Advertisement
Research ArticleImmunology Free access | 10.1172/JCI38332
An aberrant prostate antigen–specific immune response causes prostatitis in mice and is associated with chronic prostatitis in humans
Yafei Hou,1 Jason DeVoss,2 Vinh Dao,1 Serena Kwek,1 Jeffrey P. Simko,3 Douglas G. McNeel,4 Mark S. Anderson,2 and Lawrence Fong1
1Division of Hematology/Oncology, Department of Medicine, 2Diabetes Center, and 3Department of Pathology, UCSF, San Francisco, California, USA. 4University of Wisconsin Paul P. Carbone Comprehensive Cancer Center, Madison, Wisconsin, USA.
Address correspondence to: Lawrence Fong, Division of Hematology/Oncology, University of California, San Francisco, 513 Parnassus Avenue, Box 0511, San Francisco, California 94143, USA. Phone: (415) 514-3424; Fax: (415) 476-0459; E-mail: [email protected].
Find articles by Hou, Y. in: JCI | PubMed | Google Scholar
1Division of Hematology/Oncology, Department of Medicine, 2Diabetes Center, and 3Department of Pathology, UCSF, San Francisco, California, USA. 4University of Wisconsin Paul P. Carbone Comprehensive Cancer Center, Madison, Wisconsin, USA.
Address correspondence to: Lawrence Fong, Division of Hematology/Oncology, University of California, San Francisco, 513 Parnassus Avenue, Box 0511, San Francisco, California 94143, USA. Phone: (415) 514-3424; Fax: (415) 476-0459; E-mail: [email protected].
Find articles by DeVoss, J. in: JCI | PubMed | Google Scholar
1Division of Hematology/Oncology, Department of Medicine, 2Diabetes Center, and 3Department of Pathology, UCSF, San Francisco, California, USA. 4University of Wisconsin Paul P. Carbone Comprehensive Cancer Center, Madison, Wisconsin, USA.
Address correspondence to: Lawrence Fong, Division of Hematology/Oncology, University of California, San Francisco, 513 Parnassus Avenue, Box 0511, San Francisco, California 94143, USA. Phone: (415) 514-3424; Fax: (415) 476-0459; E-mail: [email protected].
Find articles by Dao, V. in: JCI | PubMed | Google Scholar
1Division of Hematology/Oncology, Department of Medicine, 2Diabetes Center, and 3Department of Pathology, UCSF, San Francisco, California, USA. 4University of Wisconsin Paul P. Carbone Comprehensive Cancer Center, Madison, Wisconsin, USA.
Address correspondence to: Lawrence Fong, Division of Hematology/Oncology, University of California, San Francisco, 513 Parnassus Avenue, Box 0511, San Francisco, California 94143, USA. Phone: (415) 514-3424; Fax: (415) 476-0459; E-mail: [email protected].
Find articles by Kwek, S. in: JCI | PubMed | Google Scholar
1Division of Hematology/Oncology, Department of Medicine, 2Diabetes Center, and 3Department of Pathology, UCSF, San Francisco, California, USA. 4University of Wisconsin Paul P. Carbone Comprehensive Cancer Center, Madison, Wisconsin, USA.
Address correspondence to: Lawrence Fong, Division of Hematology/Oncology, University of California, San Francisco, 513 Parnassus Avenue, Box 0511, San Francisco, California 94143, USA. Phone: (415) 514-3424; Fax: (415) 476-0459; E-mail: [email protected].
Find articles by Simko, J. in: JCI | PubMed | Google Scholar
1Division of Hematology/Oncology, Department of Medicine, 2Diabetes Center, and 3Department of Pathology, UCSF, San Francisco, California, USA. 4University of Wisconsin Paul P. Carbone Comprehensive Cancer Center, Madison, Wisconsin, USA.
Address correspondence to: Lawrence Fong, Division of Hematology/Oncology, University of California, San Francisco, 513 Parnassus Avenue, Box 0511, San Francisco, California 94143, USA. Phone: (415) 514-3424; Fax: (415) 476-0459; E-mail: [email protected].
Find articles by McNeel, D. in: JCI | PubMed | Google Scholar
1Division of Hematology/Oncology, Department of Medicine, 2Diabetes Center, and 3Department of Pathology, UCSF, San Francisco, California, USA. 4University of Wisconsin Paul P. Carbone Comprehensive Cancer Center, Madison, Wisconsin, USA.
Address correspondence to: Lawrence Fong, Division of Hematology/Oncology, University of California, San Francisco, 513 Parnassus Avenue, Box 0511, San Francisco, California 94143, USA. Phone: (415) 514-3424; Fax: (415) 476-0459; E-mail: [email protected].
Find articles by Anderson, M. in: JCI | PubMed | Google Scholar
1Division of Hematology/Oncology, Department of Medicine, 2Diabetes Center, and 3Department of Pathology, UCSF, San Francisco, California, USA. 4University of Wisconsin Paul P. Carbone Comprehensive Cancer Center, Madison, Wisconsin, USA.
Address correspondence to: Lawrence Fong, Division of Hematology/Oncology, University of California, San Francisco, 513 Parnassus Avenue, Box 0511, San Francisco, California 94143, USA. Phone: (415) 514-3424; Fax: (415) 476-0459; E-mail: [email protected].
Find articles by Fong, L. in: JCI | PubMed | Google Scholar
Published June 1, 2009 - More info
J Clin Invest. 2009;119(7):2031–2041. https://doi.org/10.1172/JCI38332.
© 2009 The American Society for Clinical Investigation
Received: December 15, 2008; Accepted: April 8, 2009
-
Abstract
Chronic prostatitis is a common disease of unclear etiology and has no specific treatment. Mice deficient in the expression of the autoimmune regulator (Aire) gene, which are defective in thymic expression of self antigens and central tolerance, develop spontaneous prostatitis. In this study, we found that Aire-deficient mice developed spontaneous B and T cell immune responses to a prostate autoantigen, seminal vesicle secretory protein 2 (SVS2), which we believe to be novel. We show that thymic expression of this self antigen was Aire dependent. Moreover, prostatitis was induced in WT mice through immunization with SVS2, demonstrating that immunity to SVS2 was sufficient to induce prostatitis. The clinical relevance of this antigen was highlighted by our observation that patients with chronic prostatitis possessed specific autoantibodies against the human SVS2-like seminal vesicle protein semenogelin. These results provide direct evidence that spontaneous chronic prostatitis is an autoimmune disease and is regulated by both central and peripheral tolerance. Moreover, SVS2 and semenogelin are among the relevant autoantigens in mice and humans, respectively.
-
Introduction
Prostatitis is a highly prevalent disease in men (1, 2). While acute prostatitis is commonly caused by bacterial infection, most patients with chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS) have no evidence of urinary tract infection. Moreover, a substantial proportion of men can have noninfectious CP in the form of asymptomatic inflammatory prostatitis that is diagnosed on prostate biopsy performed to evaluate for prostate cancer. Prostatitis is in fact the most common nonmalignant diagnosis in patients being evaluated for elevated serum levels of prostate-specific antigen (PSA), a biomarker used to screen for prostate cancer (3). The diagnosis of this syndrome relies solely on reported pain in the perineum, rectum, and/or prostate by affected men. CPPS often relapses and remits without clear triggers. Therapy for CPPS is nonspecific and usually involves empiric treatment with antibiotics whose efficacy is not clear. The etiology of CPPS is unknown. Proposed mechanisms of disease include urine reflux–inducing chemical and physical trauma, dietary factors, or alterations in the hormonal milieu, such as high levels of estrogens (4). However, accumulating evidence suggests that CPPS could be an autoimmune disease of the prostate (5). In some patients with noninfectious CPPS, elevated levels of inflammatory cytokines in seminal plasma or expressed prostate secretion have been detected (4, 6). Antigen-specific antibody and cellular responses to prostate antigens such as PSA, prostatic acid phosphatase, MAD-PRO-34, and seminal plasma have also been reported (7–9). To further elucidate the pathogenesis of human CPPS, a few rodent models of experimental autoimmune prostatitis have been developed and characterized (10). Immunization of rats or mice with prostate extracts or proteins expressed in the prostate, such as prostatic acid phosphatase, induces specific immune responses to prostate antigens and, in some models, induces prostatitis characterized by lymphocyte infiltration (11–13). Prostatitis can also be induced in mice with thymectomy on day 3 of life (14). In this model, regulatory T cells induced in vivo by the presence of specific prostate-derived self antigens are thought to inhibit prostatitis in the thymectomized mice, which supports the notion that prostatitis can be autoimmune in nature (15). While these models share some histologic features with CPPS in humans, induced prostatitis may not accurately reflect spontaneous disease. Spontaneous prostatitis has been described in several aged rat strains and even in aged NOD mice, but the mechanism by which these strains render the host susceptible to prostatitis is unclear (16, 17). To our knowledge, none of the prostate antigens identified in these mouse models have been shown to date to be relevant for the human disease.
Autoimmunity results from the loss of immunologic tolerance, which is usually maintained through central and peripheral mechanisms. The autoimmune regulator (Aire) gene is expressed primarily in lymphoid organs and plays a critical role in the development of central tolerance (18, 19). Within the thymus, Aire is expressed primarily by medullary epithelial cells. There, Aire regulates the ectopic transcription of peripheral tissue antigens and thereby controls central tolerance to self antigens by inducing the negative selection of autoreactive T cells. Aire-deficient KO mice have a functional immune system with diverse T and B cell repertoires, but spontaneously develop multiorgan autoimmunity, as seen by inflammatory infiltrates within different tissues and serum autoantibodies (20). Interestingly, Aire-KO male mice develop spontaneous CP. While autoantigens in this model have been identified (15, 20), Aire-dependent autoantigens that mediate prostatitis have yet to be identified.
In the present study, we determined the role of central tolerance in the development of spontaneous prostatitis. We identified a prostate-specific protein, seminal vesicle secretory protein 2 (SVS2), against which reactive CD4+ T cells and antibodies developed spontaneously in Aire-KO mice. We demonstrate, for the first time to our knowledge, that thymic expression of this autoantigen was Aire dependent. We also show that immunization with SVS2 broke tolerance to this antigen and induced autoimmune prostatitis in Aire-sufficient WT mice, demonstrating that central tolerance to this antigen was incomplete in WT mice. Furthermore, we show that patients with CPPS possessed immune responses to semenogelin (Sg), the human counterpart to SVS2. These results may provide new approaches to the diagnosis and treatment of CPPS.
-
Results
Aire-KO mice develop spontaneous lymphocytic infiltration in the prostate. Aire-KO mice spontaneously develop multiorgan autoimmunity, including that of the eye, salivary glands, ovaries, and stomach (20). Inflammation in the prostate was also observed in most Aire-KO mice (21), but to our knowledge, the nature of immune recognition for autoimmune prostatitis in Aire-KO mice has not previously been reported. We first examined the histological changes in the prostates of Aire-KO mice. In Aire-KO mice, the inflammation observed included cellular extravasation into the interstitial tissue between the glands as well as infiltration into glandular epithelium of both the dorsal and ventral prostate glands, leading to effacement of the usual glandular architecture in severe cases (Figure 1A). Moderate to severe prostate inflammation (scored as shown in Supplemental Table 1; supplemental material available online with this article; doi:10.1172/JCI38332DS1), including intraepithelial infiltration of prostate glands by lymphocytes, was observed in 5 of 7 male Aire-KO C57BL/6 (B6) mice at 10–20 weeks of age, but was completely absent in all WT littermate controls (Figure 1B). Immunohistochemistry performed on Aire-KO prostate glands demonstrated predominance of CD4+ T cells among the infiltrating cells in the prostates (Figure 1C). CD8+ T cells were also observed, but were fewer in number than CD4+ T cells. Nevertheless, both CD4+ and CD8+ T cells infiltrated the glandular epithelia of these prostates. CD19+ B cells were seen at low frequency; when present, they resided within the stroma of the prostate tissue. In contrast, WT mice lacked lymphocytes within either the glandular cell layer or the stroma (Figure 1C).
 Figure 1
Figure 1Spontaneous development of autoimmune prostatitis in the Aire-KO mouse. (A) H&E staining of representative prostate sections from Aire-KO and WT mice. The urethra (u) and ductus deferens (dd), 2 ducts that course through the prostate glands, are denoted. Scale bars: 300 μm. (B) Mononuclear infiltration in H&E-stained prostate sections from 7 Aire-KO and 5 WT littermate mice on B6 background (18–20 weeks old), graded blindly by a pathologist using a scoring system adapted from grading used in evaluating human prostate tissues (see Supplemental Table 1). Data points represent individual mice, and significance of differences was assessed by 2-tailed Student’s t test. (C) Immunohistochemical staining for CD4, CD8a, CD19, and anti-rat IgG in cryostatic sections of prostates from 20-week-old Aire-KO and WT mice. Staining is from 1 representative each of 6 Aire-KO and 5 WT mice. Scale bars: 150 μm.
Aire-KO mice develop spontaneous immune responses to SVS2. In order to define the relevant prostate autoantigens that mediate this prostatitis, we screened sera of Aire-KO mice for autoantibodies to proteins present in the protein lysates derived from mouse prostate tissue. Sera of Aire-KO and WT B6/NOD F2 mice (generated as described in Methods) were used in Western blots to determine whether autoantibodies were present. Aire-KO B6/NOD F2 mouse sera were used to facilitate the identification of relevant autoantigens because these mice develop a more aggressive autoimmune phenotype (21). Autoantibodies against prostate-derived proteins were detected in most Aire-KO sera (Figure 2A). Some sera showed broad reactivity, whereas others showed more limited and/or weaker reactivity, consistent with heterogeneity in the immune response. Nevertheless, common bands were also evident across multiple mice, which indicates that there were immune responses to proteins shared by the majority of the Aire-KO mice. These common bands within the prostate lysate were not evident in immunoblots to lysates from other tissues — such as the eye, salivary glands, or lacrimal glands — in which Aire-KO mice also develop spontaneous inflammation (Supplemental Figure 1A). In an effort to purify and identify the targeted antigens, sera from Aire-KO mice with strong autoreactivity to the lysates and from WT mice were used to make immunoprecipitation columns. These columns were used to enrich for antibody-binding proteins from pooled prostate tissue extracts. Enriched proteins from both Aire-KO sera– and WT sera–coupled columns were concentrated and subjected to gel electrophoresis. Multiple distinct bands visualized by silver staining that were unique to the Aire-KO sera column were excised and analyzed by mass spectroscopy (Supplemental Figure 1B). We successfully identified SVS2 (GenBank accession no. 78070545), with 29% sequence coverage of the protein, through peptide mass fingerprinting (PMF) from both the 26- and 38-kDa bands (Figure 2B). SVS2 is an androgen-regulated protein expressed by the prostate and secreted into seminal fluid, where it serves as a major clotting protein involved in the formation of the copulatory plug (22).
 Figure 2
Figure 2Aire-KO mice possess spontaneous autoantibodies to SVS2. (A) Immunoblotting of whole prostate extracts was performed with the sera from male Aire-KO and WT mice on the B6/NOD F2 background. Each lane of the multiscreen immunoblot represents the reactivity of sera from an individual mouse, denoted by number. Gel bands stained by sera from multiple Aire-KO mice were excised and analyzed by mass spectroscopy. (B) Multiple microsequenced peptides were derived from SVS2. The peptide sequences identified through PMF are denoted by underline and were predominantly within the N terminus of the protein. (C) Immunoblots of recombinant MBP-SVS2 and purified SVS2 from semen plasma with sera from Aire-KO and WT B6/NOD F2 mice. MBP and OVA were negative controls. (D) Anti-SVS2–positive sera from Aire-KO mice were preincubated with either MBP-SVS2 or MBP before being used in the immunoblots against the prostate extract. WT and heterozygous Aire+/– B6/NOD F2 sera were used as controls.
To confirm that SVS2 was the protein recognized by autoantibodies in the Aire-KO mice, we generated purified SVS2 through 2 approaches. Mouse-derived SVS2 protein was purified from the seminal vesicle fluid according to a protocol previously used to purify human Sg (23), a protein that has structural similarities to mouse SVS2 protein. This purification procedure yielded the expected 38-kDa protein. Alternatively, recombinant SVS2 was produced as an 80-kDa fusion protein with maltose-binding protein (MBP) expressed in E. coli. The recombinant MBP-SVS2 fusion protein was purified from bacteria lysate with an amylose column. Recombinant MBP was also expressed and purified from E. coli and used as a control in subsequent experiments.
Sera from Aire-KO mice contained autoantibodies that recognized both the purified, semen-derived protein and the recombinant protein by Western blot (Figure 2C). Aire-KO sera did not bind to the control MBP or to another irrelevant protein, OVA. The absence of SVS2 reactivity in the WT sera further supports the importance of Aire regulation in autoreactivity to this antigen. To confirm that the prostate extract–reactive autoantibodies were specific to SVS2, we preincubated the Aire-KO sera with varying concentrations of MBP-SVS2 before immunoblotting the prostate extracts. Increasing concentrations of MBP-SVS2 abrogated immunoreactivity to the bands, while preincubation with MBP did not alter this immunoreactivity (Figure 2D). We therefore conclude that SVS2 is a prostate autoantigen targeted by autoantibodies in Aire-KO mice. Moreover, these results also indicate that some of the recognized lower-MW proteins represent fragments of SVS2.
SVS2 expression in the thymus is Aire dependent. To determine the distribution of SVS2 expression, RNA was isolated from different tissues and assessed by real-time PCR to quantify the expression of SVS2 mRNA. Consistent with prior reports (24), in Aire-KO mice, SVS2 was specifically expressed in the prostate gland, but was essentially undetectable in other organs tested, including tissues in which spontaneous inflammation can develop (Figure 3A). Next, we sought to determine whether SVS2 is expressed in the thymus and whether its thymic expression is Aire dependent. We purified CD45– thymic stromal cells from WT and Aire-KO mice and analyzed the expression of SVS2 by quantitative real-time PCR. SVS2 was expressed at substantially higher levels in the WT thymus than in the Aire-KO thymus (Figure 3B). This result demonstrated that thymic expression of the SVS2 gene was indeed Aire dependent.
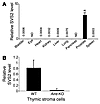 Figure 3
Figure 3SVS2 is highly expressed in the prostate and is an Aire-dependent antigen expressed in the thymus. (A) Expression levels of SVS2 were quantitated by RT-PCR on cDNA prepared from diverse tissues. Values are normalized to cyclophilin expression for each tissue and expressed in arbitrary units; exact values are shown above bars. Error bars denote SD. ND, not detectable. (B) Quantitative real-time PCR assay for the SVS2 expression in the CD45– thymic stroma cells of Aire-KO and WT mice. Values (mean ± SD) are normalized to cyclophilin and are representative of 2 experiments performed.
Autoimmune prostatitis is mediated by CD4+ T cells. Adoptive transfer experiments were performed to determine the immune effectors that could mediate prostatitis. Splenocytes from Aire-KO B6 mice were pooled; depleted of CD4+ T cells, CD8+ T cells, or CD19+ B cells; and adoptively transferred into immunodeficient RAG-KO B6 mice. Mice that received CD8+ T cell–depleted or CD19+ B cell–depleted splenocytes developed prostate inflammation with pathological characteristics similar to those of Aire-KO mice (Figure 4A). Adoptive transfer of CD4+ T cell–depleted splenocytes, however, failed to induce this level of prostatitis (Figure 4A). These results indicate that autoimmune T cells mediate the spontaneous prostatitis seen in Aire-KO mice, in which CD4+ T cells play a dominant role.
 Figure 4
Figure 4Spontaneous T cell responses to SVS2 in Aire-KO mice. (A) Splenocytes derived from Aire-KO B6 mice were depleted of CD4+ or CD8+ T cells or of CD19+ B cells by FACS. Sorted cells (5 × 106 cells) were adoptively transferred into male RAG-KO B6 mice. H&E staining of representative frozen prostate sections demonstrate that cells depleted of CD8+ T cells and CD19+ B cells, but not CD4+ T cells, were capable of transferring disease into RAG-KO mice. Results are representative of 2 separate adoptive transfer experiments, each with 2 mice per group. Scale bars: 150 μm. (B) Splenocytes (200,000 per well) from Aire-KO and WT mice were assessed for T cell responses against MBP-SVS2 (10 μg/ml) or MBP (10 μg/ml) by IFN-γ ELISPOT. Values are mean ± SD. SFU, spot-forming unit. (C) Results from individual Aire-KO and WT mice (n = 7 per group) of the experiment described in B. (D) IFN-γ ELISPOT assays were also performed with purified semen-derived SVS2 (10 μg/ml) and OVA (10 μg/ml) (n = 5 per group). In C and D, data points represent individual mice, horizontal bars denote means, and significance of differences was assessed by 2-tailed Student’s t test.
Based on these results, we next sought to determine whether Aire-KO mice possess endogenous SVS2-reactive T cells. We assessed splenocytes from Aire-KO and WT mice for antigen-specific responses by IFN-γ ELISPOT. This approach allowed us to detect specific responses to MBP-SVS2. While there was heterogeneity in the immune responses to MBP-SVS2 in Aire-KO mice, these responses were significantly different from the lack of response observed in WT mice (Figure 4, B and C). Because Aire-KO mice also had higher background reactivity to MBP than did WT mice, we assessed additional mice for immune responses to nonrecombinant, purified proteins. Again, we detected spontaneous T cell responses to semen-derived SVS2 only in the Aire-KO mice (Figure 4D).
To confirm that SVS2-reactive T cells in fact mediate prostatitis, we generated an SVS2-reactive CD4+ T cell line by stimulating splenocytes from male Aire-KO mice in vitro with purified SVS2. Upon restimulation with SVS2-MBP, the SVS2-reactive T cell line produced IFN-γ, but not IL-17 or IL-10 (Figure 5A). An OVA-reactive T cell line was generated in parallel as a control (Figure 5B). Adoptive transfer of the SVS2-reactive T cell line into RAG-KO B6 mice induced prostatitis (pathology score of 4; see Supplemental Table 1) in 3 of 3 treated mice, whereas adoptive transfer of the OVA-specific T cell line resulted in no inflammation (Figure 5C).
 Figure 5
Figure 5Functional capacity of SVS2 T cell lines. (A) SVS2-specific T cell lines were generated from Aire-KO male mice with semen-derived SVS2. After 2 rounds of in vitro restimulation with SVS2, this T cell line was restimulated in vitro with MBP-SVS2 or MBP, stained for CD4, and stained intracellularly for IFN-γ. Gated CD4+ T cells were also assessed for IL-10 and IL-17. (B) An OVA-specific T cell line was induced from male Aire-KO mice in parallel using the same approach as in A. Following 2 rounds of in vitro restimulation with OVA, this line was stained for CD4 and stained intracellularly for IFN-γ after restimulation with OVA or MBP. In A and B, numbers represent the percentage of cells within the respective gates. (C) Cells of the SVS2-specific T cell line or OVA-specific T cell line (1 × 106 cells) were adoptively transferred into male RAG-KO B6 mice. Prostates were assessed by immunohistochemistry for anti-CD3 staining. Results are representative of 3 mice per group. Scale bars: 150 μm.
Tolerance to SVS2 is broken in WT B6 mice. The results from the Aire-KO mice indicated that central tolerance plays a major role in preventing autoimmune prostatitis. In order to determine whether central tolerance to SVS2 is complete, we examined whether SVS2 is immunogenic in WT B6 mice by immunizing them with MBP-SVS2. In doing so, we could also determine whether inducing an immune response to SVS2 is sufficient for triggering prostatitis. WT mice were challenged with either MBP-SVS2 or MBP, emulsified in CFA. After 2 booster immunizations with the corresponding protein in incomplete Freund adjuvant (IFA), sera, splenocytes, and tissues were harvested for analysis. To minimize cross-reactivity to potential contaminants in the recombinant antigens, semen-derived SVS2 was used to perform the in vitro assays. Autoantibodies against the semen-derived SVS2 were detected in the sera of 7 of 14 mice (Figure 6A). None of the 14 MBP-immunized mice displayed autoantibody activity against SVS2 (Figure 6B). The pooled splenocytes from immunized mice were also assessed for T cell responses by IFN-γ ELISPOT. The splenocytes from MBP-SVS2–immunized mice demonstrated specific reactivity to the semen-derived SVS2, while the splenocytes of MBP-immunized mice had no specific immune response against SVS2 (Figure 6C). Depletion of CD4+ T cells from the MBP-SVS2–immunized splenocytes by fluorescence-activated cell sorting (FACS) eliminated the SVS2-specific response, while depletion of CD8+ T cells had essentially no effect. These results demonstrate that tolerance to this self antigen was broken and that both antibody and CD4+ T cell responses were induced in Aire-sufficient mice.
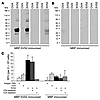 Figure 6
Figure 6Induction of SVS2-specific antibody and T cell response by immunizing WT B6 mice with MBP-SVS2. (A and B) WT B6 mice were immunized with either (A) MBP-SVS2 or (B) MBP emulsified in CFA. Sera from individual immunized B6 mice were used to immunoblot semen-purified SVS2 or OVA. Each blot represents an individual animal. For each experiment, shown is 1 group of 4 mice, representative of 14 total mice immunized. (C) The pooled splenocytes from 4 mice that were immunized with MBP-SVS2 or MBP were assessed by IFN-γ ELISPOT for reactivity to purified SVS2 (10 μg/ml), OVA (10 μg/ml), or PBS. Splenocytes depleted of CD8+ or CD4+ T cells were also assessed. Values are mean ± SD. Results are representative of 2 experiments.
SVS2 immunization induces prostatitis in WT B6 mice. Next, we examined the prostates of immunized mice for the induction of prostatitis. Although the severity of inflammation was less than that seen in Aire-KO mice, 10 of 14 MBP-SVS2–immunized mice nevertheless had intraprostatic CD3+ T cell infiltration (Figure 7, A and B). These results demonstrate that tolerance to SVS2 was broken in WT mice, with pathologic consequences.
 Figure 7
Figure 7Induction of prostatitis in WT B6 mice with SVS2 immunization. (A) Male B6 mice were immunized s.c. with MBP-SVS2 or MBP emulsified in CFA. After 2 booster immunizations with the respective proteins in IFA, the prostates were analyzed by immunohistochemical staining for CD3+ cells in frozen prostate sections. Scale bars: 150 μm. (B) Lymphocyte infiltration in the prostate of each immunized mouse in A was scored blindly by a pathologist. (C) Pooled splenocytes from the mice immunized with MBP-SVS2 or MBP were adoptively transferred (AT) i.v. into RAG-KO mice. After 4 weeks, the prostates were analyzed by immunohistochemical staining for CD3+ cells. Scale bars: 150 μm. (D) Lymphocyte infiltration in the prostate of each mouse in C was scored by a pathologist. In B and D, data points represent individual mice, and significance of differences was determined by 2-tailed Student’s t test.
To confirm that the induced prostatitis is T cell mediated, we performed adoptive transfer experiments. Pooled splenocytes from MBP-SVS2–immunized mice were also adoptively transferred into RAG-KO B6 mice. MBP-immunized splenocytes were adoptively transferred into RAG-KO B6 mice as controls. At 4 weeks after transfer, the prostates of recipient mice were assessed for prostatitis. The intraprostatic CD3+ T cell infiltrates into the glandular epithelium were observed in all mice that received SVS2-MBP–immunized splenocytes (Figure 7C). None of the mice that received MBP-immunized splenocytes displayed any prostatitis (Figure 7, C and D). These results further support the role of the T cell response induced by SVS2 in mediating disease.
CPPS patients develop an autoimmune response to human Sg. While no clear human homolog for SVS2 exists, SVS2 is a member of a family of prostate-specific proteins collectively known as rapidly evolving substrates for transglutaminase (24). Similar prostate-specific proteins also exist in humans. By the same method used to purify SVS2 from mouse semen, we purified one such protein, Sg, from human semen plasma and examined whether CPPS patients possess antibodies to this autoantigen. As expected, the Sg preparations contained both isoforms: the 52-kDa Sg1 and the 71-kDa Sg2. Sera from 26 of 39 patients with the clinical diagnosis of CPPS had autoantibodies to these proteins, in contrast to 8 of 39 sera from age-matched normal males (Fisher exact test, 2-sided P < 0.0001; Figure 8A). As part of their clinical management, 16 of the 39 assessed CPPS patients had undergone prostate biopsies; of those, 9 were found to have prostatitis. Interestingly, all patients with biopsy-proven prostatitis had autoantibodies to Sg (Table 1). The specificity of these autoantibodies was confirmed against recombinant human MBP-Sg1 and MBP-Sg2 fusion proteins (Figure 8B).
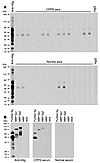 Figure 8
Figure 8CPPS patients possess autoimmune response to human Sg. (A) Immunoblotting of purified Sg was performed with the sera from CPPS patients and age-matched male blood donors. Each lane of the multiscreen immunoblot represents sera from an individual sample. Shown are 15 representative sera from each group. The 52- and 71-kDa bands denote Sg1 and Sg2, respectively. Rabbit anti-human Sg (anti-hSg) and human nonspecific IgG (hIgG) were used as positive and negative controls, respectively. (B) Immunoblots of recombinant MBP-Sg1, MBP-Sg1, and Sg purified from semen plasma were used to detect IgG antibodies within sera from a patient with CPPS and an age-matched normal male donor. Polyclonal rabbit anti-human Sg Ab was used as positive control. MBP and BSA denote irrelevant protein controls.
-
Discussion
Through the identification of an Aire-dependent autoantigen, we demonstrated that loss of thymic expression of SVS2 led to the development of spontaneous peripheral SVS2-specific T cells. These T cells produced IFN-γ, not IL-10 or IL-17, in response to antigen, consistent with a Th1-dominant immune response. Moreover, adoptive transfer of SVS2-reactive T cells was sufficient to induce autoimmune prostatitis. These results demonstrated that central tolerance may play an important role in preventing spontaneous autoimmune prostatitis and were consistent with other studies showing that Aire maintains self tolerance by regulating peripheral-tissue antigen expression in the thymus (25, 26). In our model, we found that CD4+ T cells were the primary mediators of this prostatitis, which was also consistent with findings in other inflamed tissues in Aire-KO mice (25).
We identified SVS2 as a target of spontaneous autoantibodies Aire-KO. Other autoantigens have been implicated in rodent prostatitis, such as prostate steroid binding protein (PSBP) in immunized rats and NOD mice (12, 17, 27) and EAPA-1 and EAPA-2 identified in day-3 thymectomized mice (15). Nevertheless, these models do not clearly demonstrate that prostatitis is an aberrant autoimmune response. Our results represent the first identification to our knowledge of an Aire-dependent prostate autoantigen.
Despite the high prevalence of spontaneous prostatitis and SVS2-specific antibody responses in Aire-KO males, only a subset of mice had detectable SVS2-specific T cell responses. This heterogeneity could reflect limitations in the sensitivity of detecting SVS2-specific T cells, particularly since we assessed immune responses in the spleen, as we were unable to dissect lymph nodes draining the prostate. Peripheral tolerance could also play a role in suppressing immune responses to this antigen, potentially through an Aire-dependent (28) or -independent mechanism, such as by autoantigen-specific Tregs (29). Nevertheless, the fact that Aire-KO mice developed spontaneous prostatitis with such high prevalence indicated that Aire-independent mechanisms of peripheral tolerance are insufficient to protect against autoimmunity. Again, other antigens could play a role in inducing prostatitis, while immune responses to SVS2 could involve a bystander antigen recognized through epitope spreading. Nevertheless, by demonstrating induction of prostatitis in Aire-sufficient mice through immunization with SVS2, we showed that SVS2 can in fact be an autoantigen involved in the initiation of autoimmune prostatitis. As a result, our findings also indicate that central tolerance to SVS2 is incomplete in these WT mice and support a role of peripheral tolerance in suppressing spontaneous autoimmune prostatitis, not unlike what is seen with other previously described autoantigens such as myelin basic protein and proteolipid protein (17). While high-avidity autoreactive T cells would presumably be deleted from the repertoire in these WT mice, the T cells that do escape the thymus would presumably possess low-avidity TCR for this antigen. The lower histologic scores for the inflammation in immunized WT mouse prostates compared with the spontaneous prostatitis in Aire-KO mice are therefore not surprising. Nevertheless, the inflammation we observed in immunized WT mice was localized to the glandular epithelium, where SVS2 is expressed; in fact, this inflammation more closely resembled the pathologic prostatitis seen in humans.
Prostatitis has not been described as a typical clinical manifestation in men with autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy (APECED), which is caused by mutations in Aire (30, 31). Nevertheless, CP is frequently asymptomatic, so this clinical manifestation may not yet be appreciated. Moreover, there can also be considerable heterogeneity in the clinical presentation and immune responses in these individuals, as has been seen with autoantibody reactivity against an identified autoantigen (32).
The diagnosis of human CPPS relies upon patient symptoms, and not on any objective measure of disease. When these patients undergo prostate biopsies, marked prostate inflammation is found in anywhere from 33% to 88% of patients (33, 34). While this variability may result from the different biopsy techniques (35), a substantial proportion of patients with symptoms nevertheless have negative biopsies. The identification and characterization of the autoantigen involved in the disease could resolve some of the controversy concerning the autoimmune mechanism underlying CPPS. While antibody responses to several other prostate antigens have been previously described (7–9), these antibodies were detected infrequently. To our knowledge, human homologs for the other identified autoantigens in rodent models of prostatitis (e.g., prostatic steroid-binding protein and spermine-binding protein) have not been identified. Although the direct human homolog of rodent SVS2 has not been identified, we focused on SVS2-like seminal vesicle proteins, such as Sg, that have previously been characterized in human seminal plasma and have the same pattern of tissue expression (36–38). In spite of the amino acid sequence disparity between SVS2 and Sg, these proteins share similarities in gene structure, 3-dimensional structure, and/or functional domains; they are collectively referred to as the rapidly evolving substrates for transglutaminase; and Sg was purified with the same approach used to purify SVS2 (24).
Interestingly, we found that two-thirds of sera from patients with CPPS possessed autoantibody against human Sg. However, when we narrowed our assessment to patients that had undergone prostate biopsy, all of the patients with biopsy-proved prostatitis possessed autoantibodies to Sg. Antibodies to this antigen were detected in patients without prostatitis, but their presence could stem from prior inflammation in the prostate that was no longer reflected in the biopsies. In addition, 20% of our normal male blood donors possessed anti-Sg autoantibodies. These individuals were assessed for prostatitis symptoms as part of routine blood donor screening and not based on a prostate symptom–directed history. Inflammation within the prostate can be present in asymptomatic men. Nevertheless, our data demonstrated that autoimmune recognition of Sg could be a feature of CPPS. Detecting immune responses to this autoantigen may be useful in the diagnosis of CPPS, but this will need to be formally tested prospectively. More importantly, our demonstration of this mechanism underlying CPPS in humans, which we believe to be novel, may lead to therapeutic approaches for this enigmatic disease, specifically, targeting Sg as an autoantigen and treating CPPS as an autoimmune disease. Moreover, identification of the relevant autoantigens in CPPS could provide future opportunities for antigen-specific treatments. Because prostatitis can also lead to spuriously elevated PSA levels in affected individuals, diagnosing CPPS could potentially improve the clinical utility of this blood test, which is used to diagnose prostate cancer. Finally, because CPPS may also promote the development of prostate cancer (39), the diagnosis and treatment of CPPS could possibly affect the incidence of this common malignancy.
-
Methods
Mice and reagents. Aire-KO mice were generated as previously described (25). The Aire-KO male mice used in these experiments were backcrossed into the B6 and NOD Lt/J backgrounds for more than 10 generations and generated using heterozygous breeders. WT B6 mice used in the experiments were littermates of the Aire-KO mice. Aire-KO B6/NOD F2 mice were derived from intercrosses of Aire-KO B6 and Aire-KO NOD Lt/J mice (40). All mice were housed in a pathogen-free barrier facility at UCSF. Experiments complied with the Animal Welfare Act and NIH guidelines for the ethical care and use of animals in biomedical research and were approved by the UCSF Animal Care and Use Committee. Tissue culture experiments were performed in RPMI 1640 media (Cambrex) supplemented with 10% fetal calf serum (BioWhittaker), penicillin/streptomycin (Sigma), and nonessential amino acids (Cambrex).
Antigens. The natural mouse SVS2 and human Sg were purified from mouse and human semen plasma, respectively, as described previously (23). In brief, fresh semen plasma were collected in 40 mmol/l Tris/HCL buffer (pH 9.7) with 4 M urea, 25 mmol/l EDTA, 30 mmol/l DTT, 3 mmol/l benzamidine, and 0.5 mmol/l Pefabloc (Boehringer) at 4°C and was applied on a heparin-sepharose column (0.7 × 2.5 cm; GE Healthcare) equilibrated with 0.1 mol/l Tris (pH 8.5), 2 M urea, 5 mmol/l EDTA, 3 mmol/l DTT, 3 mmol/l benzamidine, and 0.2 mmol/l Pefabloc buffer. After application of the sample, the column was washed 5 times with 2 ml equilibration buffer, then 4 ml of 0.1 M NaCl. The column was eluted with 4 ml of 0.25 M NaCl buffer. The eluant was desalted and concentrated with 30-kDa–cutoff Centricons (Millipore). The recombinant MBP-SVS2 and MBP-Sg1 proteins were made according to the protocol of the pMal Protein Fusion and Purification System (New England Biolabs Inc.) with the following modifications. SVS2 gene that was cloned from mouse prostate and Sg1 gene that was cloned from human prostate biopsy were subcloned in frame with MBP and transformed into TOP10 chemically competent cells (Invitrogen). Plasmid DNA isolated was transformed into BL21 (DE3)pLysS bacteria to express the fusion protein. Eluted proteins were concentrated with Amicon Centricon columns with a 10-kDa cutoff and washed 2 times with PBS. For ELISPOT assays, purified MBP, MBP-SVS2, and MBP-Sg1 proteins were passed through a ProteoSpin endotoxin removal kit (Norgen Biotek Corp.). Protein concentrations were measured with BioRad Dc assay (BioRad) using BSA as the standard.
Immunoprecipitation. Immunoprecipitation of autoantigens was performed using protein G agarose coupled to WT or Aire-KO sera as described previously (41). In brief, whole prostate tissues were homogenized in 0.15 M NaCl, 0.05 M Tris (pH 8), and 0.1% CHAPS (Sigma-Aldrich). Protein agarose G–coupled columns were washed in 30 ml PBS, and prostate extracts from RAG-KO mice prepared in CHAPS buffer were passed through the matrix. Columns were washed with 30 ml PBS and again with 30 ml of 10 mM phosphate (pH 6.8). Eluates were collected by passing 0.5 ml of 100 mM glycine (pH 2.5) over the column and collecting the flow through. Eluates from multiple runs were pooled and concentrated in a centrifugal protein concentrator (Vivaspin).
In-gel digestion and PMF. SVS2 was identified by provisional PMF as previously described (25). In brief, gel bands were excised, destained (stain-stripped) 3 times in 50% acetonitrile and 25 mM ammonium bicarbonate (pH 8), dehydrated with 100% acetonitrile, and dried in a Speed-Vac (Savant). Excised gel fragments were digested for 16 h at 37°C. Peptides were extracted, and PMF was used for preliminary protein identification. Mass spectra were produced by MALDI-TOF (Voyager DE STR; Applied Biosystems), representing protonated molecular ions (MH+) of tryptic peptides from the proteins present in each gel spot. The mass spectra were internally mass calibrated using 2 trypsin autolysis products present in the digest mixture. Preliminary protein identities were established by matching the experimentally determined peptide masses to those produced by an in silico tryptic digestion of the Swiss-Prot protein database (http://us.expasy.org/sprot/) within the window of experimental mass measurement accuracy. The PMF data-searching algorithm (available through MS-Fit; http://prospector.ucsf.edu/cgi-bin/msform.cgi?form=msfitstandard) was used to perform the database searches.
Immunoblotting. Mouse or human sera were screened for the presence of autoantibodies by Western blotting. Rabbit anti-human Sg antibody (Santa Cruz Biotechnology Inc.) was used as a positive control. Secondary goat anti-mouse, goat anti-rabbit HRP antibody (Upstate Biotechnologies) and goat anti-human IgG HRP antibody (Invitrogen) were used in the respective assays. The resulting films developed with either Upstate Visualizer or ECL chemiluminescent substrate. For competition studies, Aire-KO B6/NOD F2 sera were preincubated with serial dilutions of MBP-SVS2 or MBP in TBS-T with 1% nonfat dry milk for 2 hours at room temperature before use as the primary reagent to blot the membranes. The concentrations of protein used in these experiments were 30, 7.5, 1.88, and 0.47 μg.
Real-time PCR. Real-time PCR was performed on cDNA prepared from DNAse-treated RNA derived from dissected tissues or FACS-purified CD45– thymic stroma cells, as previously described (42). Aire- and cyclophilin-specific primers and probes were used as previously described (25). SVS2 Taqman primers and Fam-labeled MGB probes were purchased from Applied Biosystems (catalog no. Mn01251795_g1). Reactions were run on the HT7900 sequence detection system (Applied Biosystems).
ELISPOT analysis. Splenocytes were harvested from Aire-KO mice, WT B6 mice, or immunized WT B6 mice, and the release of IFN-γ was measured by ELISPOT assay. Briefly, MultiScreenHTS-IP plates (Millipore) were coated overnight with 2 μg/ml anti-mouse IFN-γ mAb or anti-human IFN-γ mAb (BD Biosciences) at 4°C. The plates were washed with PBS and blocked with RPMI medium containing 10% FCS for 2 hours at 37°C. Mouse splenocytes were added to each well with the different antigens and incubated for 18 hours in RPMI complete medium. The plates were washed with PBS before adding 2 μg/ml biotin-labeled anti-mouse or anti-human IFN-γ mAb (2 μg/ml; BD Biosciences) and incubated overnight at 4°C. After washing and further incubation with horseradish peroxide–conjugated avidin (diluted 1:100; BD Biosciences) for 1 hour at room temperature, the plates were washed and developed using 3-amino-9-ethylcarbazole substrate solution (BD Biosciences). Positive spots displayed in the plate membranes were examined using an automated ELISPOT reader (AID; Autoimmun Diagnostika GmbH). The number of spot-forming cells was the average number of spots in duplicate or triplicate wells.
Generation of antigen-specific T cell lines. Aire-KO B6 male mice were immunized with the purified SVS2 (200 μg/mouse) emulsified in an equal volume of CFA (Difco). The antigens were injected s.c. into the footpads. Mice were boosted twice with the same amount of antigen emulsified in IFA at 10-day intervals. At 10 days after the second boost, single-cell suspensions from draining lymph nodes of SVS2-immunized mice were incubated with purified SVS2 (50 μg/ml). After 24 hours, mouse IL-2 (50 U/ml), IL-7 (10 ng/ml), and IL-15 (10 ng/ml; all from Peprotech) were supplemented into the media. Subsequent restimulations occurred at weekly intervals by the addition of purified SVS2-pulsed, irradiated B6 splenocytes to the cultures followed by the same cytokine supplementation 24 hours later. The OVA-specific T cell lines were generated in parallel in separate mice through the same procedure. After 2 cycles of restimulation, the dead cells were removed by centrifugation with Ficoll (Histopaque-1083; Sigma-Aldrich), and the cultured cells were harvested for further analysis and adoptive transfer experiments.
Intracellular cytokine staining of antigen-specific T cells. Antigen-specific T cell lines were incubated with the irradiated B6 splenocytes pulsed with MBP-SVS2 (50 μg/ml) for 24 hours. GolgiStop buffer (BD Biosciences) was added at a final concentration of 1 μg/ml for the last 4 hours of incubation. The cells were then harvested for intracellular cytokine staining. Briefly, the cells were first stained with allophycocyanin-conjugated anti-mouse CD4 antibody (BD Biosciences) and fixed with 4% formaldehyde in PBS for 10 min. After subsequent permeabilization with 1× BD perm/wash buffer (BD Biosciences) for 15 minutes, the cells were stained with PE-conjugated anti-mouse IFN-γ (eBioscience), PE-conjugated anti-mouse IL-10 (Biolegend), or FITC-conjugated anti-mouse IL-17a (BD Biosciences) antibodies. The cells were assessed by flow cytometry and analyzed with FlowJo (version 8.0.3; TreeStar).
Adoptive transfer experiments. Splenocytes were harvested from Aire-KO B6 mice or immunized B6 mice. CD4+ or CD8+ T cells or CD19+ B cells were depleted using FACS. Briefly, cells were stained with PE-conjugated anti-CD4, -CD8, or -CD19 antibodies (BD Biosciences — Pharmingen) for 30 minutes on ice, and then the PE-negative CD4-, CD8-, and CD19-depleted cells were collected (FACSAria; BD Biosciences). Sorted depleted cells (5 × 106 cells) were injected i.v. into RAG-KO B6 mice. Animals were aged 40 days after the transfer, sacrificed, and analyzed. In additional experiments, 1 × 106 cells from either SVS2-reactive or OVA-reactive T cell lines were injected i.v. into RAG-KO B6 mice. Animals were aged 20 days after the transfer, sacrificed, and analyzed.
SVS2 immunization experiments. Male B6 mice at 25 weeks of age were immunized with MBP-SVS2 (200 μg/mouse) or MBP (200 μg/mouse). Both proteins were emulsified in an equal volume of CFA (Difco) supplemented with Mycobacterium tuberculosis H37Ra. The antigens were injected s.c. on the back. Mice were boosted twice with the same amount of antigen emulsified in IFA at 2-week intervals. At 2 weeks following the second boost, tissues and sera were harvested for further analysis or adoptive transfer experiments.
Histopathology. For H&E staining, prostate tissues were either fixed in 10% neutral-buffered formalin and embedded in paraffin or frozen in OCT tissue embedding compound (Tissue Tek) at –80°C. After sectioning, tissues were stained with H&E (Sigma-Aldrich). For immunohistochemical staining, prostates were snap-frozen in OCT, sectioned at 6 μm, and processed for staining using Vectastain Elite ABC Kit (Vector Laboratories) with biotinylated anti-rat antibody against rat anti-mouse CD3 (clone CT-CD3; Caltag Laboratories), CD4 (clone H129.19; BD Biosciences — Pharmingen), CD8a (clone 53-6.7; BD Biosciences — Pharmingen), or CD19 (clone 1D3; BD Biosciences — Pharmingen), followed by streptavidin peroxidase conjugate. Colorimetric detection was visualized using DAB substrate (Pierce). Histology was assessed by a pathologist blinded to mouse phenotype or treatment, who scored lymphocytic infiltrates as described in Supplemental Table 1.
Human subjects. Sera were obtained from patients with clinical histories of CPPS at the University of Washington Medical Center. The study protocol was reviewed and approved by the University of Washington Institutional Review Board, and individuals provided informed consent prior to participation. Clinical assessment of prostatitis in the prostate biopsies was performed by the center’s pathology department. Control sera were obtained from male volunteer blood donors without histories of prostatitis symptoms. Sera were stored in aliquots at –80°C until used.
Statistics. For the mouse study, differences between groups were evaluated using 2-tailed Student’s t test, and differences were considered statistically significant at P < 0.05. For human studies, differences between groups were evaluated by Fisher exact test, and differences were considered statistically significant at P < 0.05.
-
Acknowledgments
This work was supported by NIH grants AI056388 and CA102303. The authors thank Marcella Fasso and Dil Kapadia for critical review of the manuscript; the UCSF Biomolecular Resource Center Mass Spectrometry Facility and the Sandler Family Foundation for mass spectrometry; and the UCSF Prostate SPORE Tissue Core for providing guidance in preparing tissue sections and staining.
Address correspondence to: Lawrence Fong, Division of Hematology/Oncology, University of California, San Francisco, 513 Parnassus Avenue, Box 0511, San Francisco, California 94143, USA. Phone: (415) 514-3424; Fax: (415) 476-0459; E-mail: [email protected].
-
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Nonstandard abbreviations used:Aire, autoimmune regulator; B6, C57BL/6; CP, chronic prostatitis; CPPS, chronic pelvic pain syndrome; FACS, fluorescence-activated cell sorting; IFA, incomplete Freund adjuvant; MBP, maltose-binding protein; PMF, peptide mass fingerprinting; Sg, semenogelin; SVS2, seminal vesicle secretory protein 2.
Reference information: J. Clin. Invest.119:2031–2041 (2009). doi:10.1172/JCI38332
-
References
- Collins, M.M., Stafford, R.S., O’Leary, M.P., Barry, M.J. 1998. How common is prostatitis? A national survey of physician visits. J. Urol. 159:1224-1228.
- Krieger, J.N., et al. 2008. Epidemiology of prostatitis. Int. J. Antimicrob. Agents. 31(Suppl. 1):S85-S90. View this article via: PubMed Google Scholar
- Krieger, J.N., Nyberg (Jr.), L., Nickel, J.C. 1999. NIH consensus definition and classification of prostatitis. JAMA. 282:236-237.
- De Marzo, A.M., et al. 2007. Inflammation in prostate carcinogenesis. Nat. Rev. Cancer. 7:256-269.
- Penna, G., et al. 2007. Seminal plasma cytokines and chemokines in prostate inflammation: interleukin 8 as a predictive biomarker in chronic prostatitis/chronic pelvic pain syndrome and benign prostatic hyperplasia. Eur. Urol. 51:524-533; discussion 533.
- Shoskes, D.A., Albakri, Q., Thomas, K., Cook, D. 2002. Cytokine polymorphisms in men with chronic prostatitis/chronic pelvic pain syndrome: association with diagnosis and treatment response. J. Urol. 168:331-335.
- Ponniah, S., Arah, I., Alexander, R.B. 2000. PSA is a candidate self-antigen in autoimmune chronic prostatitis/chronic pelvic pain syndrome. Prostate. 44:49-54.
- Motrich, R.D., et al. 2005. Presence of INFgamma-secreting lymphocytes specific to prostate antigens in a group of chronic prostatitis patients. Clin. Immunol. 116:149-157.
- Dunphy, E.J., Eickhoff, J.C., Muller, C.H., Berger, R.E., McNeel, D.G. 2004. Identification of antigen-specific IgG in sera from patients with chronic prostatitis. J. Clin. Immunol. 24:492-502.
- Motrich, R.D., Maccioni, M., Riera, C.M., Rivero, V.E. 2007. Autoimmune prostatitis: state of the art. Scand. J. Immunol. 66:217-227.
- Fong, L., Ruegg, C.L., Brockstedt, D., Engleman, E.G., Laus, R. 1997. Induction of tissue-specific autoimmune prostatitis with prostatic acid phosphatase immunization: implications for immunotherapy of prostate cancer. J. Immunol. 159:3113-3117. View this article via: PubMed Google Scholar
- Liu, K.J., et al. 1997. Identification of rat prostatic steroid-binding protein as a target antigen of experimental autoimmune prostatitis: implications for prostate cancer therapy. J. Immunol. 159:472-480. View this article via: PubMed Google Scholar
- Rivero, V., Carnaud, C., Riera, C.M. 2002. Prostatein or steroid binding protein (PSBP) induces experimental autoimmune prostatitis (EAP) in NOD mice. Clin. Immunol. 105:176-184.
- Taguchi, O., Nishizuka, Y. 1987. Self tolerance and localized autoimmunity. Mouse models of autoimmune disease that suggest tissue-specific suppressor T cells are involved in self tolerance. J. Exp. Med. 165:146-156.
- Setiady, Y.Y., et al. 2006. Physiologic self antigens rapidly capacitate autoimmune disease-specific polyclonal CD4+ CD25+ regulatory T cells. Blood. 107:1056-1062.
- Vykhovanets, E.V., Resnick, M.I., MacLennan, G.T., Gupta, S. 2007. Experimental rodent models of prostatitis: limitations and potential. Prostate Cancer Prostatic Dis. 10:15-29.
- Penna, G., et al. 2007. Spontaneous and prostatic steroid binding protein peptide-induced autoimmune prostatitis in the nonobese diabetic mouse. J. Immunol. 179:1559-1567. View this article via: PubMed Google Scholar
- Mathis, D., Benoist, C. 2007. A decade of AIRE. Nat. Rev. Immunol. 7:645-650.
- Kyewski, B., Derbinski, J. 2004. Self-representation in the thymus: an extended view. Nat. Rev. Immunol. 4:688-698.
- Anderson, M.S., et al. 2002. Projection of an immunological self shadow within the thymus by the aire protein. Science. 298:1395-1401.
- Jiang, W., Anderson, M.S., Bronson, R., Mathis, D., Benoist, C. 2005. Modifier loci condition autoimmunity provoked by Aire deficiency. J. Exp. Med. 202:805-815.
- Lundwall, A., Peter, A., Lovgren, J., Lilja, H., Malm, J. 1997. Chemical characterization of the predominant proteins secreted by mouse seminal vesicles. Eur. J. Biochem. 249:39-44.
- Malm, J., Hellman, J., Magnusson, H., Laurell, C.B., Lilja, H. 1996. Isolation and characterization of the major gel proteins in human semen, semenogelin I and semenogelin II. Eur. J. Biochem. 238:48-53.
- Lundwall, A., Lazure, C. 1995. A novel gene family encoding proteins with highly differing structure because of a rapidly evolving exon. FEBS Lett. 374:53-56.
- DeVoss, J., et al. 2006. Spontaneous autoimmunity prevented by thymic expression of a single self-antigen. J. Exp. Med. 203:2727-2735.
- Gavanescu, I., Kessler, B., Ploegh, H., Benoist, C., Mathis, D. 2007. Loss of Aire-dependent thymic expression of a peripheral tissue antigen renders it a target of autoimmunity. Proc. Natl. Acad. Sci. U. S. A. 104:4583-4587.
- Maccioni, M., Rivero, V.E., Riera, C.M. 1998. Prostatein (or rat prostatic steroid binding protein) is a major autoantigen in experimental autoimmune prostatitis. Clin. Exp. Immunol. 112:159-165.
- Gardner, J.M., et al. 2008. Deletional tolerance mediated by extrathymic Aire-expressing cells. Science. 321:843-847.
- Samy, E.T., et al. 2006. The role of physiological self-antigen in the acquisition and maintenance of regulatory T-cell function. Immunol. Rev. 212:170-184.
- Nagamine, K., et al. 1997. Positional cloning of the APECED gene. Nat. Genet. 17:393-398.
- Consortium, F.-G.A., 1997. An autoimmune disease, APECED, caused by mutations in a novel gene featuring two PHD-type zinc-finger domains. Nat. Genet. 17:399-403.
- Alimohammadi, M., et al. 2008. Autoimmune polyendocrine syndrome type 1 and NALP5, a parathyroid autoantigen. N. Engl. J. Med. 358:1018-1028.
- True, L.D., Berger, R.E., Rothman, I., Ross, S.O., Krieger, J.N. 1999. Prostate histopathology and the chronic prostatitis/chronic pelvic pain syndrome: a prospective biopsy study. J. Urol. 162:2014-2018.
- Doble, A., Walker, M.M., Harris, J.R., Taylor-Robinson, D., Witherow, R.O. 1990. Intraprostatic antibody deposition in chronic abacterial prostatitis. Br. J. Urol. 65:598-605.
- Batstone, G.R., Doble, A., Gaston, J.S. 2002. Autoimmune T cell responses to seminal plasma in chronic pelvic pain syndrome (CPPS). Clin. Exp. Immunol. 128:302-307.
- Lilja, H., Laurell, C.B. 1985. The predominant protein in human seminal coagulate. Scand. J. Clin. Lab. Invest. 45:635-641. View this article via: PubMed Google Scholar
- Chaistitvanich, N., Boonsaeng, V. 1983. Molecular structure of human seminal coagulum: the role of disulfide bonds. Andrologia. 15:446-451. View this article via: PubMed Google Scholar
- Lundwall, A., Bjartell, A., Olsson, A.Y., Malm, J. 2002. Semenogelin I and II, the predominant human seminal plasma proteins, are also expressed in non-genital tissues. Mol. Hum. Reprod. 8:805-810.
- Haverkamp, J., Charbonneau, B., Ratliff, T.L. 2008. Prostate inflammation and its potential impact on prostate cancer: a current review. J. Cell. Biochem. 103:1344-1353.
- Kuchroo, V.K., et al. 2002. T cell response in experimental autoimmune encephalomyelitis (EAE): role of self and cross-reactive antigens in shaping, tuning, and regulating the autopathogenic T cell repertoire. Annu. Rev. Immunol. 20:101-123.
- Harlow, E., and Lane, D. 1999.Using antibodies: a laboratory manual. Cold Spring Harbor Laboratory Press. Cold Spring Harbor, New York, USA. 495 pp.
- Gray, D.H., Chidgey, A.P., Boyd, R.L. 2002. Analysis of thymic stromal cell populations using flow cytometry. J. Immunol. Methods. 260:15-28. View this article via: PubMed Google Scholar
-
Version history
- Version 1 (June 1, 2009): No description
- Version 2 (July 1, 2009): No description



Copyright © 2024 American Society for Clinical Investigation
ISSN: 0021-9738 (print), 1558-8238 (online)









