Abstract
Free full text

Inhibition of MEK with trametinib enhances the efficacy of anti-PD-L1 inhibitor by regulating anti-tumor immunity in head and neck squamous cell carcinoma
ABSTRACT
Major histocompatibility complex (MHC) class I downregulation is the primary immune evasion mechanism associated with failure in anti-PD-1/PD-L1 blockade therapies for cancer. Here, we examined the role of MEK signaling pathway inhibition in head and neck squamous cell carcinoma (HNSCC) both in vitro and in vivo. We found that trametinib, a small molecule inhibitor of MEK, significantly enhanced MHC class I and PD-L1 expression in human HNSCC cell lines, and this occurred via STAT3 activation. Trametinib also further upregulated the increase in CXCL9 and CXCL10 expression caused by IFN-γ in HNSCC cells, which is associated with T cell infiltration in tumor tissues. Finally, we evaluated the therapeutic efficacy of trametinib combined with an anti-PD-L1 monoclonal antibody in vivo, using SCCVII mouse syngeneic tumor model for HNSCC. While neither PD-L1 blockade nor trametinib treatment alone affected tumor growth, the combined therapy significantly delayed tumor growth. Our results indicate that in the combined therapy trametinib increases CD8+ T cell infiltration in the tumor site and upregulates antigen presentation, and this may be associated with enhanced PD-L1 blockade efficacy. Furthermore, our results suggest that this combination would therapeutically benefit patients with HNSCC.
Synopsis
Our results demonstrate that immune evasion mechanisms in HNSCC could be counteracted by combination therapy with a MEK inhibitor and anti-PD-1/PD-L1 mAb.
Introduction
Cancer immune evasion, which is characterized by disrupted antigen presentation and dysfunction of anti-tumor immune cells, such as cytotoxic T lymphocytes,1,2 affect the carcinogenesis process, as well as metastasis and the recurrence of head and neck squamous cell carcinoma (HNSCC).2,3 To override the suppression of anti-tumor immune response, immune checkpoint inhibitors (ICIs) have been developed to target the PD-1/PD-L1 pathway that have shown remarkable and durable therapeutic efficacy in HNSCC.4,5 However, only approximately 15–20% of patients respond to this treatment strategy. Therefore, additional therapies are needed that can be used in combination with ICIs to increase their efficacy and response rate.6
Several anticancer agents have been shown to modulate the immune system via off-target effects, suppressing tumor growth by enhancing anti-tumor immunity in the preclinical stages.7–12 These agents act through multiple mechanisms, which include i) the release of danger signals and inflammatory cytokines by necrotic/apoptotic cells, ii) upregulation of tumor antigen/antigen processing machinery components, iii) depletion or reprogramming of suppressive immune cell subsets, such as tumor-associated macrophage (TAM), myeloid-derived suppressor cell and regulatory T cells (Tregs) and iv) induction of tumor infiltration by CD8+ T cells. Moreover, the effects of several of these anticancer agents on ICI efficacy are currently being assessed in clinical trials.13–15
Importantly, of these agents, MAPK pathway inhibitors have been reported to counteract immune evasion mechanisms in the tumor microenvironment, as oncogenic mutations in this pathway are associated with immunosuppression.11,16–22 Inhibitors of components of the MAPK pathway (such as BRAF and/or MEK) increased the expression of tumor-associated antigen (TAA) and T cell infiltration, thereby potentiating anti-tumor T cell immune responses. Meanwhile, PD-L1 expression appears to be variably associated with the MAPK pathway, depending on the experimental context; specifically, MAPK pathway inhibitors can up- or down-regulate PD-L1 expression in tumor cells.11,17,23 Given that studies have been done almost in melanoma which have frequent BRAF mutations, clinical trials evaluating the efficacy of a MAPK pathway inhibitor in combination with ICIs have been performed mostly on RAS/RAF-mutated solid cancer patients.13
Frequently, overexpression of EGFR and activation of its downstream MAPK pathway are observed in HNSCC patients.24,25 Therefore, the aim of this study was to investigate the effects of the MEK inhibitor trametinib on antigen presentation and immune checkpoint expression in human HNSCC cell lines in vitro. Furthermore, we used a syngeneic mouse squamous cell carcinoma model (SCCVII) to evaluate the therapeutic efficacy of combining a MEK inhibitor with anti-PD-L1 blockade.
Materials and methods
Cell lines and cell culture
Human HNSCC cell lines SNU-1041, SNU-1066, SNU-1076 cells (purchased from Korean Cell Line Bank, Seoul, Korea) and HN31 cells (obtained from John F. Ensley, Wayne State University) 26 were maintained in RPMI 1640 supplemented with 10% FBS and 10 µg/ml gentamycin. Detroit 562 and FaDu cells (purchased from American Type Culture Collection) were maintained in EMEM supplemented with 10% FBS, 100 U/ml penicillin and 100 µg/ml streptomycin. Murine SCC cell line SCCVII cells 27 were maintained in RPMI 1640 supplemented with 10% FBS, 10 mM HEPES, 1 mM sodium pyruvate, 10 µM MEM non-essential amino acids, 2 mM L-glutamine, 55 µM 2-mercaptoethanol, 100 U/ml penicillin and 100 µg/ml streptomycin. Cell lines, except mouse SCCVII cells, were authenticated using PowerPlex 18D System (Promega). All cells were cultured for less than three months and routinely tested for mycoplasma negative.
Reagents and antibodies
Trametinib (from Selleckchem) was dissolved in DMSO for in vitro experiments and suspended in a mixture of 0.5% hydroxypropyl methyl cellulose and 0.2% TWEEN 80 for in vivo injections. For in vivo experiments, we used a rat anti-mouse PD-L1 monoclonal antibody (mAb) (10F.9G2) and a rat IgG2b isotype control (LTF-2) from BioXCell. Both the recombinant human (R&D Systems) and mouse IFN-γ (PeproTech) were reconstituted with 0.1% BSA in PBS and stored at −80°C before use in experiments. Primary antibodies against p-STAT1 (Tyr701), STAT1, STAT2, p-STAT3 (Ser727), STAT3, STAT5, p-STAT6 (Tyr641), STAT6, p-p44/42 MAPK (Thr202/Tyr204), p44/42 MAPK, CD274 and GAPDH (Cell Signaling Technology) were used for Western blotting. Antibodies against mouse CD8 and CD274 (Cell Signaling Technology) were used for immunohistochemistry (IHC). Fluorochrome-labeled mAbs for the following markers were used in flow cytometry: anti-human HLA-ABC (G46–2.6), anti-human CD274 (MIH1), anti-mouse CD274 (MIH5), anti-mouse CD25 (PC61), anti-mouse CD8 (53–6.7), anti-mouse CD4 (RM4–5) and anti-human CXCL9 (B8–11) (BD Biosciences); anti-mouse CD45 (30-F11), anti-mouse CD3 (eBio500A2), anti-mouse Foxp3 (NRRF-30) and anti-mouse CXCL9 (MIG-2F5.5) (Thermo Fisher Scientific); anti-mouse H-2Kk (36–7–5), anti-mouse CD11b (M1/70), anti-mouse F4/80 (BM8), anti-mouse CD279 (RMP1–30), anti-mouse Ly-6G (1A8), anti-mouse CXCR3 (CXCR3–173) and anti-human CXCL10 (J034D6) (BioLegend). Protein transport inhibitor, GolgiStop (BD Biosciences), was used to block CXCL9 and CXCL10 secretion for 12 hours before intracellular flow cytometry staining.
Cell viability assay
A total of 2.5 x 103 cells (human cell lines) and 1.2 x 103 cells (mouse SCCVII cell line) per well were dispensed into 96-well culture plates. After cells had adhered to the plate, they were treated with trametinib for 72 hours. Cell growth inhibition was analyzed using the EZ-Cytox cell viability assay (Dogen, Seoul, Korea) and CellTiter Glo-Luminescent cell viability assay (Promega). The absorbance was measured with a microplate reader (BioTek) at 450 nm for EZ-Cytox cell viability assay. The luminescent signal was measured with a luminescence counter (Perkin Elmer) for Cell Titer Glo-Luminescent cell viability assay.
Flow cytometry
For surface staining, cells were incubated with fluorochrome-conjugated mAbs for 20 minutes at 4°C. For intracellular Foxp3 staining, Foxp3/Transcription factor staining buffer set (Thermo Fisher Scientific), and for intracellular CXCL9 and CXCL10 staining, Fixation/Permeabilization solution kit (BD Biosciences) was used according to the manufacturer’s instructions. For ex vivo experiments, freshly isolated cells were pre-incubated with anti-mouse CD16/CD32 mAb (2.4G2, BD Biosciences) to block the binding of antibody to FcγIII/II receptor and then stained with fixable viability dye (Thermo Fisher Scientific) prior to antibody staining to exclude dead cells. Data were acquired using FACSCalibur or FACSCanto II (BD Biosciences) and analyzed with FlowJo software (Tree Star, Inc.)
Western blotting
Cells were resuspended with cell lysis buffer (Cell Signaling Technology) containing protease inhibitor cocktail (Sigma), PMSF (Sigma) and PhosSTOP (Merck) at 4°C for 20 minutes. After centrifuging at 13,000 rpm at 4°C for 15 minutes, the supernatant was harvested. Equal amounts of proteins were separated on an SDS-polyacrylamide gel (Thermo Fisher Scientific) and transferred to PVDF membrane (Bio-Rad), which was then blocked with 5% skim milk at room temperature for one hour, probed with diluted primary antibodies at 4°C overnight, and with diluted secondary antibodies conjugated to HRP at room temperature for 2 hours. The signals were developed using ECL detection reagent (GE Healthcare) and visualized with ImageQuant LAS 4000 mini (GE Healthcare). GAPDH was used as a loading control.
Sirna transfection
SNU-1041 cells were transfected with STAT1-, STAT3-, STAT6- or non-targeting siRNAs (Santa Cruz) using Lipofectamine RNAiMAX transfection reagent (Thermo Fisher Scientific) according to the manufacturer’s instruction. Transfected cells were treated with trametinib for 72 hours followed by flow cytometry and Western blotting.
Quantitative real-time RT-PCR (qRT-PCR)
Total RNA from cultured cells and mouse ex vivo isolated cells were extracted using RNeasy Mini Kit (Qiagen) and reverse transcribed into cDNA with Superscript III first-strand synthesis system (Thermo Fisher Scientific). Quantification of gene expression was conducted using Power SYBR green PCR Master Mix and StepOnePlus Real-Time PCR system (Thermo Fisher Scientific). GAPDH was used as an internal reference gene. Primer sequences we used are listed in Supplementary Table S1.
Enzyme-linked immunosorbent assay (ELISA)
Soluble CXCL9 and CXCL10 levels in culture supernatants were measured using DuoSet ELISA (R&D Systems) according to the manufacturer’s instructions.
Immunohistochemistry
Formalin-fixed paraffin-embedded tissue sections (4 µm) were deparaffinized, rehydrated and subjected to heat-induced antigen retrieval with Tris-EDTA buffer (for PD-L1 analysis) or citrate buffer (for CD8 analysis). Sections were incubated in 10% normal goat serum at room temperature for one hour, in diluted primary antibodies at 4°C overnight, in peroxidase blocking solution (0.3% H2O2 in PBS) at room temperature for 15 minutes, and finally in diluted secondary antibodies conjugated to HRP at room temperature for 30 minutes. Signals were developed using the DAB chromogen (Dako) and visualized with a Bright field and Fluorescence Slide Scanner (Leica).
In vivo SCCVII syngeneic model
Mouse SCCVII cells (5 x 105) were injected subcutaneously into the flank of seven-week-old, female, C3H/HeN and BALB/c nude mice (Orient Bio Inc., Seongnam, Korea). Tumor volume was measured using a caliper and calculated as: (length x width2)/2. The mice were randomized to four groups and drug treatments began when tumor volume reached 100 ± 25 (mean ± SD) mm3. Trametinib (1 mg/kg) was administered by oral gavage daily, and anti-PD-L1 mAb (10 mg/kg) was intraperitoneal injected twice per weekly. Mice were euthanized when tumor size exceeded 1,000 mm3. Tumors, draining lymph nodes (dLNs) and spleens were collected on day 5 for pharmacodynamic analysis. The Institutional Animal Care and Use Committee of Seoul National University Hospital approved all animal studies (approval number: #16–0166-S1A1, #17–0117-S1A0 and #18–0062-S1A0).
Statistical analysis
Data are represented as the mean ± SD and were analyzed by GraphPad Prism (GraphPad Software). An unpaired two-tailed student’s t test was used to determine differences between groups. All P values less than 0.05 were considered statistically significant.
Results
The MEK inhibitor trametinib increases MHC class i and PD-L1 expression in human HNSCC cell lines with moderate cellular cytotoxicity
We first tested the susceptibility of six human HNSCC cell lines to the MEK inhibitor trametinib (Figure 1A). These cell lines do not harbor activating mutations in RAS or RAF with the exception of HN31, which harbor an HRAS G12D mutation.28 Growth inhibition by trametinib treatment occurred variably in these cell lines with moderate sensitivity (range of IC50: 10 nM ~ 100 nM, in four of six cells). Based on this, we selected a sub-lethal concentration of trametinib (50 nM), which was used to investigate immunologic changes without lethal damage. As shown in Figure 1B, trametinib treatment increased MHC class I expression in all cell lines. In addition, trametinib further enhanced exogenous IFN-γ-induced MHC class I upregulation. We also observed that trametinib slightly increased basal and IFN-γ-induced PD-L1 expression in all cell lines, except in SNU-1066, in which trametinib treatment diminished IFN-γ-induced PD-L1 upregulation (Figures 1C and D).
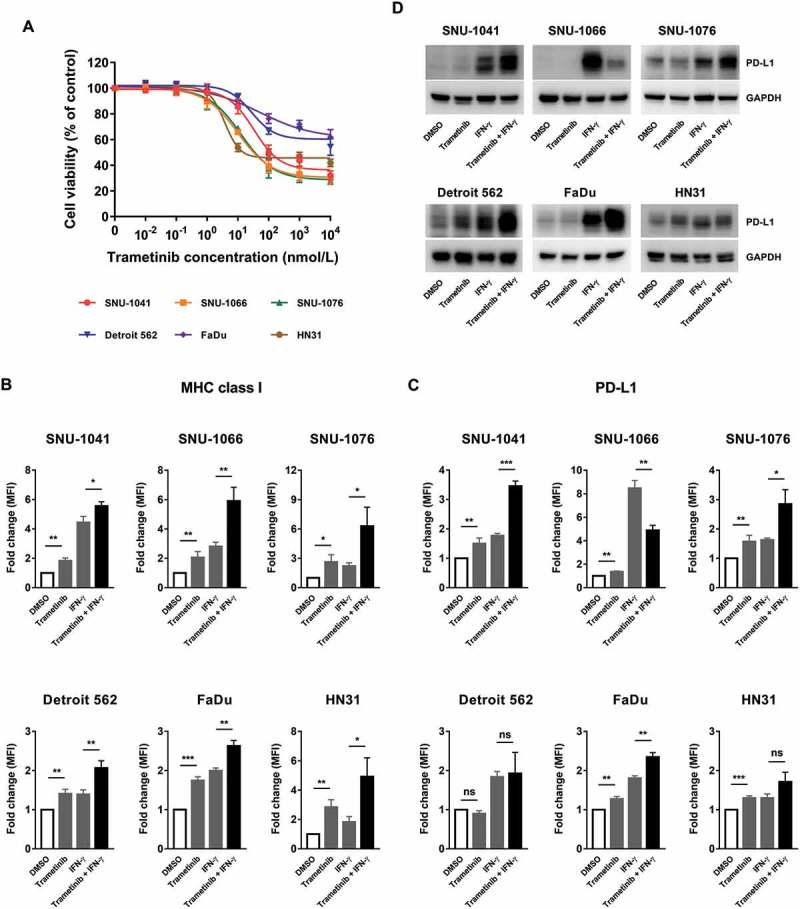
Trametinib exerts moderate cytotoxic effect on human HNSCC cell lines and increases MHC class I and PD-L1 expression in surviving cells. (A) Cells were incubated with 10 pM – 10 µM of trametinib for 72 hours and cell growth inhibition was analyzed using a cell viability assay. Data are shown as the mean ± SD from three independent experiments. (B and C) Cells were treated with trametinib (50 nM), IFN-γ (1 ng/ml; 10 ng/ml to examine PD-L1 expression in SNU-1066 cells) or the combination of trametinib and IFN-γ (pretreated with DMSO or trametinib one hour prior to the addition of IFN-γ) for 72 hours. Expression of MHC class I (B) and PD-L1 (C) were assessed by flow cytometry. The fold change in mean fluorescence intensity (MFI) was averaged from three independent experiments. Error bars indicate SD. (D) Changes in PD-L1 expression shown in Figure 1C were confirmed by Western blotting. Data show one of three independent experiments with comparable results.
Upregulation of MHC class i and PD-L1 by trametinib is stat3-dependent
Next, we investigated the effects of trametinib on STAT signaling pathway, as it is known to regulate MHC class I and/or PD-L1 expression.29–32 First, we confirmed that trametinib suppresses Erk1/2 phosphorylation at a concentration of 50 nM we used (Supplementary Fig. S1A). When we assessed change in STAT phosphorylation upon trametinib and IFN-γ treatment, except for STAT4, whose expression is restricted to myeloid cells, thymus and testes, we found that treatment with trametinib increased p-STAT1 expression, and this effect was enhanced in the presence of exogenous IFN-γ (Figure 2A). While total STAT1 expression was low, expression was increased after IFN-γ treatment, via input from a positive feedback loop, and this was further enhanced by trametinib in some cells. Furthermore, trametinib treatment activated STAT3 and STAT6 was shown to be activated by trametinib treatment in three of six cell lines. Phosphorylated STAT2 and STAT5 were not detected in any condition of any cell line we used (data not shown).

STAT3 depletion abolishes the effect of trametinib on MHC class I and PD-L1 expression in SNU-1041 cells. (A) Cells were treated with trametinib (50 nM), IFN-γ (1 ng/ml) or the combination of trametinib and IFN-γ (pretreated with DMSO or trametinib one hour prior to the addition of IFN-γ) for 72 hours. Levels of p-STAT1, p-STAT3 and p-STAT6 were examined by Western blotting. Data are representative of three independent experiments. (B) SNU-1041 cells were transfected with siControl, siSTAT1, siSTAT3 or siSTAT6. MHC class I and PD-L1 expression were analyzed in siRNA-transfected cells after trametinib treatment for 72 hours using flow cytometry. left; bar graphs showing the fold change in MFI from three independent experiments (error bars indicate SD), right; representative histogram showing unstained (dashed line), DMSO (gray) and trametinib (black) treatment. (C) SNU-1041 cells were transfected as in (B). Expression of all STAT family members, except STAT4, was examined by Western blotting in untransfected cells (No Tfx), siControl, siSTAT1, siSTAT3 or siSTAT6 transfectants, respectively. Representative Western blot from three independent experiments were shown.
To determine which signaling molecule was responsible for the upregulation of MHC class I and PD-L1 by trametinib, we knocked down STAT1, STAT3 and STAT6 expression with siRNA transfections. For these experiments, we used SNU-1041 cells, as the change in MHC class I and PD-L1 expression was most prominent in these cells (Figure 1B-D). After confirming that each STAT expression was efficiently and specifically downregulated at the protein level (Figure 2C), we found that, interestingly, STAT3 knock-down, but not STAT1 or STAT6, abolished trametinib-induced MHC class I and PD-L1 upregulation (Figure 2B). We observed that STATs were not involved in these effects in the remaining three siRNA-transfectable cell lines (data not shown).
T cell-recruiting chemokines CXCL9 and CXCL10 are upregulated by trametinib, which synergizes with ifn-γ in human HNSCC cell lines
Increased antigen presentation through MHC class I molecules can be recognized by CD8+ T cells when they are present within the tumor microenvironment. Therefore, we next examined trametinib’s effects on levels of the T cell chemoattractants CXCL9 and CXCL10, which are also induced by IFN-γ, in human HNSCC cell lines. As we expected, trametinib treatment significantly induced CXCL9 and CXCL10 mRNA expression (Figure 3A and B) as well as protein expression (Figure 3C, D and Supplementary Fig. S2). Moreover, chemokine expression was even further increased by combined exposure to both trametinib and exogenous IFN-γ. These in vitro findings suggest that trametinib treatment facilitates anti-tumor immunity by increasing T cell infiltration and antigen presentation in the tumor; however, PD-L1 blockade is also required, as trametinib induces PD-L1 expression, which may be also upregulated by T cell-derived IFN-γ.
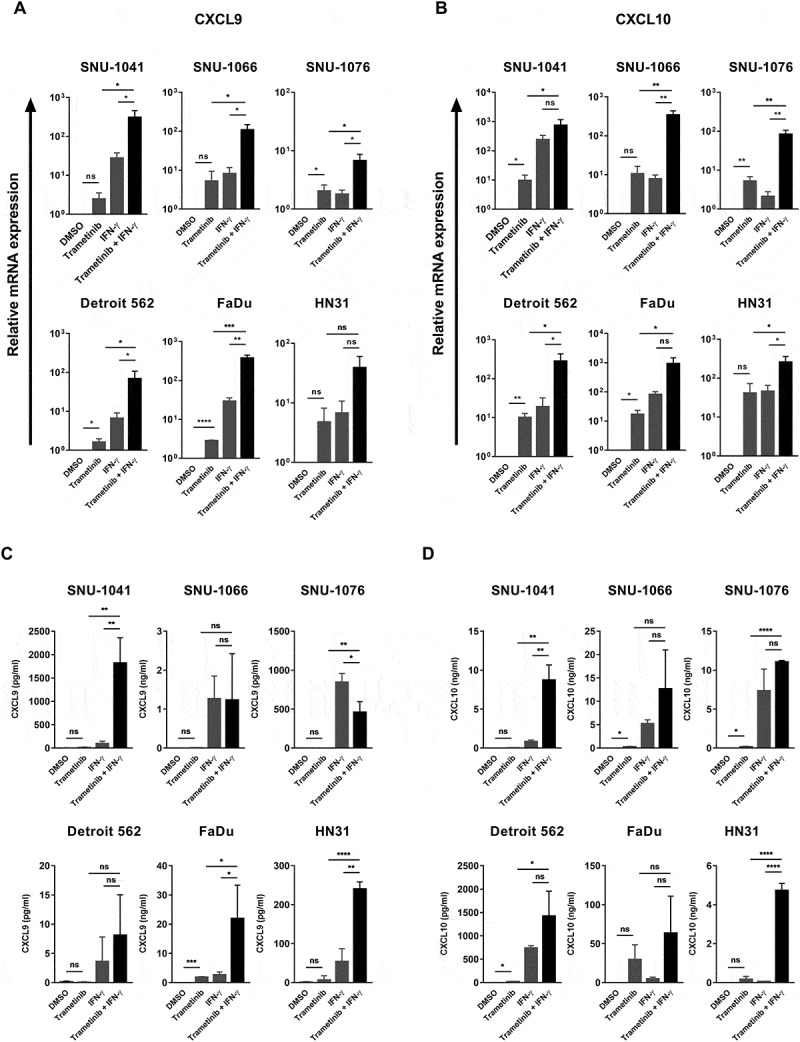
Trametinib enhances T cell chemoattractants level and synergizes with IFN-γ in human HNSCC cell lines. Cells were treated with trametinib (50 nM), IFN-γ (5 ng/ml) or the combination of trametinib and IFN-γ (pretreated with DMSO or trametinib one hour prior to the addition of IFN-γ) for 72 hours. Expression of CXCL9 (A) and CXCL10 (B) were determined by qRT-PCR. Results represent gene expression changes in log10 scale ± SD of three independent experiments. Protein levels of CXCL9 (C) and CXCL10 (D) were measured in culture supernatants by ELISA. Data represents mean ± SD from three independent experiments.
Trametinib exhibited similar effects in an SCCVII mouse cell line
Prior to investigating the therapeutic efficacy of combining trametinib with an anti-PD-L1 mAb in an SCCVII mouse squamous cell carcinoma model, we validated our human HNSCC cell lines observations in SCCVII mouse cells. Trametinib treatment moderately inhibited cell growth with an IC50 value of 10 ± 1.2 nM (Figure 4A). In addition, we found that expression of the MHC-encoded class I molecule H-2Kk was highly induced in live cells in the presence of trametinib, whereas expression of PD-L1 was not changed (Figure 4B). However, trametinib synergistically induced both PD-L1 and H-2Kk expression in the presence of exogenous IFN-γ.

In vitro evaluation of the immunomodulating activity of trametinib in an SCCVII mouse cell line. (A) SCCVII cells were incubated with 10 pM – 10 µM of trametinib for 72 hours and cell growth inhibition was determined using a cell viability assay. Data are the mean ± SD from three independent experiments. (B-D) SCCVII cells were treated with trametinib (30 nM), IFN-γ (1 ng/ml, B; 5 ng/ml, C and D) or the combination of trametinib and IFN-γ (pretreated with DMSO or trametinib one hour prior to the addition of IFN-γ) for 72 hours. (B) The expression of H-2Kk (left) and PD-L1 (right) was assessed by flow cytometry. The fold change in MFI was averaged from three independent experiments. Error bars indicate SD. (C) Changes in CXCL9 and CXCL10 gene expression were determined by qRT-PCR. Data show the mean ± SD from three independent experiments in log10 scale. (D) CXCL9 protein levels were analyzed by intracellular flow cytometry after treatment with GolgiStop for the last 12 hours of 72 hours. The fold change in percentage of cells expressing CXCL9 was averaged from three independent experiments. Error bars indicate SD. (E) Cells were treated with the indicated doses of trametinib. After two hours, the expression of phospho- and total-Erk1/2 was assessed by Western blotting. Data show one of three independent experiments with comparable results.
Interestingly, expression of CXCL9 and CXCL10 was elevated by trametinib with or without IFN-γ (Figures 4C and D). Erk1/2 activation was suppressed at the concentration of trametinib we used (Figure 4E). Therefore, we concluded that the SCCVII tumor model was an appropriate model to test our hypothesis, as we found that trametinib increased the antigenicity and immunogenicity of tumor cells by upregulating MHC class I, CXCL9 and CXCL10. However, the concurrent induction of PD-L1 indicated that co-treatment of trametinib with an anti-PD-L1 mAb would be necessary.
Combined therapy with trametinib and an anti-pd-l1 mab delays mouse tumor growth and increases the number of CD8+ tumor-infiltrating t cells
To investigate the in vivo efficacy of a combined therapy, compared to each treatment alone, SCCVII cells were implanted subcutaneously in the flank of C3H syngeneic mice. We found that the trametinib dose used was sufficient to inhibit Erk1/2 activation in tumor tissues (Supplementary Fig. S1B). However, as shown in Figure 5A, trametinib treatment alone did not delay tumor growth, and anti-PD-L1 mAb monotherapy was also ineffective. In contrast, concurrent combination treatment with trametinib and an anti-PD-L1 mAb resulted in a delay of tumor growth.
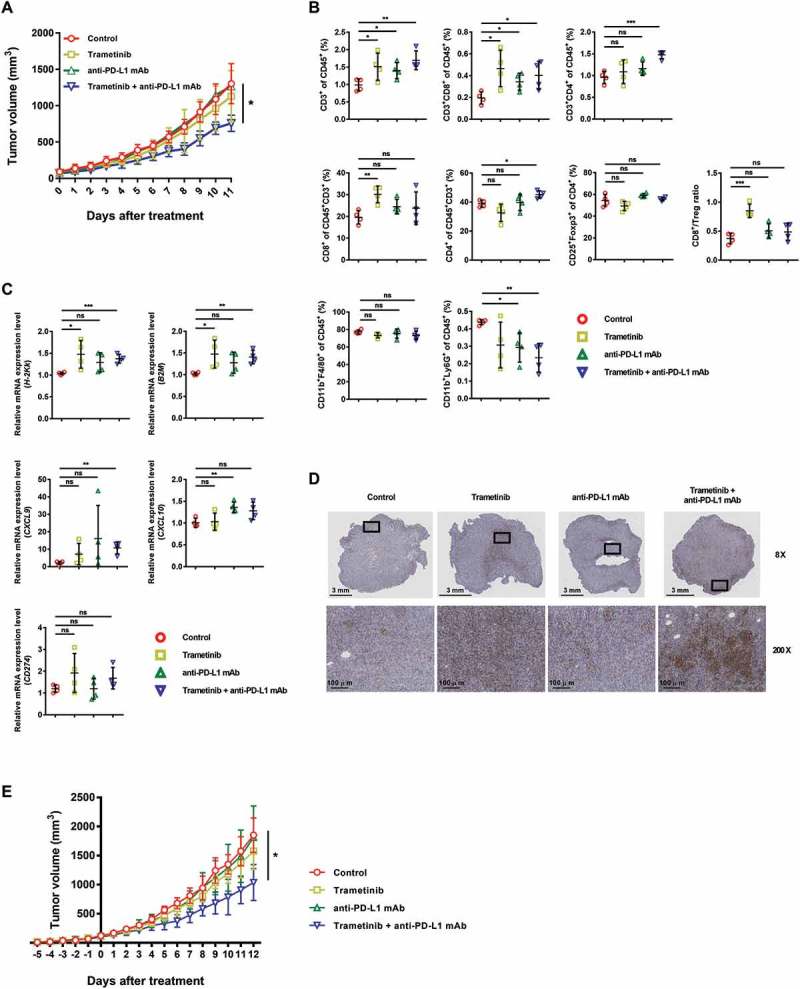
Anti-tumor activity of combined treatment with trametinib and an anti-PD-L1 mAb in an SCCVII syngeneic mouse tumor model. (A) Tumor-bearing C3H mice (n = 5–6) were treated with trametinib (1 mg/kg) by oral gavage once daily and/or an anti-PD-L1 mAb (10 mg/kg) by intraperitoneal (i.p.) injection twice weekly. Control mice received vehicle or isotype control. (B-D) Cells were obtained from tumor tissues for pharmacodynamic analysis after five days of treatment. (B) Immune subsets constituting tumor tissue were analyzed using flow cytometry (n = 4). Cells were subjected first to gating to eliminate debris, doublets and dead cells, and further analyzed by gating CD45+ cells. (C) Transcript levels of immune-related molecules were measured by performing qRT-PCR (n = 4). (D) Representative IHC images show PD-L1 expression within tumor tissue (n = 3). Rectangles (top) indicate the enlarged areas (bottom). (E) Tumor-bearing C3H mice (n = 5–7) were given trametinib (day −5) and then an anti-PD-L1 mAb five days later (day 0).
Analysis of immune subsets within the tumor microenvironment on the fifth day after treatment revealed an increase in infiltrating CD8+ T cells in trametinib-treated mice (Figure 5B; Supplementary Fig. S3A). CD3+CD4+CD25+Foxp3+ Treg cells were slightly decreased by trametinib treatment, but this was not statistically significant. TAMs (CD11b+F4/80+) were not affected by any treatments. The number of neutrophils (CD11b+Ly6G+) was decreased by exposure to an anti-PD-L1 mAb, both with or without trametinib. Increased CD3+CD8+ tumor infiltrating lymphocytes (TILs) were found in mice that received the combined trametinib and an anti-PD-L1 mAb treatment, and this was correlated with longer survival, while there were no significant alterations in other immune subsets (Supplementary Fig. S3B).
The T cell activation marker CD25 was elevated in CD8+ TILs by trametinib treatment (Supplementary Fig. S4A), and this was also associated with the longer survival observed in mice who received the combination therapy (Supplementary Fig. S4B). Other activation markers, PD-1, CD69 and T-bet, showed no significant difference between groups (Supplementary Fig. S4A). Interestingly, treatment with an anti-PD-L1 mAb resulted in a decrease in the total T cells, an increase in CD25+ – and PD-1+ – T cells, and an increased proportion of Treg cells in CD4 T cells in the secondary lymphoid organs, whereas trametinib-induced immunological changes were limited to tumor sites (Supplementary Fig. S5). This indicates that treatment with an anti-PD-L1 mAb regulated systemic immunity. We did not observe any adverse effects associated with immune activation from any of the treatments.
Finally, we confirmed our in vitro findings in vivo, five days after treatment. Consistent with the in vitro data, the mRNA expression of H-2Kk, as well as its associated molecule β2m, were increased by trametinib treatment (Figure 5C). CXCL9 and CXCL10 transcripts tended to be increased by trametinib and/or an anti-PD-L1 mAb treatment, despite variations among mice. As expected, expression of CXCR3, which is a receptor for CXCL9 and CXCL10, increased in splenic T cells after treatment with an anti-PD-L1 mAb, which activate systemic immunity as observed in Supplementary Fig. S5, because CXCR3 expression is highly increased in activated T cells (Supplementary Fig. S4C and D).33 Meanwhile, trametinib was not shown to be involved in increasing CXCR3 expression, but at least to have no negative effects either alone or in combination with an anti-PD-L1 mAb. An increase in PD-L1 expression in trametinib-treated mice was also observed (Figures 5C and D and Supplementary Fig. S6A). The specificity of PD-L1 IHC was investigated using spleens from naïve and tumor-bearing mice (Supplementary Fig. S6B). When we performed the same experiment as in Figure 5A using T cell-deficient BALB/c nude mice, there was no tumor growth retardation by the combination of trametinib and an anti-PD-L1 mAb, demonstrating it was attributed to T cells consistent with the above results (Supplementary Fig. S7A). These data indicate that trametinib-induced immunological changes enhance CD8+ T cell responses; however, blockade of the PD-L1 pathway is required for effective control of the tumor growth.
Sequential treatment of trametinib and an anti-pd-l1 mab inhibited tumor growth in a manner similar to the concurrent administration
Finally, we changed the treatment schedule from concurrent administration to sequential treatment to evaluate the immunomodulating effect of trametinib followed by an anti-PD-L1 mAb therapy. Trametinib treatment was given when the tumor grew to be palpable (at day −5), and an anti-PD-L1 mAb was administered five days later (day 0). However, we found that sequential treatment did not improve the effect on tumor growth, compared with concurrent administration (Figure 5E). Moreover, similar to the previous experiments, trametinib or anti-PD-L1 mAb alone had no benefits in tumor control, and delayed tumor growth was seen only when they were combined. Again, overall immunological changes following combined therapy, including increased CD8+ T cell infiltration in the tumor, were the same from endpoint analysis (Supplementary Fig. S7B). It was demonstrated that, in this murine squamous cell carcinoma model, combining trametinib and an anti-PD-L1 mAb is a more potent intervention than either treatment alone, regardless of treatment sequence, although no remission was observed.
Discussion
In this study, we found that treatment with the MEK inhibitor trametinib increases the expression of IFN-γ-induced immune molecules, including MHC class I, PD-L1, CXCL9 and CXCL10, which are associated with anti-tumor immunity. Inefficient tumor antigen presentation and recognition, through the loss or downregulation of MHC class I molecules on tumor cells is a well-known immune evasion mechanism. Although recent advances in anti-PD-1/PD-L1 blocking immunotherapies have shown remarkable clinical benefit, there are many non-responders as well as relapsed patients associated with downregulated MHC class I expression.34,35 When tumors harbor alterations involved in antigen processing and presentation, several strategies can be explored to restore their expression,36,37 including epigenetic modulation, IFN-γ pathway activation and inhibition of oncogenic signaling. In HNSCC, expression of MHC class I and β2m is also downregulated mostly in a reversible manner.38 Here, we demonstrated that the MEK inhibitor trametinib increases MHC class I expression at a concentration with moderate cellular cytotoxicity in human HNSCC cell lines. Simultaneous upregulation of PD-L1 expression by trametinib, with or without IFN-γ, was also observed in most cell lines tested.
MHC class I and PD-L1 are well-known downstream targets of IFN-γ signaling through the STAT1 pathway.29 PD-L1 expression is also regulated by various signaling pathways, including the PI3K/Akt, MAPK, STAT3 and NF-κB pathways, both intrinsically (congeneric) and extrinsically (interaction with stromal cells).39 Trametinib treatment acts through both the STAT1 and STAT3 pathways regulating MHC class I and PD-L1 expression. To this end, we found that trametinib-induced STAT3 activation upregulated MHC class I and PD-L1 in the SNU-1041 cell line. It is noteworthy that STAT3 is involved in the upregulation of MHC class I expression, because many signals involved in immune suppression and tumor promotion are associated with STAT3 activation.40,41
Based on the presence of effectors (CD8+ T cells) and suppressors (PD-L1+ cells), tumor microenvironments can be classified into four categories.42,43 Using this classification system, type II (TIL−PD-L1−) and III (TIL−PD-L1+) tumors are predicted to respond poorly to anti-PD-1/PD-L1 mAb therapy. Therefore, it is important to attract CD8+ T cells to tumors, as this may increase the therapeutic efficacy of immune checkpoint blockade therapies such as anti-PD-1/PD-L1 mAbs. Here, we observed that trametinib increases the expression of T cell recruitment chemokines (CXCL9 and CXCL10) in all human HNSCC cell lines examined, which may be further substantiated in the presence of T-cell derived IFN-γ.
A limitation of this study is that the mouse SCCVII tumor model lacks known TAAs; therefore, we were able to assess total CD8+ T cell population. In addition, this was not the first study to evaluate the efficacy of a combining a MAPK pathway inhibitor and an anti-PD-1/PD-L1 mAb to enhance anti-tumor immune responses. However, our study is still meaningful in that most previous reports have examined this combination in MAPK mutant systems.19,20,44 Moreover, combination therapies using a MEK inhibitor and an anti-PD-1/PD-L1 mAb that are being examined in active clinical trials are primarily being performed in patients with BRAF mutant melanoma and KRAS mutant lung and colorectal cancer (NCT01988896). Based on our results, we propose that the combination of a MEK inhibitor and an anti-PD-1/PD-L1 mAb would be therapeutically beneficial for HNSCC patients without MEK pathway mutations. In addition, further immunological studies using other small molecule inhibitors, especially those that target non-oncogene addiction, are required.
Funding Statement
This study was supported by a grant of the Korea Health Technology R&D Project “Strategic Center of Cell and Bio Therapy for Heart, Diabetes & Cancer” [grant number: HI17C2085] through the Korea Health Industry Development Institute (KHIDI), and a grant of the National R&D Program for Cancer Control [1420140] funded by the Ministry of Health & Welfare (MHW), Republic of Korea.
Acknowledgments
We thank to pathologist Kyeong Cheon Jung, and Sehui Kim for their help to interpret immunohistochemistry.
Authors’ Contributions
Conception and design: S-H Kang, B Keam, Y-O Ahn
Development of methodology: S-H Kang, B Keam, Y-O Ahn
Acquisition of data (provided animals, acquired and managed patients, provided facilities, etc.): S-H Kang, B Keam, Y-O Ahn, H-R Park, M Kim, TM Kim, D-W Kim, DS Heo
Analysis and interpretation of data (e.g., statistical analysis, biostatistics, computational analysis): S-H Kang, B Keam, Y-O Ahn, H-R Park, M Kim, TM Kim, D-W Kim, DS Heo
Writing, review, and/or revision of the manuscript: S-H Kang, B Keam, Y-O Ahn
Administrative, technical, or material support (i.e., reporting or organizing data, constructing databases): S-H Kang, H-R Park
Study supervision: B Keam, Y-O Ahn
Supplementary Material
Supplemental data for this article can be accessed here.
References
Articles from Oncoimmunology are provided here courtesy of Taylor & Francis
Full text links
Read article at publisher's site: https://doi.org/10.1080/2162402x.2018.1515057
Read article for free, from open access legal sources, via Unpaywall:
https://www.tandfonline.com/doi/pdf/10.1080/2162402X.2018.1515057?needAccess=true
Citations & impact
Impact metrics
Citations of article over time
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1080/2162402x.2018.1515057
Article citations
Current Landscape of Cancer Immunotherapy: Harnessing the Immune Arsenal to Overcome Immune Evasion.
Biology (Basel), 13(5):307, 28 Apr 2024
Cited by: 1 article | PMID: 38785789 | PMCID: PMC11118874
Review Free full text in Europe PMC
STING pathway as a cancer immunotherapy: Progress and challenges in activating anti-tumor immunity.
Mol Biol Rep, 51(1):487, 05 Apr 2024
Cited by: 0 articles | PMID: 38578532
Review
Therapeutic Advances and Challenges for the Management of HPV-Associated Oropharyngeal Cancer.
Int J Mol Sci, 25(7):4009, 03 Apr 2024
Cited by: 0 articles | PMID: 38612819 | PMCID: PMC11012756
Review Free full text in Europe PMC
Immune escape of head and neck cancer mediated by the impaired MHC-I antigen presentation pathway.
Oncogene, 43(6):388-394, 04 Jan 2024
Cited by: 1 article | PMID: 38177410
Review
Targeting MHC-I molecules for cancer: function, mechanism, and therapeutic prospects.
Mol Cancer, 22(1):194, 02 Dec 2023
Cited by: 13 articles | PMID: 38041084 | PMCID: PMC10693139
Review Free full text in Europe PMC
Go to all (43) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Clinical Trials
- (1 citation) ClinicalTrials.gov - NCT01988896
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Combination of downregulating FEN1 and PD-1 blockade enhances antitumor activity of CD8+ T cells against HNSCC cells in vitro.
J Oral Pathol Med, 52(9):834-842, 20 Sep 2023
Cited by: 0 articles | PMID: 37728572
TIGIT/CD155 blockade enhances anti-PD-L1 therapy in head and neck squamous cell carcinoma by targeting myeloid-derived suppressor cells.
Oral Oncol, 121:105472, 30 Jul 2021
Cited by: 21 articles | PMID: 34333450
The let-7 family of microRNAs suppresses immune evasion in head and neck squamous cell carcinoma by promoting PD-L1 degradation.
Cell Commun Signal, 17(1):173, 27 Dec 2019
Cited by: 31 articles | PMID: 31881947 | PMCID: PMC6935121
Current Understanding of the Mechanisms Underlying Immune Evasion From PD-1/PD-L1 Immune Checkpoint Blockade in Head and Neck Cancer.
Front Oncol, 10:268, 28 Feb 2020
Cited by: 61 articles | PMID: 32185135 | PMCID: PMC7058818
Review Free full text in Europe PMC
Funding
Funders who supported this work.
Korea Health Industry Development Institute, Republic of Korea (1)
Grant ID: HI17C2085
Ministry of Health & Welfare, Republic of Korea (1)
Grant ID: 1420140

