Since the Covid-19 pandemic, hand sanitisers and sprays have become staple features in cities around the world.
But our efforts to create 'sterile urban environments' in offices, schools, and shopping centres may be backfiring, according to a new study.
Instead, we may be creating an army of disinfectant-resistant superbugs.
Researchers at Xi'an Jiaotong-Liverpool University in China took swab samples from indoor surfaces all around Hong Kong, as well as from human skin.
They found that microbes – tiny living beings too small to see without a microscope – are consuming chemicals in cleaning products to survive.
Although the study focused on samples from Hong Kong, the team believe that similar findings could be observed in other cities.
The study comes shortly after scientists from Darwin Bioprospecting Excellence SL discovered radiation-resistant microbes breeding inside microwaves.

Researchers found that microbes – tiny living beings too small to see without a microscope – are consuming chemicals in cleaning products to survive. Clockwise from top left: Streptococcus, microbial biofilm of mixed species from the human body, Bacillus and Malassezia lopophilis
For the study, published in Microbiome, the researchers collected 738 samples from a variety of environments, including subways, residences, public facilities, piers and human skin in Hong Kong.
Lead author Dr Xinzhao Tong says her study focused on 'built environments' –human-made structures that form the setting for human activity.
'Examples of built environments include residential buildings, offices, and public transport systems like subways or metro stations,' Dr Tong told MailOnline.
'Our use of cleaning and other manufactured products creates a unique setting that puts selective pressures on microbes, which they must adapt to or be eliminated.'
To understand how they have adapted to urban conditions, the team analysed the microbes' genomic content in the lab using a method called 'shotgun metagenomic sequencing'.
'Shotgun metagenomic sequencing involves extracting genomic DNA from microbes and sequencing it to analyze their genetic material at the gene level,' Dr Tong told MailOnline.
'Each gene encodes a specific function, which helps us understand the strategies of microbes used to adapt and survive in the built environment.'
Overall, the research team identified 363 microbial strains that have not been previously identified that live on our skin and the environment around us.

With the addition of hand sanitizers and sprays, indoor spaces have barely been the same since the Covid pandemic. Pictured, a shopping mall in Hong Kong
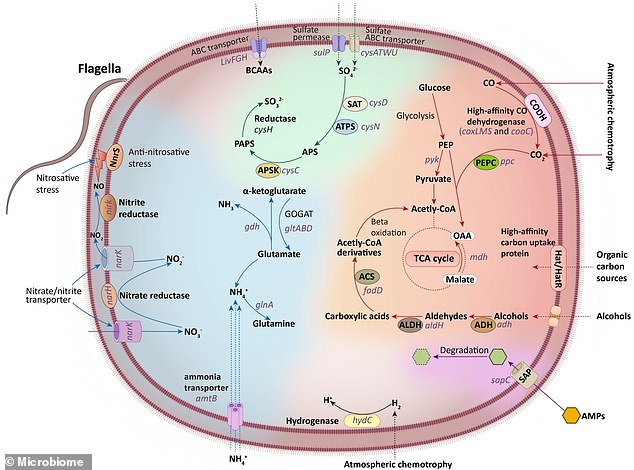
This figure summarizes the fundamental metabolic pathways of Eremiobacterota, including carbon, nitrogen, and sulfur metabolism, based on the functions encoded by its genes
Some of these strains carried genes for metabolising chemicals in common cleaning products, such as alcohol and ammonium ions, using them as carbon and energy sources.
This includes a strain of Candidatus Eremiobacterota - a bacteria previously only reported in Antarctic desert soil.
'We found a novel microbial strain of Eremiobacterota, well-adapted to residential areas and human skin, that can metabolize alcohol – a key ingredient in many hand disinfectants,' Dr Tong told MailOnline.
'This strain can utilize alcohol as a carbon source, which then feeds into its carbon metabolism for energy production.
'Additionally, the strain can metabolize inorganic salts, such as nitrate, sulfate, and ammonium, which may be present in some of the cleaning agents.'
None of the microbes identified in the study were found to be pathogenic – i.e. capable of causing human diseases.
However, the team discovered a novel strain in the genus Pseudomonas that carries a high number of genes associated with antimicrobial resistance (AMR).
AMR is when bacteria and other microbes adapt and evolve in response to modern chemicals designed to kill them, becoming ultra-strong 'superbugs'.

Pictured, Micrococcus luteus, a bacterium that's typically non-pathogenic but can cause opportunistic infections in people who have a weakened immune system (file photo)
This ongoing health crisis could turn everyday injuries and routine surgeries into life and death events, undo decades of medical progress and potentially kill millions every year.
The team also identified 11 previously uncharacterised strains of Micrococcus luteus, a bacterium that's typically non-pathogenic but can cause opportunistic infections in people who have a weakened immune system.
'Long-term exposure to suboptimal concentrations of disinfectants could drive microbial evolution, leading some microbes to develop resistance and even use disinfectants as an energy source,' Dr Tong told MailOnline.
'However, the specific microbes present might vary depending on the local environment.'
The team is now investigating pathogenic microbes in intensive care units, which are exposed to 'stringent and extensive' disinfectant routines.
'While cleaning products can effectively neutralize or eliminate most harmful microbes, the disinfectants we use in daily life are often applied less frequently and are milder than those used in clinical settings,' Dr Tong added.
'As a result, some microbes may adapt and develop increased resistance to these milder disinfectants, allowing them to survive over time.
'However, additional experimental evidence is needed to confirm this idea.'