
Volume (thermodynamics)
In thermodynamics, the volume of a system is an important extensive parameter for describing its thermodynamic state. The specific volume, an intensive property, is the system's volume per unit of mass. Volume is a function of state and is interdependent with other thermodynamic properties such as pressure and temperature. For example, volume is related to the pressure and temperature of an ideal gas by the ideal gas law.
The physical volume of a system may or may not coincide with a control volume used to analyze the system.
Overview
The volume of a thermodynamic system typically refers to the volume of the working fluid, such as, for example, the fluid within a piston. Changes to this volume may be made through an application of work, or may be used to produce work. An isochoric process however operates at a constant-volume, thus no work can be produced. Many other thermodynamic processes will result in a change in volume. A polytropic process, in particular, causes changes to the system so that the quantity  is constant (where
is constant (where  is pressure,
is pressure,  is volume, and
is volume, and 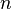 is the polytropic index, a constant). Note that for specific polytropic indexes a polytropic process will be equivalent to a constant-property process. For instance, for very large values of
is the polytropic index, a constant). Note that for specific polytropic indexes a polytropic process will be equivalent to a constant-property process. For instance, for very large values of 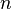 approaching infinity, the process becomes constant-volume.
approaching infinity, the process becomes constant-volume.
Podcasts:
-
by Pitboss
-
by Pitboss
-
by Pitboss
-
by Pitboss
-
by Pitboss
-
by Pitboss
-
by Pitboss
-
by Pitboss
-
by Pitboss
-
by Pitboss
-
by Pitboss
-
by Booty Bass
-
by Booty Bass
Banerio
by: PitbossLet's be honest, simple and plain
No one's as ugly as the singer from Bane
Bucked ass teeth, the color of toffee
You should use Crest instead of gargling with coffee
I look at you and it hurts my eyes
You look like you've been bobbing for french fries
Crumb cake complexion, busted as hell
Is that your teeth, or is your tongue just in jail?
You might get mad, and you'll wanna' smack me
But don't hate me, hate your abundance of acne
So, I hear you have game
How.....who knows?
Proof positive
Latest News for: btps
BTP Short Term and BTPs€I Indexed to euro-zone Inflation Issuance (Ministry of Economy and Finance of the Italian Republic)
Public Technologies 21 Mar 2025- 1

