DLVO theory
The DLVO theory is named after Boris Derjaguin and Lev Landau, Evert Verwey and Theodoor Overbeek.
The theory explains the aggregation of aqueous dispersions quantitatively and describes the force between charged surfaces interacting through a liquid medium.
It combines the effects of the van der Waals attraction and the electrostatic repulsion due to the so-called double layer of counterions.
The electrostatic part of the DLVO interaction is computed in the mean field approximation in the limit of low surface potentials - that is when the potential energy of an elementary charge on the surface is much smaller than the thermal energy scale,  .
For two spheres of radius
.
For two spheres of radius  each having a charge
each having a charge  (expressed in units of the elementary charge)
separated by a center-to-center distance
(expressed in units of the elementary charge)
separated by a center-to-center distance  in a fluid of dielectric constant
in a fluid of dielectric constant  containing a concentration
containing a concentration 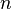 of monovalent ions,
the electrostatic potential takes the form of a screened-Coulomb or Yukawa repulsion,
of monovalent ions,
the electrostatic potential takes the form of a screened-Coulomb or Yukawa repulsion,
where  is the Bjerrum length,
is the Bjerrum length,
 is the Debye–Hückel screening length,
which is given by
is the Debye–Hückel screening length,
which is given by  , and
, and
 is the thermal energy scale at absolute temperature
is the thermal energy scale at absolute temperature 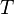 .
.
Podcasts:

