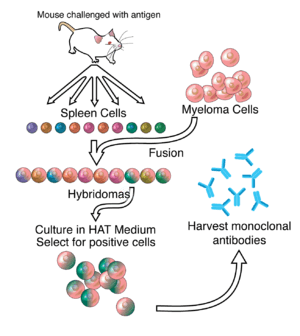
ZMapp
ZMapp is an experimental biopharmaceutical drug comprising three chimeric monoclonal antibodies under development as a treatment for Ebola virus disease. Two of the three components were originally developed at the Public Health Agency of Canada's National Microbiology Laboratory (NML), and the third at the U.S. Army Medical Research Institute of Infectious Diseases; the cocktail was optimized by Dr. Gary Kobinger, the recently-departed branch chief of the NML and is undergoing further development under license by Mapp Biopharmaceutical. Zmapp was first tested in humans during the 2014 West Africa Ebola virus outbreak, but has not been subjected to a randomized controlled trial to determine whether it works, and whether it is safe enough to allow on the market.
Medical use
ZMapp is under development as a treatment for Ebola virus disease. It was first used experimentally to treat some people with Ebola virus disease during the 2014 West African Ebola outbreak, but as of August 2014 it had not yet been tested in a clinical trial to support widespread usage in humans; it is not known whether it is effective to treat the disease, nor if it is safe.
Podcasts:

-
by Neil Diamond
-
by Screeching Weasel
-
by Ringo Starr
-
by Goldfinger
-
by Echo & The Bunnymen
-
by Babasonicos
-
by Amorphis
-
by Cranberries
-
by Bryan Ferry
-
by Caetano Veloso
-
by E-40
-
by Talking Heads
-
by Sham 69
-
by Mr. Big
-
by Soulfly
-
by Nellie McKay
-
by Brutal Truth
-
by Dolores O'Riordan
-
by Family Force 5
-
by Don Omar
-
by The Cranberries
-
by Mortuary Drape
-
by Calabrese
-
by Death SS
-
by Jay Brannan
-
by Cyclefly
-
by Elis Regina
-
by War Rocket Ajax
-
by Natalia Kills
-
by Dave Stewart
-
by Spragga Benz
-
by Impetigo
-
by Pintandwefall
-
by Veloso Caetano
-
by Adam
-
by Young Bari
-
by Anguish Unsaid
-
by Miser
-
by Versailles
-
by Andrew Spencer
-
by Antioch Arrow
-
by Sarah Jezebel Deva
-
by Two Ton Shoe
-
by Four Fingers
-
by Cinderella Effect
Say Maybe
by: Neil DiamondWhy make me plead
For what I need
And I need you
Why, why make me weep
Cry in my sleep
Cause I need you
Say maybe
Won't you even say sometime, baby
Won't you never say lovin' words again
Say baby
Don't you know I'm a fool about you
If I tried, I could live without you
For maybe a day (maybe a day)
I, I've been around
Finally found
And I need you
And I, I know what I got
And I got a lot
