Advertisement
Research Article Free access | 10.1172/JCI35197
Islet transplantation in patients with autoimmune diabetes induces homeostatic cytokines that expand autoreactive memory T cells
Paolo Monti,1 Miriam Scirpoli,1 Paola Maffi,2 Nadia Ghidoli,1 Francesca De Taddeo,2 Federico Bertuzzi,2 Lorenzo Piemonti,1 Marika Falcone,1 Antonio Secchi,2 and Ezio Bonifacio1,3
1Immunology of Diabetes Unit and 2Clinical Transplant Unit, Telethon–Juvenile Diabetes Research Foundation Center for Beta Cell Replacement, San Raffaele Scientific Institute, Milan, Italy. 3Deutsche Forschungsgemeinschaft Research Center and Cluster of Excellence for Regenerative Therapies, Dresden University of Technology, Dresden, Germany.
Address correspondence to: Ezio Bonifacio, Center for Regenerative Therapies Dresden, Dresden University of Technology, Tatzberg 47/49, 01307 Dresden, Germany. Phone: 49-0-351-463-40092; Fax: 49-0-351-463-40090; E-mail: [email protected].
Find articles by Monti, P. in: JCI | PubMed | Google Scholar
1Immunology of Diabetes Unit and 2Clinical Transplant Unit, Telethon–Juvenile Diabetes Research Foundation Center for Beta Cell Replacement, San Raffaele Scientific Institute, Milan, Italy. 3Deutsche Forschungsgemeinschaft Research Center and Cluster of Excellence for Regenerative Therapies, Dresden University of Technology, Dresden, Germany.
Address correspondence to: Ezio Bonifacio, Center for Regenerative Therapies Dresden, Dresden University of Technology, Tatzberg 47/49, 01307 Dresden, Germany. Phone: 49-0-351-463-40092; Fax: 49-0-351-463-40090; E-mail: [email protected].
Find articles by Scirpoli, M. in: JCI | PubMed | Google Scholar
1Immunology of Diabetes Unit and 2Clinical Transplant Unit, Telethon–Juvenile Diabetes Research Foundation Center for Beta Cell Replacement, San Raffaele Scientific Institute, Milan, Italy. 3Deutsche Forschungsgemeinschaft Research Center and Cluster of Excellence for Regenerative Therapies, Dresden University of Technology, Dresden, Germany.
Address correspondence to: Ezio Bonifacio, Center for Regenerative Therapies Dresden, Dresden University of Technology, Tatzberg 47/49, 01307 Dresden, Germany. Phone: 49-0-351-463-40092; Fax: 49-0-351-463-40090; E-mail: [email protected].
Find articles by Maffi, P. in: JCI | PubMed | Google Scholar
1Immunology of Diabetes Unit and 2Clinical Transplant Unit, Telethon–Juvenile Diabetes Research Foundation Center for Beta Cell Replacement, San Raffaele Scientific Institute, Milan, Italy. 3Deutsche Forschungsgemeinschaft Research Center and Cluster of Excellence for Regenerative Therapies, Dresden University of Technology, Dresden, Germany.
Address correspondence to: Ezio Bonifacio, Center for Regenerative Therapies Dresden, Dresden University of Technology, Tatzberg 47/49, 01307 Dresden, Germany. Phone: 49-0-351-463-40092; Fax: 49-0-351-463-40090; E-mail: [email protected].
Find articles by Ghidoli, N. in: JCI | PubMed | Google Scholar
1Immunology of Diabetes Unit and 2Clinical Transplant Unit, Telethon–Juvenile Diabetes Research Foundation Center for Beta Cell Replacement, San Raffaele Scientific Institute, Milan, Italy. 3Deutsche Forschungsgemeinschaft Research Center and Cluster of Excellence for Regenerative Therapies, Dresden University of Technology, Dresden, Germany.
Address correspondence to: Ezio Bonifacio, Center for Regenerative Therapies Dresden, Dresden University of Technology, Tatzberg 47/49, 01307 Dresden, Germany. Phone: 49-0-351-463-40092; Fax: 49-0-351-463-40090; E-mail: [email protected].
Find articles by De Taddeo, F. in: JCI | PubMed | Google Scholar
1Immunology of Diabetes Unit and 2Clinical Transplant Unit, Telethon–Juvenile Diabetes Research Foundation Center for Beta Cell Replacement, San Raffaele Scientific Institute, Milan, Italy. 3Deutsche Forschungsgemeinschaft Research Center and Cluster of Excellence for Regenerative Therapies, Dresden University of Technology, Dresden, Germany.
Address correspondence to: Ezio Bonifacio, Center for Regenerative Therapies Dresden, Dresden University of Technology, Tatzberg 47/49, 01307 Dresden, Germany. Phone: 49-0-351-463-40092; Fax: 49-0-351-463-40090; E-mail: [email protected].
Find articles by Bertuzzi, F. in: JCI | PubMed | Google Scholar
1Immunology of Diabetes Unit and 2Clinical Transplant Unit, Telethon–Juvenile Diabetes Research Foundation Center for Beta Cell Replacement, San Raffaele Scientific Institute, Milan, Italy. 3Deutsche Forschungsgemeinschaft Research Center and Cluster of Excellence for Regenerative Therapies, Dresden University of Technology, Dresden, Germany.
Address correspondence to: Ezio Bonifacio, Center for Regenerative Therapies Dresden, Dresden University of Technology, Tatzberg 47/49, 01307 Dresden, Germany. Phone: 49-0-351-463-40092; Fax: 49-0-351-463-40090; E-mail: [email protected].
Find articles by Piemonti, L. in: JCI | PubMed | Google Scholar
1Immunology of Diabetes Unit and 2Clinical Transplant Unit, Telethon–Juvenile Diabetes Research Foundation Center for Beta Cell Replacement, San Raffaele Scientific Institute, Milan, Italy. 3Deutsche Forschungsgemeinschaft Research Center and Cluster of Excellence for Regenerative Therapies, Dresden University of Technology, Dresden, Germany.
Address correspondence to: Ezio Bonifacio, Center for Regenerative Therapies Dresden, Dresden University of Technology, Tatzberg 47/49, 01307 Dresden, Germany. Phone: 49-0-351-463-40092; Fax: 49-0-351-463-40090; E-mail: [email protected].
Find articles by Falcone, M. in: JCI | PubMed | Google Scholar
1Immunology of Diabetes Unit and 2Clinical Transplant Unit, Telethon–Juvenile Diabetes Research Foundation Center for Beta Cell Replacement, San Raffaele Scientific Institute, Milan, Italy. 3Deutsche Forschungsgemeinschaft Research Center and Cluster of Excellence for Regenerative Therapies, Dresden University of Technology, Dresden, Germany.
Address correspondence to: Ezio Bonifacio, Center for Regenerative Therapies Dresden, Dresden University of Technology, Tatzberg 47/49, 01307 Dresden, Germany. Phone: 49-0-351-463-40092; Fax: 49-0-351-463-40090; E-mail: [email protected].
Find articles by Secchi, A. in: JCI | PubMed | Google Scholar
1Immunology of Diabetes Unit and 2Clinical Transplant Unit, Telethon–Juvenile Diabetes Research Foundation Center for Beta Cell Replacement, San Raffaele Scientific Institute, Milan, Italy. 3Deutsche Forschungsgemeinschaft Research Center and Cluster of Excellence for Regenerative Therapies, Dresden University of Technology, Dresden, Germany.
Address correspondence to: Ezio Bonifacio, Center for Regenerative Therapies Dresden, Dresden University of Technology, Tatzberg 47/49, 01307 Dresden, Germany. Phone: 49-0-351-463-40092; Fax: 49-0-351-463-40090; E-mail: [email protected].
Find articles by Bonifacio, E. in: JCI | PubMed | Google Scholar
Published April 22, 2008 - More info
J Clin Invest. 2008;118(5):1806–1814. https://doi.org/10.1172/JCI35197.
© 2008 The American Society for Clinical Investigation
Received: January 30, 2008; Accepted: March 19, 2008
Related article:
Abstract
Islet transplantation can temporarily cure type 1 diabetes mellitus (T1DM) but requires simultaneous immunosuppression to avoid allograft rejection. In this issue of the JCI, Monti et al. report that immune conditioning via use of the Edmonton protocol — a treatment approach in which T1DM patients infused with pancreatic islets from multiple cadaveric donors simultaneously receive immunosuppressive drugs — results in lymphopenia that is associated with elevated serum levels of the homeostatic cytokines IL-7 and IL-15, which causes in vivo expansion of the autoreactive CD8+ T cell population (see the related article beginning on page 1806). Reemergence of autoreactivity is likely the main culprit underlying long-term islet graft failure, and new strategies will need to be tested to circumvent this homeostatic expansion and recurrent autoreactivity.
Authors
Tom Van Belle, Matthias von Herrath
-
Abstract
Successful transplantation requires the prevention of allograft rejection and, in the case of transplantation to treat autoimmune disease, the suppression of autoimmune responses. The standard immunosuppressive treatment regimen given to patients with autoimmune type 1 diabetes who have received an islet transplant results in the loss of T cells. In many other situations, the immune system responds to T cell loss through cytokine-dependant homeostatic proliferation of any remaining T cells. Here we show that T cell loss after islet transplantation in patients with autoimmune type 1 diabetes was associated with both increased serum concentrations of IL-7 and IL-15 and in vivo proliferation of memory CD45RO+ T cells, highly enriched in autoreactive glutamic acid decarboxylase 65–specific T cell clones. Immunosuppression with FK506 and rapamycin after transplantation resulted in a chronic homeostatic expansion of T cells, which acquired effector function after immunosuppression was removed. In contrast, the cytostatic drug mycophenolate mofetil efficiently blocked homeostatic T cell expansion. We propose that the increased production of cytokines that induce homeostatic expansion could contribute to recurrent autoimmunity in transplanted patients with autoimmune disease and that therapy that prevents the expansion of autoreactive T cells will improve the outcome of islet transplantation.
-
Introduction
Lymphocyte loss is a hallmark of T cell depletion therapy and certain infections. The immune system can sense T cell loss and responds with a vigorous cytokine-dependent expansion of the remaining T cells in the periphery, a process known as homeostatic proliferation (1). Homeostatic proliferation is largely controlled by cytokines of the common γ chain receptor family. IL-7 is required for expansion of CD4 cells (2), and expansion of CD8 cells is promoted by IL-7 and IL-15 (3, 4).
Homeostatic proliferation affects the T cell repertoire by increasing the size of clonal populations. Homeostatic proliferation of peripheral naive T cells requires the presence of specific peptide, whereas memory T cells can expand independently of T cell receptor engagement (5–7). Cells that undergo homeostatic proliferation develop properties that are remarkably similar to antigen-expanded memory cells (8, 9). As a consequence, homeostatic proliferation is suggested to promote T cell–mediated pathologies, including autoimmunity (10, 11), and to hinder tolerance induction in transplantation (12).
Islet transplantation in patients with type 1 diabetes mellitus (T1DM) is performed in the presence of a memory autoimmune response, and immunosuppression must control islet graft rejection caused by alloimmunity and autoimmunity. An increase in autoimmunity to islet autoantigens after islet transplantation has previously been observed (13, 14), and the presence of high-titer autoantibodies is associated with poor islet graft survival (15). Thus, mechanisms that expand autoreactivity can occur in the presence of a heavily compromised immune system. Studies in the autoimmune nonobese diabetic (NOD) mouse model showed that autoimmunity and diabetes are promoted by a chronic state of lymphopenia and consequent homeostatic expansion of autoreactive T cells (16). Conversely, common γ chain blockade in NOD mice substantially reduces a population of memory-like autoreactive T cells (17). We therefore asked whether mechanisms akin to homeostatic T cell proliferation are active after islet transplantation and could expand the islet-autoreactive T cell pool. We studied patients with T1DM who received islet allografts under immunosuppression composed of anti–IL-2 receptor (anti–IL-2R) mAb induction therapy followed by low-dose FK506 (tacrolimus) and rapamycin (sirolimus) maintenance therapy as described in the Edmonton protocol (18). The findings in this clinical model demonstrated that a reduction in peripheral lymphocyte count was associated with a chronic elevation of circulating IL-7 and IL-15 and in vivo T cell proliferation that led to the expansion of autoantigen-specific T cells.
-
Results
Reduced blood lymphocyte counts after islet transplantation with immunosuppression. All 13 patients who received islet allografts using the Edmonton protocol experienced a significant, immediate decrease in blood lymphocyte counts after transplant (pretransplant, mean 2,068 cells/μl; 1 d after transplant, mean 1,364 cells/μl; P < 0.0001; Figure 1A and Supplemental Figure 1; supplemental material available online with this article; doi: 10.1172/JCI35197DS1). Reductions ranged between 15% and 63% of pretransplant values (mean, 33%). Moreover, reductions were seen after each islet infusion (mean reduction after second and third infusions, 33%). Reductions in lymphocyte counts after transplant were similar in patients who received rapamycin pretreatment or the Edmonton protocol, and lymphocyte counts were unaffected during rapamycin pretreatment (data not shown). Lymphocyte counts partially recovered, but, with the exception of a few patients, did not return to pretransplant levels (6 mo after last infusion, mean 1,610 cells/μl; P = 0.04 versus pretransplant). Reduced counts after transplant were observed for CD3+, CD4+, and CD8+ lymphocytes, whereas CD19+ lymphocyte counts were minimally affected (Figure 1B).
 Figure 1
Figure 1Reduction of blood lymphocyte counts following islet transplantation. (A) Total blood lymphocyte counts (mean ± SEM) from 13 patients who received islet transplantation with anti–IL-2R induction plus rapamycin and FK506 maintenance therapy. Results are shown for the first 30 d after the first islet infusion and after the second islet infusion in patients who received 2 or more infusions (n = 11). *P < 0.05, #P < 0.01, ΧP < 0.001 versus pretransplant. (B) A single representative case (patient hSR-058-ITA-Ed08 per standard Edmonton protocol) in whom sequential counts for CD3+ T cells, CD4+ T cells, CD8+ T cells, and CD19+ B cells are shown.
In vivo proliferation of lymphocytes after islet transplantation. In order to determine whether the reduction in lymphocyte count following transplantation promoted homeostatic-like proliferation, we examined fixed freshly isolated PBMCs for the cell proliferation–associated antigen Ki-67 (Figure 2A). Between 0.01% and 0.1% of lymphocytes from normal control subjects stained positive for Ki-67. The percentage of Ki-67+ cells in patients with T1DM prior to islet transplantation (median, 0.03%; interquartile range [IQR], 0.01%–0.1%) was similar to that in control subjects. Rapamycin pretreatment, given to 4 patients, did not increase the percentage of Ki-67+ cells. After islet transplantation, the percentage of Ki-67+ cells rose significantly in all patients. The percentage of Ki-67+ cells was already significantly increased within the first weeks after transplantation (mean, 1.4%; range 0.3%–2.7%; P = 0.001 versus pretransplant) and remained elevated throughout follow-up long after cessation of anti–IL-2R treatment (mean, 1.6%; range, 0.55%–2.6%; P = 0.0001 versus pretransplant). Although CD3+ lymphocyte counts returned to pretransplant values in some patients, the percentage of Ki-67+ cells remained increased (Figure 2B) unless immunosuppression was stopped or changed (see below). In vivo proliferation after islet transplantation was confirmed by propidium iodide (PI) staining to distinguish cells in S-G2-M phase (DNA content, >1n to 2n) from cells in G0-G1 phase (DNA content, 1n; data not shown). Almost all Ki-67+ lymphocytes had surface CD3 and CD45RO, consistent with memory or memory-like T cells (Figure 2C). Both CD4+ and CD8+ cells were identified in the Ki-67+ fraction. IL-7 receptor (CD127) was detected on the majority of the Ki-67+ cells.
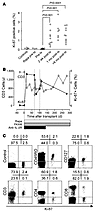 Figure 2
Figure 2Detection and characterization of in vivo proliferating T cells following transplantation. (A) Percentage of fixed peripheral blood lymphocytes expressing Ki-67, a nuclear marker of ongoing proliferation, in 5 normal nondiabetic subjects (controls), 3 patients with type 1 diabetes who received rapamycin (Rapa) as monotherapy prior to islet transplantation (tx), and 10 patients with type 1 diabetes before and after islet transplantation. In 2 of these patients, immunosuppression was stopped as a result of graft failure. Significant increases between medians are indicated. (B) A single representative case (from 10 studied) of sequential CD3+ T cell counts and the frequency of Ki-67+ lymphocytes after islet transplantation. (C) Phenotype of peripheral blood Ki-67+ cells in representative patient hSR-056-ITA-Ed07 following islet transplantation. Cells were stained for Ki-67 together with an isotype control, CD45RO, the IL-7 receptor CD127, CD3, CD4, or CD8. Numbers denote percentage of cells in the respective quadrants.
Homeostatic cytokines IL-7 and IL-15 are increased after transplantation. In antigen-driven immune responses, T cell proliferation is promoted by T cell autocrine production of IL-2. In a lymphopenic environment, the expansion of peripheral T cells is mainly driven by signaling through the common γ chain by homeostatic cytokines such as IL-7 and IL-15. IL-2, IL-7, and IL-15 were undetectable (<10 ng/l) in sera from normal control subjects and in patients with T1DM before transplant with or without rapamycin pretreatment (Figure 3). Serum IL-7 concentration was significantly increased in samples taken 2 weeks after islet transplantation in all 10 patients examined (median, 27 ng/l; IQR, 18–36 ng/l; P < 0.0001) and remained increased throughout the post-transplant period (P < 0.0001). Serum IL-15 concentration also increased after transplantation (P = 0.002). Serum IL-2 concentration was not significantly increased after islet transplantation and showed sporadic elevations in individual patients. These data suggest that the in vivo proliferation was likely to be mediated through homeostatic cytokines such as IL-7 or IL-15.
 Figure 3
Figure 3Changes in serum cytokine concentrations following islet transplantation. Increases were observed after transplantation for IL-7 and IL-15, but not IL-2. *P = 0.002, **P < 0.0001 versus pretransplant.
Rapamycin, but not mycophenolate mofetil, is permissive for homeostatic proliferation. Two patients who received islet transplantation changed from rapamycin plus FK506 to mycophenolate mofetil (MMF) plus FK506 maintenance therapy because of rapamycin intolerance during the course of follow-up (Figure 4A and Supplemental Figure 2). In both cases, the change to MMF was associated with a marked reduction in Ki-67 positivity without change in the increased IL-7 levels, which indicates that MMF did not suppress compensatory cytokine production but blocked cell proliferation. Both patients remained insulin independent for more than 2 years after islet transplantation. To provide additional support to this hypothesis, we examined Ki-67 positivity and cytokine concentrations in 3 patients who received pancreas plus kidney transplants with antithymocyte globulin induction therapy and MMF plus cyclosporine A (CyA) or FK506 maintenance therapy. In each case, and despite lymphopenia (550, 850, and 1,079 lymphocytes/μl), the percentage of Ki-67+ cells was not increased (0.01%, 0.1%, and 0.1%), whereas serum IL-7 concentrations were significantly elevated (30, 52, and 57 ng/ml; P = 0.007 versus nondiabetic control; data not shown).
 Figure 4
Figure 4Effect of immunosuppressive drugs on homeostatic lymphocyte expansion. (A) Sequential values of lymphocyte Ki-67 positivity and serum IL-7 concentrations relative to time after transplant in patient hSR-059-ITA-Ed09, whose treatment alternated between rapamycin and MMF because of rapamycin intolerance. Transfer to MMF did not affect the increased serum IL-7 concentrations, but returned the levels of proliferating Ki-67+ cells to pretransplant values. (B) In vitro proliferation of washed CFSE-labeled PBMCs before and after transplant. FACS scans of proliferation (CFSE dilution) are shown for cells stained for CD4, CD8, and CD19. Numbers denote percentage of cells in the respective quadrants. Upper left and right quadrants show cells remaining in the culture that had or had not proliferated, respectively. Data are from 1 representative case of 10 patients studied (3 pretreated with rapamycin before islet transplantation; 4 islet-transplanted per Edmonton protocol; 3 pancreas and kidney transplant). (C) In vitro culture of post-transplant washed CFSE-labeled PBMCs from patient hSR-058-ITA-Ed08 in the presence of immunosuppressive drugs demonstrated the ability of increasing doses of rapamycin, MMF, FK506, and CyA to inhibit proliferation of post-transplant PBMCs. Doses are expressed as fold of therapeutic target concentrations. Also shown are FACS histograms of CFSE dilution in the absence (Ctrl) or presence of therapeutic concentrations of rapamycin, FK506, CyA, MMF, or rapamycin and FK506 combined. The percentage of cells that had proliferated at the end of culture is indicated.
Vigorous proliferation in post-transplant lymphocytes is sensitive to MMF, but not rapamycin. Both Ki-67 positivity and serum concentrations of IL-7 returned to pretransplant levels when immunosuppression was removed from patients. We therefore cultured patient PBMCs in vitro in the absence of immunosuppression (Figure 4B). Washed PBMCs from healthy control subjects or patients with T1DM before transplantation did not proliferate in vitro. In contrast, washed PBMCs from patients on immunosuppression after islet transplantation showed vigorous in vitro proliferation in the absence of antigenic stimulation or addition of cytokines. After 7 d, 10%–80% of the total cells remaining in culture had undergone proliferation. Proliferating cells included CD4+, CD8+, and CD19+ cells. Cells that were left in culture for 3 d before labeling with CFSE did not show further proliferation (data not shown), which indicates that the in vitro proliferating cells were responsive to stimuli received in vivo, presumably homeostatic cytokines. Consistent with these data, no IL-7 was detected in supernatants of 3-d cultures.
The in vivo data on Ki-67 positivity indicated that immunosuppressive drugs varied in their ability to control lymphocyte proliferation in response to increased cytokines. In order to determine which drugs could block homeostatic proliferation, we cultured PBMCs obtained after islet transplantation in the absence and presence of increasing concentrations of different immunosuppressive drugs and quantified proliferation (Figure 4C). At therapeutic concentrations, 10 μg/ml MMF — but not 10 ng/ml rapamycin, 10 ng/ml FK506, or 100 ng/ml CyA — markedly inhibited the in vitro proliferation. Dose-dependent inhibition was observed for each drug, but only MMF inhibited proliferation by more than 50%. The combination of rapamycin plus FK506 at therapeutic concentrations was only partially effective in blocking proliferation.
Homeostatic proliferation can enrich autoreactive T cell populations. Because in vivo proliferating cells had a memory T cell phenotype (CD45RO+) and transplanted patients had an autoimmune disease, we asked whether autoreactive T cells could be preferentially expanded by homeostatic mechanisms. First, we used PE-labeled HLA-A*0201 pentamers loaded with GAD65114–122 peptide to identify glutamic acid decarboxylase 65–autoreactive (GAD65-autoreactive) CD8+ cells (19) in all 5 patients with the HLA-A*0201 allele (Figure 5A). Uncultured cells from post-transplant samples were immediately stained with anti–Ki-67 and HLA-A*0201–GAD65114–122 pentamers. In all cases, there was an enrichment of HLA-A*0201–GAD65114–122+ cells in the Ki-67+ fraction. Of the total CD8+ lymphocytes, 1.65% ± 0.25% were Ki-67+ and 0.31% ± 0.13% were HLA-A*0201–GAD65114–122+ (1 × 106 cells were acquired for pentamer quantification). The proportion of HLA-A*0201–GAD65114–122+ cells within the Ki-67+CD8+ cell fraction ranged from 2.6% (0.04/1.53; patient hSR-059-ITA-Ed09) to 8.3% (0.15/1.79; patient hSR-063-ITA-Ed06), with a median of 5.6%. In comparison, the proportion of HLA-A*0201–GAD65114–122+ cells within the Ki-67–CD8+ cell fraction ranged from 0.16% (0.16/98.54; patient hSR-064-ITA-Rp04) to 0.26% (0.27/98.47; patient hSR-059-ITA-Ed09), with a median of 0.21% (P = 0.005). Enrichment of HLA-A*0201–GAD65114–122+ cells within the Ki-67+CD8+ cell fraction was therefore 10- to 34-fold (data not shown). Virtually all HLA-A*0201–GAD65114–122+Ki-67+ cells expressed CD45RO (data not shown). CD45RO+HLA-A*0201–GAD65114–122+ cells were undetectable in nondiabetic control subjects (ref. 19 and data not shown). Enrichment was not specific for the GAD65-reactive T cells, because flu-reactive (i.e., HLA*0201–influenza A matrix protein58–66) T cells were also enriched 12- to 29-fold in this Ki-67+ fraction (data not shown). Thus it appears that homeostatic proliferation after islet transplantation preferentially expands memory T cells that include autoreactive clones in patients with T1DM.
 Figure 5
Figure 5Expansion of autoreactive T cell clones during homeostatic proliferation. (A) Direct labeling of cells from 5 patients and 1 control subject with PE-labeled HLA-A*0201–GAD65114–122 pentamers (pmr) and Ki-67 (gated on CD8+ cells). Numbers denote percentage of cells in the respective quadrants. Pentamer-positive cells were enriched in the Ki-67+ cell population. Direct labeling was also shown using HLA-A*0201–influenza A matrix protein58–66 (FLU) pentamers as a positive control and HLA-A*0201–HIV-1 gag p1776–84 pentamers as a negative control. (B) Sequential quantification of autoreactive HLA-A*0201–GAD65114–122+CD8+ T cells in patient hSR-055-ITA-Ed06, who was strongly positive for GAD autoantibodies at the time of islet transplantation. The percentage of positive cells (region denoted R3) after in vitro culture increased progressively following transplantation, as shown. The patient ceased taking immunosuppressive therapy at day 56 after transplant as a result of graft failure. (C) In vitro expansion of HLA-A*0201–GAD65114–122+CD45RO+CD8+ cells from patient hSR-055-ITA-Ed06 taken before transplant by culture with cognate peptide GAD65114–122 plus IL-7. The percentage of pentamer-positive cells in the upper right quadrant is indicated. The same experiment was also performed using cells from a nondiabetic control subject.
We subsequently selected 2 HLA-A*0201–positive patients who were also GAD65 autoantibody–positive and monitored HLA-A*0201–GAD65114–122+ cells over time (Figure 5B). Patient hSR-055-ITA-Ed06 had increasing numbers of HLA-A*0201–GAD65114–122+CD8+ T cells during follow-up, and there was further expansion of these cells as well as GAD65 autoantibodies after immunosuppression was removed because of a nonfunctioning graft at day 56 after transplantation (Figure 5B). The second patient, hSR-055-ITA-Ed09, also had increased HLA-A*0201–GAD65114–122+ cells after transplantation (pretransplant, 0.03%; after transplant, 0.26%; data not shown).
In order to determine whether IL-7 is responsible for the expansion of the autoreactive memory T cells in vivo, we cultured PBMCs depleted of CD45RO–CD8+ T cells from patient hSR-055-ITA-Ed06, obtained prior to islet transplantation, with peptide and/or 10 ng/ml IL-7 for 10 d and subsequently enumerated HLA-A*0201–GAD65114–122+CD8+ T cells (Figure 5C). Culture of cells with peptide alone resulted in a 7-fold enrichment of HLA-A*0201–GAD65114–122+CD45RO+CD8+ T cells relative to the remaining CD45RO+CD8+ T cells (0.69% versus 0.10% of cells cultured without peptide or IL-7). Addition of IL-7 further increased enrichment of the HLA-A*0201–GAD65114–122+ cells more than 2-fold (1.65%). Thus, high circulating IL-7 could expand and enrich preexisting GAD65-autoreactive memory T cells in the presence of antigen. Of interest, HLA-A*0201–GAD65114–122+ cells from patient hSR-055-ITA-Ed09 were also enriched 3-fold by IL-7 in the absence of peptide (0.32% versus 0.1% of cells cultured without peptide or IL-7). Importantly, neither IL-7 nor IL-7 plus peptide enriched HLA-A*0201–GAD65114–122+CD45RO+CD8+ T cells from control subjects, consistent with the absence of true memory GAD65-autoreactive T cells in controls.
Effector function after homeostatic proliferation can be modulated by immunosuppression. In order to determine whether the in vivo proliferating cells acquired effector T cell function, we simultaneously examined Ki-67 and intracellular expression of IFN-γ in lymphocytes from patient hSR-055-ITA-Ed06, after transplantation and during treatment with FK506 plus rapamycin (Figure 6A). IFN-γ+ cells were rare (0.05%) and were found in a minority of the Ki-67+ cell population. This indicates that the majority of in vivo proliferating cells did not acquire T effector function when patients were under immunosuppression. Removal of immunosuppression by washing cells in medium and subsequent 48-h culture of cells in medium alone resulted in both the expansion of Ki-67+ cells and the acquisition of intracellular IFN-γ staining. High concentrations of IFN-γ and lesser IL-4 were also detected in supernatants (data not shown). Thus it appeared that the in vivo proliferating cells were expanding effector memory cells, but that the rapamycin plus FK506 therapy inhibited effector function.
 Figure 6
Figure 6Effector function of proliferating cells is affected by immunosuppression. (A) IFN-γ production in cells relative to Ki-67 positivity. The percentage of cells in IFN-γ+ and Ki-67+ quadrants is shown. Freshly isolated Ki-67+ cells did not produce IFN-γ, but spontaneously became IFN-γ+ following removal of immunosuppressive drugs and 48-h in vitro culture without stimulation. The experiment was performed on cells obtained from patient hSR-055-ITA-Ed06 125 d after the first islet infusion, 55 d after the last daclizumab dose, and while receiving rapamycin and FK506 maintenance therapy. (B) Effect of immunosuppressive drugs on in vitro activation of a GAD65-specific CFSE-labeled T cell clone in the presence of the cognate peptide and IL-7. Activation was measured by proliferation (reduction of CFSE) and IFN-γ production. Numbers denote percentage of cells in the respective quadrants. Used in combination, rapamycin plus MMF blocked both proliferation and IFN-γ production.
We subsequently performed experiments on the GAD65-reactive T cell clone BRI-4.13 to determine whether IL-7 promotes T effector function in autoreactive memory T cells and which immunosuppressive drugs modulate effector function (Figure 6B). Incubation of the CFSE-labeled clone with peptide plus IL-7 induced proliferation (CSFE dilution) and marked expression of IFN-γ. Consistent with the in vivo findings, the presence of rapamycin did not block proliferation, but markedly inhibited IFN-γ production. Whereas FK506 did not affect proliferation, MMF was able to block it, and both only partially reduced IFN-γ production. Only the combination of rapamycin and MMF was effective in blocking both proliferation and IFN-γ production of the autoreactive T cell clone.
-
Discussion
The T cell repertoire, including the presence of autoreactive clones, is determined early in life by thymus-dependent pathways, but the size of clonal populations can be modified later in peripheral lymphoid compartments as a consequence of encountering antigens and by homeostatic mechanisms (1, 20). By examining in vivo and in vitro expansion of lymphocytes from autoimmune patients under immunosuppression after receiving islet allografts, we were able to identify homeostatic mechanisms that expand and enrich autoreactive memory T cells and to determine empirically how the expansion and effector function of these cells could be modulated using immunosuppression. These findings are relevant to the control of transplant rejection and autoimmunity.
Peripheral T cell expansion plays a central role in both maintenance and regeneration of T cells. Primary T cell development occurs largely via thymopoiesis, but efficient seeding of the neonatal peripheral T cell pool requires post-thymic expansion. In humans, there is marked homeostatic expansion of T cells in neonates, which declines rapidly soon after birth to negligible levels in adults (21, 22). In adults, a slow basal turnover of memory T cells is likely to continue in order to avoid attrition. Conditioning for transplantation usually involves nonspecific targeting of lymphocytes to deplete them or inhibit their function. The Edmonton islet transplantation protocol includes induction with a nondepleting anti–IL-2R. We observed an acute, moderate reduction of circulating lymphocyte numbers in all patients and at each islet infusion that partially reverted to pretransplant values soon after transplantation. Even with a relatively modest reduction in circulating lymphocyte numbers, increased lymphocyte turnover was seen. This finding indicates that there are sensitive mechanisms controlling lymphocyte homeostasis in adults in vivo.
The clinical condition in which we observed increased T cell turnover included chronic immunosuppression. Immunosuppression per se was unlikely to trigger the increased turnover, because we did not observe an increase in T cell cycling in patients who were receiving conditioning with rapamycin alone prior to transplantation. It is likely that immunosuppression impeded the return to homeostasis, thereby resulting in a chronic state of recovery that facilitated the detection of in vivo lymphocyte proliferation. Our findings are consistent with the detection of increased lymphocyte turnover in patients with immunodeficiency caused by HIV infection (22). Therefore, we propose that the human immune system, like that of the mouse, rapidly responds to lymphocyte loss in order to maintain the size of the T cell compartment.
Mechanisms of peripheral T cell expansion are supported by cytokines signaling through the common γ chain both in mice and in humans (23). The cytokines IL-7 and IL-15 play a prominent and essential role in increasing lymphocyte numbers to optimal ranges. Consistent with this, we observed elevated circulating levels of IL-7 and IL-15 after transplantation, and most cells undergoing proliferation expressed the IL-7 receptor CD127. We also observed increased serum IL-7 concentrations in conditions in which lymphocyte numbers were low but proliferation was prevented through cytostatic immunosuppression. Thus, a mechanism of cytokine release by stromal cells in response to decreased lymphocyte numbers operates in adults.
Control of cytokine-mediated homeostatic proliferation was effectively achieved by the cytostatic drug MMF. Rapamycin, FK506, and CyA hindered, but were permissive to, proliferation. Removal of the drugs in vitro resulted in proliferation of lymphocytes. In vitro proliferation was vigorous and, in the absence of stimuli, ceased within a few days. This was also the case in patients who were treated with MMF and had high circulating IL-7 concentrations, but in whom in vivo proliferation was not increased (data not shown). Islet transplantation patients who stopped immunosuppression quickly returned to a state in which no increased basal turnover of lymphocytes was observed, which suggests that the removal of immunosuppression released IL-7–stimulated cells to rapidly expand and return lymphocyte numbers to homeostasis. Thus, under conditions of lymphocyte consumption, IL-7 and IL-15 are likely to be produced by stromal cells and leukocytes and bind to receptors on lymphocytes. However, proliferation of lymphocytes can be temporarily controlled by cytostatic immunosuppressive drugs and kept in a chronic intermediate state of increased proliferation by other immunosuppressive drugs. It should be noted that our in vitro data suggest that the level of control provided by immunosuppression is likely to be dose dependent.
Evidence from mice suggests that homeostatic mechanisms could expand autoreactive T cells. In the NOD mouse model of autoimmune diabetes, islet-specific memory T cells are sensitive to a γ chain blockade that prevents diabetes (17), and homeostatic proliferation is a major pathway for expansion of autoreactive T cells (16). Hence, the model of islet transplantation in patients with autoimmune diabetes provided a unique opportunity to assess whether homeostatic proliferation expands autoreactive T cells in humans. We studied CD8+ T cells against an epitope of GAD65, previously shown to be recognized by patients with T1DM (19, 24, 25). We have previously shown that pentamers loaded with this peptide can recognize GAD65-specific T cells (19). By direct staining without in vitro priming, we found that the pentamer-positive GAD65114–122-autoreactive CD8+ cells were enriched around 30-fold in the Ki-67+ proliferating cells. We then showed that pentamer-positive GAD65114–122-autoreactive CD8+ T cells increased progressively following transplantation in a patient who was GAD65 autoantibody–positive at the time of transplantation. Finally, we showed that in vitro culture of pretransplant cells with IL-7 plus GAD65 peptide, a situation analogous to what we observed after islet transplantation, markedly expanded pentamer-positive GAD65114–122-autoreactive memory CD8+ cells. Importantly, the in vitro experiments were performed using cells that were depleted of the naive CD8+ T cell population, thus confirming that bona fide memory autoreactive T cells could be expanded by increased IL-7. This evidence indicates that the homeostatic proliferation occurring after islet transplantation under the Edmonton protocol can lead to increased numbers of autoreactive T cells. It also implies that conditions that lead to homeostatic proliferation could exacerbate autoimmunity and precipitate disease. Conditions that promote homeostatic proliferation may include certain infections previously reported to be associated with T1DM (26–28).
Expansion of autoreactive T cells does not necessarily imply that they have effector function. Indeed, after islet transplantation, proliferating T cells did not produce IFN-γ, which suggests that they were not activated. However, release from immunosuppression in vitro led to both expansion and IFN-γ production, implying that the cells were fully capable of effector function but that rapamycin and/or FK506 were able to suppress IFN-γ production. This observation is consistent with studies suggesting that rapamycin inhibits Th1 cell function, in particular IFN-γ production (29). In vitro, we found that only rapamycin inhibited IFN-γ production of a memory GAD65-autoreactive CD4+ T cell clone isolated from a patient with T1DM, which is in line with an ability of rapamycin to modulate Th1 immunity (30). Autoimmunity is a potential cause of pancreas and islet transplant failure in patients with T1DM (13–15, 31, 32). Therefore, strategies to prevent both expansion and effector function of memory autoreactive T cells are likely to improve the outcome of islet transplantation. Combining rapamycin with the cytostatic properties of MMF was extremely effective in controlling both expansion and IFN-γ production of IL-7 plus antigen-stimulated autoreactive T cells in vitro. This drug combination, previously used successfully for single donor islet transplants (33), could therefore be particularly suited for transplantation in patients with autoimmunity.
Chronic rejection resulting from alloimmune and autoimmune reaction to islet allografts is a problem for the long-term success of islet transplantation (34). Our findings in patients with T1DM indicate that T cells can expand under conditions of homeostatic proliferation. Although we have no direct evidence that alloreactive T cells may be expanded by homeostatic proliferation, a population of expanding allospecific CD4+CD25+CD45RO+CD127+ T lymphocytes has previously been described in kidney and liver transplant recipients (35). We therefore suggest that homeostatic proliferation can expand pathogenetic T cells and that such expansion could contribute to chronic or acute β cell loss, particularly if immunosuppression drug levels fall below those necessary to control effector function of expanded memory T cells. We propose that transplantation of cells that are targeted by autoimmunity in patients with autoimmune disease such as T1DM requires specific immunosuppression to avoid expansion of autoreactive T cells. Induction therapies that reduce memory T cell numbers, such as anti-CD45RB (36) or CAMPATH 1H (37), may be more appropriate than blocking IL-2–mediated proliferation. In addition, the combination of rapamycin plus MMF may prevent the expansion and activation of remaining autoreactive Th1 effector cells. Similar strategies may also be effective in controlling autoimmunity per se.
-
Methods
Patients. Thirteen patients with T1DM who had fasting C-peptide concentrations less than 0.5 ng/ml received islet allografts between 2001 and 2003. Ten patients underwent transplantation of islets alone with induction therapy of anti–IL-2R antibody (Zenapax; Roche) and maintenance therapy of rapamycin (Rapamune, 10–15 ng/ml circulating target levels; Wyeth-Ayerst) and FK506 (Prograf, 3–5 ng/ml circulating target levels; Fujizawa-Astellas Pharma) per the previously described Edmonton protocol (18). The remaining 3 patients underwent rapamycin monotherapy (10–12 ng/ml circulating target levels) for 6–27 weeks before their first islet infusion, according to the rapamycin pretreatment Edmonton protocol. Patients received between 1 and 3 islet infusions (median total islets, 777,450 islet equivalents; IQR, 456,000–839,500) and were followed for a total of 94–1,000 d. The median age of patients was 36 yr (IQR, 29.5–38.5 yr), and 6 were male. In addition, we investigated 5 control subjects, 5 T1DM patients at diabetes onset, and 3 patients with T1DM who received pancreas and kidney transplant with rabbit antithymocyte globulin induction therapy (Thymoglobuline, 75 mg over 7 d; Sangstat) — of these, 2 received FK506 (10–15 ng/ml circulating target trough levels) plus MMF (CellCept, 2 mg/d; Roche) maintenance therapy and the third received CyA (Sandimmune, 15 mg/kg/d; Novartis) plus MMF maintenance therapy. Blood was obtained by venepuncture from patients and controls. The protocols were approved by the ethical committee of the Istituto Scientifico Ospedale San Raffaele. Informed consent for the studies was obtained from all patients.
In vivo proliferation. PBMCs isolated over Ficoll-Hypaque gradients (BioWhittaker, Cambrex Bio Science) were immediately pelleted in 80% ethanol and left at 4°C overnight. The percentage of proliferating cells was determined by expression of Ki-67 and cell cycle analysis. For Ki-67 expression, ethanol-fixed PBMCs were washed twice in PBS and stained using a FITC-conjugated anti–Ki-67 antibody (clone B56; BD Biosciences — Pharmingen). The percentage of positive cells was determined by flow cytometry. Phenotypic analysis of proliferating cells was performed using the following fluorochrome-conjugated mAbs from BD Biosciences — Pharmingen: anti-CD3–PE (HIT-3a), anti-CD4–PerCP (SK3), anti-CD8–PerCP (RPA-T8), anti-CD45RO (UCHL1), and anti-CD127 (HIL-7R-MR1). For cell cycle analysis, ethanol-fixed PBMCs were washed twice in PBS and then incubated for 30 min in PBS containing 10 μg/ml PI and 50 μg/ml RNase A (Sigma-Aldrich). PI content was assessed by flow cytometry. Cell debris and doublets were excluded on the basis of side versus forward scatter.
In vitro proliferation. In vitro proliferation was determined using the CFSE (Invitrogen) dilution assay as previously described (38). PBMCs were isolated and washed twice in PBS. PBMCs at 1 × 107 cells/ml in PBS were incubated at 37°C for 5 min with 0.2 μM CFSE. Staining was stopped by adding RPMI 1640 containing 5% pooled human serum (Sigma-Aldrich) at 4°C, followed by 1 wash in PBS. Stained cells were resuspended at 1 × 106 cells/ml in RPMI 1640 supplemented with 5% pooled human serum (Sigma-Aldrich), 2 mM glutamine, 100 U/ml penicillin, and 100 U/ml streptomycin. Cells were seeded at 2 × 105 cells/well in 96-well round-bottomed plates, incubated for 5 d, harvested, and labeled with the following mAbs from BD Biosciences — Pharmingen: anti-CD4–PerCP (SK3), anti-CD8–PerCP (RPA-T8), or anti-CD19–PE (SJ25C1). Cell division analysis was performed on a BD Biosciences FACScan flow cytometer, and WinMDI software was used for data analysis. Experiments were also performed in the presence of rapamycin at 1, 10, and 100 ng/ml (Sigma-Aldrich), FK506 at 1, 10, and 100 ng/ml (Calbiochem-Merck), MMF at 1, 10, and 100 μg/ml (Roche Laboratories), or CyA at 1, 10, and 100 μg/ml (Sandoz Pharma).
Cytokine measurements. Cytokines were measured in serum samples (50 μl) or supernatants (50 μl) from cell cultures. IL-7 and IL-15 were quantified by the Duoset ELISA system (R&D Systems), and IL-2 was quantified using the Endogen kit (Pierce Biotechnology) according to the manufacturers’ instructions. Detection ranges were between 10 and 2,000 pg/ml.
Pentamer staining and IFN-γ intracellular staining. HLA-A*0201 PE-labeled pentamers loaded with GAD65114–122 peptide (VMNILLQYV), influenza A matrix protein58–66 (GILGFVFTL), or HIV-1 gag p1776–84 (SLYNTVATL) were purchased from ProImmune, and cells were stained directly with the pentamers. Cells (5 × 106) were fixed in cold ethanol, stained with anti–Ki-67–FITC, washed in PBS, and stained with PE-labeled HLA-A*0201–GAD65114–122 pentamers, anti-CD8–PerCP, and anti-CD45RO–APC. At least 1 × 106 cells were acquired and examined by flow cytometry. Production of IFN-γ was measured by intracellular staining using a commercial kit (BD Biosciences — Pharmingen) followed by FACS analysis.
In vitro expansion of autoreactive T cells. In order to study expansion of memory CD8+ T cells, PBMCs were depleted of CD45RO–CD8+ naive cells using magnetic beads (Miltenyi Biotech) according to the manufacturer’s instructions. CD8+ cells were separated by negative selection and further divided into CD45RO+ and CD45RO– fractions. The CD8– fraction containing monocytes and B cells for optimal antigen presentation was also collected. CD45RO+CD8+ cells were mixed at a 1:1 ratio with the CD8– fraction and cultured for 7 d in the presence of 2 μg/ml GAD65114–122 peptide with or without 10 ng/ml IL-7. Cells were harvested and stained with pentamers at 4°C for 1 h. To test the expansion of autoreactive T cells in the presence of immunosuppressive drugs we used BRI-4.13 CD4 T cell clone specific for GAD65555–567 peptide (provided by G.T. Nepom, Benaroya Research Institute at Virginia Mason, Seattle, Washington, USA). T cell clone labeled with CFSE was cultured for 7 d in the presence of 2 μg/ml peptide and 10 ng/ml IL-7 in the presence of rapamycin, FK506, and/or CyA. Cells were than harvested, stained for IFN-γ, and analyzed by FACS.
Statistics. The paired t test was used to compare lymphocyte counts at different time points. The Mann-Whitney U test was used to compare the proportion of Ki-67+ cells and cytokine levels between groups. For all analyses, a 2-tailed P value of 0.05 was considered significant. Statistical analyses were performed using the Statistical Package for Social Science (SPSS version 11.0).
-
Acknowledgments
This work was supported by grants from Telethon Italy and the Juvenile Diabetes Research Foundation (JT-01) and the Italian Ministry of Health (RF2002 no. 198 and RF2004 no. 123). P. Monti is a fellow of the Vita e Salute San Raffaele University PhD Programme in Molecular Medicine. The authors thank Ennio La Rocca for providing samples from patients who received pancreas transplants and Polly Bingley for critical reading of the manuscript.
Address correspondence to: Ezio Bonifacio, Center for Regenerative Therapies Dresden, Dresden University of Technology, Tatzberg 47/49, 01307 Dresden, Germany. Phone: 49-0-351-463-40092; Fax: 49-0-351-463-40090; E-mail: [email protected].
-
Footnotes
Nonstandard abbreviations used: CyA, cyclosporine A; GAD, glutamic acid decarboxylase; IL-2R, IL-2 receptor; IQR, interquartile range; MMF, mycophenolate mofetil; NOD, nonobese diabetic; T1DM, type 1 diabetes mellitus.
Conflict of interest: The authors have declared that no conflict of interest exists.
Reference information: J. Clin. Invest.118:1806–1814 (2008). doi:10.1172/JCI35197
See the related article at Immunosuppression in islet transplantation.
-
References
- Prlic, M., Jameson, S.C. 2002. Homeostatic expansion versus antigen-driven proliferation: common ends by different means? Microbes Infect. 4:531-537.
- Kondrack, R.M., et al. 2003. Interleukin 7 regulates the survival and generation of memory CD4 cells. J. Exp. Med. 198:1797-1806.
- Schluns, K.S., Kieper, W.C., Jameson, S.C., Lefrancois, L. 2000. Interleukin-7 mediates the homeostasis of naive and memory CD8 T cells in vivo. Nat. Immunol. 1:426-432.
- Zhang, X., Sun, S., Hwang, I., Tough, D.F., Sprent, J. 1998. Potent and selective stimulation of memory-phenotype CD8+ T cells in vivo by IL-15. Immunity. 8:591-599.
- Ernst, B., Lee, D.S., Chang, J.M., Sprent, J., Surh, C.D. 1999. The peptide ligands mediating positive selection in the thymus control T cell survival and homeostatic proliferation in the periphery. Immunity. 11:173-181.
- Murali-Krishna, K., et al. 1999. Persistence of memory CD8 T cells in MHC class I-deficient mice. Science. 286:1377-1381.
- Swain, S.L., Hu, H., Huston, G. 1999. Class II-independent generation of CD4 memory T cells from effectors. Science. 286:1381-1383.
- Gudmundsdottir, H., Turka, L.A. 2001. A closer look at homeostatic proliferation of CD4+ T cells: costimulatory requirements and role in memory formation. J. Immunol. 167:3699-3707. View this article via: PubMed Google Scholar
- Geginat, J., Lanzavecchia, A., Sallusto, F. 2003. Proliferation and differentiation potential of human CD8+ memory T-cell subsets in response to antigen or homeostatic cytokines. Blood. 101:4260-4266.
- Baccala, R., Theofilopoulos, A.N. 2005. The new paradigm of T-cell homeostatic proliferation-induced autoimmunity. Trends Immunol. 26:5-8.
- Khoruts, A., Fraser, J.M. 2005. A causal link between lymphopenia and autoimmunity. Immunol. Lett. 98:23-31.
- Taylor, D.K., Neujahr, D., Turka, L.A. 2004. Heterologous immunity and homeostatic proliferation as barriers to tolerance. Curr. Opin. Immunol. 16:558-564.
- Braghi, S., et al. 2000. Modulation of humoral islet autoimmunity by pancreas allotransplantation influences allograft outcome in patients with type 1 diabetes. Diabetes. 49:218-224.
- Roep, B.O., et al. 1999. Auto- and alloimmune reactivity to human islet allografts transplanted into type 1 diabetic patients. Diabetes. 48:484-490.
- Bosi, E., et al. 2001. Autoantibody response to islet transplantation in type 1 diabetes. Diabetes. 50:2464-2471.
- King, C., Ilic, A., Koelsch, K., Sarvetnick, N. 2004. Homeostatic expansion of T cells during immune insufficiency generates autoimmunity. Cell. 117:265-277.
- Demirci, G., Strom, T.B., Li, X.C. 2003. Islet allograft rejection in nonobese diabetic mice involves the common gamma-chain and CD28/CD154-dependent and -independent mechanisms. J. Immunol. 171:3878-3885. View this article via: PubMed Google Scholar
- Shapiro, A.M., et al. 2000. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N. Engl. J. Med. 343:230-238.
- Monti, P., et al. 2007. Evidence for in vivo primed and expanded autoreactive T cells as a specific feature of patients with type 1 diabetes. J. Immunol. 179:5785-5792. View this article via: PubMed Google Scholar
- Goldrath, A.W., Bevan, M.J. 1999. Selecting and maintaining a diverse T-cell repertoire. Nature. 402:255-262.
- Schonland, S.O., et al. 2003. Homeostatic control of T-cell generation in neonates. Blood. 102:1428-1434.
- Szabolcs, P., et al. 2003. Coexistent naive phenotype and higher cycling rate of cord blood T cells as compared to adult peripheral blood. Exp. Hematol. 31:708-714.
- Boyman, O., Purton, J.F., Surh, C.D., Sprent, J. 2007. Cytokines and T-cell homeostasis. Curr. Opin. Immunol. 19:320-326.
- Panina-Bordignon, P., et al. 1995. Cytotoxic T cells specific for glutamic acid decarboxylase in autoimmune diabetes. J. Exp. Med. 181:1923-1927.
- Mallone, R., et al. 2007. CD8+ T-cell responses identify beta-cell autoimmunity in human type 1 diabetes. Diabetes. 56:613-621.
- Peacock, C.D., Kim, S.K., Welsh, R.M. 2003. Attrition of virus-specific memory CD8+ T cells during reconstitution of lymphopenic environments. J. Immunol. 171:655-663. View this article via: PubMed Google Scholar
- Permar, S.R., et al. 2003. Increased thymic output during acute measles virus infection. J. Virol. 77:7872-7879.
- Hernan, M.A., Zhang, S.M., Lipworth, L., Olek, M.J., Ascherio, A. 2001. Multiple sclerosis and age at infection with common viruses. Epidemiology. 12:301-306.
- Chiang, P.H., et al. 2002. Inhibition of IL-12 signaling Stat4/IFN-gamma pathway by rapamycin is associated with impaired dendritic [correction of dendritc] cell function. Transplant Proc. 34:1394-1395.
- Kusaba, H., et al. 2005. Interleukin-12-induced interferon-gamma production by human peripheral blood T cells is regulated by mammalian target of rapamycin (mTOR). J. Biol. Chem. 280:1037-1043.
- Wong, C.P., Li, L., Frelinger, J.A., Tisch, R. 2006. Early autoimmune destruction of islet grafts is associated with a restricted repertoire of IGRP-specific CD8+ T cells in diabetic nonobese diabetic mice. J. Immunol. 176:1637-1644. View this article via: PubMed Google Scholar
- Shi, Q., et al. 2004. Long-term islet graft survival in NOD mice by abrogation of recurrent autoimmunity. Diabetes. 53:2338-2345.
- Hering, B.J. 2005. Achieving and maintaining insulin independence in human islet transplant recipients. Transplantation. 79:1296-1297.
- Shapiro, A.M., et al. 2005. Strategic opportunities in clinical islet transplantation. Transplantation. 79:1304-1307.
- Codarri, L., et al. 2007. Expansion and tissue infiltration of an allospecific CD4+CD25+CD45RO+IL-7Ralphahigh cell population in solid organ transplant recipients. J. Exp. Med. 204:1533-1541.
- Gregori, S., et al. 2005. An anti-CD45RO/RB monoclonal antibody modulates T cell responses via induction of apoptosis and generation of regulatory T cells. J. Exp. Med. 201:1293-1305.
- Kirk, A.D., et al. 2003. Results from a human renal allograft tolerance trial evaluating the humanized CD52-specific monoclonal antibody alemtuzumab (CAMPATH-1H). Transplantation. 76:120-129.
- Lyons, A.B. 2000. Analysing cell division in vivo and in vitro using flow cytometric measurement of CFSE dye dilution. J. Immunol. Methods. 243:147-154.
-
Version history
- Version 1 (April 22, 2008): No description
- Version 2 (May 1, 2008): No description



Copyright © 2024 American Society for Clinical Investigation
ISSN: 0021-9738 (print), 1558-8238 (online)