Abstract
Background
The incidence of syncope exhibits a daily pattern with more occurrences in the morning, possibly due to influences from the endogenous circadian system and/or the daily pattern of behavioral/emotional stimuli. This study tested the hypothesis that the circadian system modulates cardiovascular responses to postural stress, leading to increased susceptibility to syncope at specific times of day.
Methods and Results
Twelve subjects underwent a 13-day in-laboratory protocol, in which subjects’ sleep-wake cycles were adjusted to 20 hours for 12 cycles. A 15-minute title-table test (60° head-up) was performed ~4.5 hours after scheduled awakening in each cycle so that twelve tests in each subject were distributed evenly across the circadian cycle. Out of 144 tests, signs/symptoms of presyncope were observed in 21 tests in 6 subjects. These presyncope events displayed a clear circadian rhythm (P=0.028) with 17 cases (81%) in the circadian phase range corresponding to ~22:30-10:30 (4.25 times of the probability from the other half of the circadian cycle). Significant circadian rhythms were also observed in hemodynamic and autonomic function markers (blood pressure, heart rate, epinephrine, norepinephrine, and indices of cardiac vagal tone) that may underlie the circadian rhythm of presyncope susceptibility.
Conclusion
The circadian system affects cardiovascular responses to postural stressors resulting in greater susceptibility to presyncope during the biological night. This finding suggests that night-shift workers and people with disrupted sleep at night may have great risk of syncope due to their exposure to postural stressors during the biological night.
Keywords: syncope, circadian rhythm, cardiovascular response
Syncope is a sudden and transient loss of consciousness due to transient global cerebral hypoperfusion.1 As a common presenting problem in health care settings, syncope accounts for ~1% of emergency room visits.1,2 Syncope may cause major morbidity such as fractures and motor vehicle accidents (~6% of patients) and minor injuries such as laceration and bruises (~29% of patients)1. The impacts of syncope on public safety (e.g., syncope while driving) has also attracted recent interest.3,4
The most common type of syncope is vasovagal syncope (VVS), which is associated with hypotension and/or bradycardia, and neurally mediated by vagal excess and sympathetic withdrawal. 1,5 VVS accounts for >77% of reported syncope episodes and occurs more frequently in young individuals.2 The incidence of VVS displays a daily pattern with a broad peak in the morning (6:00-12:00).6-8 The peak conceivably could be caused by the day/night distribution of behavioral stressors that may trigger VVS, such as standing up in the morning following an overnight sleep with nocturnal diuresis and redistribution of body fluids9. Alternatively, the daily pattern of syncope could be influenced by the circadian pacemaker that coordinates/generates endogenous rhythms of ~24 hours in numerous neurophysiological processes including cardiac autonomic function,10,11 possibly making the cardiovascular system more vulnerable to stressors at specific circadian times. Hossmann et al. explored this possibility by examining sympathetic and adrenergic vascular responses of 5 healthy subjects every 3 hours across a 24-hour period.12 The study demonstrated clearly 24-hour rhythms in sympathetic responses to tilt with greater responses at 9:00 and 21:00.12 However, the observed rhythms were likely a combined effect of the endogenous circadian cycle and the sleep-wake cycle because (i) the two oscillations are normally synchronized; and (ii) subjects were allowed to sleep between 23:00-7:00 in which subjects were only awoken ~5 minutes before each test and kept awake throughout the ~30-minute test so that the influences of sleep or sleep inertia cannot be excluded.13 Therefore, the independent influences of the endogenous circadian system on vasovagal response are still unknown.
An important tool for the VVS diagnosis is the head-up tilt-table test.1 By introducing orthostatic stress, the tilt-table test is widely used to reproduce syncope in patients susceptible to hypotension and bradycardia.1 Autonomic cardiovascular regulation plays a major role in the pathogenesis of VVS.14 We hypothesize that the endogenous circadian system contributes to the daily pattern of vasovagal episodes via influences on the autonomic control, resulting in different physiological responses to the same postural stressor at different circadian times. To test this hypothesis, we examined symptomatic, hemodynamic and autonomic responses to tilt-table testing at different circadian times (see Methods).
Methods
Subjects
We studied 12 healthy subjects (6 female; 20-42 years old) who had no medical disorders, syncopal attacks, orthostatic hypotension (OH) or impaired autonomic function (see details in Supplemental Methods). Subjects were recruited by putting advertisements for healthy volunteers in local New England newspapers. To ensure regular oscillations of the circadian pacemaker, subjects reported no shift work for the prior three years and not crossing more than one time zone for the prior three months. Additionally, subjects maintained regular sleep-wake cycles with 8-hour sleep opportunity per night for 2-3 weeks before undergoing a 13-day in-laboratory protocol. Subjects were free of drugs (including caffeine, alcohol, and nicotine) prior to and throughout the study. The study was approved by the local Institutional Review Board. Subjects provided informed consent prior to participation.
Experimental protocol
To assess endogenous circadian influences on vasovagal responses, we utilized a “forced desynchrony” protocol (FD) with each subject living in a private and constant-temperature laboratory room free of time cues (Figure 1). Following two baseline days (the same sleep-wake schedule as the preceding 2-3 weeks at home), subjects underwent twelve 20-hour sleep-wake cycles (FD phase) with 6 hours and 40 minutes of scheduled sleep each cycle. This protocol during the FD phase was performed in dim light (~0 lux during scheduled sleep and ~1.8 lux during wakefulness) to avoid circadian phase resetting effects of light. Thus, the circadian pacemaker oscillates at its inherent rate (~24 hours), decoupled from the 20-hour sleep-wake cycles. The same behaviors, including tilt-table testing and mild steady–state cycle exercise, were repeated in each sleep-wake cycle so all behaviors became uniformly distributed across the circadian cycle by the end of the protocol. The responses to exercise across the circadian cycle in these subjects have been published elesewhere.15.
Figure 1.

A Graphical representation of the forced desynchrony protocol. Solid black areas indicate scheduled sleep, light-grey is wakefulness in dim light (~1.8 lux), hatched is wakefulness on baseline days in normal room light (~90 lux), and dark-grey bars indicate scheduled tilt tests.
Tilt-table test
A 15-minute passive head-up tilt test was performed at ~4.5 h after scheduled waking for each sleep-wake cycle. Prior to the test, subjects remained in bed in the semi-recumbent position (45° upper body) for ~4 h during which the subjects ate breakfast (~3 h prior to tilt), rested and completed computerized questionnaires/tests. Thereafter, subjects were gently slid from the bed to the adjacent tilt-table, and remained relaxed in the horizontal and supine position throughout a 25-minute baseline period. Then subjects were tilted to 60° from the horizontal position (head-up with foot plate support) using a motorized tilting mechanism (Model T7605, Metron Medical Australia Pty Ltd, Carrum Downs, Victoria, Australia), and maintained this posture for up to 15 minutes. Note that there was a tilt test during the baseline day so that subjects were partially habituated before the FD phase.
The safety procedures involved monitoring (i) systolic blood pressure (SBP) via an oscillometric arm cuff sphygmomanometer (Dinamap, Critikon INC, Tampa, FL) at least every 3 minutes during the tilt phase, (ii) beat-to-beat SBP changes from finger plethysmography (Portapres, TNO-Biomedical, Amsterdam, Netherlands), (iii) ECG for heart rate (HR) and arrhythmias, and (iv) symptoms (see Data acquisition). Tests were aborted whenever any of the following signs and/or symptoms appeared (criteria for presyncope):
sustained low SBP of <80 mmHg (detected via sphygmomanometer), or 15 mmHg below baseline (excluding cases with stable SBP>100 mmHg);
precipitous SBP decrease of >15 mmHg in <60 seconds (detected via finger plethysmography), leading to low SBP<80 mmHg or 15 mmHg below baseline;
HR decrease >20 bpm from baseline (bradycardia) or asystole for ≥5 sec;
other symptoms of imminent syncope including feeling faint, nauseous, tunnel vision/blacking out, and being unresponsive to questions, or subjects’ request of being tilted down due to these symptoms.
In case of presyncope, the table was rapidly lowered to the horizontal position and the subject maintained the supine posture. If vitals did not normalize immediately or symptoms did not disappear within 30 minutes after tilting down, medical assistance would be called (no such case occurred).
Data acquisition
Blood pressure (BP) was continuously measured using finger plethysmography. This device cannot accurately estimate absolute levels of brachial artery pressure and a cross-reference was made to a more accurate automatic oscillometric cuff sphygmomanometer every 5 minutes during baseline and at least every 3 minutes during tilt.
ECG waveforms were continuously recorded at 256 Hz using an ambulatory recording device (Vitaport, Temec Instruments, Kerkrade, Netherlands) to assess autonomic nervous system activity.
Core body temperature (CBT) was sampled every minute throughout using a rectal temperature sensor (Yellow Springs Instrument Company, OH, USA) to provide a marker of the circadian phase.
Epinephrine and norepinephrine were measured from blood samples collected following ~10 minute baseline and 5-10 minutes tilt in each test using chemiluminescent assays (sensitivity = 1 pg/mL and 40 pg/mL for epinephrine and norepinephrine, respectively) as markers of sympathetic activity. Blood was sampled via an indwelling catheter in a forearm vein that was in place throughout the entire study. Only 5 cc of blood was drawn each time.
The degrees of nausea, “feeling hot”, and general discomfort were rated, separately, by subjects at least every 3 minutes during tilt using an 11-point rating scale with 0 indicating no symptoms and 10 indicating extreme symptoms. In each test, the maximum scores were used as indices of the subjective tilt response.
Data Analysis
To assess tilt responses, cardiovascular variables during the baseline and the stable tilt conditions were compared. Data during the first minute after tilting up and the last minute before tilting down were excluded to avoid influences of the postural transitions.
Circadian phase estimation
The endogenous circadian cycle affects CBT with a period of ~24 hours and, thus, can be estimated by nonlinear regression of individual CBT recordings (Supplemental Figure I).16 Using the obtained circadian period of each subject (mean = 24.09 hours; range: 23.8-24.6 hours) and assigning phase 0° to the time of each subject’s minimum CBT (mean ~4:30), all data were assigned the respective circadian phases (0°-360°) (Supplemental Figure II).
Heart rate variability (HRV) analysis
To assess autonomic nervous system activity, the time domain HRV analysis was performed. R waves in the ECG were identified using a QRS wave detector based on amplitude and the first derivative of the ECG waveform,17 and were visually scanned by a trained technician to ensure that only normal R waves were included. Mean and standard deviation of normal-to-normal beat intervals (SDNN) were calculated along with the following indices of cardiac parasympathetic tone: square root of the mean of squares of differences between adjacent NN intervals (RMSSD), and percentage of differences between adjacent normal-to-normal intervals that are >50 msec (pNN50). The frequency-domain HRV analysis was also performed (Supplemental Methods and Figures III-IV).
Blood pressure analysis
Using Beatscope® Software (version 1.1, Finapres Medical Systems), beat-to-beat SBP, diastolic BP (DBP), HR, stroke volume (SV), cardiac output (CO), ejection time (EJT) and total peripheral resistance (TPR) were derived from BP waveforms (finger plethysmography). The low-frequency power of SBP was also obtained as an additional index of sympathetic activity changes (Supplemental Materials and Figures V-VI).
Statistical Analysis
All tilt tests within a subject were treated as repeated measures without causal relationship among adjacent tests with the assumption that 20 hours is sufficient for full recovery should presyncope have occurred in a prior test. Four types of statistical analyses were performed: (i) To address the primary goal of assessing the circadian distribution of presyncope events, a generalized linear mixed model (GLMM) was used with presyncopeas the response (present or absent), circadian bin as a fixed effect (divided into six 60° bins), and subject as a random factor for intercept (Supplemental Table I). In this primary analysis, test outcomes of all subjects (total 144 tests) were included. (ii) In a secondary analysis, subjects were divided into two groups: those with presyncope (presyncopal group) and those who did not experience presycnope (non-presyncopal group). To assess effects of tilt, group, and their interactions on continuous physiological variables, mixed model ANOVAs were performed with subject as a random effect for intercept (Supplemental Table II). (iii) Similar mixed models were applied to assess different tilt responses between trials with and without presyncope within the presyncopal group (72 tests; Supplemental Table III). (iv) Cosinor analyses 18 using mixed models were applied to test effects on physiological variables of circadian phase and its interactions with tilt (see Supplemental Methods and Table IV). Actual circadian phases of data (instead of 60° bins) were used in the cosinor analyses.
Results
Presyncope during tilt-table testing
Twenty-one cases of presyncope occurred in 6 subjects. In all 21 cases, signs/symptoms of presyncope disappeared almost instantaneously after tilting down, and BP and subjective symptoms normalized within 3 minutes after tilting down. No cases of fully-developed syncope occurred presumably because the tests were aborted when clear signs/symptoms of presyncope appeared. Figure 2 showed a typical presyncope event that is clearly a vasovagal event, indicated by a delayed and precipitous decrease in SBP occurring after ~12 minutes of tilt, along with bradycardia at the end of the test. In other presyncope events, the phase of reflex bradycardia and/or vasodepressor effects accompanying VVS were not always as pronounced because we aborted the tests early to avoid syncope and to reduce the burden on the volunteers during this prolonged and intensive study. In addition to the SBP decrease, significant falls in SV and CO, without a fall in TPR were observed just before presyncope occurred (Supplemental Figure XII).
Figure 2.
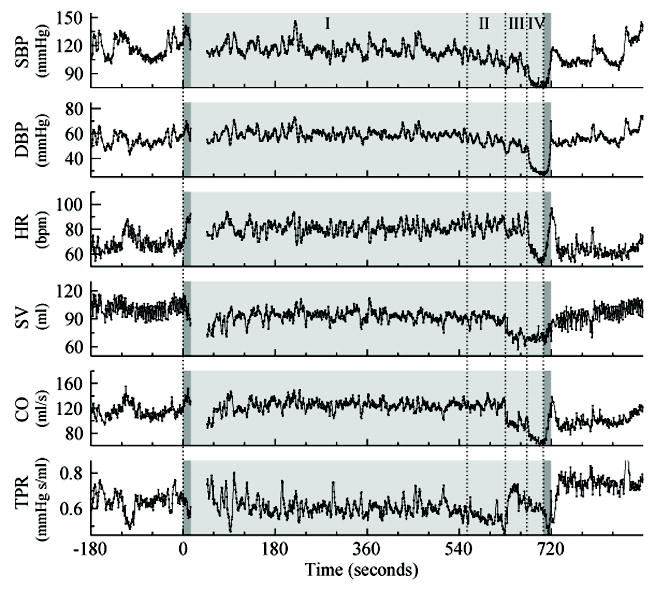
Systolic blood pressure (SBP), diastolic blood pressure (DBP), heart rate (HR), stroke volume (SV), cardiac output (CO) and total peripheral resistance (TPR) estimated via finger plethysmography in one subject during a tilt test with presyncope. Data are shown for the 3-minute baseline, the tilt test (light-grey region), and the 3-minute recovery period. Two dark-grey highlighted regions are the transitions during tilting up and tilting down. Data are missing at ~15-45 seconds due to a recalibration. The sequence of changes during tilt showed four distinct phases, separated by dashed vertical lines: I (0-555 seconds). Immediately upon tilting HR increased and SV decreased while SBP, DBP and TPR were relatively stable. All variable were relatively stable during this phase; II (555-630 seconds). TPR, SBP and DBP were steadily decreasing with maintained HR, SV and CO; III (630-672 seconds). SV and CO decreased abruptly, TPR increased rapidly and then decreased steadily, HR was maintained, and SBP and DBP were steadily decreasing; IV (672-710 seconds). SBP, DBP, HR and CO decreased abruptly with no compensatory increase in SV. Note that TPR continued decreasing precipitously at the beginning of the transition of tilting down when HR increased/recovered quickly. After returning to the supine posture TPR quickly recovered and remained elevated above baseline for ~10 minutes (3 minutes shown); HR and DBP normalized within 30 seconds; and SBP, SV and CO normalized after ~ 2 minutes.
Endogenous circadian rhythm in presyncope events
The 21 presyncope events did not occur randomly across the circadian cycle but displayed a significant circadian rhythm (GLMM P=0.028, Figure 3B) with a peak at the circadian phase bin centered around 0° (corresponding to ~4:30). Figure 3A shows an example subject with tilt tests aborted consistently during the biological night, and Figure 3B shows the group average probability of presyncope occurrence at different circadian phases. The mean probability across the biological night and early morning (270°-90°; corresponding to 22:30-10:30) is ~16.8%, ~9 times of the probability from the other half of the circadian cycle (Figure 3B). There was no training effect on presyncope occurrences through the protocol (11 presyncope events in the first 6 cycles and 10 in the last 6 cycles). The distribution of presyncope events at different cycles confirmed a strong circadian influence indicated by two peaks located at cycles 3 and 9 when tilt tests were performed during the biological night (~3:45) (Supplemental Figure VII).
Figure 3.

Circadian variations in susceptibility to presyncope. A. SBP recordings (finger plethysmography) of a representative subject with presyncope. Data are shown for the 10 minutes of baseline, the tilt test (grey highlighted region), and the recovery period. Four presyncope events (Cycles 2, 8-10) occurred at 300° (1 case), 0° (1 case), and 60° (2 cases). All presyncope cases were associated with precipitous SBP drops. B. Probability of presyncope occurrences across all circadian phases. Results were obtained from the generalized linear mixed model (Supplemental Table I), and are double plotted to better visualize rhythmicity with circadian phase on the lower abscissa and the corresponding habitual time of day on the upper abscissa. Error bars are standard errors. Grey bars indicate the average habitual sleep period in home environment.
Classification of presyncope events
The presyncope events could be divided into two categories: (i) 17 cases were associated with a precipitous SBP drop (detected with finger plethysmography) leading to either 15 mmHg below baseline (detected with sphygmomanometry; 4 cases), SBP<80 mmHg (1 case), or both (12 cases). Sphygmomanometric SBP within 2 minutes before tilting down was 81.1±5.1 (SE) mmHg in these 17 trials (baseline SBP: 99.8±3.2 mmHg). Twelve of these 17 cases (including the one with only SBP<80 mmHg) were also associated with symptoms of imminent syncope (Criterion 4). (ii) 4 cases were associated with only symptoms of imminent syncope without hypotension, i.e., SBP=89.0±4.6 mmHg when being tilted down (baseline SBP=97.4±2.1 mmHg). No presyncope cases were based on a sustained low SBP (15 mmHg below baseline and <100 mmHg) (Criterion 1). No cases of serious arrhythmias or asystole occurred.
Both VVS and syncope due to orthostatic hypotension (OH) can be triggered by orthostatic stress, and their manifestations often overlap.1 We classified all 21 presyncope events as vasovagal presyncope rather than OH for the following reasons. (i) From a pathophysiological point of view, the difference between VVS and OH is that OH is due to autonomic function failure1,19 while VVS is due to intermittently inappropriate cardiovascular reflexes in response to a trigger. Here all subjects were healthy adults without a history of OH or impaired autonomic function. Thus, OH was not expected in this group. (ii) All presyncope events occurred after >4 minutes of tilt (Mean±SE: 10.7±0.6 minutes; Supplemental Figure VIII). This is different from classical OH induced syncope/presyncope that occurs within 3 minutes of standing or tilt.1 (iii) Delayed OH can cause syncope/presyncope after 3 minutes of tilt. However, delayed OH was characterized by progressive decreases of SBP and TPR after tilt-up.20 None of the 21 cases fit such a description of delayed OH.
Cardiovascular responses to head-up tilt
To determine whether there were any underlying physiological differences that could explain the sporadic occurrence of presyncope, comparisons were performed: (i) between non-presyncopal and presyncopal subjects, and (ii) between the 21 presyncope trials and the 51 trials without presyncope in presyncopal subjects. The following results only reflected the responses during the stable tilt period.
General effects of head-up tilt
In responses to tilt, subjects showed decreased SBP, increased DBP, increased HR, increased sympathetic activity (epinephrine and norepinephrine levels), and decreased vagal tone (SDNN, RMSSD and pNN50) (Figure 4). The tilt effects on autonomic nervous activity were confirmed by the frequency-domain HRV analysis and the SBP spectral analysis (Supplemental Figures III and V).
Figure 4.

Physiological responses to head-up tilt, and their differences between the non-presyncopal (circles) and the presyncopal (squares) groups. Data are presented as Mean±SE. Shown are P values for tilt effects, mean group differences, and the interaction between group and tilt stressor. Results were obtained from the mixed model ANOVAs (Supplemental Table II). Here “NS” indicates P >0.1.
Group differences
Presyncopal subjects had reduced tilt responses in all variables except for epinephrine and HR compared to the non-presyncopal subjects (see P values for “Tilt x Group” in Figure 4). There were no significant differences in group means of all variables except that SBP was lower in the presyncopal group.
Physiological responses in presyncope trials
Within the presyncopal subjects, the SBP drop during the presyncope trials was larger than that during the trials without presyncope (Figure 5A). The tilt-induced increases of DBP and norepinephrine were less in the presyncope trials though the reductions did not reach significance (DBP: mixed model ANOVA P=0.05; norepinephrine: P=0.08; Figures 5D-E).
Figure 5.

Physiological responses to head-up tilt, and their differences between 51 completed trials (squares) and 21 aborted trials (triangles) within the presyncopal group. Data are presented as Mean±SE, where Mean was obtained by averaging the individual means for presyncopal trials and non-presyncopal trials separately and SE indicates between-subject error. Shown are P values for the effects of presyncope and its interaction with tilt effect. Results were obtained from the mixed model ANOVAs (Supplemental Table III). Here “NS” indicates P >0.1.
Circadian rhythmicity
There were overall (both conditions) significant circadian rhythms in all reported physiological variables: SBP, HR, epinephrine and norepinephrine were lowest and cardiac parasympathetic markers (SDNN, pNN50 and RMSSD) were highest during the biological night (285°–45° or ~21:30–5:30) (Figure 6 and Table 1). The circadian influences were smaller than the tilt effects on all variables except for SBP which yielded similarly sized effects.
Figure 6.

Circadian influences on indices of hemodynamics and autonomic activity and their responses to head-up tilt. The data (symbols) and the cosinor fits (lines) are plotted separately for baseline (squares and continuous lines) and head-up tilt (circles and dashed lines). Grey bars indicate the average habitual sleep period in home environment. Data are presented as Mean±SE across subjects. Shown are the P values for circadian influences and interaction between tilt and circadian influences. Results were obtained from the cosinor analyses using mixed-model ANOVAs (see Supplemental Methods) and are double plotted to better visualize rhythmicity with circadian phase on the lower abscissa and the corresponding habitual time of day on the upper abscissa.
Table 1.
Peak/trough phase locations and amplitudes of circadian rhythms. Data are presented as Mean±SE. Results were calculated from the cosinor fits (Figure 6) that were obtained from the cosinor analyses using the mixed model ANOVAs (Supplemental Table IV).
| Baseline | Tilt | P (circadian) | ||
|---|---|---|---|---|
| SBP (mmHg) | Peak phase | 231.7°±11.8° | 254.1°±9.6° | 0.0079 |
| Trough phase | 340.7°±15.2° | 357.7°±9.4° | ||
|
| ||||
| Peak-to-trough amplitude | 4.6±1.5 | 5.6±1.5 | ||
|
| ||||
| Tilt effect | -2.6±0.6 | |||
|
| ||||
| DBP (mmHg) | Peak phase | 233.3°±26.4° | 275.5°±9.1° | 0.012 |
| Trough phase | 119.2°±27.4° | 173.6°±10.6° | ||
|
| ||||
| Peak-to-trough amplitude | 1.9 ±1.1 | 3.9 ±1.1 | ||
|
| ||||
| Tilt effect | 9.3 ±0.4 | |||
|
| ||||
| HR (bpm) | Peak phase | 189.8°±24.4° | 177.0°±10.9° | <0.0001 |
| Trough phase | 318.1°±22.5° | 55.0°±18.2° | ||
|
| ||||
| Peak-to-trough amplitude | 5.9±2.2 | 8.9±2.2 | ||
|
| ||||
| Tilt effect | 25.9±0.9 | |||
|
| ||||
| SDNN (msec) | Peak phase | 35.6°±8.9° | 345.9°±19.0° | <0.0001 |
| Trough phase | 265.0°±21.4° | 228.4°±14.1° | ||
|
| ||||
| Peak-to-trough amplitude | 25.3±4.8 | 15.2±5.0 | ||
|
| ||||
| Tilt effect | -25.5 ±2.0 | |||
|
| ||||
| Epinephrine (pg/ml) | Peak phase | 217.2°±25.5° | 208.7°±11.7° | <0.0001 |
| Trough phase | 328.7°±20.4° | 329.3°±9.0° | ||
|
| ||||
| Peak-to-trough amplitude | 10.6±5.5 | 28.6±5.4 | ||
|
| ||||
| Tilt effect | 25.0±2.2 | |||
|
| ||||
| Norepinephrine (pg/ml) | Peak phase | 120.9°±28.9° | 202.3°±13.4° | 0.00023 |
| Trough phase | 352.2°±36.9° | 324.5°±15.3° | ||
|
| ||||
| Peak-to-trough amplitude | 40.7±20.8 | 81.7±21.1 | ||
|
| ||||
| Tilt effect | 163.9±8.7 | |||
|
| ||||
| RMSSD (msec) | Peak phase | 8.8°±25.3° | 113.8°±26.6° | 0.00048 |
| Trough phase | 203.6°±29.7° | 221.7°±21.1° | ||
|
| ||||
| Peak-to-trough amplitude | 17.9 ±4.2 | 8.6±5.0 | ||
|
| ||||
| Tilt effect | -41.5±2.0 | |||
|
| ||||
| pNN50 (%) | Peak phase | 5.7°±69.1° | 96.8°±48.5° | 0.0026 |
| Trough phase | 188.3°±21.7° | 220°±43.7° | ||
|
| ||||
| Peak-to-trough amplitude | 10.6 ±2.8 | 4.1±3.3 | ||
|
| ||||
| Tilt effect | -31.9±1.4 | |||
During the biological night, the tilt-induced epinephrine increase was smaller and the tilt-induced decreases in RMSSD and pNN50 were larger than during the biological day (Figure 6). For instance, the epinephrine increase at 330° (~15.0 pg/ml) was much smaller than the increase at ~200° (~33.1 pg/ml).
Subjective responses to head-up tilt
A different subjective scoring system was used in the first two subjects (one presyncopal and one non-presyncopal) so those results were excluded from the group analyses of subjective tilt responses. There were no group mean differences between presyncopal and non-presyncopal subjects in their maximum scores of “nausea”, “feeling hot” and “general discomfort” (Supplemental Figure XIII). Within the presyncopal subjects, all subjective responses were significantly higher when presyncope occurred: nausea: 0.9±0.5 (SE) without presyncope vs. 4.6±0.6 for presyncope trials; “feeling hot”: 1.6±0.5 vs. 4.1±0.6 respectively; general discomfort: 1.1±0.5 vs. 4.6±0.6 respectively (mixed model ANOVA p<0.0001 for all three measures). Significant circadian rhythms were observed in all subjective measures with a trough at ~130°–200° (~13:30–17:50) and a peak at ~340°–10° (~3:10–5:10). These circadian rhythms showed no significant group differences except that nausea displayed a larger circadian variation in the presyncopal group (cosinor analyses P=0.0017; Supplemental Figure XIII).
Discussion
We found in healthy subjects that the susceptibility to presyncope due to head-up tilt was much higher during circadian phases corresponding to the biological night (equivalent to ~22:30–10:30 in these subjects). The symptoms of nausea and general discomfort during tilt also became worse across the biological night. These findings provide strong evidence that the circadian system modulates the vasovagal responses, yielding different responses to the same stressor at different circadian times. We also found significant circadian rhythms in indices of hemodynamics and autonomic activity such as SBP, DBP, HR, epinephrine, norepinephrine, and HRV-derived parasympathetic markers which likely underlie the circadian modulation of vasovagal responses.
Blood pressure regulation during tilt-table testing
Arterial BP while upright is predominantly maintained through the regulation of the sympathetic outflow leading to increased HR, cardiac contractility and peripheral vasoconstriction. We found that SBP was lower in presyncopal subjects than non-presyncope subjects (Figure 4A), suggesting a threshold effect whereby lower baseline SBP is more likely to be associated with presyncope. Moreover, SBP is lower during the biological night, thereby increasing the likelihood of presyncope at that time. A previous study suggested that a steep fall in cardiac output is the main mechanism in the initiation of a vasovagal faint21. Our study supports such cardiac output-mediated mechanism of the initiation of hypotension, as cardiac output and stroke volume decreased significantly while total peripheral resistance showed no significant decrease before tilted down before the occurrences of presyncope (Supplemental Figure XII).
Changes in neurohumoral factors can be important mechanisms underlying development of syncope/presyncope during head-up tilt. As demonstrated in this study, circadian influences on epinephrine and norepinephrine may contribute to the observed circadian rhythm of presyncope, e.g., lower epinephrine during the biological night when presyncope occurred more frequently. The mechanistic link between endogenous circadian rhythms of neurohumoral factors and presyncope is yet to be elucidated.
Tilt-table test reproducibility
The head-up tilt-table test is widely used for the VVS diagnosis.1 However, reproducibility of the test is still a concern. The long-term reproducibility of positive tilt responses (with presyncope/syncope) varies from 62% to 85%22 and one-day reproducibility may be as low as 35%.23 The current study likely represents one of the best controlled tilt-table data sets because all tests were performed at the same time after waking-up, with all scheduled events rigorously controlled for all test cycles. We showed that is the circadian time when tests are performed is a very important source of variation e.g., the chance of experiencing presyncope at the circadian phase 0° (~4:30) was >20 times larger than that at 180° (~16:30). Thus, conceivably nocturnal tilt tests could potentially be a sensitive method to reveal individuals at higher risks for syncope. The observed presyncope events might be interpreted as “false positive” responses since these subjects had no history of syncope. However, other studies have suggested that vasovagal susceptibility is probably present in all healthy humans.24 Thus, it is possible that subjects might be asymptomatic mostly because they have never previously experienced orthostatic stress during nighttime.
Limitations
While an endogenous circadian rhythm in presyncope susceptibility is very clear, there are certain limitations regarding the underlying mechanism:
We studied healthy young subjects without a history of syncope. While VVS can occur in young and ostensibly healthy people5, it is important to validate our findings in populations more susceptible to VVS.
The underlying mechanism causing presyncope/syncope may be different during passive postural changes (as in this study) compared to standing up actively when the leg muscle contractions help maintain venous return and SBP.25
The four presyncope events based on only symptoms of imminent syncope without hypotension might not have developed into syncope. However, the circadian rhythm of presyncope events remains after excluding the 4 cases (Supplemental Figure IX).
It is conceivable that more presyncope/syncope events would have occurred if tilt was extended beyond 15 minutes. However, the peak of presyncope incidence occurred at 11-12 minutes of tilt (Supplemental Figure VIII). Thus, the choice of the maximum tilt duration is unlikely to have affected the observed circadian rhythm of presyncope events.
Among our criteria for presyncope, using a relatively arbitrary absolute SBP threshold for aborting the tilt test (Criterion 2: SBP< 80 mmHg) could artificially introduce more aborted tests when baseline SBP was lower. However, this is unlikely as only one presyncope event was based on this threshold criterion alone, and this one case did not occur in the biological night.
Additionally, the subjective responses in all trials before pre-syncope occurred also displayed similarly phased circadian rhythms as the rhythm in presyncope. Thus, the circadian rhythm of presyncope distribution is likely to reflect a real effect of the circadian system upon VVS susceptibility.
Potential implications
This study provides direct evidence that the circadian pacemaker influences vasovagal responses to head-up tilt, leading to higher susceptibility to presyncope overnight. Such a vulnerable time window may not be a concern for people with normal sleep-wake schedules who would sleep through the vulnerable period without exposure to postural stressors. However, the vulnerable time window may have a greater impact on individuals who have to remain awake or wake up during the nighttime, such as shift workers, military personnel, emergency workers, airline pilots, truck drivers, parents of infants, and people with nocturia, insomnia or other sleep disorders. Such populations may be at a higher risk for syncope, which could have important consequences on personal and public safety.
Although most syncope events occur during the daytime, previous studies also documented vasovagal syncope during the normal hours of sleep (e.g., 10PM-7AM) in non-intoxicated adults, who wake up feeling faint and may briefly lose consciousness in bed or immediately upon standing26. The occurrence of such nocturnal syncope (termed “sleep syncope”) has been a puzzle. Jardine et al. hypothesized that “sleep syncope” may be linked to different cardiovascular responses to afferent stimuli during sleep (e.g., the centrally mediated increase in vagal activity during the deeper phases of non-REM sleep)27. Our finding of the highest presyncope risk at ~4:30AM mediated by the circadian system (Figure 3B) suggests that the endogenous circadian system may be also mechanistically involved in the pathophysiology of “sleep syncope”. The possible underlying mechanisms include the observed circadian-modulated increase in parasympathetic nervous activity, decreased sympathetic nervous system activity, decreased SBP (Figure 6), and decreased cardiac output (Supplemental Figure XI) during the biological night.
Supplementary Material
Acknowledgments
We thank Dr. Wei Wang for statistical consultation.
Funding/Support This research was supported by National Institutes of Health grants R01-HL76409, K24-HL76446, NCRR-GCRC-MO1-RR02635, P30-HL101299, K99HL102241, and a KL2 Medical Research Investigator Training (MeRIT) grant (5 KL2 RR025757-02) awarded via Harvard Catalyst | The Harvard Clinical and Translational Science Center.
Footnotes
Disclosures None
References
- 1.The European Society of Cardiology Guidelines for the diagnosis and management of syncope reviewed by Angel Moya, MD, FESC, Chair of the Guideline Taskforce with J. Taylor, MPhil. Eur Heart J. 2009;30:2539–2540. doi: 10.1093/eurheartj/ehp393. [DOI] [PubMed] [Google Scholar]
- 2.Olde Nordkamp LR, van Dijk N, Ganzeboom KS, Reitsma JB, Luitse JS, Dekker LR, Shen WK, Wieling W. Syncope prevalence in the ED compared to general practice and population: a strong selection process. Am J Emerg Med. 2009;27:271–279. doi: 10.1016/j.ajem.2008.02.022. [DOI] [PubMed] [Google Scholar]
- 3.Curtis AB, Epstein AE. Syncope while driving: how safe is safe? Circulation. 2009;120:921–923. doi: 10.1161/CIRCULATIONAHA.109.890335. [DOI] [PubMed] [Google Scholar]
- 4.Sorajja D, Nesbitt GC, Hodge DO, Low PA, Hammill SC, Gersh BJ, Shen WK. Syncope while driving: clinical characteristics, causes, and prognosis. Circulation. 2009;120:928–934. doi: 10.1161/CIRCULATIONAHA.108.827626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wieling W, Smit AA, de Jong-de Vos van Steenwijk CC, van Lieshout JJ, Karemaker JM. Pathophysiological mechanisms underlying vasovagal syncope in young subjects. Pacing Clin Electrophysiol. 1997;20:2034–2038. doi: 10.1111/j.1540-8159.1997.tb03622.x. [DOI] [PubMed] [Google Scholar]
- 6.Mineda Y, Sumiyoshi M, Tokano T, Yasuda M, Nakazato K, Nakazato Y, Nakata Y, Yamaguchi H. Circadian variation of vasovagal syncope. J Cardiovasc Electrophysiol. 2000;11:1078–1080. doi: 10.1111/j.1540-8167.2000.tb01751.x. [DOI] [PubMed] [Google Scholar]
- 7.van Dijk N, Boer MC, De Santo T, Grovale N, Aerts AJ, Boersma L, Wieling W. Daily, weekly, monthly, and seasonal patterns in the occurrence of vasovagal syncope in an older population. Europace. 2007;9:823–828. doi: 10.1093/europace/eum104. [DOI] [PubMed] [Google Scholar]
- 8.Zoghi M, Duygu H, Gungor H, Nalbantgil S, Ozerkan F, Akilli A, Akin M. Circadian and infradian rhythms of vasovagal syncope in young and middle-aged subjects. Pacing Clin Electrophysiol. 2008;31:1581–1584. doi: 10.1111/j.1540-8159.2008.01229.x. [DOI] [PubMed] [Google Scholar]
- 9.Omboni S, Smit AA, van Lieshout JJ, Settels JJ, Langewouters GJ, Wieling W. Mechanisms underlying the impairment in orthostatic tolerance after nocturnal recumbency in patients with autonomic failure. Clin Sci (Lond) 2001;101:609–618. [PubMed] [Google Scholar]
- 10.Hilton MF, Umali MU, Czeisler CA, Wyatt JK, Shea SA. Endogenous circadian control of the human autonomic nervous system. Comput Cardiol. 2000;27:197–200. [PubMed] [Google Scholar]
- 11.Scheer FAJL, Ter Horst GJ, Van der Vliet J, Buijs RM. Physiological and anatomic evidence for regulation of the heart by suprachiasmatic nucleus in rats. Am J Physiol Heart Circ Physiol. 2001;280:H1391–H1399. doi: 10.1152/ajpheart.2001.280.3.H1391. [DOI] [PubMed] [Google Scholar]
- 12.Hossmann V, Fitzgerald GA, Dollery CT. Circadian rhythm of baroreflex reactivity and adrenergic vascular response. Cardiovasc Res. 1980;14:125–129. doi: 10.1093/cvr/14.3.125. [DOI] [PubMed] [Google Scholar]
- 13.Scheer FA, Shea TJ, Hilton MF, Shea SA. An endogenous circadian rhythm in sleep inertia results in greatest cognitive impairment upon awakening during the biological night. J Biol Rhythms. 2008;23:353–361. doi: 10.1177/0748730408318081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldstein DS, Holmes C, Frank SM, Naqibuddin M, Dendi R, Snader S, Calkins H. Sympathoadrenal imbalance before neurocardiogenic syncope. Am J Cardiol. 2003;91:53–58. doi: 10.1016/s0002-9149(02)02997-1. [DOI] [PubMed] [Google Scholar]
- 15.Scheer FA, Hu K, Evoniuk H, Kelly EE, Malhotra A, Hilton MF, Shea SA. Impact of the human circadian system, exercise, and their interaction on cardiovascular function. Proc Natl Acad Sci U S A. 2010;107:20541–6. doi: 10.1073/pnas.1006749107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brown EN, Czeisler CA. The statistical analysis of circadian phase and amplitude in constant-routine core-temperature data. J Biol Rhythms. 1992;7:177–202. doi: 10.1177/074873049200700301. [DOI] [PubMed] [Google Scholar]
- 17.Friesen GM, Jannett TC, Jadallah MA, Yates SL, Quint SR, Nagle HT. A comparison of the noise sensitivity of nine QRS detection algorithms. IEEE Trans Biomed Eng. 1990;37:85–98. doi: 10.1109/10.43620. [DOI] [PubMed] [Google Scholar]
- 18.Nelson W, Tong YL, Lee JK, Halberg F. Methods for cosinor-rhythmometry. Chronobiologia. 1979;6:305–323. [PubMed] [Google Scholar]
- 19.Gibbons CH, Freeman R. Delayed orthostatic hypotension: a frequent cause of orthostatic intolerance. Neurology. 2006;67:28–32. doi: 10.1212/01.wnl.0000223828.28215.0b. [DOI] [PubMed] [Google Scholar]
- 20.Podoleanu C, Maggi R, Oddone D, Solano A, Donateo P, Croci F, Carasca E, Ginghina C, Brignole M. The hemodynamic pattern of the syndrome of delayed orthostatic hypotension. J Interv Card Electrophysiol. 2009;26:143–149. doi: 10.1007/s10840-009-9429-0. [DOI] [PubMed] [Google Scholar]
- 21.Verheyden B, Liu J, van Dijk N, Westerhof BE, Reybrouck T, Aubert AE, Wieling W. Steep fall in cardiac output is main determinant of hypotension during drug-free and nitroglycerine-induced orthostatic vasovagal syncope. Heart Rhythm. 2008;5:1695–1701. doi: 10.1016/j.hrthm.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 22.Parry SW, Kenny RA. The management of vasovagal syncope. QJM. 1999;92:697–705. doi: 10.1093/qjmed/92.12.697. [DOI] [PubMed] [Google Scholar]
- 23.Brooks R, Ruskin JN, Powell AC, Newell J, Garan H, McGovern BA. Prospective evaluation of day-to-day reproducibility of upright tilt-table testing in unexplained syncope. Am J Cardiol. 1993;71:1289–1292. doi: 10.1016/0002-9149(93)90542-k. [DOI] [PubMed] [Google Scholar]
- 24.Brignole M. Vasovagal syncope and vasovagal disease. Hellenic J Cardiol. 2008;49:61–64. [PubMed] [Google Scholar]
- 25.Sherwood L. The Blood Vessels and Blood Pressure. Fundamentals of Physiology: A Human Perspective. Cengage Learning. 2005:275–314. [Google Scholar]
- 26.Krediet CT, Jardine DL, Cortelli P, Visman AG, Wieling W. Vasovagal syncope interrupting sleep? Heart. 2004;90:e25. doi: 10.1136/hrt.2003.031294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jardine DL, Krediet CT, Cortelli P, Wieling W. Fainting in your sleep? Clin Auton Res. 2006;16:76–78. doi: 10.1007/s10286-006-0314-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


