 |
|
|---|---|
| Systematic (IUPAC) name | |
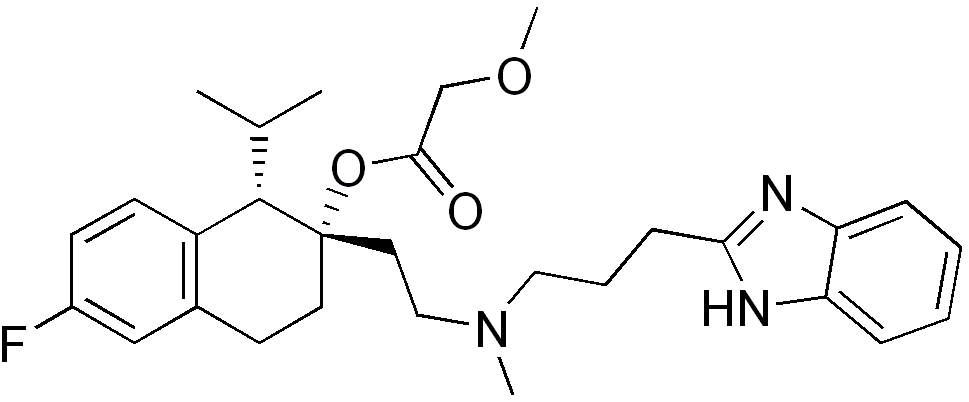
| (1S,2S)-2-(2-((3-(1H-benzo[d]imidazol-2-yl)propyl) (methyl)amino)ethyl)-6-fluoro-1-isopropyl- 1,2,3,4-tetrahydronaphthalen-2-yl 2-methoxyacetate | |
| Clinical data | |
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| MedlinePlus | a607007 |
| Pregnancy cat. | ? |
| Legal status | Withdrawn from market |
| Routes | Oral |
| Identifiers | |
| CAS number | 116644-53-2 |
| ATC code | C08CX01 |
| PubChem | CID 60662 |
| DrugBank | DB01388 |
| UNII | 27B90X776A |
| KEGG | D08217 |
| ChEMBL | CHEMBL45816 |
| Chemical data | |
| Formula | C29H38FN3O3 |
| Mol. mass | 495.63 g/mol |
| |
|
Mibefradil (Posicor) is a drug for the treatment of hypertension and chronic angina pectoris. It belongs to a group known as calcium channel blockers.
It is nonselective.[1]
On June 8, 1998, Roche announced the voluntary withdrawal of the drug from the market,[2] due to the potential for drug interactions, some of them serious, which may occur when it is taken together with some other medications.
References [link]
- ^ Bezprozvanny I, Tsien RW (September 1995). "Voltage-dependent blockade of diverse types of voltage-gated Ca2+ channels expressed in Xenopus oocytes by the Ca2+ channel antagonist mibefradil (Ro 40-5967)". Mol. Pharmacol. 48 (3): 540–9. PMID 7565636. https://molpharm.aspetjournals.org/cgi/pmidlookup?view=long&pmid=7565636.
- ^ Letter from Roche Laboratories: https://www.fda.gov/medwatch/SAFETY/1998/poscor.htm
| This drug article relating to the cardiovascular system is a stub. You can help Wikipedia by expanding it. |
https://wn.com/Mibefradil
Podcasts:
PLAYLIST TIME:

