Abstract
The ability to determine the prognosis of lean nonalcoholic fatty liver disease (NAFLD) is essential for decision making in clinical settings. Using a large community‐based Chinese cohort, we aimed to investigate NAFLD outcomes by body mass index (BMI). We used the restricted cubic splines method to investigate the dose–response relationship between BMI and outcomes in subjects with NAFLD and those without NAFLD. We included 73,907 subjects from the Kailuan cohort and grouped all subjects into four phenotypes by using NAFLD and BMI (<23 kg/m2). The probability of developing outcomes for individuals with lean NAFLD (LN), overweight/obese NAFLD (ON), overweight/obese non‐NAFLD (ONN), and lean non‐NAFLD (LNN) was estimated. We found a U‐shaped association between BMI and death but a linear positive association concerning cardiovascular disease (CVD) after adjusting for age and other covariates. Compared with the LNN group, the adjusted hazard ratios (HRs) and 95% confidence intervals (CIs) of the LN, ON, and ONN groups were 1.30 (1.14–1.49), 0.86 (0.80–0.91), 0.84 (0.80–0.89) for all‐cause death, 2.61 (1.13–6.03), 0.74 (0.44–1.26), 1.10 (0.70–1.74) for liver‐related death, 2.12 (1.46–3.08), 1.23 (0.99–1.54), 1.19 (0.98–1.43) for digestive system cancers, and 2.04 (1.40–2.96), 1.30 (1.05–1.61), 1.21 (1.01–1.46) for obesity‐related cancers. Subjects with LN had a significantly higher risk of colorectal cancer and esophagus cancer. However, the ON group had the highest CVD risk (HR, 1.39; 95% CI, 1.27–1.52). The LN group with hypertension had a higher risk of adverse outcomes, and those without hypertension had a similar risk compared to LNN. Conclusion: Subjects with LN may experience a higher risk of all‐cause death, digestive system cancers, and obesity‐related cancers than the other three groups but a lower risk of CVD than ON subjects. LN with hypertension may be a high‐risk phenotype.
INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD) is one of the most common liver diseases characterized by excessive hepatic fat accumulation and affects 25%–45% of the global population.[ 1 ] NAFLD has been reported to increase intrahepatic and extrahepatic disease risks, including cardiovascular disease (CVD), chronic kidney disease, and some specific cancers.[ 2 , 3 ] Obesity is one of the significant risk factors for NAFLD[ 1 ]; however, there is still a proportion of lean NAFLD (LN), defined as NAFLD with a normal body mass index (BMI) < 25 kg/m2 in non‐Asians or <23 kg/m2 in Asians. A meta‐analysis published in 2020 demonstrated that within the NAFLD population, 19.2% (95% confidence interval [CI], 15.9%–23.0%) of people had LN.[ 4 ]
Obesity is also a significant risk factor for all‐cause mortality,[ 5 ] CVD,[ 6 ] and many cancers.[ 7 ] However, some studies have emerged challenging this by demonstrating that overweight and obese states are associated with improved survival, known as the obesity paradox.[ 8 , 9 ] Limited data exist concerning the association between combinations of BMI and NAFLD with adverse outcomes. Further, due to limited sample size, different methods to diagnose NAFLD or obesity, diverse comparison groups, and single‐center design, the consequences of LN are controversial, even in the same ethnic group.[ 10 , 11 , 12 , 13 , 14 , 15 , 16 ] Some cohort studies have shown that nonobese NAFLD or individuals with LN have a higher risk of all‐cause mortality,[ 14 , 15 , 17 ] liver‐related mortality,[ 16 ] CVD,[ 10 ] and severe liver disease[ 16 ] than patients with NAFLD with a higher BMI; however, one study did not observe a significant difference in liver‐related diseases and all‐cause mortality.[ 13 ] Furthermore, the risk of NAFLD exhibits large interindividual variability[ 18 ] as does LN.[ 19 ] The subtype of LN with a higher risk remains unclear.
Therefore, in a large community‐based Chinese cohort, we aimed to investigate the dose–response relationship between BMI and outcomes, including all‐cause mortality, liver‐related mortality, CVD, and cancers, as well as NAFLD outcomes by BMI and the high‐risk phenotype in subjects with LN.
METHODS
Study design and participants
The Kailuan cohort (Chinese Clinical Trial registry number: ChiCTR‐TNRC‐11001489) is a prospective cohort established in the Kailuan community in Tangshan, Hebei Province, China, to explore risk factors for noncommunicable diseases in the Chinese population. We enrolled 101,510 participants (81,110 men and 20,400 women, aged 18–98 years) who completed a baseline survey from 2006 to 2007. In addition, we excluded subjects with (1) hepatitis B surface antigen‐positive or missing data (n = 5170); (2) excessive alcohol consumption (>30 g ethanol/day for men and >20 g ethanol/day for women) or missing data (n = 21,196); (3) liver cirrhosis (n = 85); (4) malignancy history (n = 292); (5) ultrasound examination or BMI data missing (n = 838); (6) implausible or extreme values of BMI and weight (BMI < 15.0 kg/m2 or >50.0 kg/m2 or weight < 30 kg) (n = 22). Finally, 73,907 subjects were enrolled (Figure S1). We performed this study according to the tenets of the Declaration of Helsinki and obtained approval from the Ethics Committee of the Kailuan General Hospital and the Institute of Basic Medicine Sciences Chinese Academy of Medical Sciences. In addition, we obtained written informed consent from all participants.
Definition of NAFLD
NAFLD was diagnosed based on hepatic steatosis on abdominal ultrasonography scan (HD‐15; Philips, the Netherlands) and absence of secondary causes of liver‐fat accumulation according to the American Association for the Study of Liver Diseases Practice Guidance.[ 20 ] As we have excluded secondary causes of liver‐fat accumulation, including hepatitis B virus infection and excessive alcohol consumption, in the study population, participants who met the criteria of fatty liver were considered to have NAFLD.
Outcome variables
The outcomes included all‐cause and liver‐related mortality, CVD, and digestive system and obesity‐related cancers. Liver‐related mortality was defined as death associated with chronic liver disease and cirrhosis, viral hepatitis, hepatobiliary malignancies, and expanded liver diagnosis, including esophageal varices, hepatic failure, toxic liver disease, other inflammatory liver diseases, and other diseases of the liver[ 21 ]; CVD included myocardial infarction, ischemic stroke, and hemorrhagic stroke; digestive system cancers included colorectal, liver, pancreatic, gastric, biliary, small intestine, and esophageal cancer; obesity‐related cancers included colorectal, liver, pancreatic, gastric, biliary, kidney, and esophageal cancer.[ 7 , 22 ] We gathered death information from provincial vital statistics offices in Hebei province, China. Cancer and CVD cases were identified by self‐reported information through questionnaires or by annual linkage with the local vital statistics data, the Tangshan medical insurance system, or the Kailuan Social Security Information System. An expert panel then reconfirmed the diagnosis by checking discharge summaries from the hospitals where participants were diagnosed. Clinical experts validated cancer diagnosis through a thorough review of medical records, including pathological, imaging, and blood biomarker findings. The diagnosis of myocardial infarction was determined by the patient's clinical symptoms, electrocardiogram, and dynamic myocardial enzyme changes following the World Health Organization's Multinational Monitoring of Trends and Determinants in Cardiovascular Disease criteria. Stroke was diagnosed based on neurologic signs, clinical symptoms, and neuroimaging tests, including computed tomographic or magnetic resonance imaging, in line with the World Health Organization criteria.[ 23 ] In addition, according to the International Classification of Diseases, 10th revision, we coded and validated cancer, CVD, and liver‐related death cases by qualified coders (Table S1). Follow‐up ended at the date of death, CVD, cancer diagnosis, or information collection termination of death (December 31, 2019), cancer (December 31, 2017), and CVD (December 31, 2019). Death causes were collected before December 31, 2016, so follow‐up ended at the date of death or information collection termination of death (December 31, 2016) when analyzing the risk of liver‐related death.
Covariates at baseline
We conducted questionnaire surveys to collect sociodemographic characteristics (age, sex, education level), lifestyle factors (smoking, alcohol intake, physical activity), and medical history. Alcohol consumption >0 g ethanol/day was defined as drinkers. We measured each subject's height and weight and calculated BMI using weight divided by height squared. Waist circumference was measured, and central obesity was defined as waist circumference ≥90 cm for men and ≥85 cm for women. Hypertension was defined as systolic blood pressure ≥140 mm Hg or diastolic blood pressure ≥90 mm Hg, medication history, or self‐reported hypertension history. In addition, we measured the serum glucose, total cholesterol, triglycerides (TGs), low‐density lipoprotein (LDL), high‐density lipoprotein (HDL), alanine aminotransferase (ALT), and C‐reactive protein (CRP) of each subject by using an auto‐analyzer (Hitachi 747; Hitachi, Tokyo, Japan) at the central laboratory of Kailuan General Hospital. We defined diabetes as fasting blood glucose concentration ≥7.0 mmol/L, history of medication, or self‐reported history. The enzyme‐linked immunosorbent assay was applied to test hepatitis B surface antigen (Shanghai Kehua Bio‐Engineering).
Statistical analyses
Characteristics are presented as median (interquartile range [IQR]) or mean ± SD for continuous variables and number (percentage) for categorical variables. We compared baseline characteristics among different groups by analysis of variance or Kruskal‐Wallis test for continuous variables and the Pearson chi‐square test for categorical variables.
We used the restricted cubic spline method to evaluate whether BMI ≥ 23.0 kg/m2, recommended for the diagnosis of Asian adults who are overweight,[ 24 ] was suitable for classification when exploring the outcomes for NAFLD by different BMI states in the Chinese population. We used the multivariable‐adjusted restricted cubic splines models, which were adjusted for sex, age groups, education level, smoking status, alcohol drinking status, physical activity, diabetes, hypertension, central obesity, TG, LDL, HDL, CRP, and ALT, to investigate the dose–response relationship between BMI and outcomes, with three knots, representing the fifth, 50th, and 95th percentiles of BMI, and BMI of 23.0 kg/m2 as a reference point. We categorized the subjects into four groups: subjects with LN (NAFLD with BMI < 23 kg/m2), subjects with overweight or obese NAFLD (ON; NAFLD with BMI ≥ 23 kg/m2), subjects who were lean but without NAFLD (LNN), and subjects who were overweight or obese without NAFLD (ONN).
We used Kaplan‐Meier analysis to estimate the cumulative incidence of events and compared the incidence difference by log‐rank test. Compared to the LNN group, we used the traditional Cox regression model to estimate hazard ratios (HRs) and 95% CIs of death for the LN, ON, and ONN groups. The Fine and Gray models were used to estimate the risk difference in CVD, digestive system cancers, obesity‐related cancers, site‐specific cancers, and liver‐related death between LNN and the other three groups by adjusting the competing risk of death. We conducted three models: model 1 adjusted for age and sex, model 2 further adjusted for education level, smoking status, alcohol drinking status, and physical activity; and model 3 further adjusted for diabetes, hypertension, central obesity, TG, LDL, HDL, CRP, and ALT. Pairwise comparisons were also conducted. We also performed subgroup analyses by dividing patients with LN into subgroups according to the differential characteristic between those who developed adverse outcomes and those who did not.
To test potential interaction, we conducted stratified analysis by age, sex, diabetes, hypertension, smoking, drinking, and central obesity. In addition, sensitivity analyses were performed to assess the robustness of the results by excluding participants who died, had incident CVD, and developed cancers within the 3 years of follow‐up. We also excluded participants with a history of stroke, ischemic heart disease, diabetes, or smoking at baseline to minimize the potential reverse causation due to the effects of preexisting conditions or smoking on baseline BMI. All data were analyzed using SAS software, version 9.4 (SAS Institute, Cary, NC), with two‐sided tests and p < 0.05 as statistically significant.
RESULTS
Baseline characteristics of the cohort
Our final cohort included 73,907 subjects (Figure S1). Baseline characteristics of study participants are summarized in Table 1. The prevalence of NAFLD was 31.4%. Participants with LN were the oldest among the four groups. The prevalence of hypertension and diabetes and diastolic blood pressure, systolic blood pressure, fasting blood glucose, total cholesterol, TG, CRP, and ALT were highest in subjects with ON and lowest in non‐NAFLD groups.
TABLE 1.
Baseline characteristics of study participants
| LNN (n = 19,605) | ONN (n = 31,105) | LN (n = 1543) | ON (n = 21,654) | p value | |
|---|---|---|---|---|---|
| Age, years | 50.6 ± 14.3 | 51.9 ± 12.4 | 53.6 ± 11.4 | 52.8 ± 11.5 | <0.001 |
| Male sex | 13,592 (69.3) | 24,165 (77.7) | 1158 (75.0) | 16,888 (78.0) | <0.001 |
| Education level | <0.001 | ||||
| Junior high school or below | 15,081 (77.0) | 25,588 (82.3) | 1272 (82.5) | 17,702 (81.8) | |
| Senior high school or higher | 4500 (23.0) | 5495 (17.7) | 269 (17.5) | 3940 (18.2) | |
| Current smoker | 5069 (25.9) | 7924 (25.5) | 399 (25.9) | 5554 (25.7) | 0.8272 |
| Drinker | 3996 (20.4) | 6776 (21.8) | 351 (22.7) | 4983 (23.0) | <0.001 |
| Physical activity | <0.001 | ||||
| Inactive | 1513 (7.7) | 2309 (7.4) | 104 (6.8) | 1483 (6.9) | |
| Moderately active | 15,311 (78.2) | 23,903 (77.0) | 1246 (80.9) | 16,954 (78.4) | |
| Active | 2754 (14.1) | 4841 (15.6) | 190 (12.3) | 3186 (14.7) | |
| SBP, mm Hg | 123.0 ± 19.5 | 131.2 ± 20.7 | 132.4 ± 20.5 | 137.8 ± 21.1 | <0.001 |
| DBP, mm Hg | 78.6 ± 10.7 | 83.6 ± 11.3 | 83.8 ± 11.1 | 87.5 ± 11.8 | <0.001 |
| WC, cm | 79.1 ± 8.9 | 87.7 ± 8.5 | 83.4 ± 8.2 | 92.8 ± 8.7 | <0.001 |
| TG, mmol/L | 1.0 (0.7–1.3) | 1.2 (0.9–1.8) | 1.5 (1.1–2.3) | 1.8 (1.2–2.7) | <0.001 |
| TC, mmol/L | 4.8 (4.2–5.4) | 4.9 (4.3–5.5) | 5.1 (4.4–5.7) | 5.1 (4.4–5.8) | <0.001 |
| LDL‐C, mmol/L | 2.2 (1.7–2.7) | 2.4 (1.9–2.9) | 2.2 (1.7–2.7) | 2.4 (1.9–2.9) | <0.001 |
| HDL‐C, mmol/L | 1.6 (1.3–1.8) | 1.5 (1.3–1.7) | 1.6 (1.4–1.9) | 1.5 (1.3–1.7) | <0.001 |
| FBG, mmol/L | 5.0 (4.5–5.4) | 5.1 (4.7–5.6) | 5.2 (4.7–6.0) | 5.3 (4.8–6.2) | <0.001 |
| CRP, mg/L | 0.5 (0.2–1.5) | 0.8 (0.3–2.0) | 0.9 (0.3–2.6) | 1.2 (0.5–3.0) | <0.001 |
| ALT, U/L | 17.2 ± 19.4 | 19.1 ± 13.5 | 23.1 ± 17.4 | 25.8 ± 20.1 | <0.001 |
| Hypertension | 5383 (27.5) | 13,761 (44.2) | 721 (46.7) | 13,082 (60.4) | <0.001 |
| Diabetes | 982 (5.0) | 2337 (7.5) | 221 (14.3) | 3688 (17.0) | <0.001 |
Note: Data are presented as mean ± SD, median (interquartile range), or the number (%) of participants with a condition. Missing numbers for education level, smoking, physical activity, SBP, DBP, WC, TG, LDL‐C, HDL‐C, FBG, CRP, and ALT data were 60, 52, 113, 291, 291, 214, 75, 128, 55, 74, 531, and 164.
Abbreviations: ALT, alanine transaminase; CRP, C‐reactive protein; DBP, diastolic blood pressure; FBG, fasting blood glucose; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; LN, lean NAFLD; LNN, lean non‐NAFLD; NAFLD, nonalcoholic fatty liver disease; ON, overweight/obese NAFLD; ONN, overweight/obese non‐NAFLD; SBP, systolic blood pressure; TC, total cholesterol; TG, triglyceride.
Dose–response relationship between BMI and risk of death, CVD, and cancers for subjects with and without NAFLD
Using multivariable‐adjusted cubic spline analyses, we found a U‐shaped association between BMI and all‐cause death in subjects with NAFLD (P overall < 0.001, P nonlinear < 0.001; Figure 1A1) and without NAFLD (P overall < 0.001, P nonlinear < 0.001; Figure 1A2), with higher mortality risks in the subjects with lower or higher BMI. Similar trends were also found in liver‐related death (Figure 1B).
FIGURE 1.
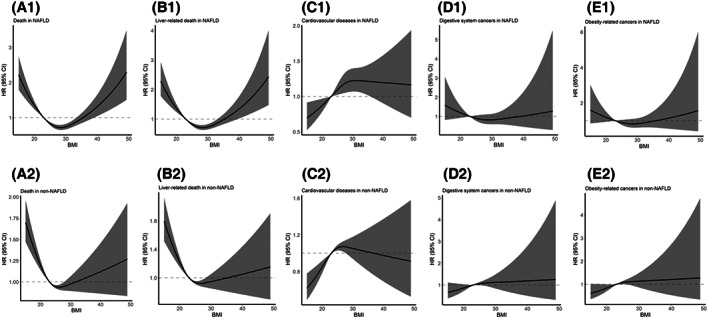
Association of BMI with all‐cause death, liver‐related death, cardiovascular disease, and cancers for the subjects with and without NAFLD in restricted cubic spline models. Multivariable adjusted HRs with 95% CI for the association of body mass index with (A) all‐cause death, (B) liver‐related death, (C) cardiovascular disease, (D) digestive system cancers, and (E) obesity‐related cancers in subjects (1) with NAFLD and (2) without NAFLD. The models were adjusted for sex, age groups, education level, smoking status, alcohol drinking status, physical activity, diabetes, hypertension, central obesity, triglycerides, low‐density lipoprotein, high‐density lipoprotein, C‐reactive protein, and alanine transaminase. For all‐cause death: in subjects with NAFLD, overall association χ 2 = 57.9, p < 0.001 and nonlinear association χ 2 = 55.5, p < 0.001; in subjects without NAFLD, overall association χ 2 = 55.5, p < 0.001 and nonlinear association χ 2 = 26.7, p < 0.001. For liver‐related death: in subjects with NAFLD, overall association χ 2 = 45.4, p < 0.001 and nonlinear association χ 2 = 43.7, p < 0.001; in subjects without NAFLD, overall association χ 2 = 51.4, p < 0.001 and nonlinear association χ 2 = 20.7, p < 0.001. For cardiovascular disease: in subjects with NAFLD, overall association χ 2 = 8.78, p = 0.012 and nonlinear association χ 2 = 3.16, p = 0.08; in subjects without NAFLD, overall association χ 2 = 19.02, p < 0.001 and nonlinear association χ 2 = 8.71, p = 0.003. For digestive system cancers: in subjects with NAFLD, overall association χ 2 = 1.86, p = 0.39 and nonlinear association χ 2 = 1.33, p = 0.25; in subjects without NAFLD, overall association χ 2 = 3.56, p = 0.17 and nonlinear association χ 2 = 0.72, p = 0.39. For obesity‐related cancers: in subjects with NAFLD, overall association χ 2 = 2.17, p = 0.34 and nonlinear association χ 2 = 1.97, p = 0.16; in subjects without NAFLD, overall association χ 2 = 5.23, p = 0.07 and nonlinear association χ 2 = 1.15, p = 0.28. BMI, body mass index; CI, confidence interval; HR, hazard ratio; NAFLD, nonalcoholic fatty liver disease.
The relationship between BMI and CVD differed between subjects with and without NAFLD. CVD risk presented a significant linear association in the NAFLD group (P overall = 0.012, P linear = 0.013; Figure 1C1), with the lowest risk in those with BMI < 23 kg/m2; however, CVD risk showed a rapid increase first and then a slightly decreasing trend in the non‐NAFLD group (P overall < 0.001, P nonlinear = 0.003; Figure 1C2).
Although results for digestive system cancers (Figure 1D) and obesity‐related cancers (Figure 1E) were not significant, a slightly increased risk in subjects with BMI < 23 kg/m2 in NAFLD was observed (P overall > 0.05, P nonlinear > 0.05).
Association between LN and ON with death
Over a median follow‐up of 13.1 (IQR, 12.8–13.2) years, 8913 deaths occurred. The mortality rate in the LN group was higher than in the other three groups (log‐rank test, p < 0.001; Figure 2A; Table S2).
FIGURE 2.
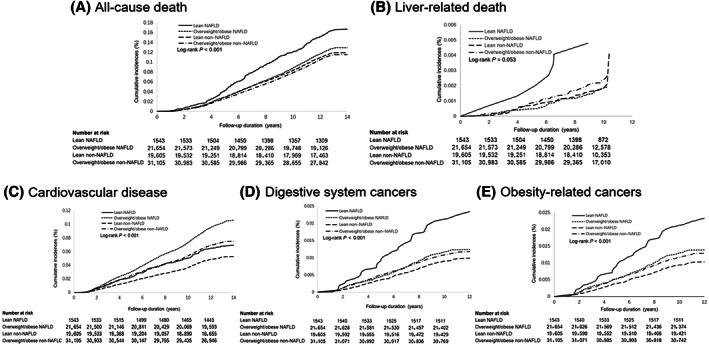
Cumulative incidence of all‐cause death, liver‐related death, cardiovascular disease, digestive system cancers, and obesity‐related cancers for subjects with different NAFLD and body mass index. Outcomes show (A) all‐cause death, (B) liver‐related death, (C) cardiovascular disease, (D) digestive system cancers, and (E) obesity‐related cancers. NAFLD: nonalcoholic fatty liver disease.
Using the LNN group as reference, LN showed a significantly higher risk of death (adjusted HR, 1.30; 95% CI, 1.14–1.49) whereas the ON (adjusted HR, 0.86; 95% CI, 0.80–0.91) and ONN (adjusted HR, 0.84; 95% CI, 0.80–0.89) groups appeared to have a decreased probability of death (Table 2). Due to the U‐shaped association between BMI and death in NAFLD and non‐NAFLD groups, we further divided the participants into three groups by BMI: <23 kg/m2 (lean), 23 to 35 kg/m2 (overweight to class I obesity), and ≥35 kg/m2 (class II+ obesity). The protective association was only found in those with overweight to class I obesity in both NAFLD and non‐NAFLD groups (Table S3), with HRs of 0.85 (95% CI, 0.80–0.91) and 0.84 (95% CI, 0.80–0.89), respectively.
TABLE 2.
Associations between NAFLD and non‐NAFLD with death, cardiovascular disease, and cancers
| Outcomes | LNN | ONN | LN | ON |
|---|---|---|---|---|
| All‐cause death | ||||
| Model 1 | 1 | 0.94 (0.89, 0.99) | 1.44 (1.26, 1.64) | 1.08 (1.02, 1.14) |
| Model 2 | 1 | 0.94 (0.90, 0.99) | 1.48 (1.29, 1.69) | 1.09 (1.04, 1.16) |
| Model 3 | 1 | 0.84 (0.80, 0.89) | 1.30 (1.14, 1.49) | 0.86 (0.80, 0.91) |
| Liver‐related death | ||||
| Model 1 | 1 | 1.27 (0.84, 1.92) | 2.60 (1.15, 5.88) | 0.94 (0.59, 1.51) |
| Model 2 | 1 | 1.27 (0.84, 1.93) | 2.67 (1.18, 6.03) | 0.96 (0.60, 1.55) |
| Model 3 | 1 | 1.10 (0.70, 1.74) | 2.61 (1.13, 6.03) | 0.74 (0.44, 1.26) |
| Cardiovascular diseases | ||||
| Model 1 | 1 | 1.40 (1.30, 1.50) | 1.28 (1.05, 1.57) | 1.96 (1.82, 2.12) |
| Model 2 | 1 | 1.40 (1.30, 1.51) | 1.30 (1.06, 1.59) | 1.99 (1.85, 2.15) |
| Model 3 | 1 | 1.19 (1.10, 1.28) | 1.07 (0.87, 1.32) | 1.39 (1.27, 1.52) |
| Digestive system cancers | ||||
| Model 1 | 1 | 1.16 (0.97, 1.39) | 2.22 (1.54, 3.20) | 1.23 (1.02, 1.48) |
| Model 2 | 1 | 1.16 (0.97, 1.39) | 2.21 (1.53, 3.19) | 1.23 (1.02, 1.48) |
| Model 3 | 1 | 1.19 (0.98, 1.43) | 2.12 (1.46, 3.08) | 1.23 (0.99, 1.54) |
| Obesity‐related cancers | ||||
| Model 1 | 1 | 1.21 (1.02, 1.43) | 2.11 (1.47, 3.04) | 1.31 (1.10, 1.57) |
| Model 2 | 1 | 1.21 (1.02, 1.44) | 2.11 (1.47, 3.04) | 1.32 (1.10, 1.58) |
| Model 3 | 1 | 1.21 (1.01, 1.46) | 2.04 (1.40, 2.96) | 1.30 (1.05, 1.61) |
Note: Data are presented as hazard ratios (95% confidence intervals). Model 1: adjusted for sex and age. Model 2: further adjusted for education level, smoking status, alcohol drinking status, and physical activity. Model 3: further adjusted for diabetes, hypertension, central obesity, triglycerides, low‐density lipoprotein cholesterol, high‐density lipoprotein cholesterol, C‐reactive protein, and alanine transaminase.
Abbreviations: LN, lean NAFLD; LNN, lean non‐NAFLD; NAFLD, nonalcoholic fatty liver disease; ON, overweight/obese NAFLD; ONN, overweight/obese non‐NAFLD.
After adjusting the covariates, a pairwise comparison (Table 3) showed that the mortality risk in the LN group increased by 52% (95% CI, 33%–74%), 54% (95% CI, 35%–76%), and 30% (95% CI, 14%–49%) compared to the ON, ONN, and LNN groups, respectively. Compared to subjects who were lean, pairwise comparisons found that subjects who were overweight or obese decreased the death risk by 16% (95% CI, 11%–20%) in the non‐NAFLD population; however, the risk reduction increased to 34% (95% CI, 25%–42%) in the NAFLD population.
TABLE 3.
Pairwise comparison of death, cardiovascular disease, and cancer risks for combinations of body mass index and NAFLD
| Lean | Overweight/obese NAFLD | Lean non‐NAFLD | Overweight/obese non‐NAFLD |
|---|---|---|---|
| All‐cause death | |||
| 1. Lean NAFLD | 1 vs. 2 | 1 vs. 3 | 1 vs. 4 |
| 1.52 (1.33, 1.74) | 1.30 (1.14, 1.49) | 1.54 (1.35, 1.76) | |
| 2 vs. 1 | 2. Overweight/obese NAFLD | 2 vs. 3 | 2 vs. 4 |
| 0.66 (0.58, 0.75) | 0.86 (0.80, 0.92) | 1.01 (0.96, 1.07) | |
| 3 vs. 1 | 3 vs. 2 | 3. Lean non‐NAFLD | 3 vs. 4 |
| 0.77 (0.67, 0.88) | 1.17 (1.09, 1.25) | 1.19 (1.12, 1.26) | |
| 4 vs. 1 | 4 vs. 2 | 4 vs. 3 | 4. Overweight/obese non‐NAFLD |
| 0.65 (0.57, 0.74) | 0.99 (0.93, 1.04) | 0.84 (0.80, 0.89) | |
| Liver‐related death | |||
| 1. Lean NAFLD | 1 vs. 2 | 1 vs. 3 | 1 vs. 4 |
| 2.77 (1.23, 6.24) | 2.67 (1.18, 6.03) | 2.10 (0.96, 4.57) | |
| 2 vs. 1 | 2. Overweight/obese NAFLD | 2 vs. 3 | 2 vs. 4 |
| 0.36 (0.16, 0.81) | 0.96 (0.60, 1.55) | 0.76 (0.51, 1.13) | |
| 3 vs. 1 | 3 vs. 2 | 3. Lean non‐NAFLD | 3 vs. 4 |
| 0.38 (0.17, 0.85) | 1.04 (0.65, 1.67) | 0.79 (0.52, 1.19) | |
| 4 vs. 1 | 4 vs. 2 | 4 vs. 3 | 4. Overweight/obese non‐NAFLD |
| 0.48 (0.22, 1.04) | 1.32 (0.89, 1.98) | 1.27 (0.84, 1.93) | |
| Cardiovascular disease | |||
| 1. Lean NAFLD | 1 vs. 2 | 1 vs. 3 | 1 vs. 4 |
| 0.77 (0.63, 0.94) | 1.07 (0.88, 1.32) | 0.91 (0.74, 1.10) | |
| 2 vs. 1 | 2. Overweight/obese NAFLD | 2 vs. 3 | 2 vs. 4 |
| 1.30 (1.06, 1.58) | 1.39 (1.27, 1.52) | 1.17 (1.10, 1.25) | |
| 3 vs. 1 | 3 vs. 2 | 3. Lean non‐NAFLD | 3 vs. 4 |
| 0.93 (0.76, 1.14) | 0.72 (0.66, 0.79) | 0.84 (0.78, 0.91) | |
| 4 vs. 1 | 4 vs. 2 | 4 vs. 3 | 4. Overweight/obese non‐NAFLD |
| 1.11 (0.91, 1.35) | 0.85 (0.80, 0.91) | 1.19 (1.10, 1.28) | |
| Digestive system cancers | |||
| 1. Lean NAFLD | 1 vs. 2 | 1 vs. 3 | 1 vs. 4 |
| 1.72 (1.18, 2.50) | 2.12 (1.46, 3.09) | 1.79 (1.24, 2.58) | |
| 2 vs. 1 | 2. Overweight/obese NAFLD | 2 vs. 3 | 2 vs. 4 |
| 0.58 (0.40, 0.85) | 1.23 (0.99, 1.54) | 1.04 (0.88, 1.24) | |
| 3 vs. 1 | 3 vs. 2 | 3. Lean non‐NAFLD | 3 vs. 4 |
| 0.47 (0.32, 0.69) | 0.81 (0.65, 1.01) | 0.84 (0.70, 1.02) | |
| 4 vs. 1 | 4 vs. 2 | 4 vs. 3 | 4. Overweight/obese non‐NAFLD |
| 0.56 (0.39, 0.81) | 0.96 (0.81, 1.14) | 1.19 (0.98, 1.43) | |
| Obesity‐related cancers | |||
| 1. Lean NAFLD | 1 vs. 2 | 1 vs. 3 | 1 vs. 4 |
| 1.57 (1.08, 2.27) | 2.04 (1.40, 2.96) | 1.68 (1.17, 2.42) | |
| 2 vs. 1 | 2. Overweight/obese NAFLD | 2 vs. 3 | 2 vs. 4 |
| 0.64 (0.40, 0.93) | 1.30 (1.05, 1.61) | 1.07 (0.91, 1.26) | |
| 3 vs. 1 | 3 vs. 2 | 3. Lean non‐NAFLD | 3 vs. 4 |
| 0.49 (0.34, 0.71) | 0.77 (0.62, 0.95) | 0.82 (0.69, 0.90) | |
| 4 vs. 1 | 4 vs. 2 | 4 vs. 3 | 4. Overweight/obese non‐NAFLD |
| 0.60 (0.41, 0.86) | 0.93 (0.79, 1.10) | 1.21 (1.01, 1.46) |
Note: Data are presented as hazard ratio (95% confidence intervals). Models were adjusted for sex, age, education level, smoking, alcohol drinking, physical activity, diabetes, hypertension, central obesity, triglycerides, low‐density lipoprotein, high‐density lipoprotein, C‐reactive protein, and alanine transaminase, except for the stratification factor.
Abbreviation: NAFLD, nonalcoholic fatty liver disease.
The LN group had the highest incidence of liver‐related death (log‐rank test, p = 0.053; Figure 2B; Table S2). After adjusting covariates, LN showed a significantly higher risk of liver‐related death compared to the LNN group (Table 2). Pairwise comparison (Table 3) showed that the LN group had a higher risk of liver‐related death than the ON and LNN groups, with HRs of 2.77 (95% CI, 1.23–6.24) for LN versus ON and 2.67 (95% CI, 1.18–6.03) for LN versus LNN.
Association between LN and ON with CVD
A total of 5618 incident CVDs developed after a median of 13.1 (IQR, 12.8–13.2) years of follow‐up. The ON group had the highest incidence of CVD, followed by the ONN group (log‐rank test, p < 0.001; Figure 2C; Table S2). After adjusting covariates, the trend remained (Table 2). Compared to the LNN group, the ON group increased CVD risk by 39% (95% CI, 27%–52%), followed by the ONN group by 19% (95% CI, 10%–28%). The increased hazard in the LN group was only observed in the univariate model (HR, 1.28; 95% CI, 1.05–1.57) and the lifestyle factors‐adjusted model (HR, 1.30; 95% CI, 1.06–1.59). However, there was no significant difference between LN and LNN groups (HR, 1.07; 95% CI, 0.87–1.32) after further adjusting for metabolism‐related factors.
Further pairwise comparison (Table 3) showed that the ON group had a higher risk than the others, with HRs being 1.39 (95% CI, 1.27–1.52) for ON versus LNN, 1.30 (95% CI, 1.06–1.58) for ON versus LN, and 1.17 (95% CI, 1.10–1.25) for ON versus ONN. The individuals who were overweight or obese had an increased risk of 30% (95% CI, 6%–58%) for CVD in the NAFLD group but 19% (95% CI, 10%–28%) in the non‐NAFLD group.
Association between LN and ON with cancers
Over a median follow‐up of 11.0 (IQR, 10.7–11.2) years, 851 incident digestive system cancers and 926 incident obesity‐related cancers developed. There was a significantly different risk of digestive system cancers among the four groups (log‐rank test, p < 0.001; Figure 2D; Table S2). Compared to the LNN group, only the LN group showed a significantly elevated digestive system cancer risk (adjusted HR, 2.12; 95% CI, 1.46–3.08); however, all three groups had increased obesity‐related cancers, with adjusted HRs increasing from 1.21 (95% CI, 1.01–1.46) in the ONN group to 1.30 (95% CI, 1.05–1.61) in the ON group and 2.04 (95% CI, 1.40–2.96) in the LN group (Table 2). Site‐specific analysis indicated a higher hazard of colorectal cancer in the LN group (HR, 2.20; 95% CI, 1.11–4.34) and the ON group (HR, 1.65; 95% CI, 1.13–2.41) than in the LNN group. In addition, subjects with LN had a significantly higher risk of esophagus cancer (HR, 6.46; 95% CI, 3.00–13.92). There were no cases in the LN group of kidney and small intestine cancer. An increased hazard was observed in the ON group (Table 4).
TABLE 4.
Associations between NAFLD and non‐NAFLD type and site‐specific cancers
| Outcomes | LNN | ONN | LN | ON |
|---|---|---|---|---|
| Biliary cancer | ||||
| Model 1 | 1 | 0.67 (0.33, 1.35) | 1.64 (0.38, 7.10) | 0.84 (0.41, 1.74) |
| Model 2 | 1 | 0.65 (0.32, 1.31) | 1.64 (0.38, 7.12) | 0.82 (0.40, 1.70) |
| Model 3 | 1 | 0.61 (0.28, 1.35) | 1.74 (0.40, 7.53) | 0.73 (0.28, 1.90) |
| Liver cancer | ||||
| Model 1 | 1 | 1.00 (0.64, 1.56) | 1.57 (0.55, 4.45) | 1.03 (0.64, 1.66) |
| Model 2 | 1 | 1.01 (0.65, 1.57) | 1.58 (0.56, 4.46) | 1.04 (0.64, 1.68) |
| Model 3 | 1 | 0.87 (0.54, 1.42) | 1.46 (0.52, 4.09) | 0.77 (0.44, 1.34) |
| Colorectal cancer | ||||
| Model 1 | 1 | 1.36 (1.00, 1.86) | 2.32 (1.22, 4.43) | 1.61 (1.17, 2.23) |
| Model 2 | 1 | 1.36 (0.99, 1.86) | 2.29 (1.20, 4.38) | 1.59 (1.15, 2.20) |
| Model 3 | 1 | 1.38 (0.98, 1.93) | 2.20 (1.11, 4.34) | 1.65 (1.13, 2.41) |
| Kidney cancer a | ||||
| Model 1 | 1 | 2.27 (1.17, 4.40) | — | 3.41 (1.76, 6.58) |
| Model 2 | 1 | 2.30 (1.19, 4.45) | — | 3.46 (1.80, 6.68) |
| Model 3 | 1 | 2.10 (1.07, 4.13) | — | 3.28 (1.64, 6.56) |
| Esophagus cancer | ||||
| Model 1 | 1 | 1.10 (0.63, 1.92) | 7.01 (3.35, 14.69) | 0.88 (0.47, 1.65) |
| Model 2 | 1 | 1.11 (0.64, 1.94) | 6.98 (3.34, 14.59) | 0.89 (0.48, 1.67) |
| Model 3 | 1 | 1.18 (0.66, 2.11) | 6.46 (3.00, 13.92) | 1.02 (0.50, 2.06) |
| Gastric cancer | ||||
| Model 1 | 1 | 1.17 (0.81, 1.68) | 1.11 (0.40, 3.09) | 1.13 (0.76, 1.67) |
| Model 2 | 1 | 1.17 (0.81, 1.69) | 1.11 (0.40, 3.09) | 1.14 (0.77, 1.69) |
| Model 3 | 1 | 1.35 (0.91, 1.99) | 1.14 (0.41, 3.22) | 1.45 (0.89, 2.36) |
| Small intestine cancer a | ||||
| Model 1 | 1 | 2.77 (0.59, 12.89) | — | 4.78 (1.06, 21.63) |
| Model 2 | 1 | 2.74 (0.58, 12.96) | — | 4.74 (1.05, 21.46) |
| Model 3 | 1 | 3.28 (0.65, 16.43) | — | 6.23 (1.24, 31.21) |
| Pancreatic cancer | ||||
| Model 1 | 1 | 1.07 (0.62, 1.87) | 1.22 (0.28, 5.21) | 0.92 (0.50, 1.70) |
| Model 2 | 1 | 1.08 (0.62, 1.88) | 1.25 (0.29, 5.38) | 0.94 (0.51, 1.74) |
| Model 3 | 1 | 1.10 (0.60, 2.02) | 1.25 (0.28, 5.59) | 0.96 (0.46, 2.01) |
Note: Data are presented as hazard ratios (95% confidence intervals). Model 1: adjusted for sex and age. Model 2: further adjusted for education level, smoking status, alcohol drinking status, and physical activity. Model 3: further adjusted for diabetes, hypertension, central obesity, triglycerides, low‐density lipoprotein cholesterol, high‐density lipoprotein cholesterol, C‐reactive protein, and alanine transaminase.
Abbreviations: LN, lean NAFLD; LNN, lean non‐NAFLD; NAFLD, nonalcoholic fatty liver disease; ON, overweight/obese NAFLD; ONN, overweight/obese non‐NAFLD.
No small intestine cancer and kidney cancer cases in the lean NAFLD group.
Further pairwise comparison (Table 3) showed that LN had the highest risks of digestive system cancers or obesity‐related cancers. NAFLD had higher risks than non‐NAFLD in subjects who were lean; however, no significant association was observed in subjects who were overweight or obese. In addition, a lower risk of obesity‐related cancers was observed in the LNN group compared to the ONN group.
Associations among the LN group and adverse outcomes
Baseline characteristics of subjects with LN with and without adverse outcomes are presented in Table S4. Subjects with LN who died or who had CVD, digestive system cancers, and obesity‐related cancers were older and had a higher prevalence of hypertension than those who did not have adverse outcomes. Therefore, we analyzed this group based on hypertension. The relationship among subjects with LN and adverse outcomes is shown in Table 5. Patients with LN with hypertension had a higher risk of death (HR, 1.62; 95% CI, 1.37–1.92), liver‐related death (HR, 2.92; 95% CI, 1.01–8.42), CVD (HR, 1.31; 95% CI, 1.01–1.70), digestive system cancers (HR, 2.63; 95% CI,1.67–4.15), and obesity‐related cancers (HR, 2.56; 95% CI, 1.62–4.03); those without hypertension had a similar risk as those with LNN.
TABLE 5.
Associations between the lean NAFLD group and death, cardiovascular disease, and cancers
| Outcomes | Lean non‐NAFLD (n = 19,605) | Lean NAFLD with hypertension (n = 721) | Lean NAFLD without hypertension (n = 822) |
|---|---|---|---|
| All‐cause death | |||
| Model 1 | 1 | 1.77 (1.50, 2.09) | 1.07 (0.87, 1.33) |
| Model 2 | 1 | 1.81 (1.54, 2.14) | 1.10 (0.89, 1.36) |
| Model 3 | 1 | 1.62 (1.37, 1.92) | 1.04 (0.84, 1.29) |
| Liver‐related death | |||
| Model 1 | 1 | 2.91 (1.03, 8.22) | 2.28 (0.70, 7.43) |
| Model 2 | 1 | 2.99 (1.06, 8.46) | 2.33 (0.71, 7.62) |
| Model 3 | 1 | 2.92 (1.01, 8.42) | 2.28 (0.69, 7.56) |
| Cardiovascular diseases | |||
| Model 1 | 1 | 1.46 (1.13, 1.90) | 1.09 (0.81, 1.48) |
| Model 2 | 1 | 1.49 (1.15, 1.93) | 1.11 (0.82, 1.50) |
| Model 3 | 1 | 1.31 (1.01, 1.70) | 1.00 (0.74, 1.36) |
| Digestive system cancers | |||
| Model 1 | 1 | 2.77 (1.78, 4.32) | 1.62 (0.90, 2.91) |
| Model 2 | 1 | 2.77 (1.78, 4.31) | 1.62 (0.90, 2.90) |
| Model 3 | 1 | 2.63 (1.67, 4.15) | 1.61 (0.89, 2.91) |
| Obesity‐related cancers | |||
| Model 1 | 1 | 2.66 (1.71, 4.14) | 1.54 (0.86, 2.75) |
| Model 2 | 1 | 2.66 (1.71, 4.14) | 1.53 (0.85, 2.75) |
| Model 3 | 1 | 2.56 (1.62, 4.03) | 1.54 (0.86, 2.78) |
Note: Data are presented as hazard ratios (95% confidence intervals). Model 1: adjusted for sex and age. Model 2: further adjusted for education level, smoking status, alcohol drinking status, and physical activity. Model 3: further adjusted for diabetes, central obesity, triglycerides, low‐density lipoprotein cholesterol, high‐density lipoprotein cholesterol, C‐reactive protein, and alanine transaminase.
Abbreviation: NAFLD, nonalcoholic fatty liver disease.
Stratified analyses
Stratified analyses by sex, age, central obesity, diabetes, hypertension, drinking, and smoking are shown in Tables S5‐1 to S5‐5. Age was observed to modify the association between NAFLD status and death (p for interaction = 0.012). Compared to the LNN group, the increased mortality risk in the LN group was more prominent in the younger population, with HR reaching up to 2.57 (95% CI, 1.59–4.18) in those aged < 45 years. The relationship between NAFLD status and CVD was also observed to be modified by sex (p for interaction = 0.014), central obesity (p for interaction = 0.062), and smoking status (p for interaction = 0.043). Men or those with central obesity had a higher liver‐related death risk in the LN group than in the LNN group. Men, those with central obesity, or current smokers had a higher CVD risk in the ON and ONN groups compared to the LNN group. No other interactions were found.
Sensitivity analyses
Conclusions remained consistent after excluding the outcomes within 3 years of follow‐up (Table S6) and the subjects with a history of stroke, ischemic heart disease, diabetes, or smoking at baseline (Table S7).
DISCUSSION
In this large community‐based prospective cohort in China, we found a U‐shaped association between BMI and death but a linear positive association concerning CVD in NAFLD and non‐NAFLD populations after adjusting for age and other covariates. After stratifying the subjects by BMI and NAFLD status, subjects with LN had more favorable biochemical features than those with ON. Compared to the participants with normal BMI and non‐NAFLD, the LN group had a higher risk of death, especially liver‐related death; however, the ON and ONN groups had a lower risk of death. In addition, the LN, ON, and ONN groups had a higher obesity‐related cancer risk, with the highest risk in the LN group, and participants with ON and ONN but not with LN had an elevated CVD hazard. Subjects with LN had a significantly higher risk of colorectal and esophagus cancer for site‐specified cancers. In contrast, colorectal, small intestine, and kidney cancer risks were increased in subjects with ON compared to those with LNN. In addition, the association between overweight or obesity on death (negative) and CVD (positive) was more prominent in the NAFLD than the non‐NAFLD group. Subgroup analysis showed patients with LN with hypertension might have higher risks of adverse outcomes.
Consistent with some studies,[ 25 ] subjects with LN in our cohort showed a healthier status in blood pressure, glucose, and blood lipids, including TG and HDL, than subjects with ON. Our findings showed a U‐shaped association between BMI and death in NAFLD and non‐NAFLD populations. Compared to the participants with normal BMI and non‐NAFLD, the LN group had a higher risk of death, but the ON and ONN groups (mainly overweight to class I obesity) had a lower risk. The results are consistent with other studies that individuals classified as normal weight or underweight may have higher all‐cause mortality,[ 26 , 27 ] representing a “lean paradox.”[ 8 ] In addition, the LN group in our population increased the all‐cause mortality risk by 52% and liver‐related mortality risk by 177% compared to the ON group, consistent with the results in other cohort studies that reported a positive association between LN and all‐cause mortality[ 14 , 15 , 17 ] and liver‐related mortality[ 28 , 29 ] compared with the ON group. Participants with LN may be individuals who are lean as characterized by a relatively low leg‐fat mass and high subcutaneous abdominal fat mass.[ 30 ] Individuals with LN may also carry a higher proportion of the patatin‐like phospholipase domain containing 3 (PNPLA3) rs738409 GG genotype,[ 31 , 32 ] which is a risk factor for liver‐related death in individuals with NAFLD.[ 33 , 34 ] In addition, individuals with LN may partly suffer from sarcopenia, which plays a role in NAFLD[ 35 ] and is associated with increased adverse outcomes, including mortality.[ 36 ] However, other cohort studies have not shown a significant difference in the risk of death between lean and non‐lean NAFLD participants.[ 13 , 16 ] This inconsistency may be partly explained by some confounders, such as genetic factors, which were not considered because genetic analyses suggest that metabolic risk appears to be determined by different pathways in subjects with normal weight and with obesity.[ 30 ]
Compared to the LNN group, we observed that the ON group had the highest CVD risk, followed by the ONN group. This could be explained by the fact that obesity and NAFLD are the determined risk factors for CVD. Furthermore, high levels of fat mass worsen most CVD risk factors, including plasma lipids, blood pressure, insulin resistance, and inflammation, among others.[ 37 ] In addition, the ON group in our population had an elevated CVD risk compared to the LN group, consistent with two cross‐sectional studies that reported a higher prevalence of early atherosclerosis[ 38 ] and CVD[ 39 ] in the ON group than in the LN group. Possible reasons might be that individuals with ON may carry a significantly higher proportion of the transmembrane 6 superfamily 2 (TM6SF2) C allele than those with LN,[ 38 , 40 ] which might cause increased hepatic very low‐density lipoprotein secretion and ultimately lead to atherogenesis.[ 41 ]
We observed that compared to LNN, the LN group in our cohort had the highest risk of obesity‐related cancers, followed by the ON and ONN groups. The marginally significantly higher obesity‐related cancer risk in the ON than in the ONN group suggests a driver role of fatty liver disease in the occurrence of obesity‐related cancers. A cohort study in the US population that reported a higher extrahepatic cancer risk in the NAFLD group than in the obesity‐only group[ 42 ] could demonstrate our hypothesis. The altered microbiome, chronic inflammation, and cytokine activation in patients with NAFLD could trigger obesity‐related cancers.[ 43 ] However, the highest risk of digestive system cancers or obesity‐related cancers in LN may indicate other mechanisms except obesity and fatty liver disease. For example, as a distinct entity shaped by the integration of signals from the diet, systemic metabolic milieu, and enterohepatic axis comprising both bile acids and gut microbiota, LN may exhibit a high rate of malignancies.[ 40 ]
Previous cohort studies on specific cancer in patients with LN mainly focused on hepatocellular carcinoma and had inconsistent results.[ 12 , 13 , 16 ] For extrahepatic cancers, the LN group was reported to have similar risks as the group with obesity in a multicenter study involving 1339 Caucasian patients with NAFLD[ 13 ] and a cohort study in Hong Kong, China.[ 12 ] We found subjects with LN had a significantly higher risk of colorectal and esophagus cancer, whereas colorectal, small intestine, and kidney cancer risks were increased in subjects with ON compared to those with LNN. Recent research has reported critical pathways that might link metabolism, low‐grade chronic inflammation,[ 44 ] gut–liver axis,[ 45 ] and cancer development with LN or ON. Due to the relatively small sample of patients with LN and low event rate of various cancers in our study, justification is needed through more cohort and mechanism studies.
Our study also showed that the association between overweight or obesity and death (negative) and CVD (positive) was more prominent in the NAFLD than the non‐NAFLD group. In addition, the hazard effect of NAFLD on mortality was only observed in subjects who were lean but not those who were overweight or obese. The results further suggest distinct characteristics of LN from ON besides obesity. Therefore, we call for further studies integrating genetics, metabolism, and environmental factors to reclassify NAFLD based on factors other than just BMI.
The association between LN, ON, ONN, and death was modified by age in our population. The risk of all‐cause death in the LN group was more robust in subjects aged < 45 years, suggesting an impact of early onset LN on adverse outcomes. Similar to our results, another study[ 23 ] reported a greater risk of death and CVD at a younger age. In addition, men or those with central obesity had a higher liver‐related death risk in the LN group than the LNN group; men, those with central obesity, or current smokers had a higher CVD risk in the ON and ONN groups compared to the LNN group. The above‐stratified analyses further indicate the need for a finer regrouping of NAFLD. Our LN group analyses showed that patients with LN with hypertension had a higher risk of death, CVD, and cancers, and those without hypertension had a similar risk compared to those with LNN, indicating LN with hypertension may be a high‐risk phenotype. The prognosis and biological mechanisms of LN and hypertension comorbidity need to be clarified.[ 46 ]
Our study has several limitations. First, we used ultrasonography instead of liver histology to diagnose NAFLD because liver biopsy is invasive and unpractical in a large community‐based cohort. This would lead to the misclassification of NAFLD as non‐NAFLD when liver fat is less than 20%.[ 2 ] Second, our cohort did not collect aspartate aminotransferase to calculate the NAFLD fibrosis score or the fibrosis‐4 score to determine NAFLD severity. The above two limitations cause difficulties in determining the histologic and fibrotic severity status in the four groups, which would help us to understand and infer the possible reasons for the results. Third, our study did not collect data regarding other secondary causes of hepatitis C virus infection, hemochromatosis, α‐1 antitrypsin deficiency, and autoimmune hepatitis, which are more likely to be present in individuals who are lean. However, considering the low prevalence of hepatitis C virus infection,[ 47 ] hemochromatosis,[ 48 ] α‐1 antitrypsin deficiency,[ 49 ] and autoimmune hepatitis[ 50 ] in China, the lack of data is unlikely to affect the results. Fourth, we collected outcomes through self‐reported questionnaires and linkage with the data set (local vital statistics data, Tangshan medical insurance system, or Kailuan Social Security Information System). The Kailuan Company deals not only with coal products but also with many other items, such as machine building, construction installation, electronic power, coking, new building materials, chemical production, bauxite, transportation, and trading. Therefore, the emigration proportion in our cohort is very low. We therefore treated the last follow‐up time as the study termination date for the subjects who were lost to follow‐up. However, this is unlikely to change our conclusions because it would lead to nondifferential misclassification considering the independence between lost to follow‐up and NAFLD status, and it would then underestimate the HR. Finally, the association between LN and adverse outcome may be due to an outcome–causal relationship. However, the conclusion remains consistent after we excluded the subject whose outcome occurred within 3 years of follow‐up and those with a history of stroke, ischemic heart disease, diabetes, or smoking at baseline, supporting the robustness of our results.
In conclusion, our study indicated that the ON group had the highest CVD risk compared to the LNN group, followed by the ONN group. However, patients with LN still showed a higher risk of all‐cause death, digestive system cancers, and obesity‐related cancers, especially colorectal and esophagus cancer, than the other three groups. LN with hypertension may be a high‐risk phenotype, and early intervention should be considered. Our results further suggest distinct characteristics of LN from ON besides obesity. Further cohort studies and multi‐omics studies are needed to reclassify NAFLD by factors other than just BMI to fulfill the objective of preventing NAFLD.
AUTHOR CONTRIBUTIONS
Li Wang and Shouling Wu designed the study and critically assessed and revised the manuscript. Jinfeng Li, Shuohua Chen, and Hongmin Liu collected the data. Shiqi Hu, Xiaojie Yuan, and Xiaomo Wang processed the data. Yanqi Lan and Ying Lu performed the statistical analyses and drafted the manuscript. Jinfeng Li, Shiqi Hu, and Yanhong Wang interpreted the data. All authors read and approved the final manuscript.
FUNDING INFORMATION
Beijing Municipal Health Commission Capital Health Development Research Project; Grant Number: 2022–1‐2021
Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences; Grant Number: 2021‐I2M‐1‐023.
CONFLICT OF INTEREST
Nothing to report.
Supporting information
Appendix S1 Supporting information
ACKNOWLEDGMENT
We are grateful to all participants in this study for their kind cooperation.
Lan Y, Lu Y, Li J, Hu S, Chen S, Wang Y, et al. Outcomes of subjects who are lean, overweight or obese with nonalcoholic fatty liver disease: A cohort study in China. Hepatol Commun. 2022;6:3393–3405. 10.1002/hep4.2081
Yanqi Lan and Ying Lu contributed equally to this work as first authors.
Contributor Information
Shouling Wu, Email: [email protected].
Li Wang, Email: [email protected].
REFERENCES
- 1. Rinella ME. Nonalcoholic fatty liver disease: a systematic review. JAMA. 2015;313(22):2263–73. Erratum in: JAMA. 2015;314(14):1521. [DOI] [PubMed] [Google Scholar]
- 2. European Association for the Study of the Liver; European Association for the Study of Diabetes; European Association for the Study of Obesity . EASL‐EASD‐EASO Clinical Practice Guidelines for the management of non‐alcoholic fatty liver disease. J Hepatol. 2016;64(6):1388–402. [DOI] [PubMed] [Google Scholar]
- 3. Kim GA, Lee HC, Choe J, Kim MJ, Lee MJ, Chang HS, et al. Association between non‐alcoholic fatty liver disease and cancer incidence rate. J Hepatol. 2018;68:140–6. [DOI] [PubMed] [Google Scholar]
- 4. Ye Q, Zou B, Yeo YH, Li J, Huang DQ, Wu Y, et al. Global prevalence, incidence, and outcomes of non‐obese or lean non‐alcoholic fatty liver disease: a systematic review and meta‐analysis. Lancet Gastroenterol Hepatol. 2020;5(8):739–52. [DOI] [PubMed] [Google Scholar]
- 5. Global BMI Mortality Collaboration , Di Angelantonio E, Bhupathiraju S, Wormser D, Gao P, Kaptoge S, et al. Body‐mass index and all‐cause mortality: individual‐participant‐data meta‐analysis of 239 prospective studies in four continents. Lancet. 2016;388(10046):776–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Khan SS, Ning H, Wilkins JT, Allen N, Carnethon M, Berry JD, et al. Association of body mass index with lifetime risk of cardiovascular disease and compression of morbidity. JAMA Cardiol. 2018;3(4):280–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kyrgiou M, Kalliala I, Markozannes G, Gunter MJ, Paraskevaidis E, Gabra H, et al. Adiposity and cancer at major anatomical sites: umbrella review of the literature. BMJ. 2017;356:j477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Elagizi A, Kachur S, Lavie CJ, Carbone S, Pandey A, Ortega FB, et al. An overview and update on obesity and the obesity paradox in cardiovascular diseases. Prog Cardiovasc Dis. 2018;61(2):142–50. [DOI] [PubMed] [Google Scholar]
- 9. Lennon H, Sperrin M, Badrick E, Renehan AG. The obesity paradox in cancer: a review. Curr Oncol Rep. 2016;18(9):56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yoshitaka H, Hamaguchi M, Kojima T, Fukuda T, Ohbora A, Fukui M. Nonoverweight nonalcoholic fatty liver disease and incident cardiovascular disease: a post hoc analysis of a cohort study. Medicine (Baltimore). 2017;96(18):e6712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Golabi P, Paik J, Fukui N, Locklear CT, de Avilla L, Younossi ZM. Patients with lean nonalcoholic fatty liver disease are metabolically abnormal and have a higher risk for mortality. Clin Diabetes. 2019;37(1):65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Leung JC, Loong TC, Wei JL, Wong GL, Chan AW, Choi PC, et al. Histological severity and clinical outcomes of nonalcoholic fatty liver disease in nonobese patients. Hepatology. 2017;65(1):54–64. [DOI] [PubMed] [Google Scholar]
- 13. Younes R, Govaere O, Petta S, Miele L, Tiniakos D, Burt A, et al. Caucasian lean subjects with non‐alcoholic fatty liver disease share long‐term prognosis of non‐lean: time for reappraisal of BMI‐driven approach? Gut. 2022;71:382–90. [DOI] [PubMed] [Google Scholar]
- 14. Zou B, Yeo YH, Nguyen VH, Cheung R, Ingelsson E, Nguyen MH. Prevalence, characteristics and mortality outcomes of obese, nonobese and lean NAFLD in the United States, 1999–2016. J Intern Med. 2020;288(1):139–51. [DOI] [PubMed] [Google Scholar]
- 15. Dela Cruz ACL , Bugianesi E, George J, Day CP, Liaquat H, Charatcharoenwitthaya P, et al. 379 Characteristics and long‐term prognosis of lean patients with nonalcoholic fatty liver disease. Gastroenterology. 2014;146(5):S909. [Google Scholar]
- 16. Hagström H, Nasr P, Ekstedt M, Hammar U, Stål P, Hultcrantz R, et al. Risk for development of severe liver disease in lean patients with nonalcoholic fatty liver disease: a long‐term follow‐up study. Hepatol Commun. 2017;2(1):48–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ahmed OT, Gidener T, Mara KC, Larson JJ, Therneau TM, Allen AM. Natural history of nonalcoholic fatty liver disease with normal body mass index: a population‐based study. Clin Gastroenterol Hepatol. 2022;20:1374–81.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Luukkonen PK, Qadri S, Ahlholm N, Porthan K, Männistö V, Sammalkorpi H, et al. Distinct contributions of metabolic dysfunction and genetic risk factors in the pathogenesis of non‐alcoholic fatty liver disease. J Hepatol. 2022;76(3):526–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vilarinho S, Ajmera V, Zheng M, Loomba R. Emerging role of genomic analysis in clinical evaluation of lean individuals with NAFLD. Hepatology. 2021;74:2241–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67(1):328–57. [DOI] [PubMed] [Google Scholar]
- 21. Asrani SK, Larson JJ, Yawn B, Therneau TM, Kim WR. Underestimation of liver‐related mortality in the United States. Gastroenterology. 2013;145(2):375–82.e1‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lauby‐Secretan B, Scoccianti C, Loomis D, Grosse Y, Bianchini F, Straif K, et al. Body fatness and cancer—viewpoint of the IARC Working Group. N Engl J Med. 2016;375(8):794–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang C, Yuan Y, Zheng M, Pan A, Wang M, Zhao M, et al. Association of age of onset of hypertension with cardiovascular diseases and mortality. J Am Coll Cardiol. 2020;75(23):2921–30. [DOI] [PubMed] [Google Scholar]
- 24. WHO Expert Consultation . Appropriate body‐mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363(9403):157–63. Erratum in: Lancet. 2004;363(9412):902. [DOI] [PubMed] [Google Scholar]
- 25. Young S, Tariq R, Provenza J, Satapathy SK, Faisal K, Choudhry A, et al. Prevalence and profile of nonalcoholic fatty liver disease in lean adults: systematic review and meta‐analysis. Hepatol Commun. 2020;4(7):953–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Flegal KM, Kit BK, Orpana H, Graubard BI. Association of all‐cause mortality with overweight and obesity using standard body mass index categories: a systematic review and meta‐analysis. JAMA. 2013;309(1):71–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Berrington de Gonzalez A, Hartge P, Cerhan JR, Flint AJ, Hannan L, MacInnis RJ, et al. Body‐mass index and mortality among 1.46 million white adults. N Engl J Med. 2010;363(23):2211–9. Erratum in: N Engl J Med. 2011;365(9):869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Feldman A, Wernly B, Strebinger G, Eder SK, Zandanell S, Niederseer D, et al. Liver‐related mortality is increased in lean subjects with non‐ alcoholic fatty liver disease compared to overweight and obese subjects. J Gastrointestin Liver Dis. 2021;30(3):366–73. [DOI] [PubMed] [Google Scholar]
- 29. Chang Y, Cho YK, Cho J, Jung HS, Yun KE, Ahn J, et al. Alcoholic and nonalcoholic fatty liver disease and liver‐related mortality: a cohort study. Am J Gastroenterol. 2019;114(4):620–9. [DOI] [PubMed] [Google Scholar]
- 30. Stefan N, Schick F, Häring HU. Causes, characteristics, and consequences of metabolically unhealthy normal weight in humans. Cell Metab. 2017;26(2):292–300. [DOI] [PubMed] [Google Scholar]
- 31. Honda Y, Yoneda M, Kessoku T, Ogawa Y, Tomeno W, Imajo K, et al. Characteristics of non‐obese non‐alcoholic fatty liver disease: effect of genetic and environmental factors. Hepatol Res. 2016;46(10):1011–8. [DOI] [PubMed] [Google Scholar]
- 32. Lin H, Wong GL, Whatling C, Chan AW, Leung HH, Tse CH, et al. Association of genetic variations with NAFLD in lean individuals. Liver Int. 2022;42:149–60. [DOI] [PubMed] [Google Scholar]
- 33. Wijarnpreecha K, Scribani M, Raymond P, Harnois DM, Keaveny AP, Ahmed A, et al. PNPLA3 gene polymorphism and liver‐ and extrahepatic cancer‐related mortality in the United States. Clin Gastroenterol Hepatol. 2021;19(5):1064–6. [DOI] [PubMed] [Google Scholar]
- 34. Grimaudo S, Pipitone RM, Pennisi G, Celsa C, Cammà C, Di Marco V, et al. Association between PNPLA3 rs738409 C>G variant and liver‐related outcomes in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2020;18(4):935–44.e3. [DOI] [PubMed] [Google Scholar]
- 35. Kim JA, Choi KM. Sarcopenia and fatty liver disease. Hepatol Int. 2019;13(6):674–87. [DOI] [PubMed] [Google Scholar]
- 36. Cruz‐Jentoft AJ, Sayer AA. Sarcopenia. Lancet. 2019;393(10191):2636–46. Erratum in: Lancet. 2019;393(10191):2590. [DOI] [PubMed] [Google Scholar]
- 37. Ortega FB, Lavie CJ, Blair SN. Obesity and cardiovascular disease. Circ Res. 2016;118(11):1752–70. [DOI] [PubMed] [Google Scholar]
- 38. Fracanzani AL, Petta S, Lombardi R, Pisano G, Russello M, Consonni D, et al. Liver and cardiovascular damage in patients with lean nonalcoholic fatty liver disease, and association with visceral obesity. Clin Gastroenterol Hepatol. 2017;15(10):1604–11.e1. [DOI] [PubMed] [Google Scholar]
- 39. Weinberg EM, Trinh HN, Firpi RJ, Bhamidimarri KR, Klein S, Durlam J, et al. Lean Americans with nonalcoholic fatty liver disease have lower rates of cirrhosis and comorbid diseases. Clin Gastroenterol Hepatol. 2021;19:996–1008.e6. [DOI] [PubMed] [Google Scholar]
- 40. Chen F, Esmaili S, Rogers GB, Bugianesi E, Petta S, Marchesini G, et al. Lean NAFLD: a distinct entity shaped by differential metabolic adaptation. Hepatology. 2020;71(4):1213–27. [DOI] [PubMed] [Google Scholar]
- 41. Hardy T, Oakley F, Anstee QM, Day CP. Nonalcoholic fatty liver disease: pathogenesis and disease spectrum. Annu Rev Pathol. 2016;11:451–96. [DOI] [PubMed] [Google Scholar]
- 42. Allen AM, Hicks SB, Mara KC, Larson JJ, Therneau TM. The risk of incident extrahepatic cancers is higher in non‐alcoholic fatty liver disease than obesity ‐ a longitudinal cohort study. J Hepatol. 2019;71(6):1229–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Adams LA, Anstee QM, Tilg H, Targher G. Non‐alcoholic fatty liver disease and its relationship with cardiovascular disease and other extrahepatic diseases. Gut. 2017;66(6):1138–53. [DOI] [PubMed] [Google Scholar]
- 44. Candido J, Hagemann T. Cancer‐related inflammation. J Clin Immunol. 2013;33(Suppl 1):S79–84. [DOI] [PubMed] [Google Scholar]
- 45. Wang R, Tang R, Li B, Ma X, Schnabl B, Tilg H. Gut microbiome, liver immunology, and liver diseases. Cell Mol Immunol. 2021;18(1):4–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ma C, Yan K, Wang Z, Zhang Q, Gao L, Xu T, et al. The association between hypertension and nonalcoholic fatty liver disease (NAFLD): literature evidence and systems biology analysis. Bioengineered. 2021;12(1):2187–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Li M, Zhuang H, Wei L. How would China achieve WHO's target of eliminating HCV by 2030? Expert Rev Anti Infect Ther. 2019;17(10):763–73. [DOI] [PubMed] [Google Scholar]
- 48. Lv T, Zhang W, Xu A, Li Y, Zhou D, Zhang B, et al. Non‐HFE mutations in haemochromatosis in China: combination of heterozygous mutations involving HJV signal peptide variants. J Med Genet. 2018;55(10):650–60. [DOI] [PubMed] [Google Scholar]
- 49. Greulich T, Nell C, Hohmann D, Grebe M, Janciauskiene S, Koczulla AR, et al. The prevalence of diagnosed α1‐antitrypsin deficiency and its comorbidities: results from a large population‐based database. Eur Respir J. 2017;49(1):1600154. [DOI] [PubMed] [Google Scholar]
- 50. Chinese Society of Hepatology, Chinese Medical Association . Guidelines on the diagnosis and management of autoimmune hepatitis (2021) [in Chinese]. Zhonghua Nei Ke Za Zhi. 2021;60(12):1038–49. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Supporting information


