Advertisement
Science in Medicine Free access | 10.1172/JCI45434
Food allergy
Julie Wang and Hugh A. Sampson
Division of Allergy and Immunology, Department of Pediatrics, Mount Sinai Hospital, New York, New York, USA.
Address correspondence to: Julie Wang, Department of Pediatrics, Box 1198, Mount Sinai School of Medicine, One Gustave L. Levy Place, New York, New York 10029, USA. Phone: 212.241.5548; Fax: 212.426.1902; E-mail: [email protected].
Find articles by Wang, J. in: JCI | PubMed | Google Scholar
Division of Allergy and Immunology, Department of Pediatrics, Mount Sinai Hospital, New York, New York, USA.
Address correspondence to: Julie Wang, Department of Pediatrics, Box 1198, Mount Sinai School of Medicine, One Gustave L. Levy Place, New York, New York 10029, USA. Phone: 212.241.5548; Fax: 212.426.1902; E-mail: [email protected].
Find articles by Sampson, H. in: JCI | PubMed | Google Scholar
Published March 1, 2011 - More info
J Clin Invest. 2011;121(3):827–835. https://doi.org/10.1172/JCI45434.
© 2011 The American Society for Clinical Investigation
-
Abstract
Food allergies affect up to 6% of young children and 3%–4% of adults. They encompass a range of disorders that may be IgE and/or non-IgE mediated, including anaphylaxis, pollen food syndrome, food-protein–induced enterocolitis syndrome, food-induced proctocolitis, eosinophilic gastroenteropathies, and atopic dermatitis. Many complex host factors and properties of foods are involved in the development of food allergy. With recent advances in the understanding of how these factors interact, the development of several novel diagnostic and therapeutic strategies is underway and showing promise.
-
Introduction
Food allergies are adverse immune reactions to food proteins that can lead to a range of symptoms. A meta-analysis focusing on milk, egg, peanut, and seafood allergy determined that the prevalence of food allergies is approximately 3.5% (1). A recent US study that utilized several national health databases and health care surveys concluded that 3.9% of US children are reported as having food allergy, with an 18% increase in prevalence between 1997 and 2007 (2). Specifically, studies on peanut allergy in the US and United Kingdom indicate that the number of children affected has doubled in the past decade, with the prevalence now over 1% (3, 4).
This review will focus on the immunopathophysiology of food allergies as well as provide an overview of therapeutic strategies currently being investigated with the aim of long-term treatment and possible cures.
-
Pathophysiology of food allergy
Oral tolerance. Food allergies may be IgE mediated (causing immediate symptoms and possible anaphylaxis), non-IgE mediated (cell-mediated reactions with more delayed symptoms), or a combination of both. Although these different forms of food allergy have varying clinical presentations, they likely share a common pathophysiology, with food antigen sensitization and Th2 skewing of the immune system. Recently, there has been increasing understanding of oral tolerance, the mechanisms by which ingested proteins are able to interact with unique populations of antigen-presenting cells leading to suppression of cellular and humoral immune responses. Advances in this area have primarily come from animal models. Food allergies are believed to be a result of either loss of oral tolerance or the failure to induce tolerance.
Oral tolerance results from complex interactions among DCs, T regulatory cells, and NKT cells as well as other immunologic components. These cells play different roles that may vary depending on their location and method of antigen presentation. For example, DCs are capable of inducing active T cell immunity via uptake, processing, and presentation of antigen to T cells, but may also induce tolerance by inducing T regulatory cells or deleting T cells (5). DCs may encounter antigen in the gastrointestinal tract by directly sampling luminal contents through dendrites extending to paracellular spaces, by directly interacting with epithelial cells, or by taking up antigen via the Peyer patches. Various antigenic properties likely determine the route of exposure and will result in different immunologic responses. Although the exact mechanisms of oral tolerance are incompletely understood, migration of DCs to mesenteric lymph nodes appears to be a critical step, since oral tolerance cannot be induced in mice lacking these structures (6).
Oral tolerance can develop with repeated low-dose exposure to antigen, which is mediated by regulatory T cells (suppressor CD8+ cells, Th3 cells, Tr1 cells, CD4+CD25+ cells, and NK1.1+ T cells) (7). These regulatory T cells migrate to lymphoid organs, where they suppress immune responses via cytokines such as IL-10 and TGF-β (Figure 1). Oral tolerance can also be induced by high-dose exposure of allergen, which is mediated by lymphocyte anergy (absence of costimulation or interactions between CD28 on T cells and CD80/86 on APCs) (8) and deletion by FAS-mediated apoptosis (9). It is possible that a combination of these effects leads to oral tolerance.
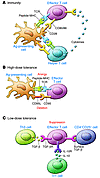 Figure 1
Figure 1Mechanisms of oral tolerance. (A) Generation of an immune response requires ligation of the T cell receptor with peptide-MHC complexes in the presence of appropriate costimulatory molecules (CD80 and CD86) and cytokines. (B) With high doses of oral antigen, T cell receptor cross-linking can occur in the absence of costimulation or in the presence of inhibitory ligands (CD95 and CD95 ligand), leading to anergy or deletion, respectively. (C) Low doses of oral antigen lead to the activation of regulatory T cells, which suppress immune responses through soluble or cell surface–associated suppressive cytokines (IL-10 and TGF-β). Adapted from ref. 7 with permission from Elsevier.
Additional components of the immune system are increasingly shown to play important roles in oral tolerance induction. Although much focus has been placed on Foxp3+ T regulatory cells, recent data suggest that other types of T cells are important in tolerance development as well, including γδ T cells (10) and NKT cells (11). Data also suggest that the gut mucosal epithelium may not only serve as a physical barrier to foreign antigen, but plays additional roles in the induction of tolerance. An example is thymic stromal lymphopoietin (TSLP), which is expressed by epithelial cells as well as stromal cells and basophils (12). TSLP is a potent inducer of Th2 responses (13) and is involved in allergic inflammation of the skin and lung, resulting in asthma and atopic dermatitis (14). In the gastrointestinal tract, TSLP appears to have a regulatory role (15). Interestingly, its presence has been shown to enhance allergic Th2 responses in the gut, but is not required for primary sensitization or tolerance to food protein (16). Currently, little is known about the role of TSLP in human food allergy, but TSLP has been identified as a candidate gene for eosinophilic esophagitis (17).
Breakdown of oral tolerance. A breakdown in the development of oral tolerance or the loss of oral tolerance is believed to lead to food allergy; however, it is currently not clear where and when this breakdown occurs. Furthermore, it is unclear how differences in breakdown lead to the various types of IgE-mediated and non–IgE-mediated food allergies, but possibilities include increased intestinal permeability, decreased oral tolerance, and defects in regulating T cell activity.
Increased intestinal permeability has been suggested as a potential cause for the breakdown in tolerance, since food-allergic infants have been found to have increased permeability compared with healthy children, as measured by urinary lactulose/mannitol ratio (18). In addition, there are several reports of food allergies developing after solid-organ transplantation, which is believed to be in part due to tacrolimus-induced increases in intestinal permeability (19, 20).
Loss of oral tolerance can occur or may be bypassed by antigen presentation via alternative routes, such as exposure through the skin or the respiratory tract. Using a mouse model, Wang et al. (21) demonstrated that exposure of protein antigen via the epicutaneous route can cause sensitization and induce a Th2 immune response. Furthermore, higher rates of peanut allergy have been found in children with atopic dermatitis who used topical creams containing peanut oil (odds ratio [OR] 6.8) (22). Respiratory exposures can also lead to food allergies, as seen in pollen-food syndrome (oral allergy syndrome) (23).
Defects in regulatory T cell activity are exemplified by the disorder of immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX), which is due to a mutation in the FOXP3 gene, a transcription factor expressed in CD4+CD25+ regulatory T cells. Atopic dermatitis and food allergies are known manifestations of this disorder (24). The importance of regulatory T cells in the development of tolerance was also demonstrated in a study of non–IgE-mediated milk allergy in which the development of tolerance to milk was associated with higher numbers of circulating CD4+CD25+ regulatory T cells (25).
-
Host factors influencing food allergy
A variety of host factors may influence the development of food allergies. One twin study (26) found a significantly higher concordance rate of peanut allergy among monozygotic twins (64%) as compared with dizygotic twins (7%), suggesting a strong genetic influence. The maturity of the gastrointestinal tract may also be a factor, as epidemiologic studies have shown a higher rate of food allergies in young children as compared with adults (1). In contrast, population-based studies suggest that early introduction of foods may be protective of food allergy. In Israel, where infants are fed a peanut snack (Bamba) starting at an early age, there is a 10-fold lower incidence of peanut allergy compared with Jewish children in Hebrew schools in London, where peanut products are not introduced until children are much older (27). The Learning Early About Peanut Allergies (LEAP) study is exploring the role of timing of peanut allergen exposure in the development of peanut allergy by randomly assigning high-risk infants to early or more delayed exposure to peanut (28). Two recent studies suggest that the role of timing of allergen exposure may vary for different foods (29, 30). Early egg exposure, by 4 to 6 months of age, appeared to be protective for egg allergy; in contrast, introduction of milk in the first 2 weeks of life was protective, while introduction between 4 and 6 months of age was associated with the highest risk of developing milk allergy. While these questionnaire-based studies are subject to recall bias and/or reverse causation, they point out that studies on one food allergen may not be applicable to other foods. Differences may also be due to variations in the form of foods being introduced (i.e., natural egg vs. baked egg) or the quantity of exposure at each age period.
Disruption of normal gut barrier functions due to alterations in gastric pH or commensal bacteria is another factor to consider. In a study of 152 patients on antacid treatment for dyspepsia, increased food allergen sensitization was seen in 25% of patients after 3 months (31). Moreover, gastric digestion has been shown to reduce the allergenicity of food proteins, such as the egg allergen ovomucoid (32). The role of commensal bacteria has been examined in several studies with conflicting results (33–35). These studies demonstrate that varying effects can be seen depending on the strain of organisms used, timing of treatment, and whether treatment is given to the mother and/or infant.
Additional host factors can modulate the clinical response of food allergy. In a study of fatal food allergic reactions, the majority of victims had underlying asthma (36, 37). Exercise, alcohol consumption, use of medication (i.e., beta blockers, angiotensin converting enzyme inhibitors, tricyclic antidepressants), and concurrent infection may increase the severity of anaphylactic reactions or diminish the efficacy of epinephrine (38–39). Recently, low serum activity of platelet-activating factor acetylhydrolase (PAF-AH) was reported to be associated with more severe food-induced anaphylaxis (40). However, low PAF-AH activity in patients with fatal anaphylaxis might also be a result of severe hypoxia followed by systemic necrosis rather than the cause of more severe reactions (41).
-
Food allergen factors influencing food allergy
Although any food can trigger an allergic response, relatively few protein families account for the majority of allergic reactions. In the US, milk, egg, peanut, tree nuts, fish, shellfish, soy, and wheat are the major food allergens (42). Seeds, particularly sesame, also appear to be an increasingly recognized allergen in many countries (43). The majority of animal food allergens can be classified into 3 protein groups, and the majority of plant food allergens can be grouped into 4 families (refs. 44, 45, and Table 1). In general, proteins with more than 62% homology to human proteins are unlikely to be allergenic (44).
The presence of specific IgE to sequential or conformational epitopes appears to distinguish between different phenotypes of food allergy. Sequential epitopes comprise consecutive amino acids along the protein backbone that are recognized by IgE antibodies, whereas conformational epitopes consist of amino acids that are brought into close proximity by protein folding and thus depend upon maintenance of the tertiary structure of the protein. Several studies have shown that IgE antibody binding predominantly to conformational epitopes is associated with transient allergy to milk and egg, whereas binding to sequential epitopes in these proteins is a marker for persistent allergy (46, 47). With maturation of gastrointestinal enzymes, decreasing intestinal permeability, and increases in antigen-specific IgA and IgG, it is hypothesized that proteins no longer penetrate the mucosal barrier and activate tissue mast cells. However, peptides of various lengths penetrate the GI tract of all individuals (48, 49), allowing peptides with intact sequential epitopes access to tissue mast cells and other cells involved in allergic reactions. Approximately 80% of milk- and egg-allergic children can tolerate extensively heated or baked forms of these foods (50, 51), which lack native conformational epitopes due to heat denaturation, implying that conformational epitopes are primarily recognized in these individuals. In addition, studies suggest that different patterns of epitope recognition and degrees of epitope diversity may correlate with clinical manifestations of allergic reactions to peanut and milk, including their natural history or severity of reactions (46, 47, 52–56). These assays are investigational and not commercially available, and the clinical utility of these tests requires confirmation.
Carbohydrates associated with these food proteins can also influence their allergenicity. For example, Maillard reaction products or advanced glycation end products that result from roasting peanuts at very high temperatures lead to increased stability and allergenicity of peanut allergens (57). This finding may in part explain the differences in prevalence of peanut allergy in the US, where peanuts are primarily consumed in the roasted form, compared with China, where boiled or fried peanuts are more common. Similar results have recently been reported for ovalbumin (58). The presence of sugar moieties naturally occurring in peanuts has also been shown to increase the allergenicity of this food. Glycosylated Ara h 1, a major peanut allergen, has been shown to act as a Th2 adjuvant by activating DCs to drive Th2 cell maturation (59). In contrast, deglycoslyated Ara h 1, or the highly homologous soy vicilin, which does not contain the carbohydrate moiety, did not activate DCs (59).
Recently, there has been evidence that carbohydrates alone can trigger IgE-mediated food allergies. Commins et al. (60) published the first report of galactose-α-1,3-galactose (α-gal) as a potential food allergen mediating adult-onset, delayed hypersensitivity reactions to red meats (beef, pork, lamb). Interestingly, these patients were from a distinct regional location in the southeastern US, raising the possibility of a sensitizing exposure that may be geographically isolated, e.g., deer tick bites. Additional studies will be needed to elucidate the mechanism for these delayed clinical symptoms as well as to establish the mode of sensitization to alpha in these patients.
-
Diagnostic tests for food allergy
Conventional diagnostic tests for IgE-mediated food allergy include skin prick testing (SPT) and serum-specific IgE testing (sIgE). Although higher levels of allergen-specific IgE and larger SPT wheal sizes are associated with increased likelihood of allergic reactions, they still lack precision and do not predict severity of allergic reactions. Predictive values for sIgE levels (using the UniCAP [Phadia]) and SPT have been published for the major food allergens, and levels above the 95% positive predicative value are highly indicative of clinical reactivity (Table 2) (61–65). Currently in the US, there are 3 commercial assays that measure sIgE levels. Each meets the WHO IgE standard for analytical sensitivity, precision, and reproducibility; however, multiple studies have demonstrated that these assays are not interchangeable or equivalent, and therefore, comparison of results using different assays may not be reliable (66–68).
The gold standard for the diagnosis of food allergy is a double-blind, placebo-controlled oral food challenge, in which the potential allergen is gradually fed in increasing doses under supervision. Open challenges (in which the food is fed in the natural form in an unblinded manner) are more practical and less time and resource intensive than the double-blind challenge and are useful when the concern for bias is low (69). Open challenges are generally used in clinical practices, and if results are equivocal, blinded challenges can be performed. Single-blind challenges are also an option when the potential for subjective symptoms and patient anxiety are high. SPT and sIgE levels are used in conjunction with the patient history to assess the risks and benefits of undergoing a food challenge, with most using at least a 50% likelihood of reaction before considering challenges (69). Food challenges should be performed in settings where medical staff and equipment are available to treat anaphylactic reactions.
Unfortunately, no standardized tests are currently available for the detection of non–IgE-mediated food allergies. Atopy patch testing has been investigated as a possible diagnostic tool to identify foods that may cause delayed symptoms (70, 71), but this test is limited by the lack of standardized reagents and methods, and there is inconsistency in interpretation of results.
Novel diagnostic tests. One limitation of current tests (SPT and sIgE) is that positive results may not be diagnostic of food allergy since cross-reactivity between proteins can give false-positive test results (e.g., wheat and grass, birch and peanut/hazelnut) (72). Component-resolved diagnostics has the potential to address these obstacles. A recent study reported the use of component resolved diagnosis to distinguish between peanut-allergic subjects and peanut-sensitized but tolerant subjects (73). By assessing IgE binding to individual recombinant proteins of peanut, grass, and potentially cross-reactive components, peanut-allergic individuals were found to have high responses to Ara h 1–3; in contrast, peanut-sensitized, but clinically tolerant subjects had high responses to grass allergens and cross-reactive carbohydrate determinants. Further analysis indicated that Ara h 2–specific IgE was the best discriminant between clinical reactivity and simple sensitivity to peanut. Similar results have been seen in studies for hazelnut and kiwi (74, 75), but further studies are necessary to establish the value of this approach.
Standard allergy tests are unable to provide an assessment of allergy severity or prognosis. Peptide microarray immunoassays, as noted above, and basophil activation tests appear to provide some of this information. Preliminary studies showed that basophil activation, as determined by antigen-induced CD63 upregulation on basophils, correlated with different phenotypes of milk allergy. In a study evaluating the effects of ingesting heat-denatured milk proteins in milk-allergic individuals, basophil reactivity was strikingly distinct between heated milk–tolerant and heated milk–reactive subjects (76).
Although further studies are needed to validate these tests, they may one day improve physicians’ ability to confidently diagnose food allergies, preventing unnecessary avoidance diets that can have nutritional as well as social consequences. In addition, they may decrease the need for costly, time-consuming, and potentially life-threatening reactions with oral food challenges.
-
Therapies for food allergy
The standard of care for treating food allergies consists of identifying the responsible food allergen and educating patients on how to avoid ingesting the food unknowingly and how to recognize and treat early signs of an allergic reaction in case of accidental ingestion. Given the increasing prevalence of food allergy and associated hospitalizations (77), this approach is clearly not optimal and there is a strong need to develop effective therapies. Several allergen-specific and allergen-nonspecific strategies are currently being investigated (Table 3).
-
Allergen-specific therapies
Allergen immunotherapy. Immunotherapy entails gradual increasing exposure to allergens in the hopes of desensitization (temporary loss of responsiveness due to continuous exposure) and/or promoting tolerance (permanent immunologic nonresponse). Immunotherapy is widely used to treat respiratory allergies, and with the success of subcutaneous immunotherapy (SCIT) for asthma and allergic rhinitis, food-specific immunotherapy has been investigated as a potential treatment for food allergy.
Although early attempts at using SCIT for food allergies resulted in unacceptably high rates of severe adverse reactions (78), oral immunotherapy (OIT) appears to be a more promising option. Although the first report of OIT was published in 1908 (79), the first double-blind, placebo-controlled OIT study for food allergy was performed 100 years later by Skripak et al. (80). Twenty children were randomized to receive daily milk OIT in 3 phases. Double-blind, placebo-controlled oral food challenges were performed before and after 13 weeks of OIT to establish thresholds of reactivity to milk. Following treatment, the threshold dose for reactions to milk increased more than 50- to 100-fold for all children on active OIT but remained unchanged in the placebo group. All children in the active OIT group experienced multiple adverse reactions; most were mild, but four children received epinephrine for treatment of more severe symptoms. Although there was no significant change in milk-specific IgE levels or SPT results, there was a significant increase in milk-specific IgG and IgG4 in the active treatment group. Importantly, the majority of participants experienced reactions during the post-OIT food challenge, indicating that complete protection from allergic reactions was not achieved. Furthermore, all successfully desensitized participants continued daily consumption of milk; therefore, it is unclear whether any OIT participants developed tolerance rather than desensitization. Jones et al. (81) reported an uncontrolled open-label peanut OIT study in which desensitization appeared successful in 93% of patients after 4–22 months of maintenance therapy. The increased tolerance was accompanied by decreased IgE levels at 12–18 months and significantly increased IgG4.
It is important to note that adverse reactions during OIT are fairly common. Nearly half of the active doses are associated with symptoms that ranged from mild localized reactions to systemic reactions requiring epinephrine (80, 81). A follow-up report from a peanut OIT trial reported that 3.5% of home doses were associated with adverse reactions, and epinephrine was required for three of the seven reactions described (82). A similar report of 15 subjects on milk OIT indicated that six reactions (in four subjects) with the home doses required treatment with epinephrine (83). Adverse reactions from OIT are common, variable, and unpredictable.
Sublingual immunotherapy (SLIT) is another potential route of administration and has been investigated for hazelnut (84, 85) and peach allergy (86). Both studies demonstrated increased tolerance after 5–6 months of treatment. Patients receiving SLIT frequently experience mild, localized adverse symptoms, but systemic symptoms sometimes occur. Like OIT, SLIT appears to desensitize patients, but whether it will lead to permanent tolerance remains to be established.
Clinical studies investigating tolerance to extensively heated (baked) milk and egg in allergic children demonstrated that 80% of milk- or egg-allergic children could tolerate baked products. Subsequent ingestion of these baked products on a regular basis in tolerant children resulted in virtually no adverse symptoms and was associated with decreased milk- and egg-specific SPT sizes and increased IgG4 levels (51). Furthermore, heated milk–tolerant children had a higher frequency of milk allergen–specific Treg cells (87), suppressed IgE-mediated basophil responses to milk allergen stimulation (76), and decreased IgE epitope diversity and lower affinity binding on peptide microarray immunoassays (56) as compared with milk-allergic children who could not tolerate baked products. These immunologic changes are similar to many of the changes seen with OIT, suggesting that ingestion of heat-denatured milk or egg may present a more natural and safer form of immunotherapy. A prospective study is underway to confirm the efficacy of this approach as a form of immunotherapy (88).
Overall, immunotherapy appears to be a promising option for the treatment of food allergy, although high rates of adverse reactions with OIT are problematic. Additional randomized, placebo-controlled trials are necessary to determine the true efficacy and safety of these methods, to standardize extracts, protocols, and durations of treatment, and to determine whether these forms of therapy can induce lasting oral tolerance, rather than simply desensitization, which necessitates continuous, almost daily treatment.
Modified recombinant vaccines. In order to decrease adverse effects of immunotherapy due to allergen activation of mast cells and basophils, modified recombinant food proteins have been engineered to decrease IgE-binding capacity while retaining the protein’s ability to stimulate T cells. Modified peanut allergens (Ara h 1, 2, 3), altered using site-directed mutagenesis, can stimulate T cells from peanut-allergic individuals to proliferate, but have greatly reduced IgE-binding capacity as compared with wild-type peanut protein (89). Heat-killed E. coli producing recombinant peanut proteins have demonstrated protective effects in a murine model of peanut anaphylaxis (90). The mechanisms hypothesized to induce this effect include activation of T regulatory cells and downregulation of Th2 cells and reduction of mast cell mediator release on reexposure to antigen (91). Human clinical trials are currently underway (92).
Other allergen-specific therapies. Several other strategies currently under investigation include immunotherapy with peptides representing T cell epitopes of major food allergens (93, 94), immunostimulatory sequence-conjugated protein immunotherapy (95, 96), and plasmid DNA immunotherapy (97, 98). Preliminary results in murine models of food allergy have shown promise, but questions remain as to whether these approaches will be equally effective in humans. For example, plasmid DNA was found to have beneficial effects in one mouse strain but worsened symptoms in another strain (98), raising concerns regarding the utility of this technique in humans.
-
Allergen-nonspecific therapies
Anti-IgE. Recombinant monoclonal humanized anti-IgE treatment is currently used for the treatment of allergic asthma and has been investigated as a possible treatment for food allergy. In 2003, a double-blind, randomized, dose-ranging trial was carried-out in 84 peanut-allergic patients (99). Patients received either Hu-901 (humanized IgG1 monoclonal antibody against IgE) or placebo for four months (Figure 2). Patients receiving the highest dose experienced significant decreases in symptoms with peanut challenge as compared with the placebo group. The median threshold of sensitivity to peanut increased from 178 mg peanut protein (equivalent to one-half of a peanut kernel) to almost nine peanut kernels (2.8 grams). Although 25% of patients had markedly improved tolerance after treatment, another 25% had no change in their threshold of reactivity, indicating that the treatment response can be variable. Investigation of another anti-IgE preparation, omalizumab (Xolair; Genentech), for the treatment of peanut allergy was initiated, but discontinued for safety concerns related to the pretreatment oral peanut challenge (100).
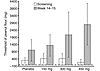 Figure 2
Figure 2Mean threshold dose of peanut flour eliciting symptoms in patients receiving Hu-901 or placebo. The mean increase in the threshold of sensitivity, as compared with that in the placebo group, reached significance only in the 450-mg group (P < 0.001); however, results of the test for trend with increasing doses were significant (P < 0.001). Data show 95% confidence intervals. Adapted from ref. 99 with permission from the Publishing Division of the Massachusetts Medical Society.
Combination therapy of anti-IgE and allergen immunotherapy is being investigated as a method to decrease adverse reactions to immunotherapy and decrease antigen-facilitated presentation by IgE-bearing antigen-presenting cells, which promotes Th2 responsiveness, in order to increase the safety and efficacy of this method (101). No data from controlled clinical trials are currently available regarding the effectiveness of this strategy.
Chinese herbal medicine and other allergen-nonspecific strategies. A nine-herb formula based on traditional Chinese medicine, named the food allergy herbal formula (FAHF-2), has been shown to be effective in preventing anaphylaxis in a murine model of peanut allergy (102). Peanut-allergic mice treated with FAHF-2 had no signs of anaphylaxis following oral peanut challenge, but all sham-treated mice had severe symptoms, decreased rectal temperatures, elevated plasma histamine, and marked vascular leakage. Decreases in peanut-specific IgE levels and Th2 cytokine production by peanut-stimulated splenocytes in vitro (IL-4, IL-5, IL-13) was seen as well as enhanced production of IFN-γ. Neutralization of IFN-γ and depletion of CD8+ T cells markedly attenuated the response to FAHF-2 (103). These protective effects lasted up to 6 months after therapy, which represents about 25% of the life span of the mouse (104). Furthermore, these effects are not peanut specific; treatment has been shown to modulate the allergic response in a murine model of multiple food allergies (105).
FAHF-2 has been shown to have multiple immunomodulatory effects, including a dose-dependent decrease in Th2 cytokine production, but increase in IFN-γ (106), indicating that it is not a general immunosuppressive. In addition, human peripheral blood mononuclear cells from peanut-allergic individuals that were stimulated with crude peanut extract in the presence of FAHF-2 had a decrease in antigen-dependent T cell proliferation. The US FDA approved a botanical drug investigational new drug (IND) for FAHF-2, and a phase I trial demonstrated that it is safe and well tolerated. A phase II trial is under way (107).
Allergic diseases are believed to develop in part because of imbalances in Th1 and Th2 cytokines. Strategies targeting cytokines (108) or TLR-9 (109) have been investigated to promote Th1-type immune responses as a potential therapeutic approach, and applications in the field of food allergies are emerging.
-
Conclusions
Food allergy continues to be a growing health concern. As we gain more insight into the immune mechanisms of oral tolerance and the complex interactions between host factors and food allergen properties, we can develop novel diagnostic tools to more accurately identify food allergies. Currently, several potential therapies are in clinical trials. The question of whether these treatments will induce only short-term desensitization or lead to long-term tolerance is under investigation. In addition to developing treatments, these studies will advance our understanding of the mechanisms of tolerance. These strategies, either alone or in combination, will hopefully provide long-term treatment options and potentially a cure for food allergy. Taken together, these next 5 years should provide several exciting advances in the field of food allergy.
-
Acknowledgments
Julie Wang is funded in part by a grant from the NIH/National Institute of Allergy and Infectious Diseases (K23 AI083883). Hugh A. Sampson is funded in part by grants from the NIH/National Institute of Allergy and Infectious Diseases (AI44236 and AI066738).
Address correspondence to: Julie Wang, Department of Pediatrics, Box 1198, Mount Sinai School of Medicine, One Gustave L. Levy Place, New York, New York 10029, USA. Phone: 212.241.5548; Fax: 212.426.1902; E-mail: [email protected].
-
Footnotes
Conflict of interest: Hugh A. Sampson is an investor in and paid consultant for Allertein Therapeutics and an investor in Herbal Springs LLC, a holding company for the patents on FAHF-2. Hugh A. Sampson receives research support from the Food Allergy Initiative, a nonprofit foundation supporting food allergy research.
Reference information: J Clin Invest. 2011;121(3):827–835. doi:10.1172/JCI45434.
-
References
- Rona RJ, et al. The prevalence of food allergy: a meta-analysis. J Allergy Clin Immunol. 2007;120(3):638–646.
- Branum AM, Lukacs SL. Food allergy among children in the United States. Pediatrics. 2009;124(6):1549–1555.View this article via: PubMed Google Scholar
- Sicherer SH, Munoz-Furlong A, Sampson HA. Prevalence of peanut and tree nut allergy in the United States determined by means of a random digit dial telephone survey: a 5-year follow-up study. J Allergy Clin Immunol. 2003;112(6):1203–1207.
- Grundy J, Matthews S, Bateman B, Dean T, Arshad SH. Rising prevalence of allergy to peanut in children: Data from 2 sequential cohorts. J Allergy Clin Immunol. 2002;110(5):784–789.
- Ilan Y. Oral tolerance: can we make it work? Hum Immunol. 2009;70(10):768–776.
- Spahn TW, et al. Mesenteric lymph nodes are critical for the induction of high-dose oral tolerance in the absence of Peyer’s patches. Eur J Immunol. 2002;32(4):1109–1113.
- Chehade M, Mayer L. Oral tolerance and its relation to food hypersensitivities. J Allergy Clin Immunol. 2005;115(1):3–12.
- Appleman LJ, Boussiotis VA. T cell anergy and costimulation. Immunol Rev. 2003;192:161–180.
- Chen Y, Inobe J, Marks R, Gonnella P, Kuchroo VK, Weiner HL. Peripheral deletion of antigen-reactive T cells in oral tolerance. Nature. 1995;376(6536):177–180.View this article via: PubMed Google Scholar
- Bol-Schoenmakers M, et al. Regulation by intestinal gd T cells during establishment of food allergic sensitization in mice [published online ahead of print September 29, 2010]. Allergy . doi: 10.1111/j.1398-9995.2010.02479.x.
- Kim HJ, Hwang SJ, Kim BK, Jung KC, Chung DH. NKT cells play critical roles in the induction of oral tolerance by inducing regulatory T cells producing IL-10 and transforming growth factor beta, and by clonally deleting antigenspecific T cells. Immunology. 2006;118(1):101–111.
- Soumelis V, et al. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat Immunol. 2002;3(7):673–680.View this article via: PubMed Google Scholar
- Liu YJ. Thymic stromal lymphopoietin: master switch for allergic inflammation. J Exp Med. 2006;203(2):269–273.
- Demehri S, Morimoto M, Holtzman MJ, Kopan R. Skin-derived TSLP triggers progression from epidermal-barrier defects to asthma. PLoS Biol. 2009;7(5):e1000067.
- Ziegler SF, Artis D. Sensing the outside world: TSLP regulates barrier immunity. Nat Immunol. 2010;11(4):289–293.
- Blázquez AB, Mayer L, Berin MC. Thymic stromal lymphopoietin is required for gastrointestinal allergy but not oral tolerance. Gastroenterology. 2010;139(4):1301–1309.
- Sherrill JD, et al. Variants of thymic stromal lymphopoietin and its receptor associate with eosinophilic esophagitis. J Allergy Clin Immunol. 2010;126(1):160–165.
- Ventura M, et al. Intestinal permeability in patients with adverse reactions to food. Dig Liver Dis. 2006;38(10):732–736.
- Levy Y, Davidovits M, Cleper R, Shapiro R. New-onset post-transplantation food allergy in children – Is it attributable only to the immunosuppressive protocol? Pediatr Transplant. 2009;13(1):63–69.
- Gabe SM, et al. The effect of tacrolimus (FK506) on intestinal barrier function and cellular energy production in humans. Gastroenterology. 1998;115(1):67–74.
- Wang LF, Lin JY, Hsieh KH, Lin RH. Epicutaneous exposure of protein antigen induces a predominant Th2-like response with high IgE production in mice. J Immunol. 1996;156(11):4077–4082.View this article via: PubMed Google Scholar
- N Engl J Med. 2003;348(11):977–985.
- Bohle B. The impact of pollen-related food allergens on pollen allergy. Allergy. 2007;62(1):3–10.View this article via: PubMed Google Scholar
- Torgerson TR, Ochs HD. Immune dysregulation, polyendocrinopathy, enteropathy, X-linked: forkhead box protein 3 mutations and lack of regulatory T cells. J Allergy Clin Immunol. 2007;120(4):744–750.
- Karlsson MR, Rugtveit J, Brandtzaeg P. Allergen-responsive CD4+CD25+ regulatory T cells in children who have outgrown cow’s milk allergy. J Exp Med. 2004;199(12):1679–1688.
- Sicherer SH, Furlong TJ, Maes HH, Desnick RJ, Sampson HA, Gelb BD. Genetics of peanut allergy: a twin study. J Allergy Clin Immunol. 2000;106(1 pt 1):53–56.View this article via: PubMed Google Scholar
- Du Toit G, et al. Early consumption of peanuts in infancy is associated with a low prevalence of peanut allergy. J Allergy Clin Immunol. 2008;122(5):984–991.
- National Institute of Allergy and Infectious Diseases (NIAID). Promoting tolerance to peanut in high-risk children (LEAP). NIH Web site. http://clinicaltrials.gov/ct2/show/NCT00329784?term=NCT00329784&rank=1 . Accessed December 13, 2010.
- Katz Y, et al. Early exposure to cow’s milk protein is protective against IgE-mediated cow’s milk protein allergy. J Allergy Clin Immunol. 2010;126(1):77–82.
- Koplin JJ, et al. Can early introduction of egg prevent egg allergy in infants? A population-based study. J Allergy Clin Immunol. 2010;126(4):807–813.
- Untersmayr E, et al. Anti-ulcer drugs promote IgE formation toward dietary antigens in adult patients. FASEB J. 2005;19(6):656–658.View this article via: PubMed Google Scholar
- Takagi K, et al. Kinetic analysis of pepsin digestion of chicken egg white ovomucoid and allergenic potential of pepsin fragments. Int Arch Allergy Immunol. 2005;136(1):23–32.
- Kukkonen K, et al. Probiotics and prebiotic galacto-oligosaccharides in the prevention of allergic diseases: a randomized, double-blind, placebo-controlled trial. J Allergy Clin Immunol. 2007;119(1):192–198.
- Abrahamsson TR, et al. Probiotics in prevention of IgE-associated eczema: a double-blind, randomized, placebo-controlled trial. J Allergy Clin Immunol. 2007;119(5):1174–1180.
- Taylor AL, Dunstan JA, Prescott SL. Probiotic supplementation for the first 6 months of life fails to reduce the risk of atopic dermatitis and increases the risk of allergen sensitization in high-risk children: a randomized controlled trial. J Allergy Clin Immunol. 2007;119(1):184–191.
- Bock SA, Munoz-Furlong A, Sampson HA. Fatalities due to anaphylactic reactions to foods. J Allergy Clin Immunol. 2001;107(1):191–193.
- Bock SA, Munoz-Furlong A, Sampson HA. Further fatalities caused by anaphylactic reactions to food, 2001–2006. J Allergy Clin Immunol. 2007;119(4):1016–1018.
- Pumphrey R. Anaphylaxis: can we tell who is at risk of a fatal reaction? Curr Opin Allergy Clin Immunol. 2004;4(4):285–290.
- Miller MM, Miller MM. Beta-blockers and anaphylaxis: are the risks overstated? J Allergy Clin Immunol. 2005;116(4):931–933.View this article via: PubMed Google Scholar
- Vadas P, et al. Platelet-activating factor, PAF acetylhydrolase, and severe anaphylaxis. N Engl J Med. 2008;358(1):28–35.
- Okamoto H, Kamatani N. Platelet-activating factor, PAF acetylhydrolase, and anaphylaxis. N Engl J Med. 2008;358(14):1516.View this article via: PubMed Google Scholar
- Sicherer SH, Sampson HA. Food allergy. J Allergy Clin Immunol. 2010;125(2 suppl 2):S116–S125.
- Cohen A, Goldberg M, Levy B, Leshno M, Katz Y. Sesame food allergy and sensitization in children: the natural history and long-term follow-up. Pediatr Allergy Immunol. 2007;18(3):217–223.
- Jenkins JA, Breiteneder H, Mills EN. Evolutionary distance from human homologs reflects allergenicity of animal food proteins. J Allergy Clin Immunol. 2007;120(6):1399–1405.
- Breiteneder H, Radauer C. A classification of plant food allergens. J Allergy Clin Immunol. 2004;113(5):821–830.
- Jarvinen KM, Beyer K, Vila L, Chatchatee P, Busse PJ, Sampson HA. B-cell epitopes as a screening instrument for persistent cow’s milk allergy. J Allergy Clin Immunol. 2002;110(2):293–297.
- Jarvinen KM, Beyer K, Vila L, Bardina L, Mishoe M, Sampson HA. Specificity of IgE antibodies to sequential epitopes of hen’s egg ovomucoid as a marker for persistence of egg allergy. Allergy. 2007;62(7):758–765.
- Walzer M. Mechanism of allergy. Bull N Y Acad Med. 1940;16(6):389–394.View this article via: PubMed Google Scholar
- Husby S. Dietary antigens: uptake and humoral immunity in man. APMIS Suppl. 1988;1:1–40.View this article via: PubMed Google Scholar
- Lemon-Mule H, Sampson HA, Sicherer SH, Shreffler WG, Noone S, Nowak-Wegrzyn A. Immunologic changes in children with egg allergy ingesting extensively heated egg. J Allergy Clin Immunol. 2008;122(5):977–983.
- Nowak-Wegrzyn A, et al. Tolerance to extensively heated milk in children with cow’s milk allergy. J Allergy Clin Immunol. 2008;122(2):342–347.
- Shreffler WG, Beyer K, Chu TH, Burks AW, Sampson HA. Microarray immunoassay: association of clinical history, in vitro IgE function, and heterogeneity of allergenic peanut epitopes. J Allergy Clin Immunol. 2004;113(4):776–782.
- Shreffler WG, Lencer DA, Bardina L, Sampson HA. IgE and IgG4 epitope mapping by microarray immunoassay reveals the diversity of immune response to the peanut allergen, Ara h 2. J Allergy Clin Immunol. 2005;116(4):893–899.
- Flinterman AE, et al. Peanut epitopes for IgE and IgG4 in peanut-sensitized children in relation to severity of peanut allergy. J Allergy Clin Immunol. 2008;121(3):737–743.
- Cerecedo I, et al. Mapping of the IgE and IgG4 sequential epitopes of milk allergens with a peptide microarray-based immunoassay. J Allergy Clin Immunol. 2008;122(3):589–594.
- Wang J, et al. Correlation of IgE/IgG4 milk epitopes and affinity of milk-specific IgE antibodies with different phenotypes of clinical milk allergy. J Allergy Clin Immunol. 2010;125(3):695–702.
- Beyer K, et al. Effects of cooking methods on peanut allergenicity. J Allergy Clin Immunol. 2001;107(6):1077–1081.View this article via: PubMed Google Scholar
- Hilmenyuk T, et al. Effects of glycation of the model food allergen ovalbumin on antigen uptake and presentation by human dendritic cells. Immunology. 2010;129(3):437–445.
- Shreffler WG, et al. The major glycoprotein allergen from Arachis hypogaea, Ara h 1, is a ligand of dendritic cell-specific ICAM-grabbing nonintegrin and acts as a Th2 adjuvant in vitro. J Immunol. 2006;177(6):3677–3685.View this article via: PubMed Google Scholar
- Commins SP, et al. Delayed anaphylaxis, angioedema, or urticaria after consumption of red meat in patients with IgE antibodies specific for galactose-alpha-1,3-galactose. J Allergy Clin Immunol. 2009;123(2):426–433.
- Sampson HA. Utility of food-specific IgE concentrations in predicting symptomatic food allergy. J Allergy Clin Immunol. 2001;107(5):891–896.
- Perry TT, Matsui EC, Kay Conover-Walker M, Wood RA. The relationship of allergen-specific IgE levels and oral food challenge outcome. J Allergy Clin Immunol. 2004;114(1):144–149.
- Garcia-Ara C, Boyano-Martinez T, Diaz-Pena JM, Martin-Munoz F, Reche-Frutos M, Martin-Esteban M. Specific IgE levels in the diagnosis of immediate hypersensitivity to cows’ milk protein in the infant. J Allergy Clin Immunol. 2001;107(1):185–190.
- Boyano Martinez T, Garcia-Ara C, Diaz-Pena JM, Munoz FM, Garcia Sanchez G, Esteban MM. Validity of specific IgE antibodies in children with egg allergy. Clin Exp Allergy. 2001;31(9):1464–1469.
- Hill DJ, Heine RG, Hosking CS. The diagnostic value of skin prick testing in children with food allergy. Pediatr Allergy Immunol. 2004;15(5):435–441.
- Wood RA, Segall N, Ahlstedt S, Williams PB. Accuracy of IgE antibody laboratory results. Ann Allergy Asthma Immunol. 2007;99(1):34–41.
- Wang J, Godbold JH, Sampson HA. Correlation of serum allergy (IgE) tests performed by different assay systems. J Allergy Clin Immunol. 2008;121(5):1219–1224.
- Hamilton RG, et al. Human IgE antibody serology: a primer for the practicing North American allergist/immunologist. J Allergy Clin Immunol. 2010;126(1):33–38.
- Nowak-Wegrzyn A, et al. Work Group report: oral food challenge testing. J Allergy Clin Immunol. 2009;123(6 suppl):S365–S383.
- Turjanmaa K, Darsow U, Niggemann B, Rance F, Vanto T, Werfel T. EAACI/GA2LEN position paper: Present status of the atopy patch test. Allergy. 2006;61(12):1377–1384.
- Mehl A, et al. The atopy patch test in the diagnostic workup of suspected food-related symptoms in children. J Allergy Clin Immunol. 2006;118(4):923–929.
- Sicherer SH. Clinical implications of cross-reactive food allergens. J Allergy Clin Immunol. 2001;108(6):881–890.
- Nicolaou N, et al. Allergy or tolerance in children sensitized to peanut: Prevalence and differentiation using component-resolved diagnostics. J Allergy Clin Immunol. 2010;125(1):191–197.View this article via: PubMed Google Scholar
- Hansen KS, et al. Component-resolved in vitro diagnosis of hazelnut allergy in europe. J Allergy Clin Immunol. 2009;123(5):1134–1141.
- Bublin M, et al. Component-resolved diagnosis of kiwifruit allergy with purified natural and recombinant kiwifruit allergens. J Allergy Clin Immunol. 2010;125(3):687–694.
- Wanich N, Nowak-Wegrzyn A, Sampson HA, Shreffler WG. Allergen-specific basophil suppression associated with clinical tolerance in patients with milk allergy. J Allergy Clin Immunol. 2009;123(4):789–794.View this article via: PubMed Google Scholar
- Ross MP, Ferguson M, Street D, Klontz K, Schroeder T, Luccioli S. Analysis of food-allergic and anaphylactic events in the National Electronic Injury Surveillance System. J Allergy Clin Immunol. 2008;121(1):166–171.
- Nelson HS, Lahr J, Rule R, Bock A, Leung D. Treatment of anaphylactic sensitivity to peanuts by immunotherapy with injections of aqueous peanut extract. J Allergy Clin Immunol. 1997;99(6 pt 1):744–75.
- Schofield AT. A case of egg poisoning. Lancet. 1908;1:716.View this article via: PubMed Google Scholar
- Skripak JM, et al. A randomized, double-blind, placebo-controlled study of milk oral immunotherapy for cow’s milk allergy. J Allergy Clin Immunol. 2008;122(6):1154–1160.
- Jones SM, et al. Clinical efficacy and immune regulation with peanut oral immunotherapy. J Allergy Clin Immunol. 2009;124(2):292–300.
- Varshney P, et al. Adverse reactions during peanut oral immunotherapy home dosing. J Allergy Clin Immunol. 2009;124(6):1351–1352.
- Narisety SD, et al. Open-label maintenance after milk oral immunotherapy for IgE-mediated cow’s milk allergy. J Allergy Clin Immunol. 2009;124(3):610–612.
- Enrique E, et al. Sublingual immunotherapy for hazelnut food allergy: a randomized, double-blind, placebo-controlled study with a standardized hazelnut extract. J Allergy Clin Immunol. 2005;116(5):1073–1079.
- Enrique E, et al. Sublingual immunotherapy for hazelnut food allergy: a follow-up study. Ann Allergy Asthma Immunol. 2008;100(3):283–284.View this article via: PubMed Google Scholar
- Fernandez-Rivas M, et al. Randomized double-blind, placebo-controlled trial of sublingual immunotherapy with a Pru p 3 quantified peach extract. Allergy. 2009;64(6):876–883.
- Shreffler WG, Wanich N, Moloney M, Nowak-Wegrzyn A, Sampson HA. Association of allergen-specific regulatory T cells with the onset of clinical tolerance to milk protein. J Allergy Clin Immunol. 2009;123(1):43–52.
- National Institute of Allergy and Infectious Diseases (NIAID). An interventional study of milk allergy. NIH Web site. http://clinicaltrials.gov/ct2/show/NCT00578656?term=NCT00578656&rank=1 . Accessed December 13, 2010.
- Bannon GA, et al. Engineering, characterization and in vitro efficacy of the major peanut allergens for use in immunotherapy. Int Arch Allergy Immunol. 2001;124(1–3):70–72.
- Li XM, et al. Persistent protective effect of heat-killed Escherichia coli producing “engineered,” recombinant peanut proteins in a murine model of peanut allergy. J Allergy Clin Immunol. 2003;112(1):159–167.
- King N, et al. Allergenic characteristics of a modified peanut allergen. Mol Nutr Food Res. 2005;49(10):963–971.
- National Institute of Allergy and Infectious Diseases (NIAID). Peanut allergy vaccine study in healthy and peanut-allergic adults. NIH Web site. http://clinicaltrials.gov/ct2/show/NCT00850668?term=NCT00850668&rank=1 . Accessed December 13, 2010.
- Hong SJ, Michael JG, Fehringer A, Leung DY. Pepsin-digested peanut contains T-cell epitopes but no IgE epitopes. J Allergy Clin Immunol. 1999;104(2 pt 1):473–478.View this article via: PubMed Google Scholar
- Li S, Li XM, Burks AW, Bannon GA, Sampson HA. Modulation of peanut allergy by peptide-based immunotherapy [abstract]. J Allergy Clin Immunol. 2001;107:S233.
- Chu RS, Targoni OS, Krieg AM, Lehmann PV, Harding CV. CpG oligodeoxynucleotides act as adjuvants that switch on T helper 1 (Th1) immunity. J Exp Med. 1997;186(10):1623–1631.
- Srivastava KD, et al. Investigation of the use of ISS-linked Ara h2 for the treatment of peanut-induced allergy [abstract]. J Allergy Clin Immunol. 2001;107(2):S233.
- Roy K, Mao HQ, Huang SK, Leong KW. Oral gene delivery with chitosan--DNA nanoparticles generates immunologic protection in a murine model of peanut allergy. Nat Med. 1999;5(4):387–391.
- Morafo V, et al. Genetic susceptibility to food allergy is linked to differential Th2-Th1 responses in C3h/HeJ and BALB/c mice. J Allergy Clin Immun. 2003;111(5):1122–1128.
- Leung DY, et al. Effect of anti-IgE therapy in patients with peanut allergy. N Engl J Med. 2003;348(11):986–993.View this article via: PubMed Google Scholar
- Sampson HA. A phase II, randomized, double-blind, parallel group, placebo-controlled oral food challenge trial of Xolair (omalizumab) in peanut allergy [abstract]. J Allergy Clin Immunol. 2007;119:S117.
- Casale TB, et al. Omalizumab pretreatment decreases acute reactions after rush immunotherapy for ragweed-induced seasonal allergic rhinitis. J Allergy Clin Immunol. 2006;117(1):134–140.
- Srivastava KD, et al. The Chinese herbal medicine formula FAHF-2 completely blocks anaphylactic reactions in a murine model of peanut allergy. J Allergy Clin Immunol. 2005;115(1):171–178.
- Srivastava KD, Qu C, Zhang T, Goldfarb J, Sampson HA, Li XM. Food Allergy Herbal Formula-2 silences peanut-induced anaphylaxis for a prolonged posttreatment period via IFN-gamma-producing CD8+ T cells. J Allergy Clin Immunol. 2009;123(2):443–451.
- Srivastava KD, Zhang T, Qu C, Sampson HA, Li XM. Silencing peanut allergy: A Chinese herbal formula, Fahf-2, completely blocks peanut-induced anaphylaxis for up to 6 months following therapy in a murine model of peanut allergy. J Allergy Clin Immunol. 2006;117(2):S328.
- Srivastava KD, Sampson HA, Li XM. The traditional Chinese medicine formula FAHF-2 provides complete protection from anaphylaxis in a murine model of multiple food allergy. J Allergy Clin Immunol. 2005;115:171–178.
- Ko J, Busse PJ, Shek L, Noone SA, Sampson HA, Li XM. Effect of Chinese herbal formulas on T-cell responses in patients with peanut allergy or asthma [abstract]. J Allergy Clin Immunol. 2005;115:S34.
- Li X-M. Therapeutic effect of chinese herbal medicine on food allergy (FAHF-2). NIH Web site. http://clinicaltrials.gov/ct2/show/NCT00602160?term=NCT00602160&rank=1 . Accessed December 13, 2010.
- Cortes-Perez NG, et al. Intranasal coadministration of live lactococci producing interleukin-12 and a major cow’s milk allergen inhibits allergic reaction in mice. Clin Vaccine Immunol. 2007;14(3):226–233.View this article via: PubMed Google Scholar
- Zhu FG, Kandimalla ER, Yu D, Agrawal S. Oral administration of a synthetic agonist of Toll-like receptor 9 potently modulates peanut-induced allergy in mice. J Allergy Clin Immunol. 2007;120(3):631–637.View this article via: PubMed Google Scholar
-
Version history
- Version 1 (March 1, 2011): No description
Article tools
-
View PDF

- Download citation information
- Send a comment
- Terms of use
- Standard abbreviations
- Need help? Email the journal
Metrics
Go to
- Top
- Abstract
- Introduction
- Pathophysiology of food allergy
- Host factors influencing food allergy
- Food allergen factors influencing food allergy
- Diagnostic tests for food allergy
- Therapies for food allergy
- Allergen-specific therapies
- Allergen-nonspecific therapies
- Conclusions
- Acknowledgments
- Footnotes
- References
- Version history



Copyright © 2024 American Society for Clinical Investigation
ISSN: 0021-9738 (print), 1558-8238 (online)





