Advertisement
Article Free access | 10.1172/JCI6218
Potential mechanisms of human natural killer cell expansion in vivo during low-dose IL-2 therapy
Todd A. Fehniger,1,2 Eric M. Bluman,2 Michelle M. Porter,2 Ewa Mrózek,2 Megan A. Cooper,1 Jeffrey B. VanDeusen,1 Stanley R. Frankel,2 Wendy Stock,3 and Michael A. Caligiuri1,2
1Department of Medicine, Division of Hematology/Oncology; Department of Molecular Virology, Immunology, and Medical Genetics, Division of Human Cancer Genetics; and the Comprehensive Cancer Center, The Ohio State University, Columbus, Ohio, USA2Division of Medicine, Roswell Park Cancer Institute, Buffalo, New York, USA3Department of Medicine, University of Illinois, Chicago, Illinois, USA
Address correspondence to: Michael A. Caligiuri, Ohio State University, 458A Starling Loving Hall, 320 West 10th Avenue, Columbus, Ohio 43210, USA. Phone: (614) 293-7521; Fax: (614) 293-7522; E-mail: [email protected].
Find articles by Fehniger, T. in: JCI | PubMed | Google Scholar
1Department of Medicine, Division of Hematology/Oncology; Department of Molecular Virology, Immunology, and Medical Genetics, Division of Human Cancer Genetics; and the Comprehensive Cancer Center, The Ohio State University, Columbus, Ohio, USA2Division of Medicine, Roswell Park Cancer Institute, Buffalo, New York, USA3Department of Medicine, University of Illinois, Chicago, Illinois, USA
Address correspondence to: Michael A. Caligiuri, Ohio State University, 458A Starling Loving Hall, 320 West 10th Avenue, Columbus, Ohio 43210, USA. Phone: (614) 293-7521; Fax: (614) 293-7522; E-mail: [email protected].
Find articles by Bluman, E. in: JCI | PubMed | Google Scholar
1Department of Medicine, Division of Hematology/Oncology; Department of Molecular Virology, Immunology, and Medical Genetics, Division of Human Cancer Genetics; and the Comprehensive Cancer Center, The Ohio State University, Columbus, Ohio, USA2Division of Medicine, Roswell Park Cancer Institute, Buffalo, New York, USA3Department of Medicine, University of Illinois, Chicago, Illinois, USA
Address correspondence to: Michael A. Caligiuri, Ohio State University, 458A Starling Loving Hall, 320 West 10th Avenue, Columbus, Ohio 43210, USA. Phone: (614) 293-7521; Fax: (614) 293-7522; E-mail: [email protected].
Find articles by Porter, M. in: JCI | PubMed | Google Scholar
1Department of Medicine, Division of Hematology/Oncology; Department of Molecular Virology, Immunology, and Medical Genetics, Division of Human Cancer Genetics; and the Comprehensive Cancer Center, The Ohio State University, Columbus, Ohio, USA2Division of Medicine, Roswell Park Cancer Institute, Buffalo, New York, USA3Department of Medicine, University of Illinois, Chicago, Illinois, USA
Address correspondence to: Michael A. Caligiuri, Ohio State University, 458A Starling Loving Hall, 320 West 10th Avenue, Columbus, Ohio 43210, USA. Phone: (614) 293-7521; Fax: (614) 293-7522; E-mail: [email protected].
Find articles by Mrózek, E. in: JCI | PubMed | Google Scholar
1Department of Medicine, Division of Hematology/Oncology; Department of Molecular Virology, Immunology, and Medical Genetics, Division of Human Cancer Genetics; and the Comprehensive Cancer Center, The Ohio State University, Columbus, Ohio, USA2Division of Medicine, Roswell Park Cancer Institute, Buffalo, New York, USA3Department of Medicine, University of Illinois, Chicago, Illinois, USA
Address correspondence to: Michael A. Caligiuri, Ohio State University, 458A Starling Loving Hall, 320 West 10th Avenue, Columbus, Ohio 43210, USA. Phone: (614) 293-7521; Fax: (614) 293-7522; E-mail: [email protected].
Find articles by Cooper, M. in: JCI | PubMed | Google Scholar
1Department of Medicine, Division of Hematology/Oncology; Department of Molecular Virology, Immunology, and Medical Genetics, Division of Human Cancer Genetics; and the Comprehensive Cancer Center, The Ohio State University, Columbus, Ohio, USA2Division of Medicine, Roswell Park Cancer Institute, Buffalo, New York, USA3Department of Medicine, University of Illinois, Chicago, Illinois, USA
Address correspondence to: Michael A. Caligiuri, Ohio State University, 458A Starling Loving Hall, 320 West 10th Avenue, Columbus, Ohio 43210, USA. Phone: (614) 293-7521; Fax: (614) 293-7522; E-mail: [email protected].
Find articles by VanDeusen, J. in: JCI | PubMed | Google Scholar
1Department of Medicine, Division of Hematology/Oncology; Department of Molecular Virology, Immunology, and Medical Genetics, Division of Human Cancer Genetics; and the Comprehensive Cancer Center, The Ohio State University, Columbus, Ohio, USA2Division of Medicine, Roswell Park Cancer Institute, Buffalo, New York, USA3Department of Medicine, University of Illinois, Chicago, Illinois, USA
Address correspondence to: Michael A. Caligiuri, Ohio State University, 458A Starling Loving Hall, 320 West 10th Avenue, Columbus, Ohio 43210, USA. Phone: (614) 293-7521; Fax: (614) 293-7522; E-mail: [email protected].
Find articles by Frankel, S. in: JCI | PubMed | Google Scholar
1Department of Medicine, Division of Hematology/Oncology; Department of Molecular Virology, Immunology, and Medical Genetics, Division of Human Cancer Genetics; and the Comprehensive Cancer Center, The Ohio State University, Columbus, Ohio, USA2Division of Medicine, Roswell Park Cancer Institute, Buffalo, New York, USA3Department of Medicine, University of Illinois, Chicago, Illinois, USA
Address correspondence to: Michael A. Caligiuri, Ohio State University, 458A Starling Loving Hall, 320 West 10th Avenue, Columbus, Ohio 43210, USA. Phone: (614) 293-7521; Fax: (614) 293-7522; E-mail: [email protected].
Find articles by Stock, W. in: JCI | PubMed | Google Scholar
1Department of Medicine, Division of Hematology/Oncology; Department of Molecular Virology, Immunology, and Medical Genetics, Division of Human Cancer Genetics; and the Comprehensive Cancer Center, The Ohio State University, Columbus, Ohio, USA2Division of Medicine, Roswell Park Cancer Institute, Buffalo, New York, USA3Department of Medicine, University of Illinois, Chicago, Illinois, USA
Address correspondence to: Michael A. Caligiuri, Ohio State University, 458A Starling Loving Hall, 320 West 10th Avenue, Columbus, Ohio 43210, USA. Phone: (614) 293-7521; Fax: (614) 293-7522; E-mail: [email protected].
Find articles by Caligiuri, M. in: JCI | PubMed | Google Scholar
Published January 1, 2000 - More info
J Clin Invest. 2000;106(1):117–124. https://doi.org/10.1172/JCI6218.
© 2000 The American Society for Clinical Investigation
Received: January 6, 1999; Accepted: May 23, 2000
-
Abstract
The continuous, in vivo infusion of low-dose IL-2 selectively expands the absolute number of human natural killer (NK) cells after 4–6 weeks of therapy. The mechanism responsible for this expansion is unknown and was examined in this study. NK cells cultured at low concentrations of IL-2, comparable to those found during in vivo therapy, proliferate for 6 days and then exit the cell cycle. However, NK cells in vivo did not traverse the S/G2/M phase of the cell cycle during low-dose IL-2 therapy. Low concentrations of IL-2 delay programmed cell death of NK cells but have the same effect on resting T cells that do not expand in vivo. When CD34+ bone marrow hematopoietic progenitor cells are cultured for 21 days with low concentrations of IL-2, they differentiate into CD56+CD3− NK cells, not T cells. Thus, the selective expansion of human NK cells during continuous in vivo infusion of low-dose IL-2 likely results from enhanced NK-cell differentiation from bone marrow progenitors, combined with an IL-2–dependent delay in NK-cell death, rather than proliferation of mature NK cells in the periphery.
-
Introduction
Natural killer (NK) cells are large granular lymphocytes that secrete IFN-γ and demonstrate spontaneous cytotoxic activity against malignant and virally infected cells (1, 2). All human NK cells express the CD56 antigen, an isoform of the neural cell adhesion molecule. Approximately 10% of NK cells have high-density expression of CD56 (CD56bright) on their surface and constitutively express both the high-affinity heterotrimeric IL-2 receptor (IL-2Rαβγ) and the intermediate-affinity heterodimeric IL-2Rβγ (3, 4). Ninety percent of human NK cells have low-density expression of CD56 (CD56dim) and only express the intermediate-affinity heterodimeric IL-2Rβγ (2). In vitro, picomolar (pM) concentrations of IL-2 bind to the high-affinity IL-2R expressed on CD56bright NK cells, resulting in a strong proliferative response that is not seen in CD56dim NK cells or in resting T cells in the absence of other mitogenic signals (3).
The in vivo administration of low-dose IL-2 by prolonged continuous intravenous infusion or daily subcutaneous injection results in pM concentrations of IL-2 that selectively expand CD56bright NK cells with little change in CD56dim NK or T-cell numbers (5–10). The mechanism by which this expansion occurs in vivo is unknown. We hypothesized that activation of CD56bright NK cells via the high-affinity IL-2Rαβγ led to a proliferative expansion of the population in vivo, similar to that seen in short-term culture in vitro (3, 4). However, our preliminary in vitro analysis did not support this hypothesis (5). In the current report, we analyze human CD56bright NK-cell proliferation, apoptosis, and differentiation in vitro and perform cell-cycle analysis on human CD56bright NK cells undergoing expansion during low-dose IL-2 administration in vivo. We demonstrate that selective expansion of NK cells during prolonged exposure to low concentrations of exogenous IL-2 does not appear to result from mature NK-cell proliferation in vivo. Rather, the in vivo expansion likely results from an IL-2–dependent decrease in NK-cell death and an increase in NK-cell differentiation from hematopoietic progenitor cells (HPCs).
-
Methods
Clinical study. Patients were diagnosed with acute myeloid leukemia (AML) and were judged to be in complete remission (i.e., < 5% blasts and normal blood counts) after uniform induction and consolidation chemotherapy (11). Patients subsequently received a prolonged continuous infusion of low-dose IL-2 therapy (0.45 × 106 IU/m2/d) for 45 consecutive days. After this, patients received the same daily infusion for 11 days followed by a 24-hour infusion of 1 × 106 U/m2/d on day 12, an infusion of 2 × 106 U/m2/d on day 13, and an infusion of 3 × 106 U/m2/d on day 14. This 14-day cycle was repeated for an additional 42 days (6). Recombinant IL-2 (specific activity 1.8 × 107 IU/mg) was supplied by the Chiron Corp. (Emeryville, California, USA). Four patients with AML who received identical induction and consolidation therapy, entered complete remission, but did not receive IL-2 therapy, were also studied. All patients participating in the study signed informed consent approved by the Institutional Review Board of Roswell Park Cancer Institute.
Reagents. Rabbit anti-human IL-2 and control polysera were purchased from PeproTech (Rocky Hill, New Jersey, USA). Murine anti-human CD25 mAb and an isotype control mAb were purchased from Biosource International (Camarillo, California, USA). PE-conjugated anti-CD56 (NKH1-RD1) and anti-CD5 (T1-RD1) mAb’s were purchased from Coulter Immunology (Hialeah, Florida, USA). FITC-conjugated anti-CD3 mAb (Leu 4-FITC) was purchased from Becton Dickinson (San Jose, California, USA). Recombinant human (rh) IL-2 used for in vitro proliferation assays was obtained from Hoffman La-Roche (Nutley, New Jersey, USA; specific activity 1.53 × 107 IU/mg). rhIL-12 was a kind gift of the Genetics Institute (Cambridge, Massachusetts, USA; specific activity 4.5 × 106 U/mg). The rh c-kit ligand (KL) was a gift of Immunex Corp. (Seattle, Washington, USA).
Isolation of human NK cells. Human CD56bright NK cells were isolated to greater than 97% purity from fresh leukopacs (American Red Cross, Buffalo, New York, USA) using immunomagnetic bead depletion and cell sorting as described previously (12). For isolation of T cells, nonadhered PBMCs were stained with anti–CD5-PE and sorted with a FACStar Plus flow cytometer (Becton-Dickinson). Sorted populations were determined to be 97% pure by flow cytometric analysis.
Proliferation, viability, and enumeration assays. Sorted CD56bright NK cells were plated in 96-well plates at a concentration of 2.0 × 104 per 200 μL of medium containing RPMI-1640, 10% human AB serum (C-six Diagnostics, Mequon, Wisconsin, USA), antibiotics, and rhIL-2 at a final concentration of 100 pM (23 IU/mL). Cells were incubated at 37°C, and medium changes were performed every 72 hours by removal of 100 μL and replacement with 100 μL of identical medium containing 100 pM of rIL-2. Twenty-four hours before harvesting, 1 μCi of [3H]thymidine (3H-TdR; Amersham, Bucks, United Kingdom) was added to each well and incubated further at 37°C. Samples were then harvested and counted in a Beckman LS 1801 scintillation counter (Fullerton, California, USA). Standard vital dye exclusion and enumeration assays were performed with trypan blue and a hemocytometer on triplicate wells by an individual blinded to experimental conditions. All assays were performed in triplicate, with results presented as the mean counts per minute (cpm) ± SEM.
Quantitation of IFN-γ production. CD56bright NK cells were isolated and cultured in a combination of IL-2 and IL-12 as described elsewhere (13). Cells were cultured for either 2, 6, or 12 days in the presence of 100 pM IL-2. At these times, IL-12 (10 U/mL) and 1 nM IL-2 were added, and 48 hours after this addition (i.e., days 4, 8, and 14), 200 μL of culture supernatant were harvested from duplicate wells and frozen at –70°C until assayed for IFN-γ protein. Quantitation of human IFN-γ in these supernatants was performed using commercial ELISA kits (sensitivity 2.0 pg/mL; Biosource International), following the manufacturer’s instructions.
Cytotoxicity assays. Sorted CD56bright NK cells were cultured for the indicated periods in 96-well plates at 2 × 104 cells per, well with or without 100 pM rIL-2. At the indicated times, cytotoxicity was measured against K562 target cells in a standard 4-hour 51Cr release assay as described previously (13), with an effector-to-target ratio of 5:1. Assays were performed in triplicate, and results are presented as the percent specific lysis ± SEM.
Cell-cycle analysis. Subsets of peripheral blood lymphocytes (PBLs) were analyzed for mitotic index using Hoechst H33342 dye (Calbiochem-Novabiochem, La Jolla, California, USA), lymphocyte subset specific cell surface mAb’s, and flow cytometry. Hoechst dye is a DNA intercalator that allows determination of the fraction of cells in both the G0/G1 and S/G2/M phases of the cell cycle by the quantity of DNA present (14). Cells were centrifuged and resuspended in 2 mg Hoechst H33342/ml PBS (pH 7.1) at a concentration of 1 × 106 cells/mL, placed in a 37°C shaking water bath for 1 hour, centrifuged, and resuspended in fresh cold 2 mg/mL Hoechst H33342/PBS (pH 7.1) at a concentration of 2 × 106 cells/mL. Cells populations were next labeled with the anti-CD3-FITC or anti-CD56-PE mAb’s, washed twice and resuspended. Cell-cycle determination on distinct lymphocyte subsets was performed on a FACStar Plus by gating on the FITC- or PE-stained cells while analyzing for Hoechst dye fluorescence.
DNA fragmentation assays and cell-cycle analysis. Assessment of DNA endonucleolytic cleavage characteristic of apoptosis was carried out by nuclear staining with propidium iodide (PI) (15) and agarose gel electrophoresis (16) as described previously. To detect simultaneously the phase of cell cycle and extent of DNA cleavage as it relates to apoptosis, we assayed for BrdU incorporation in conjunction with PI staining using a modification of the method reported by Renno et al. (17). Briefly, cells were incubated in the presence of 0.05 mM BrdU for 4 hours, harvested, and fixed in 70% ethanol for 30 minutes. DNA was then partially denatured by treatment with 3 N HCl for 20 minutes, followed by neutralization with 0.1 M Na2B4O7. Cells were then stained with a FITC-coupled anti-BrdU mAb (Becton-Dickinson), resuspended in a PI solution for 5 minutes at 37°C, and analyzed by two- color flow cytometry.
Long-term culture of CD34+ HPCs. Bone marrow aspirates were obtained from normal volunteers after obtaining informed consent. CD34+CD2−CD16− HPCs were isolated to 97% purity using immunomagnetic beads followed by fluorescent activated cell sorting as described elsewhere (18). Cells were then plated at a concentration of 2 × 104 in 200 μl of RPMI-1640 with 10% human AB serum, antibiotics, and 100 pM rIL-2, with or without 7 nM of c-kit ligand (KL). One hundred microliters of culture medium was replaced with identical concentrations of factors on days 7, 14, 17, and 20. Plates were harvested on day 21 for numerical, morphologic, flow cytometric, and cytotoxic analysis as described previously (18).
-
Results
Proliferative response of CD56bright NK cells to long-term culture with rIL-2. The continuous in vivo infusion of low-dose IL-2 results in pM serum concentrations of IL-2 and significant, selective expansion of CD56bright NK cells, but only after 4–6 weeks of therapy (Table 1) (5). CD56bright NK cells exhibit a brisk proliferative response to pM concentrations of rIL-2 during short-term (72–96 hours) culture in vitro (3, 4), but proliferation in extended cultures have not been reported. CD56bright NK cells were therefore cultured for 12 days in medium containing 100 pM rIL-2, which was replaced every 72 hours. In repeated experiments, the CD56bright NK-cell population initially exhibited a substantial increase in 3H-TdR incorporation that peaked at 4–6 days, followed by a gradual decline to baseline levels on day 10 (Figure 1a). Consistent with the 3H-TdR incorporation, cell staining with Hoechst dye showed less than 2% of CD56bright NK cells in cycle on day 1 (data not shown), but more than 20% of CD56bright NK cells in the G2/M phase of the cell cycle after day 4 of culture in rIL-2. Notably, 42–59% of CD56bright NK cells were found within the hypodiploid peak, characteristic of cells undergoing programmed cell death (Figure 1a, inset). Resting T cells or CD56dim NK cells do not proliferate in response to 100 pM IL-2 alone (3).
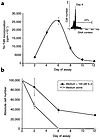 Figure 1
Figure 1(a) 12-day 3H-TdR incorporation of CD56bright NK cells cultured in 100 pM rIL-2. Freshly isolated CD56bright NK cells (>97% pure) were plated in 96-well plates and cultured in medium containing 100 pM rIL-2, as described in Methods. Cells were assayed for 3H-TdR incorporation every 48 hours. Each time point represents the mean cpm ± SEM of triplicate wells. Inset: Histogram of Hoechst-stained CD56bright NK cells cultured for 4 days with medium containing 100 pM rIL-2. Cell number is measured along the ordinate, DNA content along the abscissa. Five thousand events were collected and analyzed. Number above the region marker (22%) indicates the percentage of cells in the G2/M phase of the cell cycle. Approximately 45% of cells were found in the hypodiploid region of the histogram, usually indicative of apoptosis. (b) Enumeration of CD56bright NK cells by vital dye exclusion during 12-day culture in medium supplemented with or without 100 pM rIL-2. Values shown represent the mean ± SEM of readings done in triplicate wells. This figure is representative of four independent experiments performed on sorted CD56bright cells from four normal individuals.
Cell-enumeration assays were performed by vital dye exclusion on days 2, 6, and 12 of culture to assess further the fraction of IL-2–responsive CD56bright NK cells. No CD56bright NK cells cultured in the absence of rIL-2 survived after day 6 (Figure 1b). In contrast, cells cultured in the presence of 100 pM rIL-2 showed a 60 ± 4.3% decrease in number during the first 6 days of culture, but no significant decrease thereafter up to day 12. Thus, the significant decline in 3H-TdR incorporation seen in CD56bright NK cells between days 6 and 12 of culture in rIL-2 was not accounted for by a continual decrease in absolute cell number or increase in cell death. Indeed, the vast majority of cell death after IL-2 activation occurs during the first 6 days of culture when proliferation is maximal (Figure 1). The hypodiploid nuclei seen with Hoechst staining during the first 6 days of culture (inset, Figure 1a) depicts cleaved DNA, characteristic of apoptosis (14). This was confirmed with direct visualization of DNA by agarose gel electrophoresis, which demonstrated DNA laddering that is characteristic of apoptosis (data not shown). Thus, although a fraction of CD56bright NK cells were proliferating during the first 6 days of culture, a fraction of CD56bright NK cells were also undergoing programmed cell death.
We next examined whether the CD56bright NK cells undergoing proliferation in response to rIL-2 were also the cells undergoing programmed cell death. Cells transiting the cell cycle incorporate the thymidine analog BrdU in a manner proportional to the number of times the cell has passed through the cell cycle. Simultaneously, cellular DNA content can be evaluated by PI staining. Apoptotic nuclei stained with PI appear on the red fluorescence channel as a population showing hypodiploid DNA that is easily distinguished from the normal diploid or hyperdiploid peak of viable cells (16). Figure 2a depicts a control experiment in which CD56bright cells were analyzed after 6 days of culture in medium containing 10% HAB without IL-2. When performed with CD56bright NK cells incubated in the presence of 100 pM IL-2 for 6 days, a fraction of cells that remained quiescent during the assay were within the hypodiploid or apoptotic fraction (Figure 2b). None of the cells incorporating BrdU and therefore transiting the cell cycle were within the hypodiploid fraction. Thus, a fraction of CD56bright cells that were unresponsive to the IL-2 proliferative signal were also undergoing programmed cell death and responsible for the drop in cell number seen during early cell culture in IL-2. This suggested that the CD56bright NK cells originally proliferating in response to IL-2 during the first 6 days of culture made up the majority of cells that remained alive yet quiescent during the extended (i.e., 12-day) cell culture (Figure 1). The CD56bright NK cells that survived without cycling during the extended cell culture required IL-2 binding to the high-affinity IL-2Rαβγ to prevent programmed cell death, because the addition of neutralizing anti–IL-2 polysera or anti–IL-2Rα mAb resulted in greater than 90% cell death by day 10 in repeated experiments (data not shown).
 Figure 2
Figure 2Correlation of apoptosis and proliferation of IL-2–stimulated CD56bright NK cells as evaluated by PI/BrdU assay. Shown are representative contour plots of sorted CD56bright NK cells cultured in the absence (a) or presence (b) of 100 pM IL-2 for 6 days and assayed as described in Methods. BrdU incorporation (proliferative activity) is measured along the abscissa (FITC fluorescence). PI fluorescent intensity (DNA content) is measured along the ordinate (red fluorescence). Cells that have undergone apoptosis show a hypodiploid complement of DNA and fall below the dashed horizontal divider. Normal (viable) diploid cells are found between the dashed and solid horizontal lines. In the absence of IL-2 (a), there is no evidence of BrdU incorporation, and the vast majority of CD56bright NK cells are found within the hypodiploid fraction by day 6. In the presence of IL-2 (b), a fraction of cells proliferate, incorporate BrdU, and are therefore seen to the right of the vertical divider. Apoptosis has only occurred within the nondividing fraction of cells (left, below dashed line). This is representative of three independent experiments with similar results.
CD56bright NK cells expanded in culture are functional innate immune effector cells. We next assessed the function of CD56bright NK cells that were cultured for 12 days in the presence of IL-2. IL-2 (1 nM) plus IL-12 (10 U/mL) was added to these otherwise unmanipulated NK-cell cultures on days 2, 6, and 12, and IFN-γ production was measured in culture supernatants 2 days after the addition of IL-12 (Figure 3a). NK cells cultured in the presence of 100 pM IL-2 maintained the ability to produce IFN-γ throughout the 12-day period of culture in response to IL-2 + IL-12, whereas those cultured in medium alone had no IFN-γ production in response to IL-2 + IL-12. As another measure of viable NK-cell function, we also evaluated cytotoxic function throughout a 12-day culture in the presence or absence of 100 pM IL-2. Again, CD56bright cells cultured in the presence of IL-2 maintained significant cytotoxic activity, whereas those cultured in the absence of IL-2 did not display any ability to lyse target cells (Figure 3b).
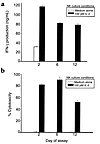 Figure 3
Figure 3CD56bright cells cultured in 100 pM IL-2 maintain functional capacity relative to culture in medium alone. (a) NK-cell production of IFN-γ in response to stimulation with IL-2 plus IL-12. Twenty thousand CD56bright cells per well were plated in the presence or absence of 100 pM IL-2. On days 2, 6, and 12, IL-2 (1 nM) and IL-12 (10 U/mL) were added, and 2 days later, the supernatants were collected and analyzed for IFN-γ by ELISA. Results depict the mean ± SEM for triplicate wells. (b) Cytotoxicity of CD56bright cells against K562 targets after 2, 6, or 12 days of culture with or without 100 pM rIL-2. Values given represent the mean ± SEM of readings from triplicate wells for each time point, at an effector-to-target ratio of 5:1. The data shown are representative of three independent experiments.
CD56bright NK cells expanded in vivo are not transiting the cell cycle. We performed cell-cycle analysis on lymphocyte subsets from five patients receiving a continuous in vivo infusion of low-dose IL-2 therapy. For these five patients, there was a significant (i.e., 7- to 36-fold; P < 0.01) and selective in vivo expansion of CD56bright NK cells during low-dose IL-2 therapy. Despite this, there was no difference in the percent of CD56− cells (i.e., T cells and B cells), CD56dim NK cells, or CD56bright NK cells traversing the G2/M phase of the cell cycle during low-dose IL-2 therapy. Further, patients receiving IL-2 showed no increase in the fraction of cells entering cell cycle compared with four similar patients not receiving IL-2 (Table 1). A graphical depiction of the selective NK-cell expansion and a histogram illustrating the fraction traversing the G2/M phase of the cell cycle from a representative patient receiving low-dose IL-2 therapy are shown in Figure 4. Thus, like the CD56bright NK cells studied in vitro, the CD56bright NK cells exposed to a prolonged continuous infusion of exogenous IL-2 in vivo are in a quiescent phase of the cell cycle. Hence, the IL-2–induced increase in the absolute number of CD56bright NK cells in vivo does not appear to result from continued proliferation of mature CD56bright cells in peripheral blood.
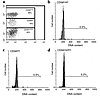 Figure 4
Figure 4CD56bright cells expanding in vivo to IL-2 are not transiting the cell cycle. (a) Dot plot of a representative patient’s lymphocytes stained with anti–CD56-PE and analyzed by flow cytometry during low-dose IL-2 therapy. Percentages located within individual gated regions indicate the fraction each subset comprises as a percentage of total cells analyzed. PE fluorescent intensity is given along the ordinate, FITC fluorescent intensity along the abscissa. Hoechst cell-cycle analyses of CD56bright NK cells (b), CD56dim NK cells (c), and CD56negative, i.e., T and B cells (d) represented in a, are shown in the right column. Cell number is measured along the ordinate, DNA content along the abscissa. Percentages noted above the region markers of each subpopulation indicate the percentage of cells in the G2/M phase of the cell cycle. A summary of data for five patients during IL-2 therapy is found in Table 1.
Low-dose IL-2 promotes the generation of CD56bright NK cells from CD34+ HPCs in vitro. The data presented thus far suggested that an IL-2–dependent delay in NK-cell death, rather than enhanced NK-cell proliferation, was contributing to the expansion of CD56bright NK cells during low-dose IL-2 therapy (Table 1). However, we found that mature T cells, which do not expand in patients receiving low-dose IL-2 therapy, showed prolonged survival similar to NK cells when cultured with 100 pM IL-2 in vitro (data not shown). We therefore investigated what role, if any, exogenous administration of low-dose IL-2 might have on the differentiation of CD56bright NK cells from bone marrow–derived HPCs. When purified CD34+ HPCs were cultured in medium containing 10% human serum and 100 pM rIL-2 for 21 days, there was a striking appearance of CD56brightCD3– NK cells compared with cells cultured in 10% human serum alone (Figure 5, top panels). These cells had large granular lymphocyte morphology and demonstrated potent NK cytotoxic activity (data not shown). Further, when low concentrations of IL-2 were combined with the c-kit ligand, a hematopoietic growth factor known to be abundantly expressed by bone marrow stromal cells and measurable in normal human serum (19), there was fourfold greater expansion of CD56brightCD3– NK cells from CD34+ HPCs, compared with cells cultured with exogenous IL-2 alone (Figure 5, middle panels). Thus, in addition to maintaining survival of CD56bright NK cells, low concentrations of exogenous IL-2 can enhance CD56bright NK-cell differentiation from CD34+ HPCs in vitro.
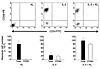 Figure 5
Figure 5Effect of low-dose IL-2, KL, or IL-2 plus KL on the development of NK cells from CD34+ HPCs. Upper panels show phenotypic evaluation of cells produced from 21-day culture of CD34+ HPCs with 100 pM IL-2, 7 nM KL, or a combination of both. Anti–CD3-FITC and anti–CD56-PE mAb’s that were subsequently analyzed by FACS are shown. Graphs below show absolute numbers of total mononuclear cells or CD56+ cells produced by CD34+ HPC cultures. Absolute numbers of CD56+ cells were obtained by multiplying absolute numbers of cells counted in trypan blue by percent CD56+ cells obtained by FACS. The data are representative of six separate experiments.
-
Discussion
We examined the potential mechanisms by which human NK cells selectively expand during prolonged continuous in vivo infusions of low-dose IL-2. Earlier work had shown that the CD56bright NK-cell subset is unique among resting lymphocytes in its constitutive expression of the high-affinity IL-2R and its brisk proliferative response to IL-2 in short-term culture (3, 4). However, the extent of CD56bright proliferation beyond 4 days of culture and characterization of CD56bright expansion in vivo has not been reported for patients receiving this therapy.
The data presented in this report show that the CD56bright NK-cell response to pM concentrations of IL-2 is heterogeneous. More than half of these freshly isolated cells undergo apoptosis in the absence of proliferation, despite the presence of IL-2. Thus, a sizable subset of these cells must be considered proliferatively unresponsive to this cytokine. The proliferative response induced by low-dose IL-2 that was seen in the remaining CD56bright NK cells was limited and was followed by an exit from the cell cycle in the absence of additional stimulation. Although there is evidence to suggest that proliferating lymphocytes are susceptible to apoptosis (17, 20), the data presented here show that it was the proliferating cells that were rescued from cell death. The reason for the halt in proliferation is not clear, but was due neither to the absence of IL-2 nor the lack of a functional IL-2Rαβγ. Indeed, if IL-2 was neutralized or if the high-affinity IL-2R was blocked, the majority of CD56bright NK cells became susceptible to programmed cell death in prolonged culture. Thus, expression of the high-affinity IL-2Rαβγ was required in both processes, i.e., initiating growth and sustaining subsequent survival, and the functional responses to IL-2 changed over time. The gradual loss of proliferation may involve the cells’ inability to replenish intracellular intermediates critical for IL-2–induced proliferation, and/or the cells’ progressive differentiation in the presence of IL-2. In contrast to proliferation, NK-cell survival could be maintained with IL-2 for weeks. IL-2 preserved NK-cell nuclear membrane and functional integrity, as evidenced by measurable cytokine production and cytotoxic activity after extended in vitro culture. However, low concentrations of IL-2 had a similar effect on preventing T-cell death in vitro. Thus, the IL-2–mediated delay in cell death observed in vitro cannot alone account for the selective in vivo expansion of CD56bright NK cells during prolonged IL-2 therapy.
Lymphocytes obtained from five patients receiving a prolonged continuous infusion of IL-2 were studied for evidence of proliferation. NK cells examined directly without in vitro culture did not show an increased fraction in the G2/M or S phase of the cell cycle, relative to T cells obtained at the same time from the same patients. NK cells obtained from patients before low-dose IL-2 therapy and from comparable patients not receiving IL-2 had similar cell-cycle profiles. This suggests that IL-2–induced proliferation of CD56bright NK cells is not responsible for the observed expansion in vivo. Although we cannot be certain that some proliferation of NK cells may occur during the first 6 days of in vivo IL-2 infusions, significant in vivo NK-cell expansion does not occur until 4–6 weeks after initiation of IL-2 (5).
The failure to detect enhanced NK-cell proliferation in vivo during IL-2 therapy leaves two other hypotheses to account for the observed alteration in NK-cell homeostasis. First, exogenous administration of IL-2 could delay NK-cell death in vivo. Indeed, patients’ NK cells that were expanded in vivo could be sustained in vitro with 100 pM IL-2 and 10% human serum. This effect appears to be the result of IL-2’s ability to prevent NK-cell apoptosis. However, this effect can also be seen with T cells, arguing against this as the only mechanism responsible for selective CD56bright NK-cell expansion. Increased NK-cell production via differentiation from BM precursors is a second, alternative mechanism explaining the selective NK-cell expansion in vivo. Indeed, studies have previously shown that relatively high concentrations of IL-2 can support NK-cell differentiation from CD34+ BM HPCs in vitro (21–24). In this report we show that BM-derived CD34+ HPCs can differentiate into CD56bright NK cells with 100 pM rIL-2 in vitro, without a stromal cell layer. This NK-cell differentiation was significantly enhanced by KL, a factor produced by BM stroma that is normally measurable in human serum (19). Additional in vitro (25) and in vivo (26) evidence suggests that endogenously produced KL and Flt3 ligand are likely to enhance IL-2–induced NK-cell differentiation from HPCs. Indeed, we have recently shown that KL or flt3 ligand induces a distinct CD34brightIL-2Rβ+ NK-cell progenitor from CD34+ HPCs that is responsive to IL-2 and IL-15 for NK-cell differentiation (25).
Similar to normal CD56bright NK cells, the majority of cells expanded in such cultures express C-lectin NK receptors (CD94/NKG2), whereas most lack the Ig-superfamily KIR (T.A. Fehniger et al., unpublished observations). The bone marrow–derived CD56bright NK cells can also express CD2, CD16, and the intracellular zeta (ζ) chain associated with these molecules (18), and can produce large amounts of IFN-γ that are comparable to CD56bright NK cells found in blood (25). Interestingly, 3–4 weeks are required for the in vitro expansion of CD56bright NK cells from CD34+ HPCs in vitro, similar to the time required for the initiation of human NK-cell expansion in vivo. T cells are not produced under either of these culture conditions (22, 24, 27, 28). Thus, it seems plausible that low-dose IL-2 therapy could serve to selectively enhance the differentiation of human NK cells from bone marrow–derived HPCs, much the same way exogenous administration of G-CSF has been shown to potentiate HPC differentiation along the myeloid lineage (29). It is also possible that the increase in absolute NK-cell number results in part from migration of NK cells from peripheral tissues. However, with the exception of the gravid uterus (30), extravascular tissue sites of CD56bright NK cells have not been well characterized, leaving this hypothesis difficult to test.
In summary, the selective in vivo expansion of CD56bright NK cells during prolonged infusion of low-dose IL-2 does not appear to result from enhanced proliferation within this lymphocyte subset. The in vitro and in vivo data provided in this report suggest that this expansion is the result of an IL-2–dependent delay in the onset of programmed cell death in combination with enhanced NK-cell differentiation from bone marrow–derived CD34+ HPCs.
-
Acknowledgments
The authors thank C. Stewart, R. Baiocchi, and T. Renno for helpful discussions during the course of this study. This work was supported by grants P30CA-16058, CA-68456, and CA-65670 from the National Institutes of Health and by the Coleman Leukemia Research Fund. T.A. Fehniger is the recipient of the Bennett and Medical Scientist Program Fellowships from The Ohio State University College of Medicine.
-
Footnotes
Todd A. Fehniger and Eric M. Bluman contributed equally to this work.
-
References
- Trinchieri, G. Biology of natural killer cells. Adv Immunol 1989. 47:187-376. View this article via: PubMed Google Scholar
- Robertson, MJ, Ritz, J. Biology and clinical relevance of human natural killer cells. Blood 1990. 76:2421-2438. View this article via: PubMed Google Scholar
- Caligiuri, MA, et al. Functional consequences of interleukin 2 receptor expression on resting human lymphocytes. Identification of a novel natural killer cell subset with high affinity receptors. J Exp Med 1990. 171:1509-1526.
- Nagler, A, Lanier, LL, Phillips, JH. Constitutive expression of high affinity interleukin 2 receptors on human CD16-natural killer cells in vivo. J Exp Med 1990. 171:1527-1533.
- Caligiuri, MA, et al. Selective modulation of human natural killer cells in vivo after prolonged infusion of low dose recombinant interleukin 2. J Clin Invest 1993. 91:123-132.
- Frankel, SR, et al. Low dose interleukin-2 (IL-2) as post remission therapy for AML increases NK cell populations. Proceedings of the American Association for Cancer Research 1994. 35:225. (Abstr.)
- Bernstein, ZP, et al. Prolonged administration of low-dose interleukin-2 in human immunodeficiency virus-associated malignancy results in selective expansion of innate immune effectors without significant clinical toxicity. Blood 1995. 86:3287-3294. View this article via: PubMed Google Scholar
- Meropol, NJ, et al. Daily subcutaneous injection of low-dose interleukin 2 expands natural killer cells in vivo without significant toxicity. Clin Cancer Res 1996. 2:669-677. View this article via: PubMed Google Scholar
- Bernstein, ZP, et al. A phase I/II study of daily subcutaneous low dose interleukin-2 in AIDS-associated lymphomas. Blood 1998. 92:625a. (Abstr.)
- Khatri, VP, et al. Ultra low dose interleukin-2 therapy promotes a type 1 cytokine profile in vivo in patients with AIDS and AIDS-associated malignancies. J Clin Invest 1998. 101:1373-1378.
- Baer, MR, et al. High-dose cytarabine, idarubicin, and granulocyte colony-stimulating factor remission induction therapy for previously untreated de novo and secondary adult acute myeloid leukemia. Semin Oncol 1993. 20:6-12. View this article via: PubMed Google Scholar
- Matos, ME, et al. Expression of a functional c-kit receptor on a subset of natural killer cells. J Exp Med 1993. 178:1079-1084.
- Carson, WE, et al. Interleukin (IL) 15 is a novel cytokine that activates human natural killer cells via components of the IL-2 receptor. J Exp Med 1994. 180:1395-1403.
- Rabinovitch, PS, Kubbies, M, Chen, YC, Schindler, D, Hoehn, H. BrdU-Hoechst flow cytometry: a unique tool for quantitative cell cycle analysis. Exp Cell Res 1988. 174:309-318.
- Baiocchi, RA, et al. Lymphomagenesis in the SCID-hu mouse involves abundant production of human interleukin-10. Blood 1995. 85:1063-1074. View this article via: PubMed Google Scholar
- Nicoletti, I, Migliorati, G, Pagliacci, MC, Grignani, F, Riccardi, C. A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J Immunol Methods 1991. 139:271-279.
- Renno, T, Hahne, M, MacDonald, HR. Proliferation is a prerequisite for bacterial superantigen-induced T cell apoptosis in vivo. J Exp Med 1995. 181:2283-2287.
- Mrozek, E, Anderson, P, Caligiuri, MA. Role of interleukin-15 in the development of human CD56+ natural killer cells from CD34+ hematopoietic progenitor cells. Blood 1996. 87:2632-2640. View this article via: PubMed Google Scholar
- Langley, KE, et al. Soluble stem cell factor in human serum. Blood 1993. 81:656-660. View this article via: PubMed Google Scholar
- Penninger, JM, Mak, TW. Signal transduction, mitotic catastrophes, and death in T-cell development. Immunol Rev 1994. 142:231-272.
- Lotzova, E, Savary, CA, Champlin, RE. Genesis of human oncolytic natural killer cells from primitive CD34+CD33- bone marrow progenitors. J Immunol 1993. 150:5263-5269. View this article via: PubMed Google Scholar
- Miller, JS, Verfaillie, C, McGlave, P. The generation of human natural killer cells from CD34+/DR- primitive progenitors in long-term bone marrow culture. Blood 1992. 80:2182-2187. View this article via: PubMed Google Scholar
- Miller, JS, Alley, KA, McGlave, P. Differentiation of natural killer (NK) cells from human primitive marrow progenitors in a stroma-based long-term culture system: identification of a CD34+7+ NK progenitor. Blood 1994. 83:2594-2601. View this article via: PubMed Google Scholar
- Shibuya, A, Nagayoshi, K, Nakamura, K, Nakauchi, H. Lymphokine requirement for the generation of natural killer cells from CD34+ hematopoietic progenitor cells. Blood 1995. 85:3538-3546. View this article via: PubMed Google Scholar
- Yu, H, et al. Flt3 ligand promotes the generation of a distinct CD34+ human natural killer cell progenitor that responds to interleukin-15. Blood 1998. 92:3647-3657. View this article via: PubMed Google Scholar
- Fehniger, TA, Carson, WE, Mrozek, E, Caligiuri, MA. Stem cell factor enhances interleukin-2-mediated expansion of murine natural killer cells in vivo. Blood 1997. 90:3647-3653. View this article via: PubMed Google Scholar
- Silva, MR, Hoffman, R, Srour, EF, Ascensao, JL. Generation of human natural killer cells from immature progenitors does not require marrow stromal cells. Blood 1994. 84:841-846. View this article via: PubMed Google Scholar
- Krajewski, CA, Mrozek, E, Caligiuri, MA. Generation and expansion of CD3-CD56+ non-MHC-restricted cytotoxic effectors from human hematopoietic precursors; requirement for c-kit ligand and interleukin-2. Proceedings of the American Society for Clinical Oncology 1994. 13:294. (Abstr.)
- Dale, DC, Liles, WC, Summer, WR, Nelson, S. Review: granulocyte colony-stimulating factor: role and relationships in infectious diseases. J Infect Dis 1995. 172:1061-1075. View this article via: PubMed Google Scholar
- Nishikawa, K, et al. Accumulation of CD16-CD56+ natural killer cells with high affinity interleukin 2 receptors in human early pregnancy decidua. Int Immunol 1991. 3:743-750.
- Trinchieri, G. Biology of natural killer cells. Adv Immunol 1989. 47:187-376.
-
Version history
- Version 1 (January 1, 2000): No description



Copyright © 2024 American Society for Clinical Investigation
ISSN: 0021-9738 (print), 1558-8238 (online)






