accessed November 4, 2021. Available at: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-lenvatinib-plus-pembrolizumab-advanced-renal-cell-carcinoma
- 44.Motzer R, Alekseev B, Rha SY, et al. Lenvatinib plus pembrolizumab or everolimus for advanced renal cell carcinoma. N Engl J Med 2021;384:1289–1300. [DOI] [PubMed] [Google Scholar]
- 45.FDA approves nivolumab plus ipilimumab combination for intermediate or poor-risk advanced renal cell carcinoma. Accessed November 4, 2021. Available at: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-nivolumab-plus-ipilimumab-combination-intermediate-or-poor-risk-advanced-renal-cell
- 46.Motzer RJ, Tannir NM, McDermott DF, et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med 2018;378:1277–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Choueiri TK, Halabi S, Sanford BL, et al. Cabozantinib versus sunitinib as initial targeted therapy for patients with metastatic renal cell carcinoma of poor or intermediate risk: the Alliance A031203 CABOSUN Trial. J Clin Oncol 2017;35:591–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Motzer RJ, Penkov K, Haanen J, et al. Avelumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med 2019;380:1103–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Choueiri TK, Motzer RJ, Rini BI, et al. Updated efficacy results from the JAVELIN Renal 101 trial: first-line avelumab plus axitinib versus sunitinib in patients with advanced renal cell carcinoma. Ann Oncol 2020;31:1030–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sternberg CN, Davis ID, Mardiak J, et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. J Clin Oncol 2010;28:1061–1068. [DOI] [PubMed] [Google Scholar]
- 51.Sternberg CN, Hawkins RE, Wagstaff J, et al. A randomised, double-blind phase III study of pazopanib in patients with advanced and/or metastatic renal cell carcinoma: final overall survival results and safety update. Eur J Cancer 2013;49:1287–1296. [DOI] [PubMed] [Google Scholar]
- 52.Motzer RJ, Hutson TE, Cella D, et al. Pazopanib versus sunitinib in metastatic renal-cell carcinoma. N Engl J Med 2013;369:722–731. [DOI] [PubMed] [Google Scholar]
- 53.Motzer RJ, Hutson TE, McCann L, et al. Overall survival in renal-cell carcinoma with pazopanib versus sunitinib. N Engl J Med 2014;370:1769–1770. [DOI] [PubMed] [Google Scholar]
- 54.Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med 2007;356:115–124. [DOI] [PubMed] [Google Scholar]
- 55.Motzer RJ, Michaelson MD, Redman BG, et al. Activity of SU11248, a multitargeted inhibitor of vascular endothelial growth factor receptor and platelet-derived growth factor receptor, in patients with metastatic renal cell carcinoma. J Clin Oncol 2006;24:16–24. [DOI] [PubMed] [Google Scholar]
- 56.Chow LQ, Eckhardt SG. Sunitinib: from rational design to clinical efficacy. J Clin Oncol 2007;25:884–896. [DOI] [PubMed] [Google Scholar]
- 57.Faivre S, Delbaldo C, Vera K, et al. Safety, pharmacokinetic, and antitumor activity of SU11248, a novel oral multitarget tyrosine kinase inhibitor, in patients with cancer. J Clin Oncol 2006;24:25–35. [DOI] [PubMed] [Google Scholar]
- 58.Motzer RJ, Hutson TE, Tomczak P, et al. Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J Clin Oncol 2009;27:3584–3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rini BI, Dorff TB, Elson P, et al. Active surveillance in metastatic renal-cell carcinoma: a prospective, phase 2 trial. Lancet Oncol 2016;17:1317–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rini BI, Escudier B, Tomczak P, et al. Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): a randomized phase 3 trial. Lancet 2011;378:1931–1939. [DOI] [PubMed] [Google Scholar]
- 61.Hutson TE, Lesovoy V, Al-Shukri S, et al. Axitinib versus sorafenib as first-line therapy in patients with metastatic renal-cell carcinoma: a randomized open-label phase 3 trial. Lancet Oncol 2013;14:1287–1294. [DOI] [PubMed] [Google Scholar]
- 62.McDermott DF, Regan MM, Clark JI, et al. Randomized phase III trial of high-dose interleukin-2 versus subcutaneous interleukin-2 and interferon in patients with metastatic renal cell carcinoma. J Clin Oncol 2005;23:133–141. [DOI] [PubMed] [Google Scholar]
- 63.Rosenberg SA, Mulé JJ, Spiess PJ, et al. Regression of established pulmonary metastases and subcutaneous tumor mediated by the systemic administration of high-dose recombinant interleukin 2. J Exp Med 1985;161:1169–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yang JC, Sherry RM, Steinberg SM, et al. Randomized study of high-dose and low-dose interleukin-2 in patients with metastatic renal cancer. J Clin Oncol 2003;21:3127–3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hudes G, Carducci M, Tomczak P, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med 2007;356:2271–2281. [DOI] [PubMed] [Google Scholar]
- 66.Choueiri TK, Escudier B, Powles T, et al. Cabozantinib versus everolimus in advanced renal-cell carcinoma. N Engl J Med 2015;373:1814–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Choueiri TK, Escudier B, Powles T, et al. Cabozantinib versus everolimus in advanced renal cell carcinoma (METEOR): final results from a randomised, open-label, phase 3 trial. Lancet Oncol 2016;17:917–927. [DOI] [PubMed] [Google Scholar]
- 68.Motzer RJ, Escudier B, Powles T, et al. Long-term follow-up of overall survival for cabozantinib versus everolimus in advanced renal cell carcinoma. Br J Cancer 2018;118:1176–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Amzal B, Fu S, Meng J, et al. Cabozantinib versus everolimus, nivolumab, axitinib, sorafenib and best supportive care: A network meta-analysis of progression-free survival and overall survival in second line treatment of advanced renal cell carcinoma. PLoS One 2017;12:e0184423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lenvatinib (LENVIMA) prescribing information. Accessed November 4, 2021. Available at: http://www.lenvima.com/pdfs/prescribing-information.pdf
- 71.FDA approves lenvatinib in combination with everolimus for advanced renal cell carcinoma. Accessed November 4, 2021. Available at: https://www.fda.gov/drugs/resources-information-approved-drugs/lenvatinib-combination-everolimus
- 72.Motzer RJ, Hutson TE, Glen H, et al. Lenvatinib, everolimus, and the combination in patients with metastatic renal cell carcinoma: a randomised, phase 2, open-label, multicentre trial. Lancet Oncol 2015;16:1473–1482. [DOI] [PubMed] [Google Scholar]
- 73.Motzer RJ, Hutson TE, Ren M, et al. Independent assessment of lenvatinib plus everolimus in patients with metastatic renal cell carcinoma. LancetOncol 2016;17:e4–e5. [DOI] [PubMed] [Google Scholar]
- 74.Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med 2015;373:1803–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Escudier B, Sharma P, McDermott DF, et al. CheckMate 025 randomized-phase 3 study: outcomes by key baseline factors and prior therapy for nivolumab versus everolimus in advanced renal cell carcinoma. Eur Urol 2017;72:962–971. [DOI] [PubMed] [Google Scholar]
- 76.Motzer RJ, Escudier B, Tomczak P, et al. Axitinib versus sorafenib as second-line treatment for advanced renal cell carcinoma: overall survival analysis and updated results from a randomised phase 3 trial. Lancet Oncol 2013;14:552–562. [DOI] [PubMed] [Google Scholar]
- 77.Apolo AB, Nadal R, Girardi DM, et al. Phase I study of cabozantinib and nivolumab alone or with ipilimumab for advanced or metastatic urothelial carcinoma and other genitourinary tumors. J Clin Oncol 2020;38:3672–3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Final results from a phase I trial and expansion cohorts of cabozantinib and nivolumab (CaboNivo) alone or with ipilimumab (CaboNivoIpi) for metastatic genitourinary tumors. ASCO; 2021. Accessed November 4, 2021. Available at: https://meetinglibrary.asco.org/record/194730/abstract [Google Scholar]
- 79.Hammers HJ, Plimack ER, Infante JR, et al. Safety and efficacy of nivolumabin combination with ipilimumab in metastatic renal cell carcinoma: the CheckMate 016 study. J Clin Oncol 2017;35:3851–3858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lee CH, Shah AY, Rasco D, et al. Lenvatinib plus pembrolizumab in patients with either treatment-naive or previously treated metastatic renal cell carcinoma (Study 111/KEYNOTE-146): a phase 1b/2 study. Lancet Oncol 2021;22:946–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hainsworth JD, Rubin MS, Arrowsmith ER, et al. Pazopanib as second-line treatment after sunitinib or bevacizumab in patients with advanced renal cell carcinoma: a Sarah Cannon Oncology Research Consortium Phase II Trial. Clin Genitourin Cancer 2013;11:270–275. [DOI] [PubMed] [Google Scholar]
- 82.Motzer RJ, Rini BI, Bukowski RM, et al. Sunitinib in patients with metastatic renal cell carcinoma. JAMA 2006;295:2516–2524. [DOI] [PubMed] [Google Scholar]
- 83.Dudek AZ, Zolnierek J, Dham A, et al. Sequential therapy with sorafenib and sunitinib in renal cell carcinoma. Cancer 2009;115:61–67. [DOI] [PubMed] [Google Scholar]
- 84.Eichelberg C, Heuer R, Chun FK, et al. Sequential use of the tyrosine kinase inhibitors sorafenib and sunitinib in metastatic renal cell carcinoma: a retrospective outcome analysis. Eur Urol 2008;54:1373–1378. [DOI] [PubMed] [Google Scholar]
- 85.Sablin MP, Negrier S, Ravaud A, et al. Sequential sorafenib and sunitinib for renal cell carcinoma. J Urol 2009;182:29–34., discussion 34. [DOI] [PubMed] [Google Scholar]
- 86.Zimmermann K, Schmittel A, Steiner U, et al. Sunitinib treatment for patients with advanced clear-cell renal-cell carcinoma after progression on sorafenib. Oncology 2009;76:350–354. [DOI] [PubMed] [Google Scholar]
- 87.Garcia JA, Hutson TE, Elson P, et al. Sorafenib in patients with metastaticrenal cell carcinoma refractory to either sunitinib or bevacizumab. Cancer 2010;116:5383–5390. [DOI] [PubMed] [Google Scholar]
- 88.FDA approves tivozanib for relapsed or refractory advanced renal cell carcinoma. Accessed November 4, 2021. Available at: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-tivozanib-relapsed-or-refractory-advanced-renal-cell-carcinoma
- 89.Rini BI, Pal SK, Escudier BJ, et al. Tivozanib versus sorafenib in patients with advanced renal cell carcinoma (TIVO-3): a phase 3, multicentre, randomised, controlled, open-label study. Lancet Oncol 2020;21:95–104. [DOI] [PubMed] [Google Scholar]
- 90.Motzer RJ, Escudier B, Oudard S, et al. Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet 2008;372:449–456. [DOI] [PubMed] [Google Scholar]
- 91.Motzer RJ, Escudier B, Oudard S, et al. Phase 3 trial of everolimus for metastatic renal cell carcinoma: final results and analysis of prognostic factors. Cancer 2010;116:4256–4265. [DOI] [PubMed] [Google Scholar]
- 92.Yang JC, Haworth L, Sherry RM, et al. A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N Engl J Med 2003;349:427–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Awada A, Hendlisz A, Gil T, et al. Phase I safety and pharmacokinetics of BAY 43–9006 administered for 21 days on/7 days off in patients with advanced, refractory solid tumours. Br J Cancer 2005;92:1855–1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Clark JW, Eder JP, Ryan D, et al. Safety and pharmacokinetics of the dual action Raf kinase and vascular endothelial growth factor receptor inhibitor, BAY 43–9006, in patients with advanced, refractory solid tumors. Clin Cancer Res 2005;11:5472–5480. [DOI] [PubMed] [Google Scholar]
- 95.Moore M, Hirte HW, Siu L, et al. Phase I study to determine the safety and pharmacokinetics of the novel Raf kinase and VEGFR inhibitor BAY43–9006, administered for 28 days on/7 days off in patients with advanced, refractory solid tumors. Ann Oncol 2005;16:1688–1694. [DOI] [PubMed] [Google Scholar]
- 96.Strumberg D, Richly H, Hilger RA, et al. Phase I clinical and pharmacokinetic study of the novel Raf kinase and vascular endothelial growth factor receptor inhibitor BAY 43–9006 in patients with advanced refractory solid tumors. J Clin Oncol 2005;23:965–972. [DOI] [PubMed] [Google Scholar]
- 97.Wilhelm SM, Carter C, Tang L, et al. BAY 43–9006 exhibits broad spectrumoral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis.Cancer Res 2004;64:7099–7109. [DOI] [PubMed] [Google Scholar]
- 98.Escudier B, Eisen T, Stadler WM, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med 2007;356:125–134. [DOI] [PubMed] [Google Scholar]
- 99.Escudier B, Eisen T, Stadler WM, et al. Sorafenib for treatment of renal cell carcinoma: final efficacy and safety results of the phase III treatment approaches in renal cancer global evaluation trial. J Clin Oncol 2009;27:3312–3318. [DOI] [PubMed] [Google Scholar]
- 100.Hutson TE, Escudier B, Esteban E, et al. Randomized phase III trial of temsirolimus versus sorafenib as second-line therapy after sunitinib in patients with metastatic renal cell carcinoma. J Clin Oncol 2014;32:760–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.de Velasco G, McKay RR, Lin X, et al. Comprehensive analysis of survival outcomes in non-clear cell renal cell carcinoma patients treated in clinical trials. Clin Genitourin Cancer 2017;15:652–660.e1. [DOI] [PubMed] [Google Scholar]
- 102.Pal SK, Tangen C, Thompson IM, Jr., et al. A comparison of sunitinib with cabozantinib, crizotinib, and savolitinib for treatment of advanced papillary renal cell carcinoma: a randomised, open-label, phase 2 trial. Lancet 2021;397:695–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Armstrong AJ, Halabi S, Eisen T, et al. Everolimus versus sunitinib for patients with metastatic non-clear cell renal cell carcinoma (ASPEN): a multicentre, open-label, randomised phase 2 trial. Lancet Oncol 2016;17:378–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tannir NM, Jonasch E, Albiges L, et al. Everolimus versus sunitinib prospective evaluation in metastatic non-clear cell renal cell carcinoma (ESPN): a randomized multicenter phase 2 trial. Eur Urol 2016;69:866–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ciccarese C, Iacovelli R, Brunelli M, et al. Addressing the best treatment for non-clear cell renal cell carcinoma: a meta-analysis of randomized clinical trials comparing VEGFR-TKis versus mTORi-targeted therapies. Eur J Cancer 2017;83:237–246. [DOI] [PubMed] [Google Scholar]
- 106.Hutson TE, Michaelson MD, Kuzel TM, et al. A single-arm, multicenter, phase 2 study of lenvatinib plus everolimus in patients with advanced non-clear cell renal cell carcinoma. Eur Urol 2021;80:162–170. [DOI] [PubMed] [Google Scholar]
- 107.Koshkin VS, Barata PC, Zhang T, et al. Clinical activity of nivolumab inpatients with non-clear cell renal cell carcinoma. J Immunother Cancer 2018;6:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.McKay RR, Bossé D, Xie W, et al. The clinical activity of PD-1/PD-L1inhibitors in metastatic non-clear cell renal cell carcinoma. Cancer Immunol Res 2018;6:758–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.McDermott DF, Lee JL, Ziobro M, et al. Open-label, single-arm, phase II study of pembrolizumab monotherapy as first-line therapy in patients with advanced non-clear cell renal cell carcinoma. J Clin Oncol 2021;39:1029–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Park I, Lee SH, Lee JL. A multicenter phase II trial of axitinib in patients with recurrent or metastatic non-clear-cell renal cell carcinoma who had failed prior treatment with temsirolimus. Clin Genitourin Cancer 2018;16:e997–e1002. [DOI] [PubMed] [Google Scholar]
- 111.Irshad T, Olencki T, Zynger DL, et al. Bevacizumab in metastatic papillary renal cell carcinoma (PRCC) [abstract]. J Clin Oncol 2011;29:e15158. [Google Scholar]
- 112.Menko FH, Maher ER, Schmidt LS, et al. Hereditary leiomyomatosis and renal cell cancer (HLRCC): renal cancer risk, surveillance and treatment.Fam Cancer 2014;13:637–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Srinivasan R, Gurram S, Al Harthy M, et al. Results from a phase II study of bevacizumab and erlotinib in subjects with advanced hereditary leiomyomatosis and renal cell cancer (HLRCC) or sporadic papillary renal cell cancer. J Clin Oncol 2020;38(15_suppl):5004–5004. [Google Scholar]
- 114.Voss MH, Molina AM, Chen YB, et al. Phase II trial and correlative genomic analysis of everolimus plus bevacizumab in advanced non-clear cell renal cell carcinoma. J Clin Oncol 2016;34:3846–3853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Buti S, Bersanelli M, Maines F, et al. First-line pazopanib in non-clear-cell renal carcinoma: the Italian retrospective multicenter PANORAMA study. Clin Genitourin Cancer 2017;15:e609–e614. [DOI] [PubMed] [Google Scholar]
- 116.Gordon MS, Hussey M, Nagle RB, et al. Phase II study of erlotinib in patients with locally advanced or metastatic papillary histology renal cell cancer: SWOG S0317. J Clin Oncol 2009;27:5788–5793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Blank CU, Bono P, Larkin JMG, et al. Safety and efficacy of everolimus in patients with non-clear cell renal cell carcinoma refractory to VEGF-targeted therapy: subgroup analysis of REACT [abstract]. J Clin Oncol 2012;30(5, suppl)402. (Abstract 402) [Google Scholar]
- 118.Koh Y, Lim HY, Ahn JH, et al. Phase II trial of everolimus for the treatmentof nonclear-cell renal cell carcinoma. Ann Oncol 2013;24:1026–1031. [DOI] [PubMed] [Google Scholar]
- 119.Escudier B, Molinie V, Bracarda S, et al. Open-label phase 2 trial of first-line everolimus monotherapy in patients with papillary metastatic renal cell carcinoma: RAPTOR final analysis. Eur J Cancer 2016;69:226–235. [DOI] [PubMed] [Google Scholar]
- 120.Jung KS, Lee SJ, Park SH, et al. Pazopanib for the treatment of non-clear cell renal cell carcinoma: a single-arm, open-label, multicenter, phase II study. Cancer Res Treat 2018;50:488–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Costello BA, Ho TH, Burbano GP, et al. A phase II efficacy trial of pazopanibin non-clear cell metastatic renal cell cancer (mRCC) PINCR [abstract]. J Clin Oncol 2020;38 (Suppl):696–696. [Google Scholar]
- 122.Dutcher JP, de Souza P, McDermott D, et al. Effect of temsirolimus versus interferon-alpha on outcome of patients with advanced renal cell carcinoma of different tumor histologies. Med Oncol 2009;26:202–209. [DOI] [PubMed] [Google Scholar]
- 123.Venugopal B, Ansari J, Aitchison M, et al. Efficacy of temsirolimus in metastatic chromophobe renal cell carcinoma. BMC Urol 2013;13:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hakimi AA, Koi PT, Milhoua PM, et al. Renal medullary carcinoma: the Bronx experience. Urology 2007;70:878–882. [DOI] [PubMed] [Google Scholar]
- 125.Watanabe IC, Billis A, Guimarães MS, et al. Renal medullary carcinoma: report of seven cases from Brazil. Mod Pathol 2007;20:914–920. [DOI] [PubMed] [Google Scholar]
- 126.Shah AY, Karam JA, Malouf GG, et al. Management and outcomes of patients with renal medullary carcinoma: a multicentre collaborative study. BJU Int 2017;120:782–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Srigley JR, Delahunt B. Uncommon and recently described renal carcinomas. Mod Pathol 2009;22(S2, Suppl 2)S2–S23. [DOI] [PubMed] [Google Scholar]
- 128.Tokuda N, Naito S, Matsuzaki O, et al. Collecting duct (Bellini duct) renal cell carcinoma: a nationwide survey in Japan. J Urol 2006;176:40–43., discussion 43. [DOI] [PubMed] [Google Scholar]
- 129.Karakiewicz PI, Trinh QD, Rioux-Leclercq N, et al. Collecting duct renal cell carcinoma: a matched analysis of 41 cases. Eur Urol 2007;52:1140–1145. [DOI] [PubMed] [Google Scholar]
- 130.Gupta R, Billis A, Shah RB, et al. Carcinoma of the collecting ducts of Bellini and renal medullary carcinoma: clinicopathologic analysis of 52 cases of rare aggressive subtypes of renal cell carcinoma with a focus on their interrelationship. Am J Surg Pathol 2012;36:1265–1278. [DOI] [PubMed] [Google Scholar]
- 131.Oudard S, Banu E, Vieillefond A, et al. Prospective multicenter phase II study of gemcitabine plus platinum salt for metastatic collecting duct carcinoma: results of a GETUG (Groupe d’Etudes des Tumeurs Uro-Génitales) study. J Urol 2007;177:1698–1702. [DOI] [PubMed] [Google Scholar]
- 132.Roubaud G, Gross-Goupil M, Wallerand H, et al. Combination of gemcitabine and doxorubicin in rapidly progressive metastatic renal cell carcinoma and/or sarcomatoid renal cell carcinoma. Oncology 2011;80:214–218. [DOI] [PubMed] [Google Scholar]
- 133.Fokas E, Henzel M, Hamm K, et al. Radiotherapy for brain metastases from renal cell cancer: should whole-brain radiotherapy be added to stereotactic radiosurgery? Analysis of 88 patients. Strahlenther Onkol 2010;186:210–217. [DOI] [PubMed] [Google Scholar]
- 134.Zekri J, Ahmed N, Coleman RE, et al. The skeletal metastatic complications of renal cell carcinoma. Int J Oncol 2001;19:379–382. [DOI] [PubMed] [Google Scholar]
- 135.Schlesinger-Raab A, Treiber U, Zaak D, et al. Metastatic renal cell carcinoma: results of a population-based study with 25 years follow-up. Eur J Cancer 2008;44:2485–2495. [DOI] [PubMed] [Google Scholar]
- 136.Roza T, Hakim L, van Poppel H, et al. Bone-targeted therapies for elderly patients with renal cell carcinoma: current and future directions. Drugs Aging 2013;30:877–886. [DOI] [PubMed] [Google Scholar]
- 137.Hunter GK, Balagamwala EH, Koyfman SA, et al. The efficacy of external beam radiotherapy and stereotactic body radiotherapy for painful spinal metastases from renal cell carcinoma. Pract Radiat Oncol 2012;2:e95–e100. [DOI] [PubMed] [Google Scholar]
- 138.Zelefsky MJ, Greco C, Motzer R, et al. Tumor control outcomes after hypofractionated and single-dose stereotactic image-guided intensity-modulated radiotherapy for extracranial metastases from renal cell carcinoma. Int J Radiat Oncol Biol Phys 2012;82:1744–1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lipton A, Zheng M, Seaman J. Zoledronic acid delays the onset of skeletal-related events and progression of skeletal disease in patients with advanced renal cell carcinoma. Cancer 2003;98:962–969. [DOI] [PubMed] [Google Scholar]
- 140.Rosen LS, Gordon D, Tchekmedyian NS, et al. Long-term efficacy and safety of zoledronic acid in the treatment of skeletal metastases in patients with nonsmall cell lung carcinoma and other solid tumors: a randomized, Phase III, double-blind, placebo-controlled trial. Cancer 2004;100:2613–2621. [DOI] [PubMed] [Google Scholar]
- 141.Henry DH, Costa L, Goldwasser F, et al. Randomized, double-blind study of denosumab versus zoledronic acid in the treatment of bone metastases in patients with advanced cancer (excluding breast and prostate cancer) or multiple myeloma. J Clin Oncol 2011;29:1125–1132. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


