Abstract
Background
Craniopharyngiomas are the most common benign histological tumours to involve the hypothalamo-pituitary region in childhood. Cystic craniopharyngiomas account for more than 90% of the tumours. The optimal treatment of cystic craniopharyngioma remains controversial. Radical resection is the treatment of choice in patients with favourable tumour localisation. When the tumour localisation is unfavourable, a gross-total or partial resection followed by radiotherapy is the main treatment option in adults. However, it presents a risk of morbidity, especially for children. Intracystic bleomycin has been utilised potentially to delay the use of radiotherapy or radical resection, to decrease morbidity. This review is the second update of a previously published Cochrane review.Objectives
To assess the benefits and harmful effects of intracystic bleomycin in children from birth to 18 years with cystic craniopharyngioma when compared to placebo (no treatment), surgical treatment (with or without adjuvant radiotherapy) or other intracystic treatments.Search methods
We searched the electronic databases CENTRAL (2016, Issue 1), MEDLINE/PubMed (from 1966 to February 2016) and EMBASE/Ovid (from 1980 to February 2016) with pre-specified terms. In addition, we searched the reference lists of relevant articles and reviews, conference proceedings (International Society for Paediatric Oncology 2005-2015) and ongoing trial databases (Register of the National Institute of Health and International Standard Randomised Controlled Trial Number (ISRCTN) register) in February 2016.Selection criteria
Randomised controlled trials (RCTs), quasi-randomised trials or controlled clinical trials (CCTs) comparing intracystic bleomycin and other treatments for cystic craniopharyngiomas in children (from birth to 18 years).Data collection and analysis
Two review authors independently performed the study selection, data extraction and 'Risk of bias' assessment. We used risk ratio (RR) for binary data and mean difference (MD) for continuous data. If one of the treatment groups experienced no events and there was only one study available for the outcome, we used the Fischer's exact test. We performed analysis according to the guidelines in the Cochrane Handbook for Systematic reviews of Interventions.Main results
We could not identify any studies in which the only difference between the treatment groups was the use of intracystic bleomycin. We did identify a RCT comparing intracystic bleomycin with intracystic phosphorus(32) ((32)P) (seven children). In this update we identified no additional studies. The included study had a high risk of bias. Survival could not be evaluated. There was no clear evidence of a difference between the treatment groups in cyst reduction (MD -0.15, 95% confidence interval (CI) -0.69 to 0.39, P value = 0.59, very low quality of evidence), neurological status (Fisher's exact P value = 0.429, very low quality of evidence), third nerve paralysis (Fischer's exact P value = 1.00, very low quality of evidence), fever (RR 2.92, 95% CI 0.73 to 11.70, P value = 0.13, very low quality of evidence) or total adverse effects (RR 1.75, 95% CI 0.68 to 4.53, P value = 0.25, very low quality of evidence). There was a significant difference in favour of the (32)P group for the occurrence of headache and vomiting (Fischer's exact P value = 0.029, very low quality of evidence for both outcomes).Authors' conclusions
Since we identified no RCTs, quasi-randomised trials or CCTs of the treatment of cystic craniopharyngiomas in children in which only the use of intracystic bleomycin differed between the treatment groups, no definitive conclusions could be made about the effects of intracystic bleomycin in these patients. Only one low-power RCT comparing intracystic bleomycin with intracystic (32)P treatment was available, but no definitive conclusions can be made about the effectiveness of these agents in children with cystic craniopharyngiomas. Based on the currently available evidence, we are not able to give recommendations for the use of intracystic bleomycin in the treatment of cystic craniopharyngiomas in children. High-quality RCTs are needed.Free full text

Intracystic bleomycin for cystic craniopharyngiomas in children
 Bo Wen Cai, Jian Guo Xu, Chao You, and Cochrane Childhood Cancer Group
Bo Wen Cai, Jian Guo Xu, Chao You, and Cochrane Childhood Cancer GroupAbstract
Background
Craniopharyngiomas are the most common benign histological tumours to involve the hypothalamo‐pituitary region in childhood. Cystic craniopharyngiomas account for more than 90% of the tumours. The optimal treatment of cystic craniopharyngioma remains controversial. Radical resection is the treatment of choice in patients with favourable tumour localisation. When the tumour localisation is unfavourable, a gross‐total or partial resection followed by radiotherapy is the main treatment option in adults. However, it presents a risk of morbidity, especially for children. Intracystic bleomycin has been utilised potentially to delay the use of radiotherapy or radical resection, to decrease morbidity. This review is the second update of a previously published Cochrane review.
Objectives
To assess the benefits and harmful effects of intracystic bleomycin in children from birth to 18 years with cystic craniopharyngioma when compared to placebo (no treatment), surgical treatment (with or without adjuvant radiotherapy) or other intracystic treatments.
Search methods
We searched the electronic databases CENTRAL (2016, Issue 1), MEDLINE/PubMed (from 1966 to February 2016) and EMBASE/Ovid (from 1980 to February 2016) with pre‐specified terms. In addition, we searched the reference lists of relevant articles and reviews, conference proceedings (International Society for Paediatric Oncology 2005‐2015) and ongoing trial databases (Register of the National Institute of Health and International Standard Randomised Controlled Trial Number (ISRCTN) register) in February 2016.
Selection criteria
Randomised controlled trials (RCTs), quasi‐randomised trials or controlled clinical trials (CCTs) comparing intracystic bleomycin and other treatments for cystic craniopharyngiomas in children (from birth to 18 years).
Data collection and analysis
Two review authors independently performed the study selection, data extraction and 'Risk of bias' assessment. We used risk ratio (RR) for binary data and mean difference (MD) for continuous data. If one of the treatment groups experienced no events and there was only one study available for the outcome, we used the Fischer's exact test. We performed analysis according to the guidelines in the Cochrane Handbook for Systematic reviews of Interventions.
Main results
We could not identify any studies in which the only difference between the treatment groups was the use of intracystic bleomycin. We did identify a RCT comparing intracystic bleomycin with intracystic phosphorus32 (32P) (seven children). In this update we identified no additional studies. The included study had a high risk of bias. Survival could not be evaluated. There was no clear evidence of a difference between the treatment groups in cyst reduction (MD ‐0.15, 95% confidence interval (CI) ‐0.69 to 0.39, P value = 0.59, very low quality of evidence), neurological status (Fisher's exact P value = 0.429, very low quality of evidence), third nerve paralysis (Fischer's exact P value = 1.00, very low quality of evidence), fever (RR 2.92, 95% CI 0.73 to 11.70, P value = 0.13, very low quality of evidence) or total adverse effects (RR 1.75, 95% CI 0.68 to 4.53, P value = 0.25, very low quality of evidence). There was a significant difference in favour of the 32P group for the occurrence of headache and vomiting (Fischer's exact P value = 0.029, very low quality of evidence for both outcomes).
Authors' conclusions
Since we identified no RCTs, quasi‐randomised trials or CCTs of the treatment of cystic craniopharyngiomas in children in which only the use of intracystic bleomycin differed between the treatment groups, no definitive conclusions could be made about the effects of intracystic bleomycin in these patients. Only one low‐power RCT comparing intracystic bleomycin with intracystic 32P treatment was available, but no definitive conclusions can be made about the effectiveness of these agents in children with cystic craniopharyngiomas. Based on the currently available evidence, we are not able to give recommendations for the use of intracystic bleomycin in the treatment of cystic craniopharyngiomas in children. High‐quality RCTs are needed.
Plain language summary
Intracystic bleomycin for children with cystic craniopharyngiomas
Craniopharyngiomas are rare, slow‐growing, benign tumours in the hypothalamic‐pituitary region of the brain. Although they are benign, i.e. the tumour lacks the ability to invade neighbouring tissue or metastasise (spread to other sites), there is considerable morbidity and disability even when the tumour can be resected completely. Cystic craniopharyngiomas are the most common type of craniopharyngiomas. They consist of a solid portion that contains fluid‐filled balloon‐like structures (cysts). Cysts are a problem because secretion of fluid into them allows the tumour to increase in size, which puts pressure on parts of the brain and can cause damage. Radical resection (removal by surgery) alone is not sufficient because the rate of recurrence is high and this procedure has a high risk of endocrinological/neurological deficits such as blindness; loss of control of appetite, urine production, emotional behaviour and physical co‐ordination; memory loss; sleep disturbances; cessation of growth and sexual development; low thyroxine levels; hydrocephalus (high pressure inside the skull); and death. While in adults radiotherapy represents a valid postoperative adjunctive (additional) therapy, in children it has a high risk of side effects including further damage to any remaining sight, with reduction of intelligence quotient (IQ) and ability to perform complex tasks in later life. Intracystic bleomycin (i.e. a type of chemotherapeutic agent injected into the cyst) has been used to potentially decrease the damage associated with cystic craniopharyngioma.
This systematic review focused on (randomised) controlled studies. We could not identify any randomised controlled trials (RCTs), quasi‐randomised trials or controlled clinical trials (CCTs) in which the only difference between the intervention and control group was the use of intracystic bleomycin. However, we did identify one RCT comparing intracystic bleomycin with intracystic phosphorus32 (32P), which is a radioactive isotope of phosphorous used for intracystic irradiation. Only seven children were included in this study. The study has a high risk of bias and the sample size is too small to detect a difference in outcomes. The therapeutic use of intracystic bleomycin in children with cystic craniopharyngiomas currently remains uncertain. Although there was no significant difference in total adverse effects between the two treatment groups, there was a significant difference in both headache and vomiting in favour of the 32P group. The quality of the evidence is, however, very low. More high‐quality studies are needed but will be difficult as so few children get these tumours.
Summary of findings
Background
Craniopharyngiomas are usually slow‐growing, benign tumours that originate from epithelial nests or from areas of squamous metaplasia located in the hypothalamic and pituitary regions. Craniopharyngiomas are the most common intracranial tumours of non‐glial origin in the paediatric population, constituting 6% to 13% of all childhood brain tumours and peaking at five to 10 years of age (Sanford 1991; Bunin 1998). Although the tumour is of a benign histological nature, there is considerable morbidity and disability even when the tumour can be resected completely.
Description of the condition
Treatment of childhood craniopharyngioma is an ongoing controversy. The currently accepted therapy is radical tumour resection. However, surgery still remains challenging because the tumour is infiltrative to the tuber cinereum and the hypothalamus. Patient series have demonstrated that gross‐total resection of craniopharyngiomas was achieved in only 50% to 80% of attempted radical resections (Sainte‐Rose 2005; Thompson 2005; Tomita 2005; Zuccaro 2005). Furthermore, even after total resection, the tumour recurrence rate is high (Yasargil 1990; Weiner 1994), especially in the residual cystic portion (Takahashi 1985). Some authors advocate external radiotherapy after partial resection of the tumour (Hetelekidis 1993; Wara 1994), which has resulted in less early morbidity, however, it may increase endocrine disturbance and mortality, especially in children (Gleeson 2003).
Description of the intervention
Bleomycin was first discovered by Hamao Umezawa in 1966 (Umezawa 1966), and was launched in Japan by Nippon Kayaku in 1969. It was found to be effective in various types of epithelial tumours and benign squamous epithelial cells. Kubo 1974 confirmed the toxicity of bleomycin to cultured craniopharyngioma cells in 1971. In 1985, Takahashi 1985 published a paper concerning seven patients with craniopharyngioma treated with intratumoural bleomycin, which indicated that the local injection of bleomycin was not as effective against craniopharyngioma of the mixed or solid type, but was markedly effective against tumours of the cystic type. After that, other research confirmed that intracystic bleomycin used to treat cystic craniopharyngioma may eliminate cysts completely with few complications (Broggi 1989; Mettolese 2001).
How the intervention might work
Bleomycin is an antitumour antibiotic secreted by Streptomyces verticillus. It is composed of two main glycopeptides, bleomycin A2 and B2. Its action is based on inhibition of DNA and RNA synthesis through the formation of metal complexes with copper and iron serving as co‐factors. These effects on the cell cycle are the G, M and S phases.
Bleomycin is very effective in squamous cell carcinomas. The squamous epithelium associated with keratinisation is characteristically found in the cyst walls of craniopharyngiomas and in its solid component. Therefore, it is believed that it can be used to treat cystic craniopharyngiomas.
Why it is important to do this review
Although some researchers have advocated bleomycin for the treatment of cystic craniopharyngioma, it is a neurotoxic drug and if leakage occurs severe complications may develop, including death. The aim of this review is to evaluate the existing evidence on intracystic bleomycin in the treatment of children with craniopharyngioma. This is an update of the previous systematic reviews evaluating the use of intracystic bleomycin for cystic craniopharyngiomas in children (Fang 2012; Zheng 2014).
Objectives
To assess the benefits and harmful effects of intracystic bleomycin in children from birth to 18 years with cystic craniopharyngioma.
Specific objectives are to compare:
intracystic bleomycin with placebo (no treatment);
intracystic bleomycin with surgical treatment (with or without adjuvant radiotherapy);
intracystic bleomycin with other intracystic treatments.
Methods
Criteria for considering studies for this review
Types of studies
We planned to include all randomised controlled trials (RCTs), quasi‐randomised trials or controlled clinical trials (CCTs) as defined by the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), which compared intracystic bleomycin with placebo/no treatment or surgical treatment (with or without adjuvant radiotherapy) or other intracystic treatments. We did not consider any other uncontrolled observational trials.
Types of participants
Participants had to meet the following criteria.
Between the ages of 0 to 18 years (age at onset of intracystic bleomycin treatment).
Diagnosed as having cystic craniopharyngioma by brain computerised tomography (CT) or magnetic resonance imaging (MRI).
Types of interventions
Intracystic bleomycin. Procedure: the catheter is inserted inside the tumour with the help of a surgical microscope or stereotactically or via a neuroendoscopic technique, and is connected to a subcutaneous reservoir. After catheter insertion, contrast agents are injected into the cyst to verify that there is no fluid leakage. Intratumoural fluid is aspired and bleomycin is injected into the cyst. The control group is given placebo/no treatment or surgical treatment (with or without adjuvant radiotherapy) or other intracystic treatments. The dose, frequency and duration can vary.
Types of outcome measures
Primary outcomes
The primary endpoint is overall survival (OS) or event‐free survival (EFS) at the end of the follow‐up.
OS is defined as the time to death from any cause. EFS is defined as the time to recurrence or progression of primary disease or death from any cause.
Secondary outcomes
We grouped time points into 'short‐term' (less than one year) or 'long‐term' (more than one year) outcomes. We evaluated the measurements as follows at pre‐specified time points.
The response of the cyst to treatment: sensitive or insensitive (as defined by the authors).
The size of the cystic component: the rate of decrease in the size of the cystic component.
Endocrine function: the comparison between pre‐ and postoperative endocrine deficits including: hypopituitarism, short stature, weight alterations, diabetes insipidus. The change is classified as improved, stable, worsened and new cases.
Neurological status: no disability; moderate disability; severe disability (the criteria of disability as defined by the authors).
Visual outcome: classified as improved, stable, worsened and new cases.
Quality of life: measured using a scale that has been validated through reporting of norms in a peer‐reviewed publication.
Adverse effects: such as death, cerebrovascular event, new neurological deficit, new hypothalamic dysfunction, new visual dysfunction, the recurrence of craniopharyngioma, headache, nausea, vomiting, transient fever, arthralgia, chronic fatigue syndrome etc.; number of patients who withdrew due to adverse events compared with placebo, no treatment, surgical treatment (with or without adjuvant radiotherapy) or other intracystic chemotherapy groups.
Search methods for identification of studies
Electronic searches
We searched the following electronic databases: the Cochrane Central Register of Controlled Trials (CENTRAL 2016, Issue 1), MEDLINE/PubMed (from 1945 to 10 February 2016) and EMBASE/Ovid (from 1980 to 10 February 2016). We have presented the sensitive search strategies used for CENTRAL, MEDLINE/PubMed and EMBASE/Ovid in Appendix 1, Appendix 2 and Appendix 3.
Searching other resources
We located information about trials not registered in CENTRAL, MEDLINE or EMBASE, either published or unpublished, by searching the reference lists of relevant articles and reviews. We scanned electronically the proceedings abstracts of the International Society for Paediatric Oncology (SIOP) (from 2005 up to and including 2015) on 3 March 2016. We searched the International Standard Randomised Controlled Trial Number (ISRCTN) register (http://www.isrctn.com) and the register of the National Institutes of Health (http://www.clinicaltrials.gov) for ongoing trials on 3 March 2016. We did not impose any language restrictions. For details of these search strategies see Appendix 4 and Appendix 5. We will update the searches every two years.
Data collection and analysis
Selection of studies
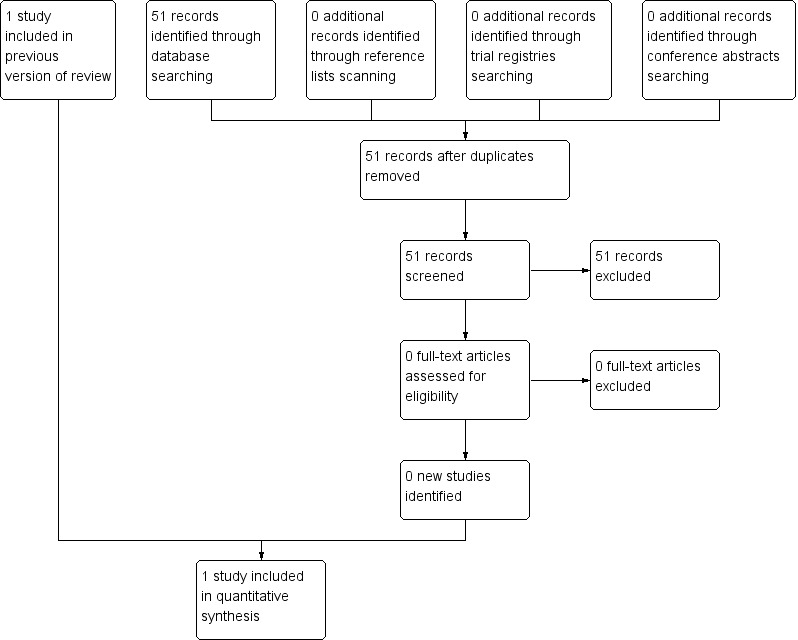
Two review authors independently screened the titles and abstracts of studies identified through the searches and selected trials that met the inclusion criteria. We retrieved full articles for further assessment. We used discussion and consultation with a third review author to resolve any disagreement. The selection process is outlined in a flow chart (see Figure 1)
Data extraction and management
For included studies, two review authors independently extracted the following information using a standard form:
General information: title, authors, published/unpublished, year of publication, language of publication, duplicate publications, study design, country, reference/source, contact address, urban/rural, sponsoring, setting.
Intervention: dose, route, timing, control intervention (placebo, no treatment, surgical treatment (with or without adjuvant radiotherapy), some other intracystic chemotherapies).
Participants: sampling, total number and number in comparison groups, sex, age, trial inclusion and exclusion criteria; withdrawals/losses to follow‐up (reasons/description), subgroups.
Outcomes: outcomes specified above, length of follow‐up, quality of reporting of outcomes.
If there were differences in data extraction, we resolved these by discussion, referring back to the original paper, or by consulting a third person.
Assessment of risk of bias in included studies
Two authors planned to assess independently the risk of bias in included RCTs and CCTs using Cochrane's 'Risk of bias' tool (Higgins 2011). The tool considers six domains of bias: sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting and other issues. For each domain, the study method is described using verbatim quotes and judged for adequacy (high, low, unclear risk of bias). We added items for the assessment of risk of bias as described in the module of Cochrane Childhood Cancer (Kremer 2010). We resolved any disagreements by discussion or using a third party arbitrator, and presented the results in the 'Risk of bias' table and also in both graph and written summary form.
Risk of bias assessment for non‐randomised clinical trials is a complex topic. However, since no non‐randomised trials (i.e. CCTs) were identified, using a modification of the Methodological Index for Non‐Randomised Studies (MINORS) was not applicable (Slim 2003).
Measures of treatment effect
For time‐to‐event data (e.g. overall survival and event‐free survival), we used the hazard ratio (HR). If HRs were not explicitly presented in the study, we planned to use Parmar's method (Parmar 1998).
For dichotomous outcomes (e.g. response of cyst to treatment, adverse events, visual outcome, neurological status and endocrine function), we calculated risk ratios (RR) with 95% confidence intervals (CI) for each trial.
For continuous outcomes (e.g. the rate of decrease of the size of the cystic component, quality of life), we intended to evaluate mean difference (MD) or standardised mean difference (SMD) with 95% CI. We only analysed one continuous outcome, reduction of cyst size, and for this we reported the MD.
Unit of analysis issues
We planned to consider each individual study included in the meta‐analysis as a unit for analysis.
Dealing with missing data
We analysed all data on an intention‐to‐treat basis. We did not contact authors for missing data.
Assessment of heterogeneity
Since only one eligible study was identified, assessing the presence of substantial heterogeneity (i.e. I² > 50% (Higgins 2011)) was not applicable.
Assessment of reporting biases
We planned to construct a funnel plot (Egger 1997), to ascertain graphically the existence of publication bias. However, as a rule of thumb, tests for funnel plot asymmetry should be used only when there are at least 10 studies included in the meta‐analysis, because when there are fewer studies the power of the tests is too low to distinguish chance from real asymmetry (Higgins 2011). Since only one RCT could be included in this review, we did not construct funnel plots.
Data synthesis
We performed data synthesis and analyses using the Cochrane Review Manager software, RevMan 5.3 (RevMan 2014). We performed the analyses according to the recommendations of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We used the Mantel‐Haenszel method to perform the analysis. Since only one RCT could be included in this review, we used a fixed‐effect model. Also, because there was only one study available, we were unable to calculate a RR if one of the treatment groups experienced no events and we therefore used the Fisher's exact test instead (in SPSS 18.0). For each comparison we prepared a 'Summary of findings' table using the GRADE profiler software (www.gradepro.org), in which we presented the following outcomes: survival, size reduction of cyst, neurological status, total number of adverse effects, fever, third nerve paralysis and headache in combination with vomiting. For each outcome two review authors independently assessed the quality of the evidence by using the five GRADE considerations, i.e. study limitations, inconsistency, indirectness, imprecision and publication bias as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) and the GradePro Handbook (Schünemann 2013).
Subgroup analysis and investigation of heterogeneity
As only one eligible study was identified and the sample size was small, subgroup analysis was not applicable. Otherwise we would have looked at:
age at onset of intracystic bleomycin treatment;
gender;
dosage (or the concentration of the dose) of treatment;
multicystic craniopharyngioma.
Sensitivity analysis
Since only one eligible study was identified, performing sensitivity analyses for the quality criteria used was not applicable.
Results
Description of studies
Results of the search
For the original review, we identified a total of 295 references through electronic searches of the Cochrane Central Register of Controlled Trials (CENTRAL) (n = 2), PubMed (n = 202) and EMBASE (n = 91). We screened one study in full text and only this randomised controlled trial was eligible for inclusion in the original review (Jiang 2002) (see Characteristics of included studies).
For the previous update, we identified an additional 68 references through electronic searches of CENTRAL (n = 0), PubMed (n = 50) and EMBASE (n = 18). From reading the titles and abstracts, we were not able to identify any eligible study from the references. No additional studies or trials was eligible for inclusion.
For this update, we identified an additional 51 references through electronic searches of CENTRAL (n = 0), PubMed (n = 39) and EMBASE (n = 12). From reading the titles and abstracts, we were not able to identify any eligible study from the references. Scanning the reference lists of relevant studies and reviews, and scanning the conference proceedings of SIOP, did not identify any other eligible studies. Scanning the ongoing trials databases did not identify any eligible (ongoing) studies. In summary, only one RCT could be included in this review. We identified no eligible CCTs or ongoing studies (See Figure 1).
Included studies
See Characteristics of included studies.
This study also included adults, but we only extracted data for children in the intracystic bleomycin and intracystic phosphorus32 (32P) groups. The total number of children was seven. Three children received intracystic bleomycin and four children received intracystic 32P. In the bleomycin group, a 1.0 ml solution of bleomycin was injected through the tube into the cysts daily for eight days. In the 32P group, 0.9% saline was injected daily into the cysts for seven days and 32P was administered to the patients in the 32P group on the eighth day. The dose of bleomycin started at 5.0 mg and was increased by 2.5 mg or 5.0 mg each day, depending on the tolerance of the patient to the drug. The largest daily dose was no more than 15 mg. The largest total dose received by any patient was 120 mg, while the least was 14.5 mg. The radioactive dose of 32P to the cyst wall was 200 Gy. The outcomes reported in the trial were size reduction of the cyst, neurological status and adverse effects (headache, fever, vomiting and third nerve paralysis). The trial did not report survival, response of the cyst to treatment, endocrine function, visual outcome or quality of life.
Excluded studies
There were no excluded studies.
Risk of bias in included studies
The risk of bias is summarised in the 'Risk of bias' summary (Figure 2) and 'Risk of bias' graph (Figure 3). For more detailed information see the 'Risk of bias' table in the Characteristics of included studies.
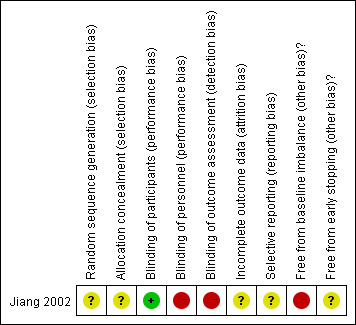
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
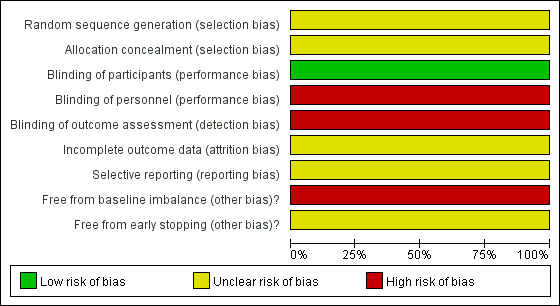
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
The generation of allocation sequence and allocation concealment was unclear.
Blinding
Blinding of participants was performed. However, blinding of personnel and outcome assessors for the clinical outcomes was not performed.
Incomplete outcome data
The numbers and reasons for dropouts and withdrawals in all intervention groups were not described. We considered the trial to have an unclear risk of bias.
Selective reporting
The protocol for this study was unavailable, therefore the trial was at unclear risk of bias.
Other potential sources of bias
Baseline imbalance
The patients in the bleomycin group were all multicystic, while the patients in the 32P group were not. Hence, the trial did have a risk of baseline imbalance.
Early stopping
The sample size calculations for the trial were not reported. Hence, it was unclear whether the trial was stopped early.
Summary of quality assessment of included trial
We considered the trial to have a high risk of performance bias, detection bias and baseline imbalance bias, and an unclear risk of selection bias, attrition bias, reporting bias and early stopping bias.
Effects of interventions
See: Table 1
Summary of findings for the main comparison
| Intracystic bleomycin compared to intracystic radiotherapy with 32P for cystic craniopharyngiomas in children | ||||||
| Patient or population: cystic craniopharyngiomas in children from birth to 18 years old Setting: inpatients from 3 hospitals in China Intervention: intracystic bleomycin Comparison: intracystic radiotherapy with 32P | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with intracystic 32P | Risk with intracystic bleomycin | |||||
| Survival | NA | NA | ‐ | ‐ | ‐ | There was no information on overall survival and event‐free survival reported in this trial |
| Change in cyst size | Mean reduction in cyst size was 65.5% | Mean reduction in cyst size was 50.7% | MD ‐15% (‐69% to 39%) | 7 (1 RCT) | ![[plus sign in circle]](/http/europepmc.org/corehtml/pmc/pmcents/x2295.gif) ![[hyphen in circle]](/http/europepmc.org/corehtml/pmc/pmcents/x229D.gif) ![[hyphen in circle]](/http/europepmc.org/corehtml/pmc/pmcents/x229D.gif) ![[hyphen in circle]](/http/europepmc.org/corehtml/pmc/pmcents/x229D.gif) VERY LOW 1 | MD was 15% reduction in cyst size; the 95% CI mean a 69% reduction to a 39% increase in cyst size |
| Neurological status | See comment | See comment | Not estimable | Not estimable | ![[plus sign in circle]](/http/europepmc.org/corehtml/pmc/pmcents/x2295.gif) ![[hyphen in circle]](/http/europepmc.org/corehtml/pmc/pmcents/x229D.gif) ![[hyphen in circle]](/http/europepmc.org/corehtml/pmc/pmcents/x229D.gif) ![[hyphen in circle]](/http/europepmc.org/corehtml/pmc/pmcents/x229D.gif) VERY LOW 1 | 1 patient in the bleomycin group had left paralysis, while none of the patients in the 32P group had severe sequelae. There was no significant difference between the 2 groups (Fisher's exact P value = 0.429). |
| Total number of adverse effects | 500 per 1000 | 875 per 1000 (340 to 1000) | RR 1.75 (0.68 to 4.53) | 7 (1 RCT) | ![[plus sign in circle]](/http/europepmc.org/corehtml/pmc/pmcents/x2295.gif) ![[hyphen in circle]](/http/europepmc.org/corehtml/pmc/pmcents/x229D.gif) ![[hyphen in circle]](/http/europepmc.org/corehtml/pmc/pmcents/x229D.gif) ![[hyphen in circle]](/http/europepmc.org/corehtml/pmc/pmcents/x229D.gif) VERY LOW 1 | Adverse effects including fever, 3rd nerve paralysis, headache and vomiting were included in this outcome measure |
| Fever | 250 per 1000 | 730 per 1000 (183 to 1000) | RR 2.92 (0.73 to 11.70) | 7 (1 RCT) | ![[plus sign in circle]](/http/europepmc.org/corehtml/pmc/pmcents/x2295.gif) ![[hyphen in circle]](/http/europepmc.org/corehtml/pmc/pmcents/x229D.gif) ![[hyphen in circle]](/http/europepmc.org/corehtml/pmc/pmcents/x229D.gif) ![[hyphen in circle]](/http/europepmc.org/corehtml/pmc/pmcents/x229D.gif) VERY LOW 1 | ‐ |
| 3rd nerve paralysis | See comment | See comment | Not estimable | 7 (1 RCT) | ![[plus sign in circle]](/http/europepmc.org/corehtml/pmc/pmcents/x2295.gif) ![[hyphen in circle]](/http/europepmc.org/corehtml/pmc/pmcents/x229D.gif) ![[hyphen in circle]](/http/europepmc.org/corehtml/pmc/pmcents/x229D.gif) ![[hyphen in circle]](/http/europepmc.org/corehtml/pmc/pmcents/x229D.gif) VERY LOW 1 | 1 patient in the 32P group had 3rd nerve paralysis, while none of the patients in the bleomycin group suffered from this. There was no significant difference between the 2 groups (Fisher's exact P value = 1.00). |
| Headache and vomiting | See comment | See comment | Not estimable | 7 (1 RCT) | ![[plus sign in circle]](/http/europepmc.org/corehtml/pmc/pmcents/x2295.gif) ![[hyphen in circle]](/http/europepmc.org/corehtml/pmc/pmcents/x229D.gif) ![[hyphen in circle]](/http/europepmc.org/corehtml/pmc/pmcents/x229D.gif) ![[hyphen in circle]](/http/europepmc.org/corehtml/pmc/pmcents/x229D.gif) VERY LOW 1 | All 3 patients in the bleomycin group had headache and vomiting, while none of the patients in the 32P group did. There was a significant difference in favour of the 32P group (Fisher's exact P value = 0.029). |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; NA: not available; RR: risk ratio; OR: odds ratio | ||||||
| GRADE Working Group grades of evidence
High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1We downgraded a total of 3 levels due to study limitations (the trial had a high risk of performance bias, detection bias and baseline imbalance bias, and an unclear risk of selection bias, attrition bias, reporting bias and early stopping bias), small sample size (the current review includes only one trial with small sample size (n = 7)), and wide confidence intervals including 'no effect', 'appreciable harm' and 'appreciable benefit'.
Overall survival or event‐free survival
There was no information on overall survival and event‐free survival reported in this trial.
The response of the cyst to treatment
There was no information about the response of the cyst to treatment.
The size of the cystic component
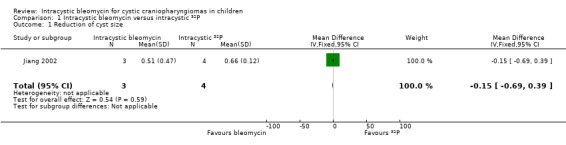
There was no significant difference in cyst size reduction between the bleomycin and phosphorus32 (32P) groups (mean difference (MD) ‐0.15, 95% confidence interval (CI) ‐0.69 to 0.39, P value = 0.59, very low quality of evidence) (see Analysis 1.1 and Table 1).
Endocrine function
The data for endocrine function could not be extracted.
Neurological status
One child in the bleomycin group had left paralysis after two episodes of epilepsy. None of the children in the 32P group had severe sequelae. There was no significant difference between the groups (Fisher's exact P value = 0.429, very low quality of evidence).
Visual outcome
There was no visual outcome reported in this trial.
Quality of life
There was no quality of life outcome reported in this trial.
Adverse effects
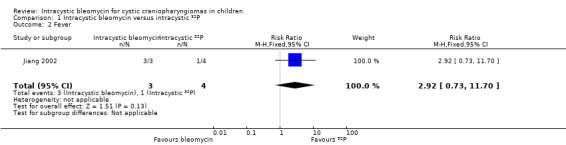
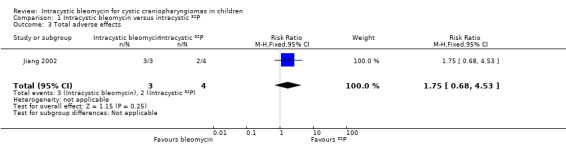
All three children in the bleomycin group had complications of fever, headache and vomiting, however, in the 32P group there was only one patient who had transient fever; none of the patients in that group suffered from headache or vomiting. There was no significant difference in fever between the two groups (risk ratio (RR) 2.92, 95% CI 0.73 to 11.70, P value = 0.13, very low quality of evidence) (see Analysis 1.2 and Table 1), but there was a significant difference in headache (Fisher's exact P value = 0.029, very low quality of evidence) and vomiting (Fisher's exact P value = 0.029, very low quality of evidence) in favour of the 32P group. A patient in the 32P group had third nerve paralysis, while none of the patients in the bleomycin group suffered from this. There was no significant difference between the two groups (Fisher's exact P value = 1.00, very low quality of evidence). In summary, there was no significant difference in total adverse effects between the groups (RR 1.75, 95% CI 0.68 to 4.53, P value = 0.25, very low quality of evidence) (see Analysis 1.3 and Table 1).
Subgroup analysis
It was not possible to perform subgroup analysis due to the small sample size.
Discussion
The occurrence of cysts is one of the typical characteristics of craniopharyngioma, occurring in more than 90% of tumours (Blacklund 1994). The treatment of cystic craniopharyngioma is controversial. Puget 2007 used a preoperative classification system to grade hypothalamic involvement and stratify treatment. For tumours that do not involve the hypothalamus and that are amenable to complete resection without hypothalamic injury, radical resection should be attempted. For tumours that appear to invade the hypothalamus on magnetic resonance imaging (MRI), especially if children with these tumours already have clinical evidence of hypothalamic dysfunction, the risks of radical resection are higher and partial resection followed by radiotherapy might be feasible. However, for any type of radiotherapy, the potential complications are higher the younger the child is, therefore intracystic treatment that delays the use of radiotherapy or is followed by resection may be beneficial. Another systematic review has concluded that intracystic interferon currently seems to have the best benefit‐risk ratio, but the evidence on which this was based was from non‐randomised controlled trials (Bartels 2012). This is an update of the previous systematic reviews evaluating the use of intracystic bleomycin for cystic craniopharyngiomas in children (Fang 2012; Zheng 2014).
There were no eligible randomised controlled trials (RCTs), quasi‐randomised trials or controlled clinical trials (CCTs) in which only the use of intracystic bleomycin in children differed between groups. Even though RCTs are the highest level of evidence, it should be recognised that data from non‐randomised studies on the use of intracystic bleomycin in cystic craniopharyngioma are available. The results are promising (Frank 1995; Hader 2000; Mettolese 2001; Park 2002; Lena 2005; Kim 2007; Hukin 2007). Most of these studies were retrospective cohort studies and they mentioned that intracystic bleomycin has potential advantages, such as inducing the shrinkage of the cyst size and allowing the delay of radiotherapy or radical resection.
There was only one eligible randomised trial that compared two different types of intracystic treatments: intracystic bleomycin and intracystic phosphorus32 (32P). No survival or duration of follow‐up data were provided to compare the two groups. There was no significant difference in the size reduction of the cyst, neurological status, fever or third nerve paralysis. There was a significant difference in the occurrence of headache and vomiting in favour of the 32P group. There was no significant difference in total adverse effects. However, the quality of the evidence is very low; the groups were small and relevant patient characteristics (i.e. characteristics of the cysts) were different between the two groups. Furthermore, there is a high risk of bias. We considered the trial to have a high risk of performance bias, detection bias and baseline imbalance bias and an unclear risk of selection bias, attrition bias, reporting bias and early stopping bias. In addition, the trial did not calculate the sample size, involved only seven patients and was therefore grossly underpowered to detect clinically important differences in outcome.
However, the role of intracystic bleomycin as a primary curative technique compared with radical therapy, partial resection plus radiotherapy, or other intracystic treatment in children with cystic craniopharyngioma can only be adequately determined through evaluation within prospective RCTs. Intracystic bleomycin is associated with a significant risk of morbidity. The acute adverse effects of intracystic bleomycin include headache, nausea, vomiting and transient mild fever, which occur in as many as 70% of patients, typically 24 hours after each instillation, and are self‐limiting (Hukin 2007). Even more serious are the reported delayed complications. There have been cases where MRI revealed signs of bleomycin leakage consistent with the extensive vasogenic oedema surrounding the cyst, which resulted in hypothalamic injury (Lafay‐Cousin 2007). The following complications have also been reported: diabetes insipidus (Park 2002), progressive panhypopituitarism (Hukin 2007), precocious puberty (Hukin 2007), and hypothalamic dysfunction resulting in transient hypersomnolence and poor memory (Park 2002), visual deficits (Mettolese 2001; Park 2002), neurocognitive deficits (Hukin 2007), sensorineural hearing loss (Broggi 1989; Frank 1995), peritumoral oedema (Hukin 2007), cerebral ischaemia (Broggi 1989), cerebral artery stenosis/occlusion (Cho 2012), hemiparesis (Jiang 2002; Park 2002), moyamoya disease after the combined treatment of intracystic bleomycin and radiotherapy (Hukin 2007), and death possibly related to a high individual and cumulative dose (Savas 2000).
Hence, surgeons have to realise that intracystic bleomycin for cystic craniopharyngioma in children currently has to be regarded as an experimental treatment that should only be performed in the context of a trial and requires close clinical monitoring. Imaging evaluation should be performed using MRI during treatment to ensure the safety of the therapy.
Comparing two intracystic treatments (with or without post‐treatment therapy) is an appropriate consideration for a future randomised trial. For example, the use of intracystic interferon alpha (IFNα) (Cavalheiro 2005; Ierardi 2007) and beta‐emitting radionuclides (Cáceres 2005), such as phosphorus32 (32P), yttrium90, rhenium186 and aurum198, are also available to provide control of the cystic tumour. Compared to the morbidity associated with intracystic bleomycin, IFNα seems to have similar advantages, but does not appear to have any significant major toxicity, even if it spills into the subarachnoid space. Therefore, further trials on intracystic treatment of cystic craniopharyngiomas are very much needed.
Authors' conclusions
What's new
| Date | Event | Description |
|---|---|---|
| 22 March 2016 | New citation required but conclusions have not changed | Unfortunately, no new studies could be included in this update of the review. As a result the conclusions have not changed. We have included a 'Summary of findings' table/GRADE assessment in this review. |
| 10 February 2016 | New search has been performed | The search for eligible studies was updated to February 2016. |
History
Protocol first published: Issue 12, 2010
Review first published: Issue 4, 2012
| Date | Event | Description |
|---|---|---|
| 16 June 2014 | New citation required but conclusions have not changed | Unfortunately, no new studies could be included in this update of the review. As a result the conclusions have not changed. |
| 16 June 2014 | New search has been performed | The search for eligible studies was updated to March 2014. |
Acknowledgements
We would like to thank the Editorial Base of Cochrane Childhood Cancer for their advice and support.
The editorial base of Cochrane Childhood Cancer is funded by 'Stichting Kinderen Kankervrij' (KIKA), the Netherlands.
Appendices
Appendix 1. Search strategy for Central Register of Controlled Trials (CENTRAL)
1. For Craniopharingioma the following text words were used:
craniopharyngioma OR craniopharingiomas OR craniopharingioma* OR Rathke's Pouch Tumor OR Rathkes Pouch Tumor OR Rathke Pouch Tumor OR Rathke's Cleft Neoplasm OR Rathkes Cleft Neoplasm OR Rathke Cleft Neoplasm OR Papillary Craniopharyngioma OR Papillary Craniopharyngiomas OR Child Craniopharyngioma OR Child Craniopharyngiomas OR Adamantinous Craniopharyngioma OR Adamantinous Craniopharyngiomas OR hypophyseal duct tumor OR hypophyseal duct tumors OR adamantinoma OR adamantinomas OR craniopharyngeal duct tumour OR adamantinomatous tumour OR dysodontogenic epithelial tumour
2. ForBleomycin the following text words were used:
bleomycin OR bleomycins OR Bleomycin* OR bleo‐cell OR bleo cell OR bellocell OR cell pharm brand of bleomycin sulfate OR bleolem OR lemery brand of bleomycin sulfate OR bleomicina OR almirall brand of bleomycin sulfate OR lundbeck brand of bleomycin sulfate OR bleomycin b2 OR bleomycin b (2) OR bleomycin sulfate OR sulfate, bleomycin OR bull brand of bleomycin sulfate OR bleomycinum mack OR mack, bleomycinum OR mack brand of bleomycin sulfate OR bleomycin bellon OR bellon, bleomycin OR bellon brand of bleomycin sulfate OR blenoxane OR bristol‐myers squibb brand of bleomycin sulfate OR bristol myers squibb brand of bleomycin sulfate OR blenoxane OR bleomycin a2 OR bleomycin a (2) OR cleocin OR bleomycin
Final search 1 and 2
[*=zero or more characters]
The search was performed in title, abstract or keywords
Appendix 2. Search strategy for MEDLINE/PubMed
1. For Craniopharingioma the following MeSH headings and text words were used:
craniopharyngioma OR craniopharingiomas OR craniopharingioma* OR Neoplasm, Rathke's Cleft OR Neoplasm, Rathkes Cleft OR Rathke's Pouch Tumor OR Rathkes Pouch Tumor OR Tumor, Rathke's Pouch OR Rathke Pouch Tumor OR Tumor, Rathke Pouch OR Rathke's Cleft Neoplasm OR Rathkes Cleft Neoplasm OR Neoplasm, Rathke Cleft OR Rathke Cleft Neoplasm OR Craniopharyngioma, Papillary OR Craniopharyngiomas, Papillary OR Papillary Craniopharyngioma OR Papillary Craniopharyngiomas OR Craniopharyngioma, Child OR Child Craniopharyngioma OR Child Craniopharyngiomas OR Craniopharyngiomas, Child OR Craniopharyngioma, Adamantinous OR Adamantinous Craniopharyngioma OR Adamantinous Craniopharyngiomas OR Craniopharyngiomas, Adamantinous OR hypophyseal duct tumor OR hypophyseal duct tumors OR adamantinoma OR adamantinomas OR Craniopharyngeal duct tumour OR Adamantinomatous tumour OR Dysodontogenic epithelial tumour
2. ForBleomycin the following MeSH headings and text words were used:
bleomycin OR bleomycins OR Bleomycin* OR bleo‐cell OR bleo cell OR bellocell OR cell pharm brand of bleomycin sulfate OR bleolem OR lemery brand of bleomycin sulfate OR bleomicina OR almirall brand of bleomycin sulfate OR lundbeck brand of bleomycin sulfate OR bleomycin b2 OR bleomycin b (2) OR bleomycin sulfate OR sulfate, bleomycin OR bull brand of bleomycin sulfate OR bleomycinum mack OR mack, bleomycinum OR mack brand of bleomycin sulfate OR bleomycin bellon OR bellon, bleomycin OR bellon brand of bleomycin sulfate OR blenoxane OR bristol‐myers squibb brand of bleomycin sulfate OR bristol myers squibb brand of bleomycin sulfate OR blenoxane OR bleomycin a2 OR bleomycin a (2) OR cleocin OR bleomycin OR 11056‐06‐7 OR 9041‐93‐4
Final search 1 and 2
[*=zero or more characters]
Appendix 3. Search strategy for EMBASE/Ovid
1. For Craniopharingioma the following Emtree terms and text words were used:
1. craniopharyngioma.mp. or exp craniopharyngioma/
2. (craniopharingiomas or craniopharingioma$).mp.
3. Rathke's Pouch Tumor.mp.
4. (Rathkes Pouch Tumor or Rathke Pouch Tumor).mp.
5. (Rathke's Cleft Neoplasm or Rathkes Cleft Neoplasm or Rathke Cleft Neoplasm).mp.
6. exp Rathke cleft cyst/
7. (Papillary Craniopharyngioma or Papillary Craniopharyngiomas).mp.
8. (Child Craniopharyngioma or Child Craniopharyngiomas).mp.
9. (Adamantinous Craniopharyngioma or Adamantinous Craniopharyngiomas).mp.
10. (hypophyseal duct tumor or hypophyseal duct tumors).mp.
11. (adamantinoma or adamantinomas).mp.
12. (craniopharyngeal duct tumour or adamantinomatous tumour or dysodontogenic epithelial tumour).mp.
13. or/1‐12
2. ForBleomycin the following Emtree terms and text words were used:
1. exp bleomycin/
2. (bleomycin or bleomycins or bleomycin$).mp.
3. (bleo‐cell or bleo cell or bellocell).mp.
4. (bleomycin sulfate or bleolem).mp.
5. bleomicina.mp.
6. (bleomycin b2 or bleomycin b 2).mp.
7. (bleomycinum mack or bleomycin bellon).mp.
8. (blenoxane or cleocin).mp.
9. (bleomycin a2 or bleomycin a 2).mp.
10. (11056‐06‐7 or 9041‐93‐4).rn.
11. or/14‐23
Final search 1 and 2
[mp = title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name; / = Emtree term; $=zero or more characters; rn= registry number]
Appendix 4. Search strategy for proceedings abstracts
The proceedings abstracts of the International Society for Paediatric Oncology (SIOP) (http://www.siop.nl/) (from 2005 to 2015) was searched for the term craniopharyngioma.
Appendix 5. Search strategy for ongoing studies
The register of the National Institutes of Health (http://www.clinicaltrials.gov) and International Standard Randomised Controlled Trial Number (ISRCTN) register (http://www.isrctn.com) were searched for craniopharyngioma.
Notes
New search for studies and content updated (no change to conclusions)
Data and analyses
Comparison 1
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Reduction of cyst size | 1 | 7 | Mean Difference (IV, Fixed, 95% CI) | ‐0.15 [‐0.69, 0.39] |
| 2 Fever | 1 | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.92 [0.73, 11.70] |
| 3 Total adverse effects | 1 | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.75 [0.68, 4.53] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Randomised clinical trial | |
| Participants | Country: China Setting: first class hospital Urban/rural: urban Number randomised: 7 Post‐randomisation dropout(s): not stated Mean age: 9.6 years Females: 2 (28.6%) Inclusion criteria: 1. Diagnosed with craniopharyngiomas according to pathology or cytological data 2. Mainly cystic or containing a solitary cyst with the volume of the cyst > 8 ml 3. Without prior radiotherapy, or with isotope radiotherapy at least 6 months previously, which was determined to have no effect 4. Patients themselves and their families agreed to receive this experimental treatment | |
| Interventions | The patients were randomised to the following groups Group 1: intracystic bleomycin (n = 3). The dose of bleomycin started at 5.0 mg and was increased by 2.5 or 5.0 mg each day. The largest daily dose was no more than 15 mg. The largest total dose received by any patient was 120 mg, while the least was 14.5 mg. Silicone tubes were inserted stereotactically into the cysts. In polycystic tumours, only the largest cysts were injected. Insertion was done with a CT‐guided Leksell‐G stereotactic system. 1.0 ml solution of bleomycin was injected through the tube into the cysts daily for 8 days in patients in group 1 Group 2: intracystic radiotherapy with 32P (n = 4); dose of 32P: 200 Gy In Group 2, 0.9% saline was injected daily into the cysts for 7 days.32P was administered to the patients in Group 2 on the 8th day | |
| Outcomes | The outcomes reported were the changes in the cyst volume, outcome and complications of the therapy. We described these as size reduction of the cyst, neurological status and adverse effects | |
| Notes | Follow‐up ranged from 7 to 24 months. The study included patients over 18 years old and we only extracted the data for children | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients were randomly allocated" Comment: the trial is described as randomised, but the method of sequence generation was not specified |
| Allocation concealment (selection bias) | Unclear risk | Quote: "patients were randomly allocated" Comment: the trial was described as randomised but the method used to conceal the allocation was not described |
| Blinding of participants (performance bias) | Low risk | Quote: "the patients were semi‐blindly (both the patients and their families did not know which therapy they received)" Comment: the blinding of patients was performed |
| Blinding of personnel (performance bias) | High risk | Comment: the blinding of personnel was not performed and the trial was at high risk of performance bias |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: the blinding of outcome assessment was not performed and the trial was at high risk of detection bias |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: the numbers and reasons for dropouts and withdrawals in all intervention groups were not described |
| Selective reporting (reporting bias) | Unclear risk | Comment: the protocol is unavailable and it is unclear whether data for survival, response of the cyst to treatment, endocrine function, visual outcome and QOL were recorded or not |
| Free from baseline imbalance (other bias)? | High risk | Comment: the patients in the bleomycin group were all polycystic, while the patients in the 32P group were not all polycystic |
| Free from early stopping (other bias)? | Unclear risk | Comment: a sample size calculation was not reported and it is not clear whether the trial was stopped early or not |
32P: phosphorus32
CT: computerised tomography
QOL: quality of life
Differences between protocol and review
We added "The dose, frequency and duration could vary" under the heading Types of interventions.
We added "or via neuroendoscopic technique" under the heading Types of interventions; this technique was not used for this procedure when we wrote the protocol and the original version of the review, but it is now.
We did not contact authors for missing data under the heading Dealing with missing data.
We deleted "We scanned the proceedings abstracts of the International Symposium of Pediatric Neuro‐Oncology (ISPNO) conferences and paediatric neurosurgical conferences (from 2005 to 2010)" under the heading Search methods for identification of studies and Results of the search.
We added "visual outcome" under the heading Secondary outcomes.
We added "cerebrovascular event" under the heading Secondary outcomes; since writing the protocol this adverse effect has been shown in non‐randomised studies.
As opposed to the earlier version of the review we now have prepared a 'Summary of findings' table and we have performed a GRADE assessment of the quality of the evidence.
Contributions of authors
S Zhang: searching for trials (this updated review), GRADE assessment and 'Summary of findings' table (this updated review).
Y Fang: searching for trials (previous publication and this updated version), GRADE assessment and 'Summary of findings' table (this updated review), 'Risk of bias' assessment (previous publication), data analyses (previous publication).
BW Cai: searching for trials (previous publication), 'Risk of bias' assessment (previous publication).
JG Xu: searching for trials (previous publication).
C You: protocol and review development, searching for trials (previous publication), 'Risk of bias' assessment (previous publication), data analyses (previous publication).
All authors approved the final version of this updated review.
Sources of support
Internal sources
No sources of support supplied
External sources
Research Grants No. 81001117, 30872646/H1618 and 30973082/H1618 from the National Natural Science Foundation, China.
Declarations of interest
S Zhang: nothing to declare.
Y Fang: nothing to declare.
BW Cai: nothing to declare.
JG Xu: nothing to declare.
C You: nothing to declare.
References
References to studies included in this review
Jiang 2002 {published data only}
- Jiang R, Liu Z, Zhu C. Preliminary exploration of the clinical effect of bleomycin on craniopharyngiomas. Stereotactic and Functional Neurosurgery 2002;78(2):84‐94. [Abstract] [Google Scholar]
Additional references
Bartels 2012
- Bartels U, Laperriere N, Bouffet E, Drake J. Intracystic therapies for cystic craniopharyngioma in childhood. Frontiers in Endocrinology (Lausanne) 2012;3:39. [Europe PMC free article] [Abstract] [Google Scholar]
Blacklund 1994
- Backlund EO. Treatment of craniopharyngiomas: the multi‐modality approach. Pediatric Neurosurgery 1994;21(Suppl 1):82‐9. [Abstract] [Google Scholar]
Broggi 1989
- Broggi G, Giorgi C, Franzini A, Servello D, Solero CL. Preliminary results of intracavitary treatment of craniopharyngioma with bleomycin. Journal of Neurosurgical Sciences 1989;33:145‐8. [Abstract] [Google Scholar]
Bunin 1998
- Bunin GR, Surawicz TS, Witman PA, Preston‐Martin S, Davis F, Bruner JM. The descriptive epidemiology of craniopharyngioma. Journal of Neurosurgery 1998;89:547‐51. [Abstract] [Google Scholar]
Cavalheiro 2005
- Cavalheiro S, Dastoli PA, Silva NS, Toledo S, Lederman H, Silva MC. Use of interferon alpha in intratumoral chemotherapy for cystic craniopharyngioma. Child's Nervous System 2005;21:719‐24. [Abstract] [Google Scholar]
Cho 2012
- Cho WS, Kim SK, Wang KC, Phi JH, Cho BK. Vasculopathy after intracystic bleomycin administration for a recurrent cystic craniopharyngioma: case report. Journal of Neurosurgery Pediatrics 2012;9:394‐9. [Abstract] [Google Scholar]
Cáceres 2005
- Cáceres A. Intracavitary therapeutic options in the management of cystic craniopharyngioma. Child's Nervous System 2005;21:705‐18. [Abstract] [Google Scholar]
Egger 1997
- Egger M, Smith GD, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315:629‐34. [Europe PMC free article] [Abstract] [Google Scholar]
Frank 1995
- Frank F, Fabrizi AP, Frank G, Fioravanti A. Stereotactic management of craniopharyngiomas. Stereotactic and Functional Neurosurgery 1995;65:176‐83. [Abstract] [Google Scholar]
Gleeson 2003
- Gleeson HK, Stoeter R, Ogilvy‐Stuart AL. Improvements in final height over 25 years in growth hormone (GH)‐deficient childhood survivors of brain tumors receiving GH replacement. Journal of Clinical Endocrinology & Metabolism 2003;88(8):3682‐9. [Abstract] [Google Scholar]
Hader 2000
- Hader WJ, Steinbok P, Hukin J, Fryer C. Intratumoral therapy with bleomycin for cystic craniopharyngiomas in children. Pediatric Neurosurgery 2000;32:211‐8. [Abstract] [Google Scholar]
Hetelekidis 1993
- Hetelekidis S, Barnes PD, Tao ML. 20‐year experience in childhood craniopharyngioma. International Journal of Radiation Oncology, Biology, Physics 1993;27(2):189‐95. [Abstract] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hukin 2007
- Hukin J, Steinbok P, Lafay‐Cousin L, Hendson G, Strother D, Mercier C, et al. Intracystic bleomycin therapy for cranio‐pharyngioma in children: the Canadian experience. Cancer 2007;109:2124‐31. [Abstract] [Google Scholar]
Ierardi 2007
- Ierardi DF, Fernandes MJ, Silva IR, Thomazini‐Gouveia J, Silva NS, Dastoli P, et al. Apoptosis in alpha interferon (IFN‐alpha) intratumoral chemotherapy for cystic craniopharyngiomas. Child's Nervous System 2007;23:1041‐6. [Abstract] [Google Scholar]
Kim 2007
- Kim SD, Park JY, Park J, Lee JB, Kim SH, Lim DJ. Radiological findings following postsurgical intratumoral bleomycin injection for cystic craniopharyngioma. Clinical Neurology and Neurosurgery 2007;109:236‐41. [Abstract] [Google Scholar]
Kremer 2010
- Kremer LCM, Dalen EC, Moher D, Caron HN. Cochrane Childhood Cancer Group. About The Cochrane Collaboration (Cochrane Review Groups (CRGs)) 2010, issue 3:Art. No.: CHILDCA.
Kubo 1974
- Kubo O, Takakura K, Miki Y. Intracystic therapy of bleomycin for craniopharyngioma effect of bleomycin for cultured craniopharyngioma cells and intracystic concentration of bleomycin. No Shinkei Geka 1974;2:683‐8. [Abstract] [Google Scholar]
Lafay‐Cousin 2007
- Lafay‐Cousin L, Bartels U, Raybaud C, Kulkarni AV, Guger S, Huang A. Neuroradiological findings of bleomycin leakage in cystic craniopharyngioma. Report of three cases. Journal of Neurosurgery 2007;107(Suppl 4):318‐23. [Abstract] [Google Scholar]
Lena 2005
- Lena G, Paz Paredes A, Scavarda D, Giusiano B. Craniopharyngioma in children: Marseille experience. Child's Nervous System 2005;21:778‐84. [Abstract] [Google Scholar]
Mettolese 2001
- Mettolese C, Stan H, Hermier M. Intracystic chemotherapy with bleomycin in the treatment of craniopharyngiomas. Child's Nervous System 2001;17:724‐30. [Abstract] [Google Scholar]
Park 2002
- Park DH, Park JY, Kim JH, Chung YG, Lee HK, Lee KC. Outcome of postoperative intratumoral bleomycin injection for cystic craniopharyngioma. Journal of Korean Medical Science 2002;17:254‐9. [Europe PMC free article] [Abstract] [Google Scholar]
Parmar 1998
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta‐analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17(24):2815‐34. [Abstract] [Google Scholar]
Puget 2007
- Puget S, Garnett M, Wray A, Grill J, Habrand JL, Bodaert N. Pediatric craniopharyngiomas: classification and treatment according to the degree of hypothalamic involvement. Journal of Neurosurgery 2007;106(Suppl 1):3‐12. [Abstract] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Sainte‐Rose 2005
- Sainte‐Rose C, Puget S, Wray A, Zerah M, Grill J, Brauner R, et al. Craniopharyngioma: the pendulum of surgical management. Child's Nervous System 2005;21:691‐5. [Abstract] [Google Scholar]
Sanford 1991
- Sanford RA, Muhlbauer MS. Craniopharyngioma in children. Neurologic Clinics 1991;9:453‐65. [Abstract] [Google Scholar]
Savas 2000
- Savas A, Erdem A, Tun K, Kanpolat Y. Fatal toxic effect of bleomycin on brain tissue after intracystic chemotherapy for a craniopharyngioma: case report. Neurosurgery 2000;46:213‐7. [Abstract] [Google Scholar]
Schünemann 2013
- Schünemann H, BrożekJ, Guyatt G, Oxman A. GradePro Handbook. Available from www.gradepro.org October 2013.
Slim 2003
- Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non‐randomized studies (minors): development and validation of a new instrument. ANZ Journal of Surgery 2003;73(9):712‐6. [Abstract] [Google Scholar]
Takahashi 1985
- Takahashi H, Nakazawa S, Shimura T. Evaluation of postoperative intratumoral injection of bleomycin for craniopharyngioma in children. Journal of Neurosurgery 1985;62:120‐7. [Abstract] [Google Scholar]
Thompson 2005
- Thompson D, Phipps K, Hayward R. Craniopharyngioma in childhood: our evidence‐based approach to management. Child's Nervous System 2005;21:660‐8. [Abstract] [Google Scholar]
Tomita 2005
- Tomita T, Bowman RM. Craniopharyngiomas in children: surgical experience at Children's Memorial Hospital. Child's Nervous System 2005;21:729‐46. [Abstract] [Google Scholar]
Umezawa 1966
- Umezawa H, Maeda K, Takeuchi Y. New antibiotics, bleomycin A and B. Journal of Antibiotics (Tokyo) 1966;19:200‐9. [Abstract] [Google Scholar]
Wara 1994
- Wara WM, Sneed PK, David A. The role of radiation therapy in the treatment of craniopharyngioma. Pediatric Neurosurgery 1994;21(Suppl 1):98‐100. [Abstract] [Google Scholar]
Weiner 1994
- Weiner HL, Wisoff JH, Rosenberg ME. Craniopharyngiomas: a clinicopathological analysis of factors predictive of recurrence and functional outcome. Neurosurgery 1994;35:1001‐11. [Abstract] [Google Scholar]
Yasargil 1990
- Yasargil MG, Curcic M, Kis M, Siegenthaler G, Teddy PJ, Roth P. Total removal of craniopharyngiomas. Approaches and long‐term results in 144 patients. Journal of Neurosurgery 1990;73:3‐11. [Abstract] [Google Scholar]
Zuccaro 2005
- Zuccaro G. Radical resection of craniopharyngioma. Child's Nervous System 2005;21:679‐90. [Abstract] [Google Scholar]
References to other published versions of this review
Fang 2010
- Fang Y, Wu B, You C. Intracystic bleomycin for cystic craniopharyngiomas in children. Cochrane Database of Systematic Reviews 2010, Issue 12. [DOI: 10.1002/14651858.CD008890] [Abstract] [CrossRef] [Google Scholar]
Fang 2012
- Fang Y, Cai BW, Zhang H, Liu W, Wu B, Xu JG, et al. Intracystic bleomycin for cystic craniopharyngiomas in children. Cochrane Database of Systematic Reviews 2012, Issue 4. [DOI: 10.1002/14651858.CD008890.pub2] [Abstract] [CrossRef] [Google Scholar]
Liu 2012
- Liu W, Fang Y, Cai B, Xu J, You C, Zhang H. Intracystic bleomycin for cystic craniopharyngiomas in children (abridged republication of Cochrane Systematic Review). Neurosurgery 2012;71(5):909‐15. [Abstract] [Google Scholar]
Zheng 2014
- Zheng J, Fang Y, Cai BW, Zhang H, Liu W, Wu B, et al. Intracystic bleomycin for cystic craniopharyngiomas in children. Cochrane Database of Systematic Reviews 2014, Issue 9. [DOI: 10.1002/14651858.CD008890.pub3] [Abstract] [CrossRef] [Google Scholar]
Articles from The Cochrane Database of Systematic Reviews are provided here courtesy of Wiley
Full text links
Read article at publisher's site: https://doi.org/10.1002/14651858.cd008890.pub4
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc6457977?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/9797953
Article citations
Craniopharyngioma: A comprehensive review of the clinical presentation, radiological findings, management, and future Perspective.
Heliyon, 10(11):e32112, 31 May 2024
Cited by: 2 articles | PMID: 38961911 | PMCID: PMC11219339
Review Free full text in Europe PMC
Integrating Systemic Therapies into the Multimodality Therapy of Patients with Craniopharyngioma.
Curr Treat Options Oncol, 25(2):261-273, 01 Feb 2024
Cited by: 1 article | PMID: 38300480 | PMCID: PMC11203386
Review Free full text in Europe PMC
Pediatric-Like Brain Tumors in Adults.
Adv Tech Stand Neurosurg, 50:147-183, 01 Jan 2024
Cited by: 1 article | PMID: 38592530
Review
Advances in the treatment of Adamantinomatous craniopharyngioma: How to balance tumor control and quality of life in the current environment: a narrative review.
Front Oncol, 13:1326595, 21 Dec 2023
Cited by: 1 article | PMID: 38188294 | PMCID: PMC10771305
Review Free full text in Europe PMC
Adamantinomatous craniopharyngioma: evolution in the management.
Childs Nerv Syst, 39(10):2613-2632, 20 Sep 2023
Cited by: 1 article | PMID: 37728836 | PMCID: PMC10613147
Review Free full text in Europe PMC
Go to all (18) article citations
Other citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Intracystic bleomycin for cystic craniopharyngiomas in children.
Cochrane Database Syst Rev, (9):CD008890, 19 Sep 2014
Cited by: 6 articles | PMID: 25233847
Review
Intracystic bleomycin for cystic craniopharyngiomas in children.
Cochrane Database Syst Rev, (4):CD008890, 18 Apr 2012
Cited by: 2 articles | PMID: 22513968
Review
Intracystic bleomycin for cystic craniopharyngiomas in children (abridged republication of cochrane systematic review).
Neurosurgery, 71(5):909-915, 01 Nov 2012
Cited by: 3 articles | PMID: 22902333
Review
Medical interventions for the prevention of platinum-induced hearing loss in children with cancer.
Cochrane Database Syst Rev, (7):CD009219, 01 Jul 2014
Cited by: 13 articles | PMID: 24984156
Review