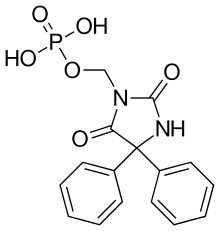
Fosphenytoin
Fosphenytoin (fosphenytoin sodium injection, previously Cerebyx, Parke-Davis; Prodilantin, Pfizer Holding France) is a water-soluble phenytoin prodrug that is administered intravenously to deliver phenytoin, potentially more safely than intravenous phenytoin. It is most commonly used in the acute treatment convulsive status epilepticus.
Fosphenytoin was developed in 1996. On 18 November 2004, Sicor (a subsidiary of Teva) received a tentative approval letter from the United States Food and Drug Administration for a generic version of fosphenytoin.
Medical uses
Fosphenytoin is approved in the United States for the short term (five days or fewer) treatment of epilepsy when more widely used means of phenytoin administration are not possible or are ill-advised, such as endotracheal intubation, status epilepticus or some other type of repeated seizures; vomiting, and/or the patient is unalert or not awake or both.
Other
In 2003, it was reported that even though anticonvulsants are often very effective in mania, and acute mania requires rapid treatment, fosphenytoin had no antimanic effect.
Podcasts:

