Advertisement
Science in Medicine Free access | 10.1172/JCI35309
Mechanisms and treatment of cardiovascular disease in Williams-Beuren syndrome
Barbara R. Pober,1 Mark Johnson,2 and Zsolt Urban3
1Department of Pediatrics, MassGeneral Hospital for Children, Department of Surgery, Children’s Hospital Boston, and Department of Pediatrics, Harvard Medical School, Boston, Massachusetts, USA. 2Division of Pediatric Cardiology, Department of Pediatrics, Washington University School of Medicine/St. Louis Children’s Hospital, St. Louis, Missouri, USA. 3Departments of Pediatrics and Genetics, Washington University School of Medicine, St. Louis, Missouri, USA.
Address correspondence to: Barbara R. Pober, MassGeneral Hospital for Children, Simches Research Building, Rm. 222, 185 Cambridge St., Boston, Massachusetts 02114, USA. Phone: (617) 726-1561; Fax: (617) 726-1566; E-mail: [email protected].
Find articles by Pober, B. in: JCI | PubMed | Google Scholar
1Department of Pediatrics, MassGeneral Hospital for Children, Department of Surgery, Children’s Hospital Boston, and Department of Pediatrics, Harvard Medical School, Boston, Massachusetts, USA. 2Division of Pediatric Cardiology, Department of Pediatrics, Washington University School of Medicine/St. Louis Children’s Hospital, St. Louis, Missouri, USA. 3Departments of Pediatrics and Genetics, Washington University School of Medicine, St. Louis, Missouri, USA.
Address correspondence to: Barbara R. Pober, MassGeneral Hospital for Children, Simches Research Building, Rm. 222, 185 Cambridge St., Boston, Massachusetts 02114, USA. Phone: (617) 726-1561; Fax: (617) 726-1566; E-mail: [email protected].
Find articles by Johnson, M. in: JCI | PubMed | Google Scholar
1Department of Pediatrics, MassGeneral Hospital for Children, Department of Surgery, Children’s Hospital Boston, and Department of Pediatrics, Harvard Medical School, Boston, Massachusetts, USA. 2Division of Pediatric Cardiology, Department of Pediatrics, Washington University School of Medicine/St. Louis Children’s Hospital, St. Louis, Missouri, USA. 3Departments of Pediatrics and Genetics, Washington University School of Medicine, St. Louis, Missouri, USA.
Address correspondence to: Barbara R. Pober, MassGeneral Hospital for Children, Simches Research Building, Rm. 222, 185 Cambridge St., Boston, Massachusetts 02114, USA. Phone: (617) 726-1561; Fax: (617) 726-1566; E-mail: [email protected].
Find articles by Urban, Z. in: JCI | PubMed | Google Scholar
Published May 1, 2008 - More info
J Clin Invest. 2008;118(5):1606–1615. https://doi.org/10.1172/JCI35309.
© 2008 The American Society for Clinical Investigation
-
Abstract
Williams-Beuren syndrome (WBS) is a microdeletion disorder caused by heterozygous loss of approximately 1.5-Mb pairs of DNA from chromosome 7. Patients with WBS have a characteristic constellation of medical and cognitive findings, with a hallmark feature of generalized arteriopathy presenting as stenoses of elastic arteries and hypertension. Human and mouse studies establish that defects in the elastin gene, leading to elastin haploinsufficiency, underlie the arteriopathy. In this review we describe potential links between elastin expression and arteriopathy, possible explanations for disease variability, and current treatment options and their limitations, and we propose several new directions for the development of nonsurgical preventative therapies based on insights from elastin biology.
Williams-Beuren syndrome (WBS, also referred to as Williams syndrome; OMIM 194050) was first recognized as a clinical syndrome, separate from other developmental disability syndromes, because of a unique constellation of cardiovascular (CV) abnormalities. In this review, we describe these characteristic abnormalities, the considerable progress that has been made in understanding their etiology and pathophysiology, and new insights gained into underlying molecular pathways. Finally, we consider currently available therapeutic approaches and opportunities to develop new treatment options.
-
Overview of WBS
In 1961, J.C.P. Williams described four patients and proposed that “the association of supravalvular stenosis with the physical and mental characteristics here described may constitute a previously unrecognized syndrome” (1). Shortly thereafter A.J. Beuren reported eleven new patients (2), and the condition has fittingly borne the eponym Williams-Beuren syndrome ever since.
WBS affects approximately 1/10,000 individuals, is found worldwide among all racial and ethnic groups, and displays multisystem medical and nonmedical problems (3–5). In 1993 the genetic cause of WBS, a chromosomal microdeletion, was reported (6), and this knowledge permitted development of a laboratory-based diagnostic test, so the diagnosis no longer rests on clinical criteria only. The most widely used method to confirm the diagnosis has been FISH, but DNA dosage techniques such as quantitative PCR, multiplex ligation-dependent probe amplification, and chromosomal microarray, also known as comparative genomic hybridization, may soon become confirmatory tests of choice.
Non-CV clinical features of WBS. Persons with WBS have a subtle but distinctive facial appearance that changes with age (Figure 1) (3). Their linear growth is usually smaller than that of siblings or healthy, age-matched controls. Though infants and young children tend to be thin, or even underweight, many WBS adults are overweight (3, 7, 8).
 Figure 1
Figure 1Distinctive facial appearance of persons with WBS. Young child with WBS at 15 months (A) and 3 years (B). Note subtle characteristic facial features including wide mouth, full cheeks, long philtrum, small nose, and delicate chin. (C) At left, the same individual depicted in A and B at 21 years of age. At right, another individual with WBS at 28 years of age. Note persistence of wide mouth, full lips, and delicate chin in adults with WBS.
Endocrine abnormalities are commonly reported and include hypercalcemia, abnormal glucose metabolism, and (subclinical) hypothyroidism. The precise frequencies, etiologies, natural histories, and best treatments of these endocrine problems remain to be determined. Other common findings include dental anomalies (small, abnormally shaped teeth, absent teeth, malocclusion), gastrointestinal dysmotility (reflux, constipation), diverticular disease, musculoskeletal anomalies (joint stiffness, scoliosis), sensorineural hearing loss, genitourinary anomalies (urinary frequency, bladder diverticuli), and neurological problems (abnormal tone, hyperreflexia, and cerebellar findings) (3, 7, 9, 10).
Persons with WBS have intellectual handicaps that generally meet the definition of mild to moderate mental retardation on standardized testing. The average full-scale IQ is reported to be 55–60, but a fairly broad range exists, extending from 40–90 (11). Particularly notable is the pattern of intellectual peaks and valleys, referred to as the Williams syndrome cognitive profile, with relative strengths in selected language domains and a prominent weakness in the visuospatial domain (12). Persons with WBS display characteristic personality and emotional traits. Their generally social and friendly demeanor coexists with anxiety (especially anticipatory anxiety), phobias, and perseverative tendencies (i.e., repetitive thoughts or behaviors) (7, 13).
Molecular genetics of WBS. The etiology of WBS, a mystery for more than 30 years after its initial description, is now known to be a contiguous gene deletion or microdeletion syndrome at chromosome 7q11.23. Although the chromosomal location is unique, the mechanism of origin is comparable with that of other microdeletion disorders, namely, a deleted genomic interval resulting from the presence of low-copy repetitive DNA (duplicons) that predispose to nonallelic homologous recombination (NAHR) (14).
The locus for WBS was found through the study of a phenotypically overlapping disorder. Specifically, disruption of the elastin (ELN) gene was identified as the cause of the condition known as familial supravalvular aortic stenosis (familial SVAS) syndrome (OMIM 185500) by application of linkage analysis and gene sequencing, and by cloning of a chromosome 7q11.2 translocation breakpoint, in selected familial SVAS kindreds (15, 16). Given similar vascular pathologies in familial SVAS and in WBS, study of the ELN gene was undertaken, and deletion of an ELN allele was identified as the cause of WBS (6). Subsequent characterization of the deleted interval, now referred to as the WBS critical region (Figure 2), reveals the following (17–19): (a) 90%–95% of patients clinically diagnosed with WBS have an approximately 1.55-Mb deletion associated with loss of 26–28 genes; (b) 5%–8% of clinically diagnosed WBS patients have a slightly larger, approximately 1.84-Mb pair deletion associated with loss of 28 genes; (c) the deleted intervals are flanked by highly homologous stretches of DNA, organized into a single centromeric duplicon and two telomeric duplicons; (d) each duplicon contains genes, pseudogenes, and clusters of related genes; (e) the duplicons predispose to NAHR through intra- or inter-chromosomal exchange during meiosis; and (f) the deletion arises with equal frequency on either the maternally or the paternally inherited chromosome 7 homolog.
 Figure 2
Figure 2The WBS critical region. The chromosome 7q11.23 microdeletion, with the loss of 26–28 genes, that is responsible for WBS. Selected genes are labeled. Duplicons predispose to NAHR. More than 90% of WBS patients have the ~1.55-Mb pair deletion extending from FKBP6 to GTF2I, while approximately 5% have the slightly larger deletion of 1.84-Mb pairs. Very rare patients have atypical deletions smaller than the common deletion. Schematics of atypical deletions are shown on right and include a very small deletion encompassing ELN and an adjacent gene; a typical centromeric breakpoint but not the common telomeric breakpoint; and a typical telomeric breakpoint but not the typical centromeric breakpoint. Not all genes are shown; see ref. 20 for a complete list of genes. WBSCR, WBS critical region.
Different breakpoints in the medial block of telomeric repeats determine whether neutrophil cytosolic factor 1 (NCF1) and general transcription factor II I repeat domain–containing 2 (GTF2IRD2) are deleted or not. Several of the deleted genes in patients with WBS are depicted in Figure 2. As this review focuses on CV aspects of WBS, our discussion will emphasize the ELN gene and consequences resulting from its functional loss. For a complete list and description of the genes constituting the WBS critical region, the reader is directed to ref. 20.
Loss of an ELN allele is the single most important genetic change responsible for the CV problems of WBS. The ELN gene encodes a precursor protein, tropoelastin. Multiple isoforms of tropoelastin are generated via alternative splicing, secreted into the extracellular matrix, deposited onto a preformed network of fibrillin microfibrils, and are cross-linked by the lysyl oxidase family of enzymes (21). This process of elastic fiber assembly requires the coordinated expression of multiple genes, and peaks during late fetal and perinatal stages of development (22).
The most common pathological consequence of chromosome 7q11.23 duplicon–mediated NAHR is WBS (e.g., deletion of the intervening sequence). However, duplication of the intervening sequence (23) has recently been detected by chromosomal microarray; affected individuals have expressive speech delay without evidence of WBS features (24). The most common nonpathological consequence of NAHR at this locus is inversion of the WBS critical region (25). Inversion carriers are asymptomatic, though they may have a slightly greater risk of meiotic NAHR producing a gamete, which, when fertilized, results in an offspring with WBS (26).
The chromosome 7q11.23 genomic architecture in the WBS critical region has genetic synteny on mouse chromosome 5 (27). The salient differences are the opposite orientation of the region and the absence of low-copy repeats in the mouse. Such synteny provides a powerful tool for genotype-phenotype analysis through the generation and characterization of genetically modified mice; a complete list of currently published WBS mouse models is provided in Supplemental Table 1 (supplemental material available online with this article; doi: 10.1172/JCI35309DS1). The Eln knockout mouse model is discussed in considerable detail below. A CV phenotype has not been appreciated in any other mouse in which a WBS critical region gene has been genetically altered, such as Fzd9–/– (28), Limk1–/– (29), Gtf2ird1–/– (30), or Cyln2–/– mice (31).
A complementary approach to elucidating the role of deleted genes in the WBS phenotype involves detailed characterization of patients with smaller atypical deletions. These patients, in whom less than 26 genes have been deleted from the WBS critical region (Figure 2), are relatively uncommon. Despite their rarity, such cases can provide valuable information. Characterization of patients with atypical deletions that variously encompass LIM1K, GTF2IRD1, and/or GTF2I suggest that loss of one or more of these genes contributes to the WBS cognitive profile (32–34). Given the combination of a relatively small number of genes in the WBS critical region and a remarkably distinct set of features, WBS has become a “model” disorder for the study of genotype-phenotype correlations in microdeletion syndromes.
-
CV clinical features of WBS
CV disease, particularly an arteriopathy consisting of stenoses of medium- and large-sized arteries, is the hallmark of WBS. In early case reports and case series, the diagnosis was primarily established by cardiologists evaluating patients for a heart murmur, which usually was diagnosed as SVAS on cardiac imaging or through catheterization and/or surgery (1, 35).
The prevalence of CV abnormalities in 423 patients from nine selected international series published in the last two decades is shown in Table 1 (36–44). Many patients have multiple CV clinical findings. Although the vascular stenoses of WBS predominantly affect the supravalvular aortic (Figure 3) and pulmonary regions, lesions located elsewhere also occur, primarily but not exclusively affecting the vascular branch points. The wide range of published prevalence reflects the age-dependent frequency of clinical features, variable study methods, especially the modality used to ascertain specific clinical features, and biases in case ascertainment. Spontaneous improvement in pulmonary arterial stenosis over time has been well established, whereas SVAS may progress especially in the first five years of life (36, 38–40, 45, 46). Prospective use of echocardiographic dimensions in one study found a 100% frequency of SVAS but only a 3% frequency of pulmonary arterial stenosis (37). A much higher frequency of pulmonary arterial stenosis (77%–83%) was found in two series of patients, all of whom had undergone at least one cardiac catheterization (36, 39). With improved noninvasive cardiac imaging, valvular abnormalities, particularly of the mitral valve, are being detected with increasing frequency (42).
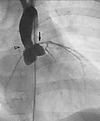 Figure 3
Figure 3Aortogram in a 4-year-old individual with WBS. SVAS (arrowhead) and mild narrowing of the proximal left main coronary artery (arrow) are shown. The gradient measured at catheterization was 40 mmHg.
A small number of patients with SVAS (as part of either WBS or familial SVAS syndrome) have a diffuse and more severe variant of arteriopathy, sometimes termed middle aortic syndrome (36, 42, 47). These patients have stenosis of the thoracic and abdominal aorta, mesenteric arteries, and renal arteries. Left ventricular hypertrophy and hypertension are common in this severe form of CV disease.
Clinically important coronary artery lesions (Figure 3) are reported infrequently but may put the patient at risk of sudden death. An autopsy series of five patients whose CV disease represented the severe end of the spectrum demonstrated obstructive coronary disease in all individuals, with vessel wall hyperplasia, fibrosis, and disorganization (48). In a catheterization study of 26 patients with WBS, coronary artery dilation or stenosis was found in 27% (39). In mixed cohorts of individuals who had either WBS or mutations in the ELN gene in conjunction with severe SVAS requiring surgery, coronary disease was found in 28%–45% (49, 50). Although coronary disease appears to be related to the severity of supravalvular aortic narrowing (39), case reports indicate that severe coronary artery disease leading to death may be the sole vascular feature in some patients with WBS (51, 52). Since the sensitivity of standard non-stress echocardiographic studies for coronary stenosis in the general population is low, perfusion imaging techniques may be needed to investigate the significance of coronary artery disease in WBS (53).
Patients with WBS are at a higher risk of sudden death. Wessel et al. found a risk of 1/1,000 patient years with five cases of sudden death, a 25- to 100-fold increase compared with the normal population (54). Death secondary to myocardial infarction has been detailed in three patients (55, 56). In another report of ten WBS patients with sudden death, pathologic findings suggest that coronary artery stenosis and severe biventricular outflow tract obstruction are mechanisms for myocardial ischemia and arrhythmia (57). Many of the deaths occurred with anesthesia/sedation (often with cardiac catheterization), suggesting that decreased cardiac output from anesthetic agents in concert with coronary artery abnormalities altered myocardial perfusion.
A wide variation in the prevalence of systemic hypertension is shown in Table 1 and is likely due the methodological variations of the type described in the first paragraph of this section. Although more commonly found in adults, hypertension can develop during childhood (58, 59). Three recent studies using 24-hour ambulatory monitoring found hypertension in 40%–70% of patients (59–61), though the etiology of hypertension is not fully known and in most cases is idiopathic. Only a small minority of patients have overt renovascular disease, such as renal artery stenosis or diffuse aortic narrowing (47, 58), but all have vascular abnormalities at the histological level, which will be detailed below.
In one study, infantile hypercalcemia predicted hypertension, but no relationship was found between blood pressure and patients reported to be frequently anxious (59). Others have pointed out that “white coat” hypertension, defined as elevated office blood pressure but normal ambulatory blood pressure (62), is frequent in the general pediatric population. Accordingly, it should be considered in WBS before diagnosing bona fide hypertension, given the high frequency of anxiety disorders in these individuals. The Coanda effect (the tendency of a jet stream to adhere to a wall) from SVAS may cause higher blood pressure only in the right arm (63). Arterial wall thickening seen with intravascular ultrasound imaging in humans has led to the hypothesis that decreased compliance of the arterial tree is a factor in hypertension in WBS (64). A later ultrasound study of the common carotid in 21 patients with WBS confirmed increased intima-media thickness but found no difference in compliance compared with controls (65).
Other cardiac defects are occasionally observed in WBS. Ventricular septal defects, typically small, were found in 0%–14% of the series described in Table 1. Single cases of more complex defects such as tetralogy of Fallot (malalignment ventricular septal defect with right ventricular outflow obstruction) and atrioventricular canal are reported (40, 66). Mild left ventricular myocardial abnormalities in young patients with WBS in the absence of clinically significant outflow tract obstruction (67) parallels the mouse model findings (68). Cerebral arterial disease with stroke are described infrequently in WBS (69, 70), but the true frequency of intracranial vascular stenoses is not known.
-
Vascular pathology
Arterial abnormalities in WBS include localized or diffuse narrowing of elastic arteries. Diagnostic imaging (64, 71) and pathological studies (72, 73) have both demonstrated generalized arterial wall thickening even in nonstenotic regions of the arterial tree (Figure 4). These nonstenotic areas are characterized by an expansion of the media, caused by up to a 2.5-fold increased number of lamellar units (72), with relatively preserved organization of elastic lamellae and smooth muscle cells.
 Figure 4
Figure 4Vascular abnormalities in WBS and in animal models of elastin deficiency. Aortic sections from a 2-year-old healthy donor (A and C) and from an age-matched WBS patient with severe SVAS (B, D, and E) were stained with Movat’s pentachrome stain to visualize elastic lamellae (black). A nonstenotic segment of the WBS aorta shows relatively preserved lamellar architecture but greatly increased medial thickness (B). In the stenotic region, the lamellae in the media are fragmented (D) and a focal area of proliferation is observed on the intimal aspect of the vessel that accompanies some SVAS lesions, particularly those of the hourglass type (E). Original magnification, ×100 (A and B); ×400 (C–E). Reproduced from American Journal of Human Genetics (73). Mice heterozygous for targeted inactivation of Eln (+/–) show increased number and decreased thickness of lamellae (G and I) compared with control (+/+) (F and H). Reproduced from ref. 68. Knockout mice rescued by a human bacterial artificial chromosome transgene (–/–BAC) (K) have only 30% of normal levels of elastin in the aorta, but thickness of the media is significantly increased relative to control (J). Reproduced with permission from Circulation Research (81). Complete loss of elastin in homozygous knockout mice (–/–) (M) show neonatal obstructive disease of the aorta. Control aorta at postnatal day 0.5 shows robust elastin staining (L). Reproduced with permission from Nature (80).
Three morphological types of SVAS are defined (74). Membranous SVAS is rare and consists of constricting semicircular valve-like membrane or membranes located at the sinotubular junction. The membrane contains small stellate cells in abundant mucopolysaccharide ground substance with few collagen and elastic fibrils but no medial elements (75). The second and third types of SVAS, the hourglass and diffuse types, share histological characteristics of disorganized lamellar architecture in the media, haphazard and fragmented elastic fibers, and focal clumping and hypertrophy of smooth muscle cells (Figure 4D) (75). In addition, enlargement of vasa vasorum has also been observed in the media and adventitia. Focal mural induration is sometimes observed. These regions, in addition to smooth muscle cells, have an extracellular matrix (Figure 4E) that is rich in mucopolysaccharides and contains endothelium-lined lacunae (74, 75).
-
Elastin haploinsufficiency as a cause of CV disease
Familial SVAS and WBS. As introduced earlier, understanding of the molecular basis of CV disease in WBS was aided by studies of a related but genetically distinct disease, familial SVAS (76). The spectrum, natural history, and pathological characteristics of CV and connective tissue lesions in patients with familial SVAS are virtually identical to those found in WBS. However, familial SVAS patients do not have neurobehavioral, metabolic, endocrine, developmental, or some of the craniofacial characteristics of WBS. These differences are due to the fact that familial SVAS is not a microdeletion syndrome but rather is caused by translocations (15), deletions (6, 16), and point mutations (77–79) that disrupt only the ELN gene. Despite significant allelic heterogeneity, all ELN mutations in familial SVAS studied to date cause loss of function at various levels of elastin biosynthesis, most commonly by eliminating the mutant mRNA via the nonsense-mediated decay pathway (79). Heterozygous loss-of-function mutations in familial SVAS and chromosomal deletion in WBS both cause reduced elastin synthesis and increased proliferation of cultured vascular smooth muscle cells and fibroblasts (73). Thus, both the clinical characteristics of, and the molecular mechanisms underlying, CV disease in WBS and familial SVAS appear to be the same and can be denoted by the term elastin arteriopathy (11).
Animal models. Animal models provide further evidence for elastin haploinsufficiency as the main cause of CV disease in WBS. Mice homozygous for targeted inactivation of Eln die from vascular occlusion (Figure 4M) associated with increased subendothelial smooth muscle cell proliferation in early postnatal life (80). Heterozygous Eln knockout mice have hypertension, increased arterial stiffness, and increased number of lamellar units (Figures 4, G and I), but thinner vessel walls than wild-type mice (72). Hypertension in Eln+/– mice is thought to be a developmental adaptation to maintain vascular caliber and perfusion, whereas the increased number of lamellar units are believed to normalize vessel wall strain (68). Both hypertension and increased number of lamellae are characteristic of the human and the animal model elastin arteriopathy. However, Eln+/– mice do not develop segmental stenoses of the arteries, and the media is thinner than in wild-type animals — clear differences from the human disease. Hypertension in Eln+/– mice is associated with elevated renin levels and can be blocked by the administration of angiotensin II receptor blockers candesartan and salarasin (68).
Transgenic expression of elastin rescues perinatal lethality in Eln–/– mice (Figure 4K) and improves hypertension and decreases vascular compliance in Eln+/– mice (81). These findings raise the possibility that upregulation of elastin at the appropriate developmental stage may be a potential strategy to prevent elastin arteriopathy. However, it is unknown whether upregulation of elastin in already diseased blood vessels (e.g., those that have developed arteriopathy) can be an effective treatment option.
-
Modifying factors
The expression of CV disease in WBS is highly variable, ranging from infantile lethality to no overt or clinically apparent CV involvement. Male sex, a known significant risk factor, is associated with earlier onset and more severe disease (82) but does not explain all of the variability in CV disease expression. Among potential genetic modifiers, variants in ELN, in the size of the WBS critical region, in the “intact” alleles on the normal chromosome 7 homolog, and elsewhere in the genome need to be considered.
Given that the developing vasculature is exquisitely sensitive to elastin dose (81), factors that influence elastin biosynthesis, including polymorphisms within ELN, are likely to have important effects on disease severity. Significant differences in elastin synthesis and cross-linking have been observed between different ethnic groups (83) suggesting a genetic basis for natural variation in elastin production. Expression of ELN has been studied in two WBS cohorts. One, enrolling only WBS patients with severe SVAS (73), found significantly reduced ELN expression compared with controls even after accounting for hemizygosity. In contrast, the second study detected normal ELN expression on average, though the authors noted high individual variability (84). Additionally, the second cohort showed a trend for higher ELN expression in patients without SVAS, but the difference did not reach statistical significance. These findings suggest that residual elastin expression protects against the development of CV disease in WBS, but adequately powered and controlled studies are needed to prove this point beyond doubt. Investigation of patients with intracranial aneurysms identified noncoding polymorphisms that affected the expression of ELN, identifying candidate risk alleles for elastin arteriopathy (85).
Reduced expression of a few nondeleted genes mapping to the duplicons flanking the WBS critical region have been documented (84). Variations in the expression of these adjacent genes may be another factor contributing to the phenotypic differences in individuals with WBS.
Among the genes within the WBS critical region, NCF1 dose has been associated with the prevalence of hypertension (18). The NCF1 gene (Figure 2) and two NCF1 pseudogenes, both inactivated by a GT dinucleotide deletion, are located in a telomeric block of low copy number repeats flanking the WBS critical region. In the general population, gene conversion events between the gene and the pseudogenes result in a natural variation of active NCF1 gene dose, ranging from 2 to 4 (86). In WBS patients, the deletion breakpoint determines whether the NCF1 gene is deleted (14), so that gene dose ranges from 1 to 4. In the WBS patients with more than one copy of NCF1, approximately 56% of the total WBS population, the risk of hypertension is increased 4-fold compared with those with only one functional NCF1 allele (18). NCF1 encodes the p47phox subunit of the NADPH oxidase, and reduced angiotensin II–mediated oxidative stress in the vasculature is proposed as the mechanism responsible for this protective effect.
-
Mechanisms of obstructive vascular disease in WBS
Both human and animal studies suggest that elastin is required for the terminal differentiation and quiescence of vascular smooth muscle cells. In elastin-null mice, increased vascular smooth muscle cell proliferation both in vivo and in organ culture occur (80). This hyperproliferative phenotype was associated with decreased stress fiber and focal adhesion formation and increased vascular smooth muscle cell migration in vitro and was suppressed by treatment with tropoelastin, the soluble precursor protein of elastin (87). Similarly, both dermal fibroblasts and aortic smooth muscle cells isolated from patients with WBS or familial SVAS showed increased proliferation inversely proportional to the amount of elastin produced. Treatment of these cells with insoluble elastin normalized the hyperproliferative phenotype (73).
The formation of segmental obstructive lesions is thought to be a two-step process, consisting of increased number of lamellar units and vessel wall thickening during fetal development, leading to a uniformly altered vascular tree, followed by postnatal injury-mediated inward remodeling (72). The preferential localization of segmental stenoses to the sinotubular junction and to branch points, areas of high turbulence, supports this notion. Interestingly, Eln+/– mice are protected from vascular remodeling following carotid artery ligation in the ipsilateral vessel while enhanced remodeling occurs in the contralateral artery (88), suggesting that elastin haploinsufficiency may differentially affect ligation-mediated and flow-mediated injury responses. Eln+/– mice show the same CV remodeling response in the renal artery clipping model of adult hypertension as do wild-type animals (89) but are protected from the age-related vessel wall thickening observed in wild-type animals (90). Further clinical studies are needed to determine whether patients with WBS are also protected from age-related vascular changes.
Although it is clear that elastin is a negative regulator of cell proliferation in development, the precise receptors and pathways mediating elastin signaling remain to be identified. Studies in different experimental systems yielded conflicting results. A specific 67-kD elastin-binding protein (EBP) localized to the cell surface has been identified as an enzymatically inactive, alternatively spliced isoform of β-galactosidase (91). EBP has been shown to mediate either increased (92) or decreased (93) cell proliferation depending on whether elastin is present in solution or in solid phase. Elastin peptides were shown to increase the proliferation of coronary artery smooth muscle cells by activating cellular Ca2+ influx through L-type Ca2+ channels and by activating the focal adhesion kinase (FAK), Src, and MAPK pathways (92) (Figure 5A). In contrast, canine coronary vascular cells plated on elastin under cyclic stretch conditions showed reduced serum-induced cell proliferation when compared with cells plated on collagen in an EBP-dependent manner (93). Other studies implicated a GPCR in transmitting elastin signals in elastin-null cells (87). This pathway involves the inhibition of adenylate cyclase and the activation of RhoA and Rho kinase, leading to increased actin polymerization (Figure 5B).
 Figure 5
Figure 5Proposed mechanisms of elastin signaling. (A) A 67-kDa EBP is thought to form a receptor complex with protective protein/cathepsin A (PP) and transmembrane sialidase/neuraminidase (Sase). Elastin peptide binding to the receptor activates the L-type Ca2+ channel and G-proteins (Gα, β, γ) to activate the MAPK pathway (92). (B) A different pathway postulates a GPCR for tropoelastin. GPCR-elastin binding depresses cAMP levels by inhibiting adenylate cyclase (AC) and leads to increased actin polymerization through the Rho kinase pathway (87). (C) Cells can also sense the elastin content indirectly by binding to elastic fiber components such as fibrillins and fibulin-5 via integrins.
Several structural proteins of the elastic fibers including fibrillin-1, fibrillin-2, and fibulin-5 have RGD sequences that serve as attachment sites for cellular integrins and could provide indirect elastin-dependent signals in the arterial wall (21) (Figure 5C). Interestingly, patients with recessive mutations in fibulin-5 develop SVAS (94) and fibulin-5–null mice show exaggerated neointima formation, as well as increased vascular cell proliferation and migration (88), providing in vivo evidence to support a role for fibulin-5 in elastin signaling. Better understanding of the pathways that connect elastin deficiency to increased vascular cell proliferation may help identify new targets for the treatment of CV disease in WBS.
-
Current treatment
The series from Table 1 describe operative or catheter-based interventions in 18% of patients for left ventricular outflow tract obstruction and in 4% of patients for right ventricular outflow tract obstruction (36–44). Patients with mild SVAS in infancy (peak catheterization gradient <20 mmHg) often remain stable and do not require intervention (36).
Operative techniques for repair of SVAS have utilized patch aortoplasty that may involve augmentation of 1–3 of the aortic sinuses (95, 96). The symmetric inverted 3-sinus patch plasty has resulted in improved outcome in one large series (49). Early mortality for repair of SVAS is 1%–9% (range of median or mean age at operation, 6–16 years), with 20-year survival of 77%–97% and long-term reduction in peak catheterization gradients in the majority of patients (36, 49, 95, 97). Although gradients maybe relieved, hypoplasia may persist in the remainder of the aortic arch (98). Diffuse hypoplasia of the aorta is a risk factor for reoperation (36, 49, 99). In patients with severe left main coronary obstruction, patch enlargement of the coronary os, excision of a fused aortic leaflet, and bypass grafting have been utilized (100).
Most patients with pulmonary arterial stenosis without significant SVAS can be observed without need for treatment in view of well-documented spontaneous improvement. For patients with persistent systemic or suprasystemic right ventricular pressure, marked asymmetry in pulmonary blood flow, or symptoms, balloon dilation angioplasty has been used to improve arterial diameter, especially in distal vessels. After catheter-based therapy, right ventricular pressure often remains elevated due to residual proximal obstruction, and the incidence of aneurysms is higher in comparison with non-WBS subjects (101). Patients with biventricular outflow obstruction with an indication for surgical relief of SVAS may undergo balloon angioplasty of pulmonary arterial stenosis prior to surgery. In a series of 33 patients with median age of operation of 4 years for biventricular obstruction, early mortality was 18% (96). Patients with middle aortic syndrome and long segment narrowing may undergo aorto-aortic bypass or patch plasty with bypass grafting of involved renal and visceral arteries (102). Catheter-based therapy is an option for some middle aortic lesions that are more localized (102).
Many patients require treatment for hypertension, but data are not available to recommend drug selection targeted for WBS. Beta blocker and calcium channel blocker drugs have been utilized frequently in several of the retrospective series (7, 40, 61, 103), and the link between infantile hypercalcemia and hypertension (59) suggests a role for calcium channel blockade. Angiotensin receptor blockade is effective in the Eln+/– mouse model. Medical treatment in WBS can be challenging so that multidrug regimens may be required for adequate control of blood pressure. Patients with hypertension resistant to drug therapy should be studied for a renovascular etiology.
-
Future therapies
Surgical treatment of vascular lesions in WBS frequently relies on the use of vascular grafts, most commonly made of artificial materials such as polyethylene terephthalate (Dacron) or expanded polytetrafluoroethylene (ePTFE). The resilience, long-term stability, and cellular attachment properties of elastin make it an attractive material to explore (104). Elastin-like polymers from recombinant tropoelastin or synthetic elastin peptides can be used to coat or functionalize graft materials or used as a scaffold for vascular cells in tissue engineering approaches. Coating of synthetic materials with elastin peptides was shown to decrease thrombogenicity in a variety of settings (105), and insoluble elastin coating significantly decreased in-stent restenosis (87) in a porcine carotid artery model. Tissue-engineered aortic grafts prepared using autologous endothelial and smooth muscle cells seeded onto biodegradable scaffolds and surrounded with intestinal submucosa showed promising long-term patency in animal models, but achieving appropriate amounts of elastin formation continues to be a limitation (106).
An alternative approach to the treatment of CV disease in WBS would involve small molecules or biologicals that can either promote elastin biosynthesis or suppress vascular smooth muscle cell proliferation and migration. Minoxidil, a K+-channel blocker and putative NO agonist (107), glucocorticoids (108), and retinoids (109) have been shown to upregulate elastin production in vivo. MMP inhibitors may be beneficial in preventing elastin degradation associated with vascular remodeling (110). A number of pulmonary vasodilatory drugs are efficacious in the treatment of primary pulmonary arterial hypertension, including calcium channel blockers, prostacyclin analogs, endothelin receptor antagonists, and NO agonists (111, 112). These agents could be evaluated as potential modulators of vascular lesions in WBS, even though the pathology of ELN arteriopathy is distinct from primary pulmonary arterial hypertension.
Nutritional intervention or nutraceuticals may theoretically be targets affecting elastin arteriopathy. Copper deficiency (113), β-aminopropionitrile (contained in certain legumes), and drugs such as amine oxidase inhibitors and penicillamine (114) all interfere with the activity of lysyl oxidases, a family of enzymes required for elastin cross-linking (115). On the other hand, dill extract has been shown to increase LOXL1 (lysyl oxidase like 1) gene expression and elastin deposition in vitro (116). Finally, ellagic and tannic acid (polyphenols found in berries and nuts) inhibit proteolytic degradation of elastin and increase elastin deposition in skin fibroblast and organ cultures (117). It remains to be shown whether any of these natural products can reverse the effects of elastin deficiency in vivo.
Better understanding of the mechanisms behind the spontaneous improvement of pulmonary vascular lesions in WBS might lead to new treatment options. Developmental, physiological, mechanical, and biochemical differences between the pulmonary and systemic arterial trees need to be considered as potential mechanisms. Benign peripheral pulmonary stenosis is a common cause of innocent murmurs detected in the first month of life. The murmur and slightly elevated flow velocity are usually gone by 6–12 months of life (118, 119). Neonatal adaptation to breathing is known to involve a rapid increase in pulmonary flow, with a more gradual growth of the branch pulmonary arteries. One can speculate that this developmental adaptation may account for some of the resolution of pulmonary stenotic lesions in infants with WBS.
Another potential therapeutic strategy involves targeted upregulation of a patient’s own genes, as has already been accomplished for HIF-1α in a patient with peripheral artery disease (120). Although approximately two dozen genes are deleted in WBS patients, their normal chromosome 7 homolog contains an intact copy of each allele. WBS is a particularly compelling model for this treatment approach, especially if transcriptional control of normal ELN gene splice variants can be retained (121, 122). An alternative approach to in situ gene therapy would involve genetic modification of autologous progenitor (or already differentiated) vascular cells ex vivo followed by re-introduction back into the affected individual, though the challenges of appropriate delivery and integration into sites of vascular lesions present a formidable challenge.
In summary, the insight that the vascular lesions in WBS are linked to hemizygosity of the ELN gene and the creation of an Eln knockout mouse with vascular pathology have provided a focus for connecting elastin biology with vascular disease. In order to translate experimental insights to treatments, newer animal models that better recapitulate the features of WBS may play an important part. For example a second-generation model with regulatable elastin expression in the vessel wall may resolve important questions such as whether re-expression of elastin, or administration of antiproliferative smooth muscle cell pharmacotherapy, can reverse the disease process, especially in instances where vascular lesions have already formed. These efforts, along with continued research to elucidate pathophysiology and disease modifiers, will hopefully result in therapies that are alternatives to surgery in that they can ameliorate or even prevent the common complications of WBS arteriopathy.
-
Acknowledgments
This work was funded in part by NIH grant HL084922 and American Heart Association grant 0655626Z, to Z. Urban. The authors gratefully acknowledge ongoing encouragement from the Chloe’s Quest Foundation and the Williams Syndrome Association.
Address correspondence to: Barbara R. Pober, MassGeneral Hospital for Children, Simches Research Building, Rm. 222, 185 Cambridge St., Boston, Massachusetts 02114, USA. Phone: (617) 726-1561; Fax: (617) 726-1566; E-mail: [email protected].
-
Footnotes
Nonstandard abbreviations used: CV, cardiovascular; EBP, elastin-binding protein; NAHR, nonallelic homologous recombination; NCF1, neutrophil cytosolic factor 1; SVAS, supravalvular aortic stenosis; WBS, Williams-Beuren syndrome.
Conflict of interest: The authors have declared that no conflict of interest exists.
Reference information: J. Clin. Invest.118:1606–1615 (2008). doi:10.1172/JCI35309.
-
References
- Williams, J.C., Barratt-Boyes, B.G., Lowe, J.B. 1961. Supravalvular aortic stenosis. Circulation. 24:1311-1318. View this article via: PubMed Google Scholar
- Beuren, A.J., Apitz, J., Harmjanz, D. 1962. Supravalvular aortic stenosis in association with mental retardation and a certain facial appearance. Circulation. 26:1235-1240. View this article via: PubMed Google Scholar
- Morris, C.A., Demsey, S.A., Leonard, C.O., Dilts, C., Blackburn, B.L. 1988. Natural history of Williams syndrome: physical characteristics. J. Pediatr. 113:318-326.
- Pober, B.R., Dykens, E.M. 1996. Williams syndrome: An overview of medical, cognitive, and behavioral features. Child Adolesc. Psychiatr. Clin. N. Am. 5:929-943.
- Stromme, P., Bjornstad, P.G., Ramstad, K. 2002. Prevalence estimation of Williams syndrome. J. Child Neurol. 17:269-271.
- Ewart, A.K., et al. 1993. Hemizygosity at the elastin locus in a developmental disorder, Williams syndrome. Nat. Genet. 5:11-16.
- Cherniske, E.M., et al. 2004. Multisystem study of 20 older adults with Williams syndrome. Am. J. Med. Genet. A. 131:255-264. View this article via: PubMed Google Scholar
- Partsch, C.J., et al. 1999. Longitudinal evaluation of growth, puberty, and bone maturation in children with Williams syndrome. J. Pediatr. 134:82-89.
- Pober, B.R. 2006. Evidence-based medical management of adults with Williams-Beuren syndrome. InWilliams–Beuren syndrome: research, evaluation, and treatment. C.A. Morris, H.M. Lenhoff, and P.P. Wang, editors. Johns Hopkins University Press. Baltimore, Maryland, USA. 125–143.
- Kaplan, P. 2006. The medical management of children with Williams-Beuren syndrome. InWilliams-Beuren syndrome: research, evaluation, and treatment. C.A. Morris, H.M. Lenhoff, and P.P. Wang, editors. Johns Hopkins University Press. Baltimore, Maryland, USA. 83–106.
- Morris, C.A., Mervis, C.B. 2000. Williams syndrome and related disorders. Annu. Rev. Genomics Hum. Genet. 1:461-484.
- Mervis, C.B., et al. 2000. The Williams syndrome cognitive profile. Brain Cogn. 44:604-628.
- Dykens, E.M. 2003. Anxiety, fears, and phobias in persons with Williams syndrome. Dev. Neuropsychol. 23:291-316.
- Bayes, M., Magano, L.F., Rivera, N., Flores, R., Perez Jurado, L.A. 2003. Mutational mechanisms of Williams-Beuren syndrome deletions. Am. J. Hum. Genet. 73:131-151.
- Curran, M.E., et al. 1993. The elastin gene is disrupted by a translocation associated with supravalvular aortic stenosis. Cell. 73:159-168.
- Olson, T.M., et al. 1993. Autosomal dominant supravalvular aortic stenosis: localization to chromosome 7. Hum. Mol. Genet. 2:869-873.
- B17Cusco, I., et al. 2008. Copy number variation at the 7q11.23 segmental duplications is a susceptibility factor for the Williams-Beuren syndrome deletion. Genome Res.In press
- Del Campo, M., et al. 2006. Hemizygosity at the NCF1 gene in patients with Williams-Beuren syndrome decreases their risk of hypertension. Am. J. Hum. Genet. 78:533-542.
- Osborne, L.R. 2006. The molecular basis of a multisystem disorder. In Williams-Beuren syndrome: research, evaluation, and treatment. C.A. Morris, H.M. Lenhoff, and P.P. Wang, editors. Johns Hopkins University Press. Baltimore, Maryland, USA. 18–58.
- DECIPHER: Syndrome report for Williams-Beuren Syndrome (WBS). 2007. https://decipher.sanger.ac.uk/perl/application?action=syndromes;syndrome_id=3;o_geneimprint_hugo=off; o_fu=on;o_2Mb_novelgene=off;o_2Mb_array=off;o_2Mb_syn=on;o_2Mb_geneimprint_hugo=off;o_trans=on;o_array=on; o_prior_genes=off;o_2Mb_hugo=off;o_clone=on;o_fc=off;o_desc=on;o_hugo=off;o_fd=on;o_cite=on;o_novelgene=off; o_karyo=on;o_pheno=on;o_2Mb_omim_hugo=off;o_ff=on;o_knowngene=on;o_omim_hugo=on;o_2Mb_knowngene=off;o_syn=on; o_2Mb_trans=off;o_prior_indiv_genes=off .
- Kielty, C.M. 2006. Elastic fibres in health and disease. Expert Rev. Mol. Med. 8:1-23. View this article via: PubMed Google Scholar
- Kelleher, C.M., McLean, S.E., Mecham, R.P. 2004. Vascular extracellular matrix and aortic development. Curr. Top. Dev. Biol. 62:153-188. View this article via: PubMed Google Scholar
- Somerville, M.J., et al. 2005. Severe expressive-language delay related to duplication of the Williams-Beuren locus. N. Engl. J. Med. 353:1694-1701.
- Berg, J.S., et al. 2007. Speech delay and autism spectrum behaviors are frequently associated with duplication of the 7q11.23 Williams-Beuren syndrome region. Genet. Med. 9:427-441. View this article via: PubMed Google Scholar
- Osborne, L.R., et al. 2001. A 1.5 million-base pair inversion polymorphism in families with Williams-Beuren syndrome. Nat. Genet. 29:321-325.
- Scherer, S.W., et al. 2005. Observation of a parental inversion variant in a rare Williams-Beuren syndrome family with two affected children. Hum. Genet. 117:383-388.
- DeSilva, U., Massa, H., Trask, B.J., Green, E.D. 1999. Comparative mapping of the region of human chromosome 7 deleted in williams syndrome. Genome Res. 9:428-436. View this article via: PubMed Google Scholar
- Ranheim, E.A., et al. 2005. Frizzled 9 knock-out mice have abnormal B-cell development. Blood. 105:2487-2494.
- Meng, Y., et al. 2002. Abnormal spine morphology and enhanced LTP in LIMK-1 knockout mice. Neuron. 35:121-133.
- Young, E.J., et al. 2007. Reduced fear and aggression and altered serotonin metabolism in Gtf2ird1-targeted mice. Genes Brain Behav. 7:224-234.
- Hoogenraad, C.C., et al. 2002. Targeted mutation of Cyln2 in the Williams syndrome critical region links CLIP-115 haploinsufficiency to neurodevelopmental abnormalities in mice. Nat. Genet. 32:116-127.
- Frangiskakis, J.M., et al. 1996. LIM-kinase1 hemizygosity implicated in impaired visuospatial constructive cognition. Cell. 86:59-69.
- Morris, C.A., et al. 2003. GTF2I hemizygosity implicated in mental retardation in Williams syndrome: genotype-phenotype analysis of five families with deletions in the Williams syndrome region. Am. J. Med. Genet. A. 123:45-59. View this article via: PubMed Google Scholar
- Morris, C.A. 2006. Genotype-phenotype correlations in Williams-Beuren syndrome. In Williams-Beuren syndrome: research, evaluation, and treatment. C.A. Morris, H.M. Lenhoff, and P.P. Wang, editors. Johns Hopkins University Press. Baltimore, Maryland, USA. 59–82.
- Beuren, A.J., Schulze, C., Eberle, P., Harmjanz, D., Apitz, J. 1964. The syndrome of supravalvular aortic stenosis, peripheral pulmonary stenosis, mental retardation and similar facial appearance. Am. J. Cardiol. 13:471-483.
- Wessel, A., Pankau, R., Kececioglu, D., Ruschewski, W., Bursch, J.H. 1994. Three decades of follow-up of aortic and pulmonary vascular lesions in the Williams-Beuren syndrome. Am. J. Med. Genet. 52:297-301.
- Hallidie-Smith, K.A., Karas, S. 1988. Cardiac anomalies in Williams-Beuren syndrome. Arch. Dis. Child. 63:809-813. View this article via: PubMed Google Scholar
- Zalzstein, E., Moes, C.A., Musewe, N.N., Freedom, R.M. 1991. Spectrum of cardiovascular anomalies in Williams-Beuren syndrome. Pediatr. Cardiol. 12:219-223.
- Kim, Y.M., Yoo, S.J., Choi, J.Y., Kim, S.H., Bae, E.J., Lee, Y.T. 1999. Natural course of supravalvar aortic stenosis and peripheral pulmonary arterial stenosis in Williams’ syndrome. Cardiol. Young. 9:37-41. View this article via: PubMed Google Scholar
- Eronen, M., et al. 2002. Cardiovascular manifestations in 75 patients with Williams syndrome. J. Med. Genet. 39:554-558.
- Amenta, S., et al. 2005. Clinical manifestations and molecular investigation of 50 patients with Williams syndrome in the Greek population. Pediatr. Res. 57:789-795.
- Scheiber, D., et al. 2006. Echocardiographic findings in patients with Williams-Beuren syndrome. Wien. Klin. Wochenschr. 118:538-554.
- Wang, C.C., et al. 2007. Outcome of pulmonary and aortic stenosis in Williams-Beuren syndrome in an Asian cohort. Acta Paediatr. 96:906-909. View this article via: PubMed Google Scholar
- Bruno, E., Rossi, N., Thuer, O., Cordoba, R., Alday, L.E. 2003. Cardiovascular findings, and clinical course, in patients with Williams syndrome. Cardiol. Young. 13:532-536. View this article via: PubMed Google Scholar
- Giddins, N.G., Finley, J.P., Nanton, M.A., Roy, D.L. 1989. The natural course of supravalvar aortic stenosis and peripheral pulmonary artery stenosis in Williams’s syndrome. Br. Heart J. 62:315-319.
- Wren, C., Oslizlok, P., Bull, C. 1990. Natural history of supravalvular aortic stenosis and pulmonary artery stenosis. J. Am. Coll. Cardiol. 15:1625-1630. View this article via: PubMed Google Scholar
- Radford, D.J., Pohlner, P.G. 2000. The middle aortic syndrome: an important feature of Williams’ syndrome. Cardiol. Young. 10:597-602. View this article via: PubMed Google Scholar
- van Son, J.A., Edwards, W.D., Danielson, G.K. 1994. Pathology of coronary arteries, myocardium, and great arteries in supravalvular aortic stenosis. Report of five cases with implications for surgical treatment. J. Thorac. Cardiovasc. Surg. 108:21-28. View this article via: PubMed Google Scholar
- Stamm, C., et al. 1999. Forty-one years of surgical experience with congenital supravalvular aortic stenosis. J. Thorac. Cardiovasc. Surg. 118:874-885.
- Stamm, C., Li, J., Ho, S.Y., Redington, A.N., Anderson, R.H. 1997. The aortic root in supravalvular aortic stenosis: the potential surgical relevance of morphologic findings. J. Thorac. Cardiovasc. Surg. 114:16-24.
- Bonnet, D., Cormier, V., Villain, E., Bonhoeffer, P., Kachaner, J. 1997. Progressive left main coronary artery obstruction leading to myocardial infarction in a child with Williams syndrome. Eur. J. Pediatr. 156:751-753.
- van Pelt, N.C., Wilson, N.J., Lear, G. 2005. Severe coronary artery disease in the absence of supravalvular stenosis in a patient with Williams syndrome. Pediatr. Cardiol. 26:665-667.
- Singh, T.P., Muzik, O., Forbes, T.F., Di Carli, M.F. 2003. Positron emission tomography myocardial perfusion imaging in children with suspected coronary abnormalities. Pediatr. Cardiol. 24:138-144.
- Wessel, A., et al. 2004. Risk of sudden death in the Williams-Beuren syndrome. Am. J. Med. Genet. A. 127:234-237. View this article via: PubMed Google Scholar
- Conway (Jr.), E.E., Noonan, J., Marion, R.W., Steeg, C.N. 1990. Myocardial infarction leading to sudden death in the Williams syndrome: report of three cases. J. Pediatr. 117:593-595.
- Terhune, P.E., Buchino, J.J., Rees, A.H. 1985. Myocardial infarction associated with supravalvular aortic stenosis. J. Pediatr. 106:251-254.
- Bird, L.M., et al. 1996. Sudden death in Williams syndrome: report of ten cases. J. Pediatr. 129:926-931.
- Rose, C., Wessel, A., Pankau, R., Partsch, C.J., Bursch, J. 2001. Anomalies of the abdominal aorta in Williams-Beuren syndrome--another cause of arterial hypertension. Eur. J. Pediatr. 160:655-658. View this article via: PubMed Google Scholar
- Broder, K., et al. 1999. Elevated ambulatory blood pressure in 20 subjects with Williams syndrome. Am. J. Med. Genet. 83:356-360.
- Wessel, A., Motz, R., Pankau, R., Bursch, J.H. 1997. Arterielle Hypertension und Blutdruckprofil bei Patienten mit Williems-Beuren-Syndrom [Arterial hypertension and blood pressure profile in patients with Williams-Beuren syndrome]. Zeitschrift für Kardiologie. 86:251-257.
- Giordano, U., et al. 2001. Exercise testing and 24-hour ambulatory blood pressure monitoring in children with Williams syndrome. Pediatr. Cardiol. 22:509-511.
- Sorof, J.M., Portman, R.J. 2000. White coat hypertension in children with elevated casual blood pressure. J. Pediatr. 137:493-497.
- French, J.W., Guntheroth, W.G. 1970. An explanation of asymmetric upper extremity blood pressures in supravalvular aortic stenosis: the Coanda effect. Circulation. 42:31-36. View this article via: PubMed Google Scholar
- Rein, A.J., Preminger, T.J., Perry, S.B., Lock, J.E., Sanders, S.P. 1993. Generalized arteriopathy in Williams syndrome: an intravascular ultrasound study. J. Am. Coll. Cardiol. 21:1727-1730. View this article via: PubMed Google Scholar
- Aggoun, Y., et al. 2000. Mechanical properties of the common carotid artery in Williams syndrome. Heart. 84:290-293.
- Nakamoto, S., Saga, T., Shinohara, T. 2003. Williams syndrome associated with complete atrioventricular septal defect. Heart. 89:e15. View this article via: PubMed Google Scholar
- Verrengia, M., et al. 2007. Left ventricle myocardial mechanics and textural properties in patients with Williams syndrome. J. Cardiovasc. Med. (Hagerstown) 8:330-336. View this article via: PubMed Google Scholar
- Faury, G., et al. 2003. Developmental adaptation of the mouse cardiovascular system to elastin haploinsufficiency. J. Clin. Invest. 112:1419-1428.
- Kaplan, P., Levinson, M., Kaplan, B.S. 1995. Cerebral artery stenoses in Williams syndrome cause strokes in childhood. J. Pediatr. 126:943-945.
- Soper, R., et al. 1995. Ischemic stroke and intracranial multifocal cerebral arteriopathy in Williams syndrome. J. Pediatr. 126:945-948.
- Sadler, L.S., Gingell, R., Martin, D.J. 1998. Carotid ultrasound examination in Williams syndrome. J. Pediatr. 132:354-356.
- Li, D.Y., et al. 1998. Novel arterial pathology in mice and humans hemizygous for elastin. J. Clin. Invest. 102:1783-1787.
- Urban, Z., et al. 2002. Connection between elastin haploinsufficiency and increased cell proliferation in patients with supravalvular aortic stenosis and Williams-Beuren syndrome. Am. J. Hum. Genet. 71:30-44.
- Perou, M.L. 1961. Congenital supravalvular aortic stenosis. A morphological study with attempt at classification. Arch. Pathol. 71:453-466. View this article via: PubMed Google Scholar
- O’Connor, W.N., et al. 1985. Supravalvular aortic stenosis. Clinical and pathologic observations in six patients. Arch. Pathol. Lab. Med. 109:179-185. View this article via: PubMed Google Scholar
- Eisenberg, R., Young, D., Jacobson, B., Boito, A. 1964. Familial supravalvular aortic stenosis. Am. J. Dis. Child. 108:341-347. View this article via: PubMed Google Scholar
- Li, D.Y., et al. 1997. Elastin point mutations cause an obstructive vascular disease, supravalvular aortic stenosis. Hum. Mol. Genet. 6:1021-1028.
- Tassabehji, M., et al. 1997. Elastin: genomic structure and point mutations in patients with supravalvular aortic stenosis. Hum. Mol. Genet. 6:1029-1036.
- Urban, Z., et al. 2000. Isolated supravalvular aortic stenosis: functional haploinsufficiency of the elastin gene as a result of nonsense-mediated decay. Hum. Genet. 106:577-588.
- Li, D.Y., et al. 1998. Elastin is an essential determinant of arterial morphogenesis. Nature. 393:276-280.
- Hirano, E., Knutsen, R.H., Sugitani, H., Ciliberto, C.H., Mecham, R.P. 2007. Functional rescue of elastin insufficiency in mice by the human elastin gene: implications for mouse models of human disease. Circ. Res. 101:523-531.
- Sadler, L.S., et al. 2001. Differences by sex in cardiovascular disease in Williams syndrome. J. Pediatr. 139:849-853.
- Urban, Z., et al. 2007. Population differences in elastin maturation in optic nerve head tissue and astrocytes. Invest. Ophthalmol. Vis. Sci. 48:3209-3215.
- Merla, G., et al. 2006. Submicroscopic deletion in patients with Williams-Beuren syndrome influences expression levels of the nonhemizygous flanking genes. Am. J. Hum. Genet. 79:332-341.
- Akagawa, H., et al. 2006. A haplotype spanning two genes, ELN and LIMK1, decreases their transcripts and confers susceptibility to intracranial aneurysms. Hum. Mol. Genet. 15:1722-1734.
- Heyworth, P.G., Noack, D., Cross, A.R. 2002. Identification of a novel NCF-1 (p47-phox) pseudogene not containing the signature GT deletion: significance for A47 degrees chronic granulomatous disease carrier detection. Blood. 100:1845-1851.
- Karnik, S.K., et al. 2003. A critical role for elastin signaling in vascular morphogenesis and disease. Development. 130:411-423.
- Spencer, J.A., et al. 2005. Altered vascular remodeling in fibulin-5-deficient mice reveals a role of fibulin-5 in smooth muscle cell proliferation and migration. Proc. Natl. Acad. Sci. U. S. A. 102:2946-2951.
- Wagenseil, J.E., Knutsen, R.H., Li, D.Y., Mecham, R.P. 2007. Elastin-insufficient mice show normal cardiovascular remodeling in 2K1C hypertension despite higher baseline pressure and unique cardiovascular architecture. Am. J. Physiol. Heart Circ. Physiol. 293:H574-H582.
- Pezet, M., et al. 2008. Elastin Haploinsufficiency Induces Alternative Aging Processes in the Aorta. Rejuvenation Res. 11:97-112.
- Privitera, S., Prody, C.A., Callahan, J.W., Hinek, A. 1998. The 67-kDa enzymatically inactive alternatively spliced variant of beta-galactosidase is identical to the elastin/laminin-binding protein. J. Biol. Chem. 273:6319-6326.
- Mochizuki, S., Brassart, B., Hinek, A. 2002. Signaling pathways transduced through the elastin receptor facilitate proliferation of arterial smooth muscle cells. J. Biol. Chem. 277:44854-44863.
- Spofford, C.M., Chilian, W.M. 2001. The elastin-laminin receptor functions as a mechanotransducer in vascular smooth muscle. Am. J. Physiol. Heart Circ. Physiol. 280:H1354-H1360. View this article via: PubMed Google Scholar
- Loeys, B., et al. 2002. Homozygosity for a missense mutation in fibulin-5 (FBLN5) results in a severe form of cutis laxa. Hum. Mol. Genet. 11:2113-2118.
- Hazekamp, M.G., et al. 1999. Brom’s three-patch technique for repair of supravalvular aortic stenosis. J. Thorac. Cardiovasc. Surg. 118:252-258.
- Stamm, C., et al. 2000. Surgery for bilateral outflow tract obstruction in elastin arteriopathy. J. Thorac. Cardiovasc. Surg. 120:755-763.
- Brown, J.W., Ruzmetov, M., Vijay, P., Turrentine, M.W. 2002. Surgical repair of congenital supravalvular aortic stenosis in children. Eur. J. Cardiothorac. Surg. 21:50-56.
- English, R.F., Colan, S.D., Kanani, P.M., Ettedgui, J.A. 2003. Growth of the aorta in children with Williams syndrome: does surgery make a difference? Pediatr. Cardiol. 24:566-568.
- Minakata, K., Nishimura, K., Nomoto, S., Matsuda, K., Ban, T. 1997. Surgical repair for supravalvular aortic stenosis: intermediate to long-term follow-up. J. Card. Surg. 12:398-402.
- Thistlethwaite, P.A., Madani, M.M., Kriett, J.M., Milhoan, K., Jamieson, S.W. 2000. Surgical management of congenital obstruction of the left main coronary artery with supravalvular aortic stenosis. J. Thorac. Cardiovasc. Surg. 120:1040-1046.
- Geggel, R.L., Gauvreau, K., Lock, J.E. 2001. Balloon dilation angioplasty of peripheral pulmonary stenosis associated with Williams syndrome. Circulation. 103:2165-2170. View this article via: PubMed Google Scholar
- Delis, K.T., Gloviczki, P. 2005. Middle aortic syndrome: from presentation to contemporary open surgical and endovascular treatment. Perspect. Vasc. Surg. Endovasc. Ther. 17:187-203.
- Wessel, A., Motz, R., Pankau, R., Bursch, J.H. 1997. Arterial hypertension and blood pressure profile in patients with Williams-Beuren syndrome [In German]. Z. Kardiol. 86:251-257.
- Daamen, W.F., Veerkamp, J.H., van Hest, J.C., van Kuppevelt, T.H. 2007. Elastin as a biomaterial for tissue engineering. Biomaterials. 28:4378-4398.
- Woodhouse, K.A., et al. 2004. Investigation of recombinant human elastin polypeptides as non-thrombogenic coatings. Biomaterials. 25:4543-4553.
- Opitz, F., et al. 2004. Tissue engineering of aortic tissue: dire consequence of suboptimal elastic fiber synthesis in vivo. Cardiovasc. Res. 63:719-730.
- Tsoporis, J., Keeley, F.W., Lee, R.M., Leenen, F.H. 1998. Arterial vasodilation and vascular connective tissue changes in spontaneously hypertensive rats. J. Cardiovasc. Pharmacol. 31:960-962.
- Pierce, R.A., Mariencheck, W.I., Sandefur, S., Crouch, E.C., Parks, W.C. 1995. Glucocorticoids upregulate tropoelastin expression during late stages of fetal lung development. Am. J. Physiol. 268:L491-L500. View this article via: PubMed Google Scholar
- McGowan, S.E., Doro, M.M., Jackson, S.K. 1997. Endogenous retinoids increase perinatal elastin gene expression in rat lung fibroblasts and fetal explants. Am. J. Physiol. 273:L410-L416. View this article via: PubMed Google Scholar
- Sluijter, J.P., de Kleijn, D.P., Pasterkamp, G. 2006. Vascular remodeling and protease inhibition — bench to bedside. Cardiovasc. Res. 69:595-603.
- Benedict, N., Seybert, A., Mathier, M.A. 2007. Evidence-based pharmacologic management of pulmonary arterial hypertension. Clin. Ther. 29:2134-2153.
- Humbert, M., Sitbon, O., Simonneau, G. 2004. Treatment of pulmonary arterial hypertension. N. Engl. J. Med. 351:1425-1436.
- Madsen, E., Gitlin, J.D. 2007. Copper deficiency. Curr. Opin. Gastroenterol. 23:187-192. View this article via: PubMed Google Scholar
- Tinker, D., Rucker, R.B. 1985. Role of selected nutrients in synthesis, accumulation, and chemical modification of connective tissue proteins. Physiol. Rev. 65:607-657. View this article via: PubMed Google Scholar
- Kagan, H.M., Li, W. 2003. Lysyl oxidase: properties, specificity, and biological roles inside and outside of the cell. J. Cell. Biochem. 88:660-672.
- Cenizo, V., et al. 2006. LOXL as a target to increase the elastin content in adult skin: a dill extract induces the LOXL gene expression. Exp. Dermatol. 15:574-581.
- Jimenez, F., Mitts, T.F., Liu, K., Wang, Y., Hinek, A. 2006. Ellagic and tannic acids protect newly synthesized elastic fibers from premature enzymatic degradation in dermal fibroblast cultures. J. Invest. Dermatol. 126:1272-1280.
- Miyake, T., Yokoyama, T. 1993. Evaluation of transient heart murmur resembling pulmonary artery stenosis in term infants by Doppler and M-mode echocardiography. Jpn. Circ. J. 57:77-83. View this article via: PubMed Google Scholar
- Danilowicz, D.A., Rudolph, A.M., Hoffman, J.I., Heymann, M. 1972. Physiologic pressure differences between main and branch pulmonary arteries in infants. Circulation. 45:410-419. View this article via: PubMed Google Scholar
- Rajagopalan, S., et al. 2007. Use of a constitutively active hypoxia-inducible factor-1alpha transgene as a therapeutic strategy in no-option critical limb ischemia patients: phase I dose-escalation experience. Circulation. 115:1234-1243. View this article via: PubMed Google Scholar
- Giordano, F.J. 2007. Therapeutic gene regulation: targeting transcription. Circulation. 115:1180-1183. View this article via: PubMed Google Scholar
- Yu, J., et al. 2006. An engineered VEGF-activating zinc finger protein transcription factor improves blood flow and limb salvage in advanced-age mice. FASEB J. 20:479-481. View this article via: PubMed Google Scholar
- Williams, J.C., Barratt-Boyes, B.G., Lowe, J.B. 1961. Supravalvular aortic stenosis. Circulation. 24:1311-1318.
-
Version history
- Version 1 (May 1, 2008): No description
Article tools
-
View PDF

- Download citation information
- Send a comment
- Terms of use
- Standard abbreviations
- Need help? Email the journal
Metrics
Go to
- Top
- Abstract
- Overview of WBS
- CV clinical features of WBS
- Vascular pathology
- Elastin haploinsufficiency as a cause of CV disease
- Modifying factors
- Mechanisms of obstructive vascular disease in WBS
- Current treatment
- Future therapies
- Supplemental material
- Acknowledgments
- Footnotes
- References
- Version history



Copyright © 2024 American Society for Clinical Investigation
ISSN: 0021-9738 (print), 1558-8238 (online)






