Halocarbon
Halocarbon compounds are chemicals in which one or more carbon atoms are linked by covalent bonds with one or more halogen atoms (fluorine, chlorine, bromine or iodine – group 17) resulting in the formation of organofluorine compounds, organochlorine compounds, organobromine compounds, and organoiodine compounds. Chlorine halocarbons are the most common and are called organochlorides.
Many synthetic organic compounds such as plastic polymers, and a few natural ones, contain halogen atoms; they are known as halogenated compounds or organohalogens. Organochlorides are the most common industrially used organohalides, although the other organohalides are used commonly in organic synthesis. Except for extremely rare cases, organohalides are not produced biologically, but many pharmaceuticals are organohalides. Notably, many pharmaceuticals such as Prozac have trifluoromethyl groups.
For information on inorganic halide chemistry, see halide.
Chemical families
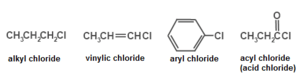
Halocarbons are typically classified in the same ways as the similarly structured organic compounds that have hydrogen atoms occupying the molecular sites of the halogen atoms in halocarbons. Among the chemical families are:

Hexafluoroethane
Hexafluoroethane is a fluorocarbon counterpart to the hydrocarbon ethane. It is a non-flammable gas negligibly soluble in water and slightly soluble in alcohol.
Physical properties
Hexafluoroethane's solid phase has two polymorphs. In the scientific literature, different phase transition temperatures have been stated. The latest works assign it at 103 K (−170 °C). Below 103 K it has a slightly disordered structure, and over the transition point, it has a body centered cubic structure.
Table of densities:
Vapor density is 4.823 (air = 1), specific gravity at 21 °C is 4.773 (air = 1) and specific volume at 21 °C is 0.1748 m3/kg.
Uses
Hexafluoroethane is used as a versatile etchant in semiconductor manufacturing. It can be used for selective etching of metal silicides and oxides versus their metal substrates and also for etching of silicon dioxide over silicon. The primary aluminium and the semiconductor manufacturing industries are the major emitters of hexafluoroethane.
Together with trifluoromethane it is used in refrigerants R508A (61%) and R508B (54%).

Perfluorobutane
Perfluorobutane (PFB) is a colorless gas. It is a simple fluorocarbon with a n-butane skeleton and all the hydrogen atoms replaced with fluorine atoms. It is used as a replacement for Halon 1301 fire extinguishers, as well as the gas component for newer generation microbubble ultrasound contrast agents. Sonazoid is one such microbubble formulation developed by Amersham Health that uses perfluorobutane for the gas core.
References
Podcasts:

