Latest News for: h-s chart
Edit
Chart Master: Charting the course for the energy sector
CNBC 09 Apr 2025
Carter Worth, Worth Charting, joins 'Fast Money' to track what the charts are saying in the energy sector as it leads to the downside in today's trading session ... .
Edit
New open source AI company Deep Cogito releases first models and they’re already topping the charts
Venture Beat 09 Apr 2025
The initial model lineup includes five base sizes. 3 billion, 8 billion, 14 billion, 32 billion, and 70 billion parameters.Read More ....
Edit
'When Life Gives You Tangerines' reclaims No. 1 spot on Netflix's weekly chart
Yonhap News 09 Apr 2025
Edit
Support the SWP Campaign in 2025! Break with the Bosses’ Parties! Chart a Course Toward Workers’ Power!
The Militant 09 Apr 2025
Alyson Kennedy, Socialist Workers Party. candidate for mayor of Fort Worth ....
Edit
‘When Life Gives You Tangerines’ No. 1 on Netflix chart anew; ‘Karma’ debuts in top 10
Manila Bulletin 09 Apr 2025
Edit
Chart Master: Key S&P 500 levels to watch
CNBC 08 Apr 2025
Carter Worth, Worth Charting, joins 'Fast Money' to talk the technicals on the S&P 500 ... .
Edit
The Quiet Part Live Ep. 62: Chart Go Sideways, Judge Orders Return of MS-13 & ...
Rumble 08 Apr 2025
Edit
BTS J-Hope’s Mona Lisa Secures No.1 Position On Billboard Chart, Leaves Behind Doechii’s Anxiety
News18 08 Apr 2025
Edit
 The Post and Courier
08 Apr 2025
The Post and Courier
08 Apr 2025
A 'cool' SC-made hard seltzer chases its way up the country music charts
 The Post and Courier
08 Apr 2025
The Post and Courier
08 Apr 2025
Country singer Russell Dickerson includes a line about a drink produced at Gallo's York County winery in "Happen To Me," which has been called a "feel-good anthem of 2025." ... .
Edit
Lil Durk’s ‘DEEP THOUGHTS’ Debuts Strong on Billboard Charts
The Source 08 Apr 2025
2 on the Billboard Rap Albums chart and No.
Edit
Interactive charts on non-financial corporations (MNB - Central Bank of Hungary)
Public Technologies 08 Apr 2025
). The text version of this document is not available ... Disclaimer ... (noodl. 123534030) .
Edit
Five things you need to know, my side hustle, and pie charts
Business Journal 08 Apr 2025
Good morning, Boston. Happy National Empanada Day.
- 1
- 2
- Next page »


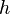 in terms of
in terms of  , pressure
, pressure 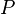 and volume
and volume 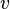 using the relationship
using the relationship  .
.