Abstract
Glucose-6-phosphate dehydrogenase (G6PD) participates in glucose metabolism and it acts as the rate-limiting enzyme of the pentose phosphate pathway (PPP). Recently, G6PD dysregulation has been found in a variety of human cancers. Through analyzing published data in The Cancer Genome Atlas (TCGA), our pilot study indicated that G6PD mRNA expression was significantly higher in advanced Fuhrman grade in clear cell renal cell carcinoma (ccRCC). These clues promoted us to further evaluate the expression profile of G6PD and its prognostic impact in patients with ccRCC. In this study, G6PD expression levels were analyzed in 149 human ccRCC and normal tissues using immunohistochemistry. The results showed that compared with that in the normal renal samples, G6PD was found highly expressed in 51.0% of ccRCC (p<0.05). High expression of G6PD was significantly correlated to tumor extent, lymph node metastasis, Fuhrman grade, and TNM stage of ccRCC (all p<0.05). Moreover, positive G6PD expression was associated with poorer overall survival in ccRCC (p<0.001). In Cox regression analyses, high expression of G6PD also could be an independent prognostic factor for overall survival in ccRCC (p=0.007). This study suggests that overexpression of G6PD is associated with advanced disease status and therefore may become an important prognosticator for poor outcomes in ccRCC, as well as a potential therapeutic target for developing effective treatment modalities.
Keywords: glucose-6-phosphate dehydrogenase, renal cell carcinoma, TCGA data mining, immunohistochemistry, prognosis biomarker
Introduction
Renal cell carcinoma (RCC), the most prevalent renal malignancy, accounts to approximately 3% of all type of tumors and its incidence continues to rise 1. Recent estimates in US have calculated that in 2016, 62,700 new kidney and renal pelvis carcinoma cases were diagnosed and of these 14,240 patients died 2. Clear cell renal cell carcinoma (ccRCC) represents the predominant histologic subtype of RCC and constitutes approximately 80~90% of all the cases 1, 3. Nowadays, ccRCC can be diagnosed at an early stage, but approximately 30% patients develop metastasis during diagnosis, and 20~40% patients will suffer from recurrence after resection 4, 5. Therefore, it is extremely important for us to further explore the molecular mechanisms of this deadly disease. Identification of novel genes that are potentially recognized as diagnostic and prognostic biomarkers and functionally involved in the ccRCC tumorigenic initiation and progression may improve early diagnosis of ccRCC and further help to develop more strategies for ccRCC treatment 6.
Glucose-6-phosphate dehydrogenase (G6PD) is the first and rate-limited enzyme of the pentose phosphate pathway (PPP) which is expressed mostly in all the cells 7. Recent studies demonstrated that G6PD was involved in the cell growth regulation and tumorigenesis. Wei-ying et al reported that overexpression of G6PD in NIH 3T3 could not only alter cell morphology and contact inhibition feature, but also gave rise to rapid growth and large fibrosarcomas in nude mice. These results imply that G6PD can act as a tumor driver gene 7. Recently, accumulated evidences reveal that elevated G6PD expression or activities have been found in a series of human cancers, including ovarian cancer 8, breast cancer 9, cervical carcinoma 10, prostate cancer 11, bladder cancer 12, etc. Moreover, G6PD overexpression is closely related to the progression of gastric cancer 13 and breast carcinoma 9, and also might be regarded as an independent predictor of poor prognosis for these cancers. Additionally, our previous reports also demonstrated that silencing G6PD expression decreased melanoma cell proliferation and enhanced apoptosis 14. However, there are still no reports that published regarding the expression profile of G6PD in human ccRCC, and what its clinical significance are, to date, unknown. Therefore, it is urgent and necessary to comprehensively investigate the expression pattern and evaluate the prognostic value of G6PD in ccRCC. Hence, in the present study, we extracted the data from “The Cancer Gene Atlas (TCGA)” to gain insight into the role of G6PD in ccRCC and additionally verified the clinicopathological significance of G6PD in ccRCC by immunohistochemical analysis.
Materials and methods
TCGA ccRCC data mining
Published mRNA expression data of 72 normal kidney tissues and 521 ccRCC cases (with Fuhrman tumor grade information) were downloaded from TCGA (https://tcga-data.nci.nih.gov/tcga/). Details for each patient that have clinical information regarding their Fuhrman grade, survival status and time to follow-up were also extracted from TCGA.
Tissue microarray and patients
75 human ccRCC tissues along with relevant normal adjacent tissue microarray sections with patient's basic parameters and overall survival information were purchased from Shanghai Outdo Biotech Co., LTD. (HKid-CRCC150CS-01). In addition, 74 ccRCC samples with clinical information (obtained from the Department of Pathology, First and Second Affiliated Hospital of Kunming Medical University with the informed consent and the approval from the Research Ethics Committee of Kunming Medical University) were also analyzed. In total, G6PD protein expression levels of tumor and relevant normal adjacent tissues were analyzed in 149 ccRCC patients. These patients were staged according to the TNM classification system of malignant tumors (7th) 15. The detailed characteristics of patients are listed in Table 1.
Table 1.
List of 149 clear cell renal cell carcinoma tissues
| Characteristics | n (%) |
|---|---|
| Gender | |
| Male | 114 (76.5%) |
| Female | 35 (23.5%) |
| Age at surgery | |
| <60 | 91 (61.1%) |
| ≥60 | 58 (38.9%) |
| Tumor extent a | |
| T1 T2 T3 |
85 (57.0%) 35 (23.5%) 20 (13.4%) |
| T4 | 9 (6.1%) |
| Lymph node metastasis a | |
| N0 ≥N1 |
122 (81.9%) 27 (18.1%) |
| Distant metastasis a | |
| M0 M1 |
141 (94.6%) 8 (5.4%) |
| Tumor max diameter (cm) | |
| <7 | 89 (59.7%) |
| ≥7 | 60 (40.3%) |
| Fuhrman grade b | |
| G1 G2 |
10 (6.8%) 64 (42.9%) |
| G3 G4 |
47 (31.5%) 28 (18.8%) |
| TNM stage a | |
| I II III |
77 (51.7%) 27 (18.1%) 28 (18.8%) |
| IV | 17 (11.4%) |
a According to 2009 TNM classification (7th) of malignant tumors by the International Union Against cancer 15.
b Based on the Fuhrman tumor grade system.
Immunohistochemistry
For immunological histological chemistry (IHC) staining, all specimen were subjected in turn to rehydration, antigen retrieval and endogenous peroxidase activity block in 3% H2O2, then a Pap pen was used to create a hydrophobic barrier around each tissue sections. After blocked with 5% bovine serum albumin (BSA), rabbit monoclonal anti-G6PD antibody (ab133525, Abcam, Cambridge, U.K.) or isotype control polyclonal rabbit IgG (X0936, DAKO A/S, Glostrup, Denmark) was used at recommended concentration of the instruction for IHC staining at 4°C overnight. Subsequently, Envision Detection Kit (GK500705, DAKO A/S) was used for the bound antibodies detection, and the sections were then counterstained with hematoxylin.
Immunohistologic analysis
A total of 10 random visual fields close to the center of each tumor core at 400 × magnification were examined under microscopy, and the immunostaining was evaluated by two independent investigators in a double-blinded fashion. G6PD expression was determined semi-quantitatively based on the staining intensity and the percentage of cells stained 13. The staining intensity was rated as follows: 1 point denoted as weak intensity; 2 points as moderate intensity; 3 points as strong intensity. The percentage of positive cells was rated as follows: 0 points as 0~25%; 2 points as 26~50%; 3 points as 51~75%; 4 points as 76~100%. The expression level of G6PD was scored as the product of staining intensity multiplied by the percentage of stained cells. Based on the final scores, specimens were categorized into negative (-), ≤5% cells were stained, regardless of intensity; weak (+), 1~4 points; moderate (++), 5~8 points; and strong (+++), 9~12 points. For the following statistical analysis, the negative and weak groups (- and +) were considered to be G6PD low expression and the moderate and strong groups (++ and +++) were considered to be G6PD high expression.
Statistical analysis
Statistical analyses were carried out using SPSS 17.0 (SPSS, Chicago, IL). For TCGA analysis, one-way ANOVA was employed to measure the statistical difference between various groups. Patients with clinical outcomes were separated into two groups based on the mean levels of G6PD mRNA expression. Log-rank test was used to measure the statistical difference between the high G6PD and low G6PD groups for Kaplan-Meier curves. For immunohistologic analysis, the correlation between G6PD expression and clinicopathological features of the patient was calculated using the χ2 test or Fisher exact test as appropriate. The Kaplan-Meier method was applied to assess the effect of G6PD overexpression on the ccRCC patient survival and the significance was evaluated by the log-rank test. Variables with p<0.05 at the univariate analysis were joined into in multivariate survival analysis by employing the Cox proportional hazards model. Differences were considered to be statistically significant at p<0.05.
Results
Data mining of the expression profile and prognostic value of G6PD in ccRCC patients
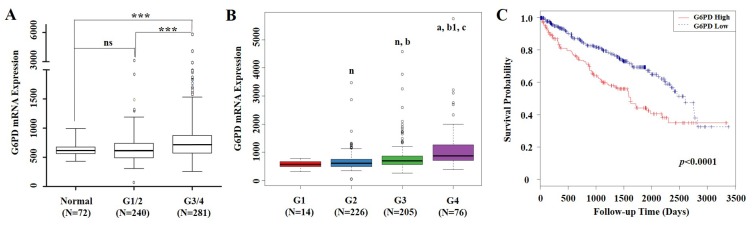
mRNA expression levels of G6PD and clinical information of 529 ccRCC cases were extracted from TCGA. From the validation dataset of these cases, we found that compared with normal renal tissues, G6PD gene was significantly upregulated in Fuhrman grade 3 and 4 (G3/4) of ccRCC specimens. Significant differences in G6PD expression were also observed between ccRCC G1/2 and G3/4 (p<0.001, ANOVA, Figure 1A). Moreover, G6PD showed a stepwise increment from G1 to G4 in ccRCC (Figure 1B). These findings prompted us to justify whether the G6PD expression was of significance in this current cohort of ccRCCs. Using the mean value of G6PD mRNA expression levels as cutoff, all 529 ccRCC cases were assigned into G6PD-low group (n=368) and G6PD-high group (n=161). Time to last follow-up was plotted in a Kaplan-Meier curve according to the different groups. These results showed that ccRCC patients who owned higher expression levels of G6PD but with a shorter survival rate (p<0.0001, log-rank test, Figure 1C), indicating that G6PD mRNA overexpression were correlated with poor outcomes in ccRCC.
Figure 1.
mRNA expression of G6PD and its association with survival in ccRCC based on TCGA data mining. (A) Box plot of G6PD mRNA levels in normal renal tissues, Fuhrman tumor grade 1 and 2 (G1/2) and G3/4 of ccRCC patients. Significant differences were observed by one-way ANOVA. (ns, not significant; ***p<0.001, vs. the control) (B) Stepwise expression of G6PD mRNA associated with significant increase in advanced Fuhrman grade in ccRCC. One-way ANOVA was applied to calculate the p value. (n, a, vs G1 group; b, b1 vs G2 group; c, vs G3 group. n, not significant; a, p=0.001; b, p=0.008; b1, c, p<0.001) (C) Kaplan-Meier analysis of G6PD mRNA expression and overall survival in ccRCC. Patients are stratified as low and high expression group by using mean G6PD mRNA expression level as cutoff. p values were calculated using a log-rank test (p<0.0001, vs. G6PD low group)
Analysis of G6PD expression in ccRCC tissues by immunohistologic staining
To verify the above results, the expression pattern of G6PD was further analyzed by IHC in 149 primary ccRCC specimens and relevant cancer-adjacent normal renal tissues. The G6PD levels in the tissue specimens were assessed by the measurement of the final staining scores. By using the same species of IgG antibody as internal control (Figure 2A), we found that G6PD was predominantly located in the cytoplasm of the renal cells (Figure 2B-D). Moreover, weak, moderate and strong positive expression of G6PD was detected in 49.0% (73/149), 28.2% (42/149) and 22.8% (34/149) of the ccRCC tissues (Figure 2B-D top panel), 62.4% (93/149), 30.2% (45/149) and 7.4% (11/149) of the noncancerous renal tissues (Figure 2B-D bottom panel). The moderate and strong groups were considered to be those which had high G6PD expression. Statistical analysis revealed that levels of G6PD were significantly increased in ccRCC tissues when compared with normal adjacent tissues. High expression of G6PD was detected in 51.0% (76/149) of the ccRCC specimens and 37.6% (56/149) of the adjacent samples (Figure 3A), and the score of G6PD expression in ccRCC and adjacent tissues was significantly different (p<0.05, χ2 test, Figure 3B).
Figure 2.
Representative images of immunohistochemical staining for G6PD in ccRCC and adjacent non-tumor renal tissues. (A) Immunohistochemistry in human ccRCC specimens and matched adjacent normal tissues by using IgG antibody as isotope negative control. (B-D) Immunohistochemistry for G6PD expression in matched adjacent normal tissues (upper row) and ccRCC tissue samples (lower row), showing weak (B), moderate (C), and strong (D) levels of G6PD staining. Images were captured using a 20 × objective lens. Scale bars=100 μm.
Figure 3.
Statistical analysis of G6PD immunoreactivity in human ccRCC and paired normal tissue. (A) Percentage of ccRCC specimens and matched adjacent normal tissues with different expression levels of G6PD was analyzed. (B) Statistical analysis of G6PD expression in ccRCC and matched adjacent normal tissues by χ2 test according to IHC staining scores. (* p<0.05, vs. matched adjacent normal tissues)
In addition, high expression levels of G6PD were also found in various ccRCC cell lines when compared with that in HK2 cell (a normal human renal tubular epithelial cell line) (Data not shown). These above results demonstrate that G6PD expression is significantly higher in ccRCC and might be associated with renal tumorigenesis.
Association of G6PD expression with clinicopathological features of ccRCC
To explore the role of G6PD in ccRCC, the correlations between G6PD expression levels and the clinicopathological parameters of the 149 ccRCC patients (Table 1) were analyzed. The results showed that the expression of G6PD was significantly associated with the tumor extent, lymph node metastasis, Fuhrman tumor grade, and TNM stage (p=0.031, p<0.001, p=0.001, and p=0.004, respectively; χ2 test, Table 2), but not significantly related to other clinicopathological features such as gender, age at surgery, or tumor max diameter (p>0.05, χ2 test, Table 2).
Table 2.
Correlation between the expression of G6PD and clinicopathologic parameters in clear cell renal cell carcinoma (n=149)
| Characteristics | Expression of G6PD | p value | |
|---|---|---|---|
| Low | High | ||
| Gender | |||
| Male | 53 | 61 | 0.270a |
| Female | 20 | 15 | |
| Age at surgery | |||
| <60 | 47 | 44 | 0.417a |
| ≥60 | 26 | 32 | |
| Tumor extent | |||
| T1/2 | 64 | 56 | 0.031a |
| T3/4 | 9 | 20 | |
| Lymph node metastasis | |||
| N0 | 68 | 54 | <0.001a |
| ≥N1 | 5 | 22 | |
| Distant metastasis | |||
| M0 | 72 | 69 | 0.063b |
| M1 | 1 | 7 | |
| Tumor max diameter (cm) | |||
| <7 | 44 | 45 | 0.895a |
| ≥7 | 29 | 31 | |
| Fuhrman grade | |||
| G1/2 | 46 | 28 | 0.001a |
| G3/4 | 27 | 48 | |
| TNM stage | |||
| I/II | 59 | 45 | 0.004a |
| III/IV | 14 | 31 | |
a, χ2 test; b, Fisher exact test.
Significant p-value is in bold.
Moreover, we found that in the T stages 1 and 2 (T1/2) of cancer tissues, 46.7% (56/120) cases exhibited strong immunopositivity, whereas 69.0% (20/29) cases exhibited strong immunopositivity in the T3/4 cancer tissues (Figure 4A left panel), and the final score of G6PD was remarkably increased in the T3/4 cancer tissues when compared with the T1/2 tissues (p<0.05, χ2 test, Figure 4A right panel). Likewise, the rate and score of G6PD expression in progressively advanced regional lymph node metastasis (p<0.001, χ2 test, Figure 4B), Fuhrman tumor grade 3 and 4 (G3/4) (p<0.01, χ2 test, Figure 4E), and TNM stage III and IV (III/IV) (p<0.01, χ2 test, Figure 4F) was also significantly different when compared with that in each control. However, the G6PD expression levels were not correlated with distant metastasis (p>0.05, Fisher exact test, Figure 4C) or tumor max diameter in ccRCC (p>0.05, χ2 test, Figure 4D). These results indicate that higher levels of G6PD might be correlated with ccRCC metastasis.
Figure 4.
Correlation between G6PD expression and clinical parameters in ccRCC. Percentage of ccRCC patients in different status (A-F left panel). G6PD expression level in different ccRCC status compared with each control according to IHC staining scores (A-F right panel). Statistical analysis was carried out with χ2 test (A, B, D-F) or Fisher exact test (C). (ns, not significant; *p<0.05, **p<0.01, ***p<0.001, vs. each control)
Correlation of G6PD expression with ccRCC prognosis
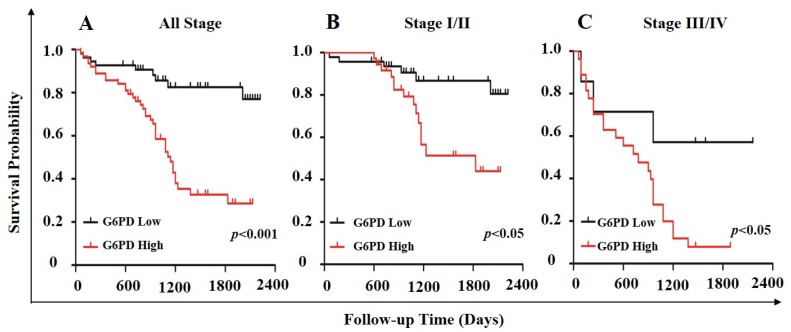
In order to further evaluate the value of G6PD in the prognosis of patients with ccRCC, Kaplan-Meier survival analysis and log-rank test were used to analyze the immunohistologic results. We divided the samples into high (n=54) and low (n=63) G6PD expression groups according to the IHC staining scores of G6PD. The results showed that the median survival of the patients with G6PD-high expression (1110 days) was significantly shorter than that of the patients with G6PD-low expression (p<0.001, log-rank test, Figure 5A). Similarly, both in stage I/II ccRCC and stage III/IV ccRCC, patients with higher G6PD expression had worse overall survival (p=0.013 and p=0.034, respectively; log-rank test, Figure 5B and Figure 5C).
Figure 5.
Association of G6PD expression level with survival in ccRCC. Stratification of ccRCC into low and high G6PD expression groups was based on an optimal threshold determined by immunohistologic analysis. Kaplan-Meier overall survival curves for all 117 patients (A; p<0.001), 83 patients with stage I/II ccRCC (B; p<0.05), and 34 patients with stage III/IV ccRCC (C; p<0.05) were shown. Significance measures were based on log-rank test of the p value (vs. G6PD low group).
Furthermore, the univariate cox regression analysis unraveled that tumor extent (T1/2, T3/4), lymph node metastasis (N0, ≥N1), distant metastasis (M0, M1), Fuhrman tumor grade (G1/2, G3/4), TNM stage (I/II, III/IV) as well as higher levels of G6PD expression were significant predictors of poorer outcome in ccRCC (all p<0.01, Table 3). The other clinical characteristics, such as gender (male, female), age at surgery (<60, ≥60) and tumor max diameter (<7 cm, ≥7 cm) were not statistically significant prognosis factors (all p>0.05, Table 3). When the G6PD level and other joined five factors were analyzed again using multivariate Cox hazard regression, the results showed that G6PD expression (p=0.007, Table 3), as well as Fuhrman grade (p<0.001, Table 3) and TNM stage (p=0.019, Table 3) were independent prognostic factors for ccRCC survival. In conclusion, these results demonstrate that higher expression of G6PD might be served as an independent prognostic factor of poor survival of ccRCC patients.
Table 3.
Cox regression analyses in clear cell renal cell carcinoma (n=117)
| Characteristics | Univariate | Multivariate | ||||||
|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | p value | HR | 95% CI | p value | |||
| Gender (Male vs Female) |
1.112 | 0.595-2.076 | 0.739 | |||||
| Age at surgery (<60 vs ≥60) |
1.019 | 0.993-1.046 | 0.150 | |||||
| Tumor extent (T1/2 vs T3/4) |
3.426 | 1.878-6.250 | <0.001 | 0.829 | 0.241-2.851 | 0.766 | ||
| Lymph node metastasis (N0 vs ≥N1) |
4.914 | 2.724-8.865 | <0.001 | 1.358 | 0.588-3.133 | 0.474 | ||
| Distant metastasis (M0 vs M1) |
3.188 | 1.347-7.544 | 0.008 | 0.890 | 0.336-2.356 | 0.815 | ||
| Tumor max diameter (<7 cm vs ≥7 cm) | 1.489 | 0.837-2.648 | 0.176 | |||||
| Fuhrman grade (G1/2 vs G3/4) |
16.091 | 4.988-51.904 | <0.001 | 9.545 | 2.835-32.132 | <0.001 | ||
| TNM stage (I/II vs III/IV) |
4.963 | 2.765-8.909 | <0.001 | 2.804 | 1.131-3.839 | 0.019 | ||
| G6PD expression (Low vs High) | 4.445 | 2.121-9.315 | <0.001 | 2.836 | 1.338-6.009 | 0.007 | ||
Abbreviations: HR, hazard ratio; CI, confidence interval; G6PD, Glucose-6-phosphate dehydrogenase.
Significant p-value is in bold.
Discussion
Each year, approximate 270,000 cases will be diagnosed as having renal cancer around the world 16-18. At present, considering the use of advanced instruments and innovative therapy, some cases of ccRCC can be diagnosed at early stages and cured by surgical resection and chemo-radiotherapy 5. However, because of metastasis and the high recurrence after surgical resection, ccRCC patients still have unfavorable prognosis and worse clinical outcomes. Although great effort has been made to for searching appropriate biomarkers for ccRCC diagnosis and prognosis, till date there is no individual biomarker that is satisfied for renal tumor classification and even fewer of them could be helpful for ccRCC prognosis or already has been commonly used in clinical validation 19-21. Therefore, identification of novel biomarkers that have diagnostic or prognostic significance may be of helpful for ccRCC earlier diagnosis and prognosis prediction, and may even become therapeutic target and improve the survival of ccRCC.
Metabolic reprogramming has been considered as a hallmark of cancer formation 22. The pentose phosphate pathway which is controlled by G6PD and always get disordered in cancers has been recognized to generate reducing agents and ribose 5-phosphate for maintenance of cancer cells' redox homeostasis and nucleotides biosynthesis 23. In ccRCC, the growth of renal cancer cells becomes faster and the intracellular reactive oxygen species (ROS) level becomes very high 24. Therefore, we hypothesized that the upregulated proliferation rate and heightened oxidative state might be controlled by the dysregulation of G6PD. In this study, to examine the role of G6PD in the pathogenesis of ccRCC, we downloaded transcriptomic and survival data from TCGA to analyze the expression levels of G6PD in ccRCC and normal renal tissues and then evaluate its correlation to the overall survival of ccRCC. We found that G6PD mRNA expression level was significantly higher in advanced pathological status in ccRCC. In addition, the statistical analysis results revealed that G6PD expression level of Fuhrman tumor grade G3 or G4 was obviously higher in ccRCC tissues when compared with that in the normal renal samples, G1 or G2 status, indicating a tumor concomitant role of G6PD which was dysregulated for the adaptation and progression of tumor cells to become more aggressive phenotypes. However, it should not be ignored that G6PD expression might be involved in the ccRCC tumorigenesis; but because of the small sample size in the Fuhrman grade G1 phase (only 14 cases), we could not draw a conclusion for its association with tumor initiation and future researches need to be done based on more large survey samples to address this hypothesis. More importantly, the results also revealed that higher expression of G6PD was associated with poor overall survival in patients with ccRCC. Our findings support that G6PD overexpression might be exploited to increase the incidence or progression of ccRCC.
To further confirm whether G6PD has the potential to become a new biomarkers for improving the diagnosis and to predict the prognosis of ccRCC patients, protein expression levels of G6PD in 149 primary ccRCC specimens (117 with follow-up data) and corresponding adjacent normal tissue samples were examined by using immunohistochemical analysis. We found that G6PD protein was expressed at higher levels in ccRCC tissues, and aberrant expression of G6PD was significantly correlated to tumor extent, lymph node metastasis, Fuhrman grade, and TNM stage of ccRCC. Positive G6PD expression was associated with shorter overall survival and it is prognostically independent for G6PD overexpression with worse overall survival in ccRCC. These results demonstrate that G6PD may be served as a prognostic biomarker to predict the prognosis of patients with ccRCC and become a potent therapeutic target for RCC treatment.
Our above immunohistochemical results found that G6PD was aberrantly high expressed in ccRCC than that of their normal counterparts which was similar to other study conclusions which used different cohorts and with distinct analytical approaches 25, 26. Moreover, we also found that G6PD expression in ccRCC was a prognostic molecular and high expression of G6PD predicted worse overall survival in ccRCC patients. But these findings are not in parallel with those from the study of German patients with ccRCC 26. In this report, the G6PD activity were higher in RCC specimens than that of the normal renal tissues, but was not much more increased in metastasizing tumors or associated with the TNM stage of RCC. However, there was a trend that G6PD activity might be higher in tumors with progression than in non-progressing RCC and the total number of patients included in this study was not very high (n=55) 26. Besides the ethnic differences of RCC patients, different experimental methods for analyzing the association between G6PD and ccRCC features may also be the reasons for different findings.
Tumor growth and development are complex and continuous processes. The regulatory mechanisms of ccRCC progression are also diversified. It has been reported that NOX4, an NADP (H) oxidase, products superoxide and has the most abundance in distal renal tubules 27. NOX4, which is highly expressed and is the main source of ROS in ccRCC cells, has been proved to contribute to the ccRCC tumorigenesis 24, 27. Furthermore, previous reports show that NOX4 suppression can abrogate ccRCC cells invasion, proliferation and the tumor formation in vivo, which demonstrate that NOX4 is of great importance for renal tumorigenesis 28. However, the molecular regulatory mechanism for NOX4 up-regulation in ccRCC remains unclear. In our pioneer study, we found that overexpression of G6PD in ACHN, a commonly used cell line for ccRCC study 29-31, could increase NOX4 expression and promote cell proliferation and migration (unpublished data). Although the molecular mechanism underlying G6PD overexpression is still not well defined in ccRCC, we have confidence that G6PD must possess multi-functions in facilitating ccRCC oncogenesis and further investigations are waiting to be carried out for testifying this hypothesis.
Collectively, by data mining the publicly-available datasets and immunohistochemical analysis, we demonstrated that G6PD was overexpressed in ccRCC and significantly correlated to advanced Fuhrman grade and TNM stage. Furthermore, G6PD expression was associated with ccRCC worse outcomes and high levels of G6PD expression could act as an independent unfavorable prognostic biomarker for ccRCC. Hence, our results indicate that G6PD may be regarded as a specific diagnostic biomarker and served as an independent predictor of poor prognosis to ccRCC patients and that the G6PD suppression represents a potential target for ccRCC treatment. Furthermore, the exact molecular mechanisms by which G6PD regulate the progression of ccRCC needs to be further elucidated.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 81460421, 81160426, 81560037, 31660246) and Department of Yunnan province - Kunming medical university of applied basic research joint special funds for major projects (No. 2013FB102 and No. 2016FB003).
Abbreviations
- G6PD
glucose-6-phosphate dehydrogenase
- PPP
pentose phosphate pathway
- ccRCC
clear cell renal cell carcinoma
- TCGA
The Cancer Gene Atlas
- IHC
immunological histological chemistry
- PBS
phosphate buffered saline
- BSA
bovine serum albumin
- ROS
reactive oxygen species
- CI
confidence interval
- HR
hazard ratio.
References
- 1.Busch J, Ralla B, Jung M. et al. Piwi-interacting RNAs as novel prognostic markers in clear cell renal cell carcinomas. Journal of experimental & clinical cancer research: CR. 2015;34:61. doi: 10.1186/s13046-015-0180-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA: a cancer journal for clinicians. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 3.Zhuang J, Tu X, Cao K. et al. The expression and role of tyrosine kinase ETK/BMX in renal cell carcinoma. Journal of experimental & clinical cancer research: CR. 2014;33:25. doi: 10.1186/1756-9966-33-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leibovich BC, Lohse CM, Crispen PL. et al. Histological subtype is an independent predictor of outcome for patients with renal cell carcinoma. The Journal of urology. 2010;183:1309–15. doi: 10.1016/j.juro.2009.12.035. [DOI] [PubMed] [Google Scholar]
- 5.Pantuck AJ, Zisman A, Belldegrun AS. The changing natural history of renal cell carcinoma. The Journal of urology. 2001;166:1611–23. [PubMed] [Google Scholar]
- 6.Li T, Cheng Y, Wang P. et al. CMTM4 is frequently downregulated and functions as a tumour suppressor in clear cell renal cell carcinoma. Journal of experimental & clinical cancer research: CR. 2015;34:122. doi: 10.1186/s13046-015-0236-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuo W, Lin J, Tang TK. Human glucose-6-phosphate dehydrogenase (G6PD) gene transforms NIH 3T3 cells and induces tumors in nude mice. International journal of cancer Journal international du cancer. 2000;85:857–64. doi: 10.1002/(sici)1097-0215(20000315)85:6<857::aid-ijc20>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 8.Yi H, Zheng X, Song J. et al. Exosomes mediated pentose phosphate pathway in ovarian cancer metastasis: a proteomics analysis. International journal of clinical and experimental pathology. 2015;8:15719–28. [PMC free article] [PubMed] [Google Scholar]
- 9.Pu H, Zhang Q, Zhao C. et al. Overexpression of G6PD is associated with high risks of recurrent metastasis and poor progression-free survival in primary breast carcinoma. World journal of surgical oncology. 2015;13:323. doi: 10.1186/s12957-015-0733-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu T, Li YS, Chen B. et al. Elevated glucose-6-phosphate dehydrogenase expression in the cervical cancer cases is associated with the cancerigenic event of high-risk human papillomaviruses. Experimental biology and medicine. 2015;240:1287–97. doi: 10.1177/1535370214565971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsouko E, Khan AS, White MA. et al. Regulation of the pentose phosphate pathway by an androgen receptor-mTOR-mediated mechanism and its role in prostate cancer cell growth. Oncogenesis. 2014;3:e103. doi: 10.1038/oncsis.2014.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang X, Wu G, Cao G. et al. Zoledronic acid inhibits the pentose phosphate pathway through attenuating the Ras-TAp73-G6PD axis in bladder cancer cells. Molecular medicine reports. 2015;12:4620–5. doi: 10.3892/mmr.2015.3995. [DOI] [PubMed] [Google Scholar]
- 13.Wang J, Yuan W, Chen Z. et al. Overexpression of G6PD is associated with poor clinical outcome in gastric cancer. Tumour biology: the journal of the International Society for Oncodevelopmental Biology and Medicine. 2012;33:95–101. doi: 10.1007/s13277-011-0251-9. [DOI] [PubMed] [Google Scholar]
- 14.Cai T, Kuang Y, Zhang C. et al. Glucose-6-phosphate dehydrogenase and NADPH oxidase 4 control STAT3 activity in melanoma cells through a pathway involving reactive oxygen species, c-SRC and SHP2. American journal of cancer research. 2015;5:1610–20. [PMC free article] [PubMed] [Google Scholar]
- 15.Sobin L GM, Wittekind C. TNM classification of malignant tumours. 7th ed. New York: Wiley; 2009. [Google Scholar]
- 16.Wang ZR, Wei JH, Zhou JC. et al. Validation of DAB2IP methylation and its relative significance in predicting outcome in renal cell carcinoma. Oncotarget. 2016;7:31508–19. doi: 10.18632/oncotarget.8971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baldewijns MM, van Vlodrop IJ, Schouten LJ. et al. Genetics and epigenetics of renal cell cancer. Biochimica et biophysica acta. 2008;1785:133–55. doi: 10.1016/j.bbcan.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 18.Ferlay J, Shin HR, Bray F. et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. International journal of cancer Journal international du cancer. 2010;127:2893–917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 19.Brooks SA, Brannon AR, Parker JS. et al. ClearCode34: A prognostic risk predictor for localized clear cell renal cell carcinoma. European urology. 2014;66:77–84. doi: 10.1016/j.eururo.2014.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gulati S, Martinez P, Joshi T. et al. Systematic evaluation of the prognostic impact and intratumour heterogeneity of clear cell renal cell carcinoma biomarkers. European urology. 2014;66:936–48. doi: 10.1016/j.eururo.2014.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mikami S, Mizuno R, Kosaka T. et al. Expression of TNF-alpha and CD44 is implicated in poor prognosis, cancer cell invasion, metastasis and resistance to the sunitinib treatment in clear cell renal cell carcinomas. International journal of cancer Journal international du cancer. 2015;136:1504–14. doi: 10.1002/ijc.29137. [DOI] [PubMed] [Google Scholar]
- 22.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 23.Zhang C, Zhang Z, Zhu Y. et al. Glucose-6-phosphate dehydrogenase: a biomarker and potential therapeutic target for cancer. Anti-cancer agents in medicinal chemistry. 2014;14:280–9. doi: 10.2174/18715206113136660337. [DOI] [PubMed] [Google Scholar]
- 24.Fitzgerald JP, Nayak B, Shanmugasundaram K. et al. Nox4 mediates renal cell carcinoma cell invasion through hypoxia-induced interleukin 6- and 8- production. PloS one. 2012;7:e30712. doi: 10.1371/journal.pone.0030712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lucarelli G, Galleggiante V, Rutigliano M. et al. Metabolomic profile of glycolysis and the pentose phosphate pathway identifies the central role of glucose-6-phosphate dehydrogenase in clear cell-renal cell carcinoma. Oncotarget. 2015;6:13371–86. doi: 10.18632/oncotarget.3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Langbein S, Frederiks WM, zur Hausen A. et al. Metastasis is promoted by a bioenergetic switch: new targets for progressive renal cell cancer. International journal of cancer Journal international du cancer. 2008;122:2422–8. doi: 10.1002/ijc.23403. [DOI] [PubMed] [Google Scholar]
- 27.Maranchie JK, Zhan Y. Nox4 is critical for hypoxia-inducible factor 2-alpha transcriptional activity in von Hippel-Lindau-deficient renal cell carcinoma. Cancer research. 2005;65:9190–3. doi: 10.1158/0008-5472.CAN-05-2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gregg JL, Turner RM 2nd, Chang G. et al. NADPH oxidase NOX4 supports renal tumorigenesis by promoting the expression and nuclear accumulation of HIF2alpha. Cancer research. 2014;74:3501–11. doi: 10.1158/0008-5472.CAN-13-2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang FQ, Zhang HM, Chen SJ. et al. MiR-506 is down-regulated in clear cell renal cell carcinoma and inhibits cell growth and metastasis via targeting FLOT1. PloS one. 2015;10:e0120258. doi: 10.1371/journal.pone.0120258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liep J, Kilic E, Meyer HA. et al. Cooperative Effect of miR-141-3p and miR-145-5p in the Regulation of Targets in Clear Cell Renal Cell Carcinoma. PloS one. 2016;11:e0157801. doi: 10.1371/journal.pone.0157801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zi X, Lusch A, Blair CA. et al. Effect of perineoplasm perinephric adipose tissues on migration of clear cell renal cell carcinoma cells: a potential role of WNT signaling. Oncotarget. 2016;7:53277–88. doi: 10.18632/oncotarget.10467. [DOI] [PMC free article] [PubMed] [Google Scholar]