A permanganate is the general name for a chemical compound containing the manganate(VII) ion, (MnO4−). Because manganese is in the +7 oxidation state, the permanganate(VII) ion is a strong oxidizing agent. The ion has tetrahedral geometry.[1] Permanganate solutions are purple in color and are stable in neutral or slightly alkaline media.
In an acidic solution, permanganate(VII) is reduced to the colourless +2 oxidation state of the manganese(II) (Mn2+) ion.
- 8 H+ + MnO4− + 5 e− → Mn2+ + 4 H2O
In a strongly basic solution, permanganate(VII) is reduced to the green +6 oxidation state of the manganate MnO42−.
- MnO4− + e− → MnO42−
In a neutral medium however, it gets reduced to the brown +4 oxidation state of manganese dioxide MnO2.
- 2 H2O + MnO4− + 3 e− → MnO2 + 4 OH−
Contents |
Production [link]
Permanganates can be produced by oxidation of manganese compounds such as manganese chloride or manganese sulfate by strong oxidizing agents, for instance, sodium hypochlorite or lead dioxide:
- 2 MnCl2 + 5 NaClO + 6 NaOH → 2 NaMnO4 + 9 NaCl+ 3 H2O
- 2 MnSO4 + 5 PbO2+ 3 H2SO4 → 2 HMnO4 + 5 PbSO4 + 2 H2O
It may also be produced by the dismutation of manganates, with manganese dioxide as a side-product:
- 3 Na2MnO4 + 2 H2O → 2 NaMnO4 + MnO2 + 4 NaOH
Properties [link]
Permanganates(VII) are salts of permanganic acid. Permanganate(VII) is a strong oxidizer, and similar to perchlorate. It is therefore in common use in qualitative analysis that involves redox reactions (permanganometry). Besides this, it is stable.
It is a useful reagent, though with organic compounds, not very selective.
Manganates(VII) are not very stable thermally. For instance, potassium permanganate decomposes at 230 °C to potassium manganate and manganese dioxide, releasing oxygen gas:
- 2 KMnO4 → K2MnO4 + MnO2 + O2
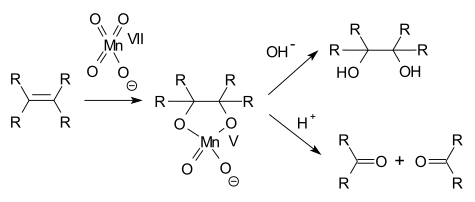
A permanganate can oxidize an amine to a nitro compound,[2][3] an alcohol to a ketone,[4] an aldehyde to a carboxylic acid,[5][6] a terminal alkene to a carboxylic acid,[7] oxalic acid to carbon dioxide,[8] and an alkene to a diol.[9] This list is not exhaustive.
In alkene oxidations one intermediate is a cyclic Mn(V) species:
Compounds [link]
- Ammonium permanganate, NH4MnO4
- Calcium permanganate, Ca(MnO4)2
- Potassium permanganate, KMnO4
- Sodium permanganate, NaMnO4
See also [link]
- Perchlorate, a similar ion with a chlorine(VII) center
- Chromate, which is isoelectronic with permanganate
References [link]
- ^ Sukalyan Dash, Sabita Patel and Bijay K. Mishra (2009). "Oxidation by permanganate: synthetic and mechanistic aspects". Tetrahedron 65 (4): 707–739. DOI:10.1016/j.tet.2008.10.038.
- ^ A. Calder, A. R. Forrester1, and S. P. Hepburn (1972), "2-methyl-2-nitrosopropane and its dimer", Org. Synth. 6: 803, https://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=CV6P0803; Coll. Vol. 52: 77
- ^ Nathan Kornblum and Willard J. Jones (1963), "4-nitro-2,2,4-trimethylpentane", Org. Synth. 5: 845, https://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=CV5P0845; Coll. Vol. 43: 87
- ^ J. W. Cornforth (1951), "Ethyl pyruvate", Org. Synth. 4: 467, https://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=CV4P0467; Coll. Vol. 31: 59
- ^ R. L. Shriner and E. C. Kleiderer (1930), "Piperonylic acid", Org. Synth. 2: 538, https://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=CV2P0538; Coll. Vol. 10: 82
- ^ John R. Ruhoff (1936), "n-heptanoic acid", Org. Synth. 2: 315, https://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=CV2P0315; Coll. Vol. 16: 39
- ^ Donald G. Lee, Shannon E. Lamb, and Victor S. Chang (1981), "Carboxylic acids from the oxidation of terminal alkenes by permanganate: nonadecanoic acid", Org. Synth. 7: 397, https://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=CV7P0397; Coll. Vol. 60: 11
- ^ Kovacs KA, Grof P, Burai L, Riedel M (2004). "Revising the Mechanism of the Permanganate/Oxalate Reaction". J. Phys. Chem. A 108 (50): 11026. DOI:10.1021/jp047061u.
- ^ E. J. Witzemann, Wm. Lloyd Evans, Henry Hass, and E. F. Schroeder (1931), "dl-glyceraldehyde ethyl acetal", Org. Synth. 2: 307, https://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=CV2P0307; Coll. Vol. 11: 52
https://wn.com/Permanganate

Manganese dioxide
Manganese(IV) oxide is the inorganic compound with the formula MnO
2. This blackish or brown solid occurs naturally as the mineral pyrolusite, which is the main ore of manganese and a component of manganese nodules. The principal use for MnO2 is for dry-cell batteries, such as the alkaline battery and the zinc-carbon battery.MnO
2 is also used as a pigment and as a precursor to other manganese compounds, such as KMnO
4. It is used as a reagent in organic synthesis, for example, for the oxidation of allylic alcohols. MnO2 in the α polymorph can incorporate a variety of atoms (as well as water molecules) in the "tunnels" or "channels" between the magnesium oxide octahedra. There is considerable interest in α-MnO2 as a possible cathode for lithium ion batteries.
Structure
Several polymorphs of MnO
2 are claimed, as well as a hydrated form. Like many other dioxides, MnO
2 crystallizes in the rutile crystal structure (this polymorph is called β-MnO
2), with three-coordinate oxide and octahedral metal centres.MnO
2 is characteristically nonstoichiometric, being deficient in oxygen. The complicated solid-state chemistry of this material is relevant to the lore of "freshly prepared" MnO
2 in organic synthesis. The α-polymorph of MnO
2 has a very open structure with ``channels" which can accommodate metal atoms such as silver or barium. α-MnO2 is often called Hollandite, after a closely related mineral.

Manganese(II) oxide
Manganese(II) oxide (systematically named manganese(2+) oxide(2−)) is an inorganic compound with chemical formula MnO. It forms green crystals. The compound is produced on a large scale as a component of fertilizers and food additives.
Structure, stoichiometry, reactivity
Like many monoxides, MnO adopts the rock salt structure, where cations and anions are both octahedrally coordinated. Also like many oxides, manganese(II) oxide is often nonstoichiometric: its composition can vary from MnO to MnO1.045.
Below 118 K MnO is antiferromagnetic. MnO has the distinction of being one of the first compounds to have its magnetic structure determined by neutron diffraction, the report appearing in 1951. This study showed that the Mn2+ ions form a face centered cubic magnetic sub-lattice where there are ferromagnetically coupled sheets that are anti-parallel with adjacent sheets.
Manganese(II) oxide undergoes the chemical reactions typical of an ionic oxide. Upon treatment with acids, it converts to the corresponding manganese(II) salt and water. Oxidation of manganese(II) oxide gives manganese(III) oxide.