- Department of Neuroscience, Carleton University, Ottawa, ON, Canada
Consolidation processes, involving synaptic and systems level changes, are suggested to stabilize memories once they are formed. At the synaptic level, dendritic structural changes are associated with long-term memory storage. At the systems level, memory storage dynamics between the hippocampus and anterior cingulate cortex (ACC) may be influenced by the number of sequentially encoded memories. The present experiment utilized Golgi-Cox staining and neuron reconstruction to examine recent and remote structural changes in the hippocampus and ACC following training on three different behavioral procedures. Rats were trained on one hippocampal-dependent task only (a water maze task), two hippocampal-dependent tasks (a water maze task followed by a radial arm maze task), or one hippocampal-dependent and one non-hippocampal-dependent task (a water maze task followed by an operant conditioning task). Rats were euthanized recently or remotely. Brains underwent Golgi-Cox processing and neurons were reconstructed using Neurolucida software (MicroBrightField, Williston, VT, USA). Rats trained on two hippocampal-dependent tasks displayed increased dendritic complexity compared to control rats, in neurons examined in both the ACC and hippocampus at recent and remote time points. Importantly, this behavioral group showed consistent, significant structural differences in the ACC compared to the control group at the recent time point. These findings suggest that taxing the demand placed upon the hippocampus, by training rats on two hippocampal-dependent tasks, engages synaptic and systems consolidation processes in the ACC at an accelerated rate for recent and remote storage of spatial memories.
Introduction
Consolidation is distinguished into two specific components, synaptic, and systems consolidation. Synaptic consolidation occurs shortly after acquisition and refers to changes at the cellular level (McGaugh, 2000; Kandel, 2001; Dudai, 2004, 2012; Frankland and Bontempi, 2005; Alberini et al., 2006). Systems consolidation refers to a gradual process of reorganization and rearrangement in the brain circuits which support the storage of memories, shifting the storage of memories from an initial encoding circuit to a circuit for long-term storage (McGaugh, 2000; Kandel, 2001; Dudai, 2004, 2012; Frankland and Bontempi, 2005; Alberini et al., 2006).
Synaptic consolidation processes involve the induction of signaling cascades and second messenger systems, protein synthesis and gene expression alterations, all of which may be important synaptic pathways in the initiation of long-term memory storage processes (Kandel, 2001; Dudai, 2004, 2012; Alberini et al., 2006). Once these pathways have been initiated, structural changes, in the form of dendritic complexity, often ensue and are proposed to provide a substrate for the storage of long-term, or remote, memories (Bailey and Kandel, 1993; Trommald et al., 1996; Yang et al., 2009). Therefore, examining differences in dendritic complexity in distinct brain regions following a behavioral task may allow one to infer the involvement of that particular brain region in storing the memory representation for the task.
The hippocampus critically contributes to the initial encoding and storage of memory representations (Scoville and Milner, 1957; Squire, 1992; Cohen and Eichenbaum, 1993). It is hypothesized that as time passes, there is a shift in the balance of neural activity from the hippocampus to cortical regions in the representation of a remote memory. Evidence for the involvement of the anterior cingulate cortex (ACC) in representing remote memories is mounting. Studies have shown increased activity in the ACC on tests for remote (~30 days after encoding) memory (Bontempi et al., 1999; Frankland et al., 2004; Maviel et al., 2004; Teixeira et al., 2006; Lopez et al., 2012; Weible et al., 2012), inactivation of the ACC has been shown to hinder performance on remote memory tests (Bontempi et al., 1999; Frankland et al., 2004; Maviel et al., 2004; Teixeira et al., 2006; Holahan and Routtenberg, 2007; Ding et al., 2008; Restivo et al., 2009; Lopez et al., 2012) and structural changes, indicative of memory storage within the ACC, have been observed at remote time points (Restivo et al., 2009; Vetere et al., 2011; Weible et al., 2012).
The present experiment investigated whether taxing the demand placed upon the hippocampus, by training rats on two hippocampal-dependent tasks, would alter systems consolidation process between the hippocampus and ACC. Rats were trained on one hippocampal-dependent task (the water maze; WM), two different hippocampal-dependent tasks (the WM followed by the radial arm maze; RAM) or one hippocampal-dependent and one non-hippocampal-dependent task (the WM followed by operant conditioning; OP). Because impaired hippocampal function does not alter OP task performance (Shull and Holloway, 1985; Gallagher and Holland, 1992; Corbit and Balleine, 2000; Carvalho et al., 2001) but does impair WM (Morris et al., 1982, 1986; Sutherland et al., 1983, 2001; D'Hooge and De Deyn, 2001; Remondes and Schuman, 2004; Clark et al., 2005a,b; Broadbent et al., 2006; Teixeira et al., 2006; Ramos, 2009; Wiltgen et al., 2010; Holahan and Routtenberg, 2011) and RAM (Jarrard, 1978; Olton et al., 1978; Olton and Papas, 1979; Bouffard and Jarrard, 1986) performance, the OP task is classified as non-hippocampal dependent and the WM and RAM are classified as hippocampal dependent. As such, sequential training on the WM and RAM tasks may result in the activation of similar neural pathways within the hippocampus and result in an accelerated systems consolidation. To examine this hypothesis, dendritic complexity was analyzed in the ACC and CA1 at recent and remote time points using the Golgi-Cox method.
Materials and Methods
Subjects
Forty-eight Long Evans rats (190–250 g) from Charles River, Quebec were used. Rats were housed individually in clear plastic cages (26 × 20 × 45 cm) and given ad libitum water under a 12-h light/dark cycle (lights on at 8:00 a.m.; rats tested during the light phase). Rats received no nesting material and no direct enrichment of any kind in their home cage. Food was restricted until rats reached 90% of their free-feeding baseline, which was maintained throughout the experiment. Prior to behavioral training, rats were given 5 chocolate pellets (45 mg) in their home cage and handled for 5 min daily. Principles of laboratory animal care were followed and all procedures were conducted in accordance with the Canadian Council on Animal Care and protocols approved by the Carleton University Animal Care Committee.
Apparatus
Water maze (WM)
The WM was located in a room within the animal housing area. The opaque, white, polypropylene pool measured 155 cm in diameter and 60 cm in height. The pool was filled to a depth of 37.5 cm with water that remained at approximately 21°C. The “escape” platform was made from clear Plexiglas and submerged approximately 2 cm below the surface of the water. Visual cues such as posters and geometric shapes were located on the walls around the room. The experimenter remained in the same position throughout all trials.
Radial arm maze (RAM)
RAM testing was done in a room within the animal housing area, located across the hall from the WM testing room. The maze was positioned 98.5 cm off the floor. Each arm measured 59 cm long and 11 cm wide. The distance between the ends of arms, where food (chocolate pellet, BioServe, New Jersey) reward was located, was 32.5 cm. Plastic inserts were placed on the sides of the maze arms to prevent animals from jumping across arms. Visual cues such as posters and geometric shapes were located on the walls around the room. The experimenter remained in the same position throughout all trials.
Operant conditioning (OP)
Rats were tested in groups of six using six operant chambers (Habitest Operant Cage, Coulbourn Instruments; 12”W X 10”D X12”H). Each chamber was housed in an insulated box to minimize external noise. Each chamber possessed a pellet dispensing system, two levers separated by a food hopper, a houselight, a grid floor and a three light-panel. OP training took place in a room outside of the animal housing area.
Behavioral Procedure
An overview of the behavioral procedure is shown in Figure 1. Rats were assigned to one of three behavioral groups: (1) Training on one hippocampal-dependent task, the WM; (2) Training on two, different, hippocampal-dependent tasks, the WM followed by the RAM; or (3) Training on one hippocampal-dependent task and one non-hippocampal-dependent task, the WM followed by OP. Sequential training on two tasks was separated by a 24 h rest period. A group of control rats received no behavioral training. Following training, rats were sacrificed recently (8 days after the end of WM training, n = 24) or remotely (37 days after the end of WM training, n = 24). Eight experimental groups resulted (n = 6 in each group): (1) Control:Recent (2) WM:Recent (3) WM/RAM:Recent (4) WM/OP:Recent (5) Control:Remote (6) WM:Remote (7) WM/RAM:Remote (8) WM/OP:Remote.
Water maze
Rats received 5 training trials per day for 5 days, with a different starting location for every trial within a day and randomized starting locations across days. The hidden platform was located in a fixed location within and across days. Rats were placed in the pool, facing the perimeter, and given a maximum of 60 s to locate the hidden platform. Rats that did not find the hidden platform within 60 s were guided to the platform by the experimenter. All rats remained on the platform for 15 s. Rats then received a 15 s rest period in a holding cage before the next trial. All movement within the pool was tracked using HVS Image 2100 Tracking System (HVS Image, Buckingham, UK). Following the final trial of each day, rats were dried with a towel and placed in a holding cage on a heating pad in the housing room for 10–15 min after which they were returned to the home cage.
Radial arm maze
Rats received one day of pretraining and 4 days of testing on the RAM. On the first trial of pretraining, chocolate pellets were located in the starting area, at the entrance to arms, within the arms as well as in the food holes located at the end of each arm. On trial 2 of pretraining, pellets were located within the arms and in food holes only. Trials 3–5 of pretraining had pellets located only in food holes.
Days 2–5 were testing days, where pellets were located in food holes at the ends of 3 of the 5 arms. Baited arms were always the same for an individual rat but differed between rats. Each rat was given 5 trials per day. Trials were a maximum of 5 min each or ended when all food reward had been collected. Rats were placed in a holding cage for 30 s between trials while arms were re-baited. Performance on the maze was manually scored. Sessions were timed, and correct and incorrect arm entries were recorded. An arm entry was defined as all four feet inside an arm.
Operant conditioning
Rats in the WM/OP condition were trained to lever-press for chocolate pellets over 5 days, 30 min per day. Upon pressing the lever to the left of the hopper two times (FR2), the house light extinguished, the panel lights above the lever changed from red to green and the pellet dispenser released one 45-mg chocolate pellet (BioServe, New Jersey) into the hopper. Presses on the right lever had no programmed consequences. Presses on the left lever were considered correct and presses on the right lever, incorrect. The number of lever presses, the number of times a rat poked its nose into the hopper and locomotor activity were recorded automatically (Graphic State Notation 2 Version 2.002-00, Coulbourn Instruments).
Golgi-Cox Procedure
The Golgi method, originally named the “Black Reaction” was developed by Camillo Golgi in 1873. The invention of the Golgi method marked a huge advancement in the field of neuroscience, largely thanks to Santiago Ramon y Cajal's use of the method. Curiously, and fortunately, the Golgi method stains only a small percentage of cells (estimates range between 1 and 5% Spacek, 1989; Milatovic et al., 2010). This is one of the great advantages of the Golgi method: if every neuron were to become impregnated and stained, it would be impossible to trace a single neuron from its beginning to end amongst all the processes from other neurons. The seemingly random staining seen in the Golgi method provides the opportunity to examine individual neurons. Even presently, it is not well understood why some neurons become impregnated with Golgi solution and why some do not. Some factors suggested to play a role include the functional or metabolic state of a neuron at the time of Golgi fixation or mechanical injury from tissue processing rendering a neuron more susceptible to impregnation (Spacek, 1989; Panese, 2010). The Golgi-Cox method used in this study is a variation on the original Golgi method. This variation uses potassium dichromate and mercuric chloride and is well-suited for analyses in rats (Glaser and Van der Loos, 1981; Gibb and Kolb, 1998). The Golgi-Cox method is considered appropriate for the purposes of studying dendritic morphology (Buell, 1982; Ranjan and Mallick, 2010).
Rats were placed into a Decapicone (Braintree) and decapitated. Brains were rapidly removed and hemisected (hemispheres were counterbalanced). One hemisphere was placed in Golgi-Cox fixative and stored at room temperature, away from light, for 14 days. Following incubation in Golgi-Cox fixative, brains underwent 3 washes in DH2O (4 h, 3 h, and 8 h; fresh DH2O each time). Next, brains underwent incubation in 10% (8 h), 20% (8 h), and 30% sucrose (minimum 4 days). 200 μm thick sections were sliced on a vibratome and mounted onto gelatinized slides. Slides were placed in a humidified, dark box for 24 h before staining.
Slides were immersed in the following solutions: DH2O (1 min), 28% Ammonium Hydroxide (40 min), DH2O (1 min), Kodak film fix A (40 min), DH2O (2 × 1 min), 50, 70, and 95% ETOH (1 min each), desiccated 100% ETOH (3 × 5 min), desiccated ETOH/Clearene/Chloroform solution (10 min), and desiccated Clearene (2 × 15 min). Slides were coverslipped with generous amounts of Permount mounting medium (Sigma) and placed in a desiccated box to dry for a minimum of 4 days.
Quantitative Neuromorphology
Three pyramidal neurons per rat were analyzed from each brain region. Four rats from each behavioral group were examined. All neuron reconstruction was performed by one experimenter who was unaware of the experimental condition. Brain regions were defined according to Paxinos and Watson (1998). The ACC area of interest was constricted in the anterior–posterior plan from approximately bregma +1.7 to +0.7 mm. The medial border was the midline of the brain and the lateral border was the corpus callosum. Neurons from layer II/III of the ACC were analyzed. The CA1 area of interest focused on the anterior aspect of the dorsal hippocampus and was constricted in the anterior–posterior plan from approximately bregma −3.1 to −4.0 mm.
Neurons to be traced were selected at random, but had to meet predetermined criteria for analysis. Neurons had to be entirely impregnated, staining had to be uniform and complete within processes, the neuron had to be relatively isolated from surrounding impregnated cells or obstructions, and the cell body had to be centrally located within the 200 μm section depth (Anderson et al., 2009). Neurons were reconstructed at 100X magnification using Neurolucida software (MicroBrightField, Williston, VT, USA). Tracings began at the cell body. Apical and basal dendrites were traced in their entirety and all visible spines were marked. For each neuron, dendritic length, total number of branches, and total number of spines were analyzed separately in apical and basal dendrites.
Statistical Analyses
Four rats from each behavioral group were examined; three neurons per rat per brain area (ACC and CA1) were analyzed. Thus, resulting in 12 neurons per behavioral condition per brain region (i.e., 96 reconstructed neurons from the ACC and 96 reconstructed neurons from the CA1). For statistical analysis, the measurements of the three analyzed neurons per rat per brain region were pooled to get one value for each rat (n = 4 per behavioral group). Separate analyses were carried out in the ACC and CA1 of the hippocampus and apical and basal dendrites were analyzed separately. Two-Way ANOVAs in each region for apical and basal dendrites were run with Behavioral group (Control, WM Only, WM/RAM, or WM/OP) and Time (Recent or Remote) as the fixed factors and the characteristic of interest (dendritic length, total number of branches, and total number of spines) as the dependent variables.
Results
Behavioral Training
Figure 2 shows acquisition data for the WM, RAM, and OP tasks. All rats received WM training. Latency to reach the hidden platform was recorded (Figure 2A). A repeated measures ANOVA with day (1–5) as the within-subject factor and behavior (WM Only, WM/RAM, or WM/OP) and time (recent or remote) as the between-subject factors revealed a main effect of day [F(4, 120) = 112.88, p < 0.001] but no main effect of behavior or time.

Figure 2. Behavioral training data. (A) All rats received water maze training. Average latencies to reach the hidden platform during the 5 days of water maze training are displayed. A main effect of day was found. Latency to reach the hidden platform decreased across training day indicating an improvement in performance. No main effect of behavior or time was found. (B) Average number of errors (unbaited arm entries and re-entries) made in the radial arm maze task during the 4 days of training. A main effect of day was found. Errors made decreased across training day indicating an improvement in performance. No main effect of time was found. (C) Average correct lever presses on the operant conditioning task during the 5 days of training. A main effect of day was found. Correct lever presses increased across training day indicating an improvement in performance. No main effect of time was found.
Two groups received training on the WM followed by training on the RAM: one assigned to receive a recent WM probe test and one assigned to receive a remote WM probe (Figure 2B). The number of errors (unbaited arm entries and re-entries) made during training was analyzed with a repeated measures ANOVA with day (2–4) as the within-subject factor and time (recent or remote) as the between-subject factor. Analyses revealed a main effect of day [F(3, 30) = 2.82, p = 0.05] but no main effect of time.
Two groups received training on the WM followed by training on the operant task: one assigned to receive a recent WM probe test and one assigned to receive a remote WM probe (Figure 2C). The number of correct lever presses made during training was analyzed with a repeated measures ANOVA with day (1–5) as the within-subject factor and time (recent or remote) as the between-subject factor. Analyses revealed a main effect of day [F(4, 40) = 12.73, p < 0.001] but no main effect of time.
Neuron Morphology
Cell body size
Cell body size in the ACC was analyzed using a Two-Way ANOVA with Behavior (Control, WM Only, WM/RAM or WM/OP) and time (recent or remote) as the independent variables and cell body size as the dependent variable. No main effect of behavior or time was found (data not shown).
Cell body size in the CA1 of the hippocampus was analyzed using a Two-Way ANOVA with Behavior (Control, WM Only, WM/RAM, or WM/OP) and time (recent or remote) as the independent variables and cell body size as the dependent variable. No main effect of behavior or time was found (data not shown).
Anterior cingulate cortex
Figure 3 displays images of Golgi impregnated neurons and representative graphics of Golgi impregnated neurons from each of the behavioral groups in the ACC.

Figure 3. (A) Images of 4X, 20X, and 100X magnified Golgi impregnated neurons in the ACC. A zoomed-in image of an apical dendrite with dendritic spines is shown in the last panel. (B) Representative graphics of Golgi impregnated neurons in the ACC, traced using Neurolucida software.
Number of branches. Figure 4 displays total number of branches in the ACC in apical and basal dendrites.

Figure 4. Total number of branches in the ACC in apical and basal dendrites. Only significant differences between behavioral groups and their respective control group are displayed. Refer to Results section for all comparison results. *Significantly different from Control:Recent group (p < 0.05); +Significantly different from Control:Remote (p < 0.05).
Apical. A Two-Way ANOVA with Behavioral group (Control, WM Only, WM/RAM, or WM/OP) and time (Recent or Remote) as the fixed factors and number of branches as the dependent variable revealed a main effect of behavior [F(3, 24) = 7.46, p < 0.001] with group WM/RAM displaying the greatest number of branches (16). Fisher's LSD post-hoc analyses revealed group WM/RAM:Recent had significantly greater number of branches compared to groups WM/OP:Remote, Control:Recent, and Control:Remote (p < 0.05). Group WM/RAM:Remote had significantly greater number of branches compared to groups WM Only:Recent, WM/OP:Recent, WM/OP:Remote, Control:Recent, and Control:Remote (p < 0.05). Group WM Only:Remote had significantly greater number of branches compared to group Control:Remote (p < 0.05).
Basal. A Two-Way ANOVA with Behavioral group (Control, WM Only, WM/RAM, or WM/OP) and time (Recent or Remote) as the fixed factors and number of branches as the dependent variable revealed a main effect of behavior [F(3, 24) = 5.98, p < 0.01], with group WM/RAM displaying the greatest number of branches (18). Fisher's LSD post-hoc analyses revealed group WM/RAM:Remote had significantly greater number of branches compared to groups from WM Only:Recent, WM Only:Remote, WM/OP:Remote, Control:Recent, and Control:Remote, (p = 0.05). Group WM/OP:Recent had significantly greater number of branches compared to group Control:Recent (p < 0.05).
Dendritic Length. Figure 5 displays total dendritic length in the ACC in apical and basal dendrites.

Figure 5. Total dendritic length in the ACC in apical and basal dendrites. Only significant differences between behavioral groups and their respective control group are displayed. Refer to Results section for all comparison results. *Significantly different from Control:recent group (p < 0.05); +Significantly different from Control:Remote (p < 0.05).
Apical. A Two-Way ANOVA with Behavioral group (Control, WM Only, WM/RAM, or WM/OP) and time (Recent or Remote) as the fixed factors and dendritic length as the dependent variable revealed a main effect of behavior [F(3, 24) = 5.00, p < 0.01], with group WM/RAM displaying the greatest dendritic length (1016 μm). Fisher's LSD post-hoc analyses revealed group WM/RAM:Recent had significantly greater dendritic length compared to groups WM/OP:Remote, Control:Recent, and Control:Remote (p < 0.05). Group WM/RAM:Remote had significantly greater dendritic length compared to groups WM/OP:Remote, Control:Recent, Control:Remote (p < 0.05). Group WM/OP:Recent had significantly greater dendritic length compared to group Control:Remote (p = 0.05).
Basal. A Two-Way ANOVA with Behavioral group (Control, WM Only, WM/RAM, or WM/OP) and time (Recent or Remote) as the fixed factors and dendritic length as the dependent variable revealed a main effect of behavior [F(3, 24) = 8.52, p < 0.001], with group WM/RAM displaying the greatest dendritic length (839 μm). Fisher's LSD post-hoc analyses revealed group WM/RAM:Recent had significantly greater dendritic length compared to groups WM Only:Recent, WM Only:Remote, WM/OP:Remote, Control:Recent, and Control:Remote (p < 0.05). Group WM/RAM:Remote had significantly greater dendritic length compared to groups WM Only:Recent, WM Only:Remote, WM/OP:Remote, Control:Recent and Control:Remote (p < 0.05).
Number of spines. Figure 6 displays total number of spines in the ACC in apical and basal dendrites.

Figure 6. Total number of spines in the ACC in apical and basal dendrites. Only significant differences between behavioral groups and their respective control group are displayed. Refer to Results section for all comparison results. *Significantly different from Control:recent group (p < 0.05); +Significantly different from Control:Remote (p < 0.05).
Apical. A Two-Way ANOVA with Behavioral group (Control, WM Only, WM/RAM, or WM/OP) and time (Recent or Remote) as the fixed factors and number of spines as the dependent variable revealed a main effect of behavior [F(3, 24) = 6.92, p < 0.01], with group WM/RAM displaying the greatest number of spines (511). Fisher's LSD post-hoc analyses revealed group WM/RAM:Recent had significantly greater number of spines compared to groups WM Only:Recent, WM Only:Remote, WM/OP:Recent, WM/OP:Remote, Control:Recent and Control:Remote (p < 0.05). Group WM/RAM:Remote had significantly greater number of spines compared to groups Control:Recent and Control:Remote (p < 0.05).
Basal. A Two-Way ANOVA with Behavioral group (Control, WM Only, WM/RAM, or WM/OP) and time (Recent or Remote) as the fixed factors and number of spines as the dependent variable revealed a main effect of behavior [F(3, 24) = 10.23, p < 0.001], with group WM/RAM displaying the greatest number of spines (385). Fisher's LSD post-hoc analyses revealed group WM/RAM:Recent had significantly greater number of spines compared to groups WM Only:Recent, WM Only:Remote, WM/OP:Recent, WM/OP:Remote, Control:Recent, Control:Remote (p < 0.05). Group WM/RAM:Remote had significantly greater number of spines compared to groups WM Only:Recent, WM Only:Remote, Control:Recent, and Control:Remote (p < 0.05). Group WM/OP:Recent had significantly greater number of spines compared to groups WM Only:Recent and Control:Recent (p < 0.05).
Spine density. Figure 7 displays spine density in the ACC in apical and basal dendrites.

Figure 7. Spine density in the ACC in apical and basal dendrites. Only significant differences between behavioral groups and their respective control group are displayed. Refer to Results section for all comparison results. *Significantly different from Control:recent group (p < 0.05).
Apical. A Two-Way ANOVA with Behavioral group (Control, WM Only, WM/RAM, or WM/OP) and time (Recent or Remote) as the fixed factors and spine density as the dependent variable revealed no main effects. Planned Fisher's LSD post-hoc comparisons revealed group WM/RAM:Recent had significantly greater spine density compared to group Control:Recent (p < 0.05).
Basal. A Two-Way ANOVA with Behavioral group (Control, WM Only, WM/RAM or WM/OP) and time (Recent or Remote) as the fixed factors and spine density as the dependent variable revealed no main effects. A significant interaction between behavior X time [F(3, 24) = 3.39, p < 0.05] was found. Fisher's LSD post-hoc comparisons revealed group WM/RAM:Recent had significantly greater spine density compared to groups WM Only:Recent, WM Only:Remote, WM/RAM:Remote, and Control:Recent (p < 0.05). Group WM/OP:Recent had significantly greater spine density compared to group Control:Recent (p < 0.05). Group Control:Remote had significantly greater spine density compared to group Control:Recent (p < 0.05).
Summary of ACC findings. Group WM/RAM displayed the greatest and most consistent dendritic complexity in the ACC. At the recent time point, group WM/RAM was the sole group to show significantly increased dendritic complexity in apical dendrites compared to the control group.
CA1 of the hippocampus
Figure 8 displays images of Golgi impregnated neurons and representative graphics of Golgi impregnated neurons from each of the behavioral groups in the CA1.

Figure 8. (A) Images of 4X, 20X, and 100X magnified Golgi impregnated neurons in the CA1. A zoomed-in image of an apical dendrite with dendritic spines is shown in the last panel. (B) Representative graphics of Golgi impregnated neurons in the CA1, traced using Neurolucida software.
Number of branches. Figure 9 displays total number of branches in the CA1 in apical and basal dendrites.
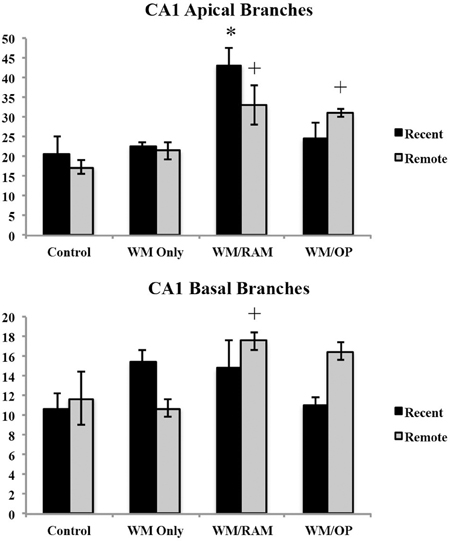
Figure 9. Total number of branches in the CA1 in apical and basal dendrites. Only significant differences between behavioral groups and their respective control group are displayed. Refer to Results section for all comparison results. *Significantly different from Control:recent group (p < 0.05); +Significantly different from Control:Remote (p < 0.05).
Apical. A Two-Way ANOVA with Behavioral group (Control, WM Only, WM/RAM, or WM/OP) and time (Recent or Remote) as the fixed factors and number of branches as the dependent variable revealed a main effect of behavior [F(3, 24) = 12.63, p < 0.001] with group WM/RAM displaying the greatest number of branches (38). Fisher's LSD post-hoc analyses revealed group WM/RAM:Recent had significantly greater number of branches compared to every other behavioral group (p < 0.05). Group WM/RAM:Remote had significantly greater number of branches compared to groups WM Only:Recent, WM Only:Remote, WM/RAM:Recent, Control:Recent, and Control:Remote (p < 0.05). Group WM/OP:Recent had significantly greater number of branches compared to group WM/OP:Remote (p < 0.05). Group WM/OP:Remote had significantly greater number of branches compared to groups WM/RAM:Recent, Control:Recent, and Control:Remote (p < 0.05).
Basal. A Two-Way ANOVA with Behavioral group (Control, WM Only, WM/RAM, or WM/OP) and time (Recent or Remote) as the fixed factors and number of branches as the dependent variable revealed a main effect of behavior [F(3, 24) = 2.98, p = 0.05] with group WM/RAM displaying the greatest number of branches (16). A significant interaction between behavior X time was found [F(3, 24) = 3.42, p < 0.05]. Fisher's LSD post-hoc analyses revealed group WM/RAM:Remote had significantly greater number of branches compared to groups WM Only:Remote, WM/OP:Recent, Control:Recent, and Control:Remote (p < 0.05). Group WM/OP:Recent had significantly fewer number of branches compared to groups WM/RAM:Remote, WM/OP:Remote (p < 0.05). Group WM/OP:Remote had significantly greater number of branches compared to group WM Only:Remote, WM/OP:Recent, and Control:Recent (p < 0.05).
Dendritic length. Figure 10 displays total dendritic length in the CA1 in apical and basal dendrites.

Figure 10. Total dendritic length in the CA1 in apical and basal dendrites. Only significant differences between behavioral groups and their respective control group are displayed. Refer to Results section for all comparison results. *Significantly different from Control:recent group (p < 0.05); +Significantly different from Control:Remote (p < 0.05).
Apical. A Two-Way ANOVA with Behavioral group (Control, WM Only, WM/RAM, or WM/OP) and time (Recent or Remote) as the fixed factors and dendritic length as the dependent variable revealed a main effect of behavior [F(3, 24) = 6.24, p < 0.01], with group WM/RAM displaying the greatest dendritic length (2259 μm). A significant interaction between behavior X time was found [F(3, 24) = 4.04, p < 0.05]. Fisher's LSD post-hoc analyses revealed group WM/RAM:Recent had significantly greater dendritic length compared to groups WM Only:Recent, WM Only:Remote, WM/OP:Recent, Control:Recent, and Control:Remote (p < 0.05). Group WM/RAM:Remote had significantly greater dendritic length compared to group Contol:Remote (p < 0.05). Group WM/OP:Remote had significantly greater dendritic length compared to groups WM Only:Recent, WM Only:Remote, WM/OP:Recent, Control:Recent, and Control:Remote (p < 0.05).
Basal. A Two-Way ANOVA with Behavioral group (Control, WM Only, WM/RAM, or WM/OP) and time (Recent or Remote) as the fixed factors and dendritic length as the dependent variable revealed no main effects. Planned Fisher's LSD post-hoc comparisons revealed group WM/OP:Remote had significantly greater dendritic length compared to groups WM Only:Remote and Control:Recent. Group Control:Recent had significantly less dendritic length compared to groups WM Only:Recent and WM/OP:Remote (p < 0.05).
Number of spines. Figure 11 displays total number of spines in the CA1 in apical and basal dendrites.

Figure 11. Total number of spines in the ACC in apical and basal dendrites. Only significant differences between behavioral groups and their respective control group are displayed. Refer to Results section for all comparison results. *Significantly different from Control:recent group (p < 0.05); +Significantly different from Control:Remote (p < 0.05).
Apical. A Two-Way ANOVA with Behavioral group (Control, WM Only, WM/RAM, or WM/OP) and time (Recent or Remote) as the fixed factors and number of spines as the dependent variable revealed a main effect of behavior [F(3, 24) = 10.81, p < 0.001], with group WM/RAM displaying the greatest number of spines (1793). Fisher's LSD post-hoc analyses revealed group WM/RAM:Recent had significantly greater number of spines compared to groups WM Only:Recent, WM Only:Remote, WM/OP:Recent, Control:Recent, and Control:Remote (p < 0.05). Group WM/RAM:Remote had significantly greater number of spines compared to group WM Only:Recent, WM Only:Remote, WM/OP:Reent, Control:Recent, and Control:Remote (p < 0.05). Group WM/OP:Recent had significantly fewer number of spines compared to groups WM/RAM:Recent, WM/RAM:Remote and WM/OP:Remote (p < 0.05). Group WM/OP:Remote had significantly greater number of spines compared to groups WM Only:Recent, WM Only:Remote, WM/OP:Recent, Control:Recent, and Control:Remote (p < 0.05).
Basal. A Two-Way ANOVA with Behavioral group (Control, WM Only, WM/RAM, or WM/OP) and time (Recent or Remote) as the fixed factors and number of spines as the dependent variable revealed a main effect of behavior [F(3, 24) = 5.07, p < 0.01], with group WM/RAM displaying the greatest number of spines (647). Fisher's LSD post-hoc analyses revealed group WM/RAM:Recent had significantly greater number of spines compared to group Control:Recent (p < 0.05). Group WM/RAM:Remote had significantly greater number of spines compared to groups WM Only:Remote, WM/OP:Recent, Control:Recent, and Control:Remote (p < 0.05). Group WM/OP:Recent had significantly fewer number of spines compared to groups WM/RAM:Remote and WM/OP:Remote (p < 0.05). Group WM/OP:Remote had significantly greater number of spines compared to groups WM Only:Remote, WM/OP:Recent, Control:Recent, and Control:Remote (p < 0.05).
Spine density. Figure 12 displays spine density in the CA1 in apical and basal dendrites.

Figure 12. Spine density in the CA1 in apical and basal dendrites. Only significant differences between behavioral groups and their respective control group are displayed. Refer to Results section for all comparison results. *Significantly different from Control:recent group (p < 0.05); +Significantly different from Control:Remote (p < 0.05).
Apical. A Two-Way ANOVA with Behavioral group (Control, WM Only, WM/RAM or WM/OP) and time (Recent or Remote) as the fixed factors and spine density as the dependent variable revealed a main effect of behavior [F(3, 24) = 3.42, p < 0.05], with group WM/RAM displaying the greatest spine density (0.812). Fisher's LSD post-hoc analyses revealed group WM/RAM:Remote had significantly greater spine density compared to groups WM Only:Recent, WM Only:Remote, WM/OP:Recent, Control:Recent, and Control:Remote (p < 0.05).
Basal. A Two-Way ANOVA with Behavioral group (Control, WM Only, WM/RAM, or WM/OP) and time (Recent or Remote) as the fixed factors and spine density as the dependent variable revealed a main effect of behavior [F(3, 24) = 3.63, p < 0.05], with group WM/RAM displaying the greatest spine density (0.812). Fisher's LSD post-hoc analyses revealed group WM/RAM:Recent had significantly greater spine density compared to group Control:Recent (p < 0.05). Group WM/RAM:Remote had significantly greater spine density compared to groups WM/OP:Recent and Control:Recent (p < 0.05).
Summary of CA1 findings. Group WM/RAM displayed the greatest dendritic complexity in the CA1. Group WM/RAM was the only group to show increased dendritic complexity at the recent time point. Group WM/RAM also showed persistent increases in the dendritic complexity characteristics analyzed as this group was found to have these characteristics significantly increased from the Control group at the remote time point as well. Group WM/OP:Remote consistently showed increases in dendritic complexity at the remote time point.
Discussion
A great deal of human research supports the idea of a time-limited role for the hippocampus in memory storage (Scoville and Milner, 1957; Kapur and Brooks, 1999; Teng and Squire, 1999; Rosenbaum et al., 2000; Manns et al., 2003; Bayley et al., 2006). Within animal research, some evidence suggests hippocampal damage results in temporally graded retrograde amnesia (Kim and Fanselow, 1992; Frankland et al., 2004; Maviel et al., 2004; Teixeira et al., 2006; Ding et al., 2008) whereas other evidence suggests hippocampal damage results in a flat, or ungraded, retrograde amnesia (Mumby et al., 1999; Sutherland et al., 2001, 2008; Clark et al., 2005a,b, 2007; Broadbent et al., 2006; Lehmann et al., 2007, 2013; Epp et al., 2008; Sparks et al., 2011). We propose that the number of memories processed over the lifetime underlies this discrepancy. In humans, there is a seemingly endless sequence of memory storage and retrieval processes, while laboratory rats and mice are typically required to retrieve and store only one memory for one behavioral task. In the absence of multiple memories competing for processing “space” in the hippocampus, a hippocampal-dependent memory may not be as likely to require, and thus form, extra-hippocampal (cortical) connections, thereby relying heavily on hippocampal involvement for an extended period of time. Processing of several hippocampal-dependent memories (resulting from multiple hippocampal-dependent tasks) may compete for hippocampal processing space, resulting in an increased reliance on cortical structures, such as the ACC.
Dendrites represent structures that serve to receive and integrate information (Jan and Jan, 2001). As such, structural changes in dendrites may partially fulfill the neural requirements necessary to maintain memory representations for the long term (Bailey and Kandel, 1993; Trommald et al., 1996). Changes in the dendritic characteristics examined (dendritic length, branching, number of spines, and spine density) collectively grouped as dendritic complexity, serve to increase the available surface area for synaptic contact, increasing the capacity for neuronal connectivity (Ramón y Cajal, 1911). Greater dendritic length and greater dendritic branching increase the number of possible synaptic contacts and information that may be received by a dendrite (Jan and Jan, 2001). Alterations in dendritic characteristics may reflect increased or decreased responses to incoming synaptic information.
Spine remodeling, which includes both spine formation and elimination, has been suggested to play an important role in structural plasticity (Yang et al., 2009). Yang et al. (2009) demonstrated that only a small proportion of new spines that result from novel experiences persist and survive. Thus, number of spines or spine density may not always be an effective indicator of memory storage or retention on its own; spine formation may represent a transient event following experience. Likely, the type of spine (thin, mushroom, etc.) is of greater importance for memory persistence. Unfortunately, spine type was not included in the analysis in the present study as technical limitations prohibited this type of examination.
Results
Examination of neuron morphology included total dendritic length, number of branches, number of spines, and spine density (Hamilton et al., 2010) in the ACC and CA1. Overall, group WM/RAM displayed the most robust increases on all indices evaluated compared to the control groups in both the ACC and CA1. This suggests a greater degree of dendritic complexity in this group. Importantly, group WM/RAM consistently showed increased dendritic complexity at the recent time point in the ACC.
In the ACC, group WM/RAM displayed longer apical and basal dendritic length and more spines, both recently and remotely, compared to the control groups. This group also displayed more branches and increased spine density compared to the control groups. Group WM/OP showed increases across most of the characteristics examined at the recent time point in the ACC, but only in basal dendrites. Group WM Only did not show increased dendritic complexity at the recent time point in the ACC. However, this group did show an increase in the number of apical branches at the remote time point.
In the CA1, group WM/RAM displayed more apical and basal spines, both recently and remotely, compared to the control groups. This group displayed longer dendritic length and more branches and spine density compared to control groups. Group WM/OP showed increases across most of the characteristics analyzed, but only at the remote time point. Group WM Only did not show increased dendritic complexity compared to the control group.
In examining published findings on dendritic complexity, it is important to note that there is a great deal of variability between laboratories (Scorcioni et al., 2004). For example, average apical dendritic lengths in the ACC in other published reports range from 800 to 1500 μm (Radley et al., 2004; Bock et al., 2005; Murmu et al., 2006; Zehle et al., 2007). The apical dendritic length in the present report ranged from 230 to 626 μm. This variance may result from age or strain differences, tissue processing protocols or neuron reconstruction software and techniques.
These results suggest competing hippocampal-dependent memories may result in more pronounced, or accelerated, increases in dendritic complexity in the ACC. Without hippocampal-dependent memories competing for hippocampal processing space, structural changes may be less distinct, or take a greater amount of time to come about. Interestingly, group WM/OP showed increases in dendritic complexity in both the ACC and CA1. As the type of OP task used in this study is not typically dependent upon the hippocampus (Shull and Holloway, 1985; Gallagher and Holland, 1992; Corbit and Balleine, 2000; Carvalho et al., 2001) we presume that there is no immediate competition between memory traces for the WM and OP tasks. However, structural changes indicative of memory storage did present themselves, suggesting that multiple memory processing may result in accelerated and/or more distinct structural changes compared to the processing of just a single memory.
The results presented in this study provide new information regarding structural changes associated with memory storage. Past studies have focused on spine density as the primary measure of structural changes (Restivo et al., 2009; Vetere et al., 2011). Specifically, Restivo et al. (2009) observed an increase in spine density in the CA1 recently, but not remotely, while an increase in spine density was observed remotely, but not recently, in the ACC. Spine density was also examined in the present experiment. Significantly increased apical spine density was noted in group WM/RAM:Recent compared to Control:Recent. In basal dendrites, group WM/RAM:Recent had a significantly higher spine density compared to Control:Recent, the WM Only groups and group WM/RAM:Remote. In the CA1, apical spine density was higher in group WM/RAM:Remote compared to Control:Remote, while basal spine density was higher in group WM/RAM:Recent compared to Control:Recent.
Our results are not consistent with the pattern of spine density found by others. A few important differences between Restivo et al. (2009), Vetere et al. (2011) and the present study should be pointed out. Firstly, the time point described as “recent” differed substantially between the current experiment and those of Restivo et al. (2009) and Vetere et al. (2011). In the present experiment, we defined recent as 8 days following the end of WM training. Restivo and Vetere defined recent as 24 h following conditioning. These represent two very different time points. It may be the case that our “recent” time point comes too late to see an increase in CA1 spine density recently, and a decrease remotely as has been noted before (Restivo et al., 2009; Vetere et al., 2011). Different behavioral training procedures were also used. Restivo et al. (2009) and Vetere et al. (2011) trained mice using a contextual fear conditioning procedure, while the present study trained rats using a water maze procedure (along with the RAM and OP in some groups). Differences in the type and duration of behavioral procedures may play a role as well. Our behavioral training procedure took 5 days (for each procedure) to train, whereas training on contextual fear conditioning can be done in a single day. This may result in differences in the timing of structural changes.
In the present study, structural changes were found in both the ACC and CA1 of the hippocampus. The only experimental difference between the different groups of rats was the behavioral training procedures they received. No probe trial was given prior to sacrifice. Thus, the morphological changes observed were not due to retrieval processes but likely associated with a memory storage process. Group WM/RAM was the only group to show consistent, significant differences in dendritic complexity at the recent time point compared to the control group in the ACC. This finding supports the hypothesis that taxing the demand placed upon the hippocampus, by training rats on two hippocampal-dependent tasks, results in structural changes indicative of memory storage, in the ACC. This is consistent with results from Wartman and Holahan (2013) that showed increased number of c-Fos positive cells in remotely probed groups compared to recently probed groups. In that report, the group trained on two hippocampal-dependent tasks (WM/RAM) and probed recently demonstrated increased c-Fos labeling in the ACC compared to the other recently probed groups, and similar to the level of c-Fos labeling noted in remotely probed groups, suggesting an accelerated reliance on the ACC.
The current study did not examine structural changes associated with training on the RAM or OP tasks alone. In future studies it would be of interest to examine the structural changes resulting from these tasks alone or reversed training on these tasks (i.e., RAM or OP training followed by WM). Future behavioral studies utilizing one-day training procedures are underway so that early structural changes in the ACC and CA1 can be examined. A one-day training procedure would also help to diminish delay differences between the end of training on a behavioral task and neuron analysis. In the present study, there was a difference in the delay between the end of behavioral training and neuron analysis in rats trained on one or two tasks. Training on the RAM or OP tasks entailed 5 days of training followed by a day of rest then sacrifice. Rats trained on only the WM task had 6 days of rest prior to sacrifice. This difference could influence dynamic dendritic characteristics such as spine density. This difference may become more substantial when examining recent vs. remote time points.
Conclusion
Neuron reconstruction data showed robust increases in dendritic complexity in the ACC and CA1 at recent and remote time points in groups trained on two hippocampal-dependent tasks. These results suggest competing hippocampal-dependent memories may result in more pronounced, or accelerated, increases in dendritic complexity in the ACC. Without hippocampal-dependent memories competing for hippocampal processing space, structural changes may be less distinct, or take a longer amount of time to come about. The introduction of a multiple memory behavioral procedure presents an innovative method through which structural changes associated with memory storage can be examined. Introducing variations in the type and number of tasks used during training provides an opportunity to examine and contrast morphological differences throughout the consolidation process, shedding light on the processes underlying remote memory storage.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This research was supported by the National Science and Engineering Research Council of Canada Discovery grants to Matthew R. Holahan (RGPIN 341673) and a Canadian Foundation for Innovation grant (335892) to Matthew R. Holahan. Brianne C. Wartman was supported by an Alexander Graham Bell Post-Graduate Scholarship from the National Science and Engineering Research Council of Canada.
References
Alberini, C. M., Milekic, M. H., and Tronel, S. (2006). Mechanisms of memory stabilization and de-stabilization. Cell. Mol. Life Sci. 63, 999–1008. doi: 10.1007/s00018-006-6025-7
Anderson, K., Bones, B., Robinson, B., Hass, C., Lee, H., Ford, K., et al. (2009). The morphology of supragranular pyramidal neurons in the human insular cortex: a quantitative Golgi study. Cereb. Cortex 19, 2131–2144. doi: 10.1093/cercor/bhn234
Bailey, C. H., and Kandel, E. R. (1993). Structural changes accompanying memory storage. Annu. Rev. Physiol. 55, 397–426. doi: 10.1146/annurev.ph.55.030193.002145
Bayley, P. J., Hopkins, R. O., and Squire, L. R. (2006). The fate of old memories after medial temporal lobe damage. J. Neurosci. 26, 13311–13317. doi: 10.1523/JNEUROSCI.4262-06.2006
Bock, J., Gruss, M., Becker, S., and braun, K. (2005). Experience-induced changes of dendritic spine densities in the prefrontal and sensory cortex: correlation with developmental time windows. Cereb. Cortex 15, 802–808. doi: 10.1093/cercor/bhh181
Bontempi, B., Laurent-Demir, C., Destrade, C., and Jaffard, R. (1999). Time-dependent reorganization of brain circuitry underlying long-term memory storage. Nature 400, 671–675. doi: 10.1038/23270
Bouffard, J. P., and Jarrard, L. E. (1986). Acquisition of a complex place task in rats with selective ibotenate lesions of hippocampal formation: combined lesions of subiculum and entorhinal cortex versus hippocampus. Behav. Neurosci. 102, 828–834. doi: 10.1037/0735-7044.102.6.828
Broadbent, N. J., Squire, L. R., and Clark, R. E. (2006). Reversible hippocampal lesions disrupt water maze performance during both recent and remote memory tests. Learn Mem. 13, 187–191. doi: 10.1101/lm.134706
Buell, S. J. (1982). Golgi-Cox and rapid Golgi methods as applied to autopsied human brain tissue: widely disparate results. J. Neuropathol. Exp. Neurol. 41, 500–507. doi: 10.1097/00005072-198209000-00003
Carvalho, O. M., Silva, A. J., and Balleine, B. W. (2001). Evidence of selective learning deficits on tests of pavlovian and instrumental conditioning in alpha-CaMKIIT286A mutant mice. Int. J. Comp. Psychol. 14, 161–174.
Clark, R. E., Broadbent, N. J., and Squire, L. R. (2005a). Hippocampus and remote spatial memory in rats. Hippocampus 15, 260–272. doi: 10.1002/hipo.20056
Clark, R. E., Broadbent, N. J., and Squire, L. R. (2005b). Impaired remote spatial memory after hippocampal lesions despite extensive training beginning early in life. Hippocampus 15, 340–346. doi: 10.1002/hipo.20076
Clark, R. E., Broadbent, N. J., and Squire, L. R. (2007). The hippocampus and spatial memory: findings with a novel modification of the water maze. J. Neurosci. 27, 6647–6654. doi: 10.1523/JNEUROSCI.0913-07.2007
Cohen, N. J., and Eichenbaum, H. (1993). Memory, Amnesia, and the Hippocampal System. Cambridge, MA: MIT Press.
Corbit, L. H., and Balleine, B. W. (2000). The role of the hippocampus in instrumental conditioning. J. Neurosci. 20, 4233–4239.
D'Hooge, R., and De Deyn, P. P. (2001). Applications of the morris water maze in the study of learning and memory. Brain Res. Rev. 36, 60–90. doi: 10.1016/S0165-0173(01)00067-4
Ding, H. K., Teixeira, C. M., and Frankland, P. W. (2008). Inactivation of the anterior cingulate cortex blocks expression of remote, but not recent, conditioned taste aversion memory. Learn Mem. 15, 290–293. doi: 10.1101/lm.905008
Dudai, Y. (2004). The neurobiology of consolidations, or, how stable is the engram? Annu. Rev. Psychol. 55, 51–86. doi: 10.1146/annurev.psych.55.090902.142050
Dudai, Y. (2012). The restless engram: consolidations never end. Annu. Rev. Neurosci. 35, 227–247. doi: 10.1146/annurev-neuro-062111-150500
Epp, J., Keith, J. R., Spanswick, S. C., Stone, J. C., Prusky, G. T., and Sutherland, R. J. (2008). Retrograde amnesia for visual memories after hippocampal damage in rats. Learn Mem. 15, 214–221. doi: 10.1101/lm.788008
Frankland, P. W., and Bontempi, B. (2005). The organization of recent and remote memories. Nat. Rev. Neurosci. 6, 119–130. doi: 10.1038/nrn1607
Frankland, P. W., Bontempi, B., Talton, L. E., Kaczmarek, L., and Silva, A. J. (2004). The involvement of the anterior cingulate cortex in remote contextual fear memory. Science 304, 881–883. doi: 10.1126/science.1094804
Gallagher, M., and Holland, P. C. (1992). Preserved configural learning and spatial learning impairment in rats with hippocampal damage. Hippocampus 2, 81–88. doi: 10.1002/hipo.450020111
Gibb, R., and Kolb, B. (1998). A method for vibratome sectioning of Golgi-Cox stained whole rat brain. J. Neurosci. Meth. 79, 1–4. doi: 10.1016/S0165-0270(97)00163-5
Glaser, E. M., and Van der Loos, H. (1981). Analysis of thick brain sections by obverse-reverse computer microscopy: application of a new, high clarity Golgi-Nissl stain. J. Neurosci. Meth. 4, 117–125. doi: 10.1016/0165-0270(81)90045-5
Hamilton, G. F., Whitcher, L. T., and Klintsova, A. Y. (2010). Postnatal binge-like alcohol exposure decreases dendritic complexity while increasing the density of mature spines in mPFC layer II/III pyramidal neurons. Synapse 64, 127–135. doi: 10.1002/syn.20711
Holahan, M. R., and Routtenberg, A. (2007). Post-translational synaptic protein modification as substrate for long-lasting, remote memory: an initial test. Hippocampus 17, 93–97. doi: 10.1002/hipo.20245
Holahan, M. R., and Routtenberg, A. (2011). Lidocaine injections targeting CA3 hippocampus impair long-term spatial memory and prevent learning-induced mossy fiber remodeling. Hippocampus 21, 532–540. doi: 10.1002/hipo.20786
Jarrard, L. E. (1978). Selective hippocampal lesions: differential effects on performance by rats of a spatial task with preoperative versus postoperative training. J. Comp. Phys. Psychol. 92, 1119–1127. doi: 10.1037/h0077516
Kandel, E. R. (2001). The molecular biology of memory storage: a dialog between genes and synapses. Biosci. Rep. 21, 565–611. doi: 10.1023/A:1014775008533
Kapur, N., and Brooks, D. J. (1999). Temporally-specific retrograde amnesia in two cases of discrete bilateral hippocampal pathology. Hippocampus 9, 247–254. doi: 10.1002/(SICI)1098-1063(1999)9:3%3C247::AID-HIPO5%3E3.0.CO;2-W
Kim, J. J., and Fanselow, M. S. (1992). Modality-specific retrograde amnesia of fear. Science 256, 675–677. doi: 10.1126/science.1585183
Lehmann, H., Lacanilao, S., and Sutherland, R. J. (2007). Complete or partial hippocampal damage produces equivalent retrograde amnesia for remote contextual fear memories. Eur. J. Neurosci. 25, 1278–1286. doi: 10.1111/j.1460-9568.2007.05374.x
Lehmann, H., Rourke, B. K., Booker, A., and Glenn, M. J. (2013). Single session contextual fear conditioning remains dependent on the hippocampus despite an increase in the number of context-shock pairings during learning. Neurobiol. Learn Mem. 106, 294–299. doi: 10.1016/j.nlm.2012.10.011
Lopez, J., Herbeaux, K., Cosquer, B., Engeln, M., Muller, C., Lazarus, C., et al. (2012). Context-dependent modulation of hippocampal and cortical recruitment during remote spatial memory retrieval. Hippocampus 22, 827–841. doi: 10.1002/hipo.20943
Manns, J. R., Hopkins, R. O., and Squire, L. R. (2003). Semantic memory and the human hippocampus. Neuron 38, 127–133. doi: 10.1016/S0896-6273(03)00146-6
Maviel, T., Durkin, T. P., Menzaghi, F., and Bontempi, B. (2004). Sites of neocortical reorganization critical for remote spatial memory. Science 305, 96–99. doi: 10.1126/science.1098180
McGaugh, J. L. (2000). Memory - a century of consolidation. Science 287, 248–251. doi: 10.1126/science.287.5451.248
Milatovic, D., Montine, T. J., Zaja-Milatovic, S., Madison, J. L., Bowman, A. B., and Aschner, M. (2010). Morphometric analysis in neurodegenerative disorders. Curr. Protoc. Toxicol. 16, 1–14. doi: 10.1002/0471140856.tx1216s43
Morris, R. G., Hagan, J. J., and Rawlins, J. N. (1986). Allocentric spatial learning by hippocampectomised rats: a further test of the spatial mapping and working memory theories of hippocampal function. Q. J. Exp. Psychol. B 38, 365–395.
Morris, R. G. M., Garrud, P., Rawlins, J. N. P., and O'Keefe, J. (1982). Place navigation impaired in rats with hippocampal lesions. Nature 297, 681–683. doi: 10.1038/297681a0
Mumby, D. G., Astur, R. S., Weisend, M. P., and Sutherland, R. J. (1999). Retrograde amnesia and selective damage to the hippocampal formation: memory for places and object discriminations. Behav. Brain Res. 106, 97–107. doi: 10.1016/S0166-4328(99)00097-2
Murmu, M., Salomon, S., Biala, Y., Weinstock, M., Braun, K., and Bock, J. (2006). Changes in spine density and dendritic complexity in the prefrontal cortex in offspring of mothers exposed to stress during pregnancy. J. Neurosci. 24, 1477–1487. doi: 10.1111/j.1460-9568.2006.05024.x
Olton, D. S., and Papas, B. C. (1979). Spatial memory and hippocampal function. Neuropsychologia 17, 669–682. doi: 10.1016/0028-3932(79)90042-3
Olton, D. S., Walker, J. A., and Gage, F. H. (1978). Hippocampal connections and spatial discrimination. Brain Res. 139, 295–308. doi: 10.1016/0006-8993(78)90930-7
Panese, E. (2010). The Golgi stain: invention, diffusion and impact on the neurosciences. J. Hist. Neurosci. 8, 132–140. doi: 10.1076/jhin.8.2.132.1847
Paxinos, G., and Watson, C. (1998). The Rat Brain in Stereotaxic Coordinates, 4th Edn. San Diego, CA: Elsevier Academic Press.
Radley, J. J., Sisti, H. M., Hao, J., Rocher, A. B., McCall, T., Hof, P. R., et al. (2004). Chronic behavioral stress induces apical dendritic reorganization in pyramidal neurons of the medial prefrontal cortex. Neuroscience 125, 1–6. doi: 10.1016/j.neuroscience.2004.01.006
Ramón y Cajal, S. (1911). Histology of the Nervous System of Man and Vertebrates, (1995 translation). Oxford: Oxford University Press.
Ramos, J. M. J. (2009). Remote spatial memory and the hippocampus: effect of early and extensive training in the radial maze. Learn. Mem. 16, 554–563. doi: 10.1101/lm.1480309
Ranjan, A., and Mallick, B. N. (2010). A modified method for consistent and reliable Golgi-Cox staining in significantly reduced time. Front. Neurol. 1:157. doi: 10.3389/fneur.2010.00157
Remondes, M., and Schuman, E. M. (2004). Role for a cortical input to hippocampal area CA1 in the consolidation of a long-term memory. Lett. Nat. 431, 699–703. doi: 10.1038/nature02965
Restivo, L., Vetere, G., Bontempi, B., and Ammassari-Teule, M. (2009). The formation of recent and remote memory is associated with time-dependent formation of dendritic spines in the hippocampus and anterior cingulate cortex. J. Neurosci. 29, 8206–8214. doi: 10.1523/JNEUROSCI.0966-09.2009
Rosenbaum, R. S., Priselac, S., Kohler, S., Black, S. E., Gao, F., Nadel, L., et al. (2000). Remote spatial memory in an amnesic person with extensive bilateral hippocampal lesions. Nat. Neurosci. 3, 1044–1048. doi: 10.1038/79867
Scorcioni, B., Lazarewicz, M. T., and Ascoli, G. A. (2004). Quantitative morphometry of hippocampal pyramidal cells: differences between anatomical classes and reconstructing laboratories. J. Comp. Neurol. 473, 177–193. doi: 10.1002/cne.20067
Scoville, W. B., and Milner, B. (1957). Loss of recent memory after bilateral hippocampal lesions. J. Neurol. Neurosurg. Psychiatry 20, 11–21. doi: 10.1136/jnnp.20.1.11
Shull, R. N., and Holloway, F. A. (1985). Behavioral effects of hippocampal system lesions on rats in an operant paradigm. Brain Res. Bull. 14, 315–322. doi: 10.1016/0361-9230(85)90191-1
Spacek, J. (1989). Dynamics of the Golgi method: a time-lapse study of the early stages of impregnation in single sections. J. Neurocytol. 18, 27–38. doi: 10.1007/BF01188421
Sparks, F. T., Lehmann, H., Hernandez, K., and Sutherland, R. J. (2011). Suppression of neurotoxic lesion-induced seizure activity: evidence for a permanent role for the hippocampus in contextual memory. PLoS ONE 6:e27426. doi: 10.1371/journal.pone.0027426
Squire, L. R. (1992). Memory and the hippocampus: a synthesis from findings with rats, monkeys, and humans. Psychol. Rev. 99, 195–231. doi: 10.1037/0033-295X.99.2.195
Sutherland, R. J., O'Brien, J., and Lehmann, H. (2008). Absence of systems consolidation of fear memories after dorsal, ventral, or complete hippocampal damage. Hippocampus 18, 710–718. doi: 10.1002/hipo.20431
Sutherland, R. J., Weisend, M. P., Mumby, D., Astur, R. S., Hanlon, F. M., Koerner, A., et al. (2001). Retrograde amnesia after hippocampal damage: recent vs. remote memories in two tasks. Hippocampus 11, 27–42. doi: 10.1002/1098-1063(2001)11:1%3C27::AID-HIPO1017%3E3.0.CO;2-4
Sutherland, R. J., Whishaw, I. Q., and Kolb, B. (1983). A behavioural analysis of partial localization following electrolytic kainite-or colchicine-induced damage to the hippocampal formation in the rat. Behav. Brain Res. 7, 133–153. doi: 10.1016/0166-4328(83)90188-2
Teixeira, C. M., Pomedli, S. R., Maei, H. R., Kee, N., and Frankland, P. W. (2006). Involvement of the anterior cingulate cortex in the expression of remote spatial memory. J. Neurosci. 26, 7555–7564. doi: 10.1523/JNEUROSCI.1068-06.2006
Teng, E., and Squire, L. R. (1999). Memory for places learned long ago is intact after hippocampal damage. Lett. Nat. 400, 675–677. doi: 10.1038/23276
Trommald, M., Hulleberg, G., and Anderson, P. (1996). Long-term potentiation is associated with new excitatory spine synapses on rat dentate granule cells. Learn. Mem. 3, 218–228. doi: 10.1101/lm.3.2-3.218
Vetere, G., Restivo, L., Cole, C. J., Ross, P. J., Ammassari-Teule, M., Josselyn, S. A., et al. (2011). Spine growth in the anterior cingulate cortex is necessary for the consolidation of contextual fear memory. Proc. Natl. Acad. Sci. U.S.A. 108, 8456–8460. doi: 10.1073/pnas.1016275108
Wartman, B. C., and Holahan, M. R. (2013). The use of sequential hippocampal-dependent and –non-dependent tasks to study the activation profile of the anterior cingulate cortex during recent and remote memory tests. Neurobiol. Learn. Mem. 106, 334–342. doi: 10.1016/j.nlm.2013.08.011
Weible, A. P., Rowland, D. C., Monaghan, C. K., Wolfgang, N. T., and Kentros, C. G. (2012). Neural correlates of long-term object memory in the mouse anterior cingulate cortex. J. Neurosci. 32, 5598–5608. doi: 10.1523/JNEUROSCI.5265-11.2012
Wiltgen, B. J., Zhou, M., Cai, Y., Balaji, J., Karlsson, M. G., Parivash, S. N., et al. (2010). The hippocampus plays a selective role in the retrieval of detailed contextual memories. Cur. Biol. 20, 1336–1344. doi: 10.1016/j.cub.2010.06.068
Yang, G., Pan, F., and Gan, W. (2009). Stably maintained dendritic spines are associated with lifelong memories. Lett. Nat. 462, 920–925. doi: 10.1038/nature08577
Keywords: hippocampus, anterior cingulate cortex, remote memory, Golgi, memory storage
Citation: Wartman BC and Holahan MR (2014) The impact of multiple memory formation on dendritic complexity in the hippocampus and anterior cingulate cortex assessed at recent and remote time points. Front. Behav. Neurosci. 8:128. doi: 10.3389/fnbeh.2014.00128
Received: 03 February 2014; Accepted: 27 March 2014;
Published online: 21 April 2014.
Edited by:
Katharina A. Braun, Otto von Guericke University Magdeburg GermanyReviewed by:
Menahem Segal, Weizman Institute for Science, IsraelJoerg Bock, Otto von Guericke University Magdeburg, Germany
Copyright © 2014 Wartman and Holahan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Brianne C. Wartman, Department of Neuroscience, Carleton University, 1125 Colonel By Drive, 325 Life Sciences Research Building, Ottawa, ON K1S 5B6, Canada e-mail: [email protected]
 Brianne C. Wartman
Brianne C. Wartman Matthew R. Holahan
Matthew R. Holahan