Abstract
Free full text

TRPM2 in the Brain: Role in Health and Disease
Abstract
Transient receptor potential (TRP) proteins have been implicated in several cell functions as non-selective cation channels, with about 30 different mammalian TRP channels having been recognized. Among them, TRP-melastatin 2 (TRPM2) is particularly involved in the response to oxidative stress and inflammation, while its activity depends on the presence of intracellular calcium (Ca2+). TRPM2 is involved in several physiological and pathological processes in the brain through the modulation of multiple signaling pathways. The aim of the present review is to provide a brief summary of the current insights of TRPM2 role in health and disease to focalize our attention on future potential neuroprotective strategies.
1. Introduction
Transient receptor potential (TRP) proteins form non-selective cation channels which are involved in several cell functions in activated form. To date, about 30 different mammalian TRP channels have been recognized, which are divided into six subfamilies: TRPA (Ankyrin), TRPC (canonical), TRPM (melastatin), TRPML (mucolipin), TRPP (polycystin) and TRPV (vanilloid) [1]. Most TRP channels have a role in sensory perception in animals and they all share structural similarities [2]. Indeed, they contain six transmembrane regions with the C- and N-termini located intracellularly. Furthermore, they function mostly as heterotetramers or homotetramers that form a central ion permeation path between the fifth and sixth regions [3]. These channels are non-selective polymodal cation channels that are located in the plasma membrane. Their stimulation produces cell depolarization, which leads to the opening or closing of voltage-dependent ion channels and subsequently, affects the modulation of the ion cellular flux. TRPs are mainly calcium (Ca2+) release channels that are found in several cell organelles, such as lysosomes, endosomes, endoplasmic reticulum, synaptic vesicles and the Golgi network [4]. As polymodal channels, TRPs can be activated by different physical and chemical stimuli, such as mechanical forces, temperature, intracellular signaling molecules and exogenous compounds. However, to date, few endogenous ligands are recognized as “TRP activators” [5,6,7]. There are three different ways of activation for these channels: activation by receptor, ligand activation and direct activation [7]. In the first case, G protein–coupled receptors (GPCRs) and receptor tyrosine kinases activate phospholipases C (PLCs) that can regulate channel activity by the hydrolysis of phosphatidylinositol bisphosphate (PIP2) or by the production of diacylglycerol (DAG) or inositol trisphosphate (IP3), which results in the liberation of intracellular Ca2+ [8,9,10]. Ligands that activate TRP channels may be exogenous or endogenous small organic molecules; purine nucleotides and their metabolites (adenosine diphosphoribose (ADP-ribose), βNAD+); or inorganic ions, especially Ca2+ and magnesium (Mg2+). Since Ca2+ is a key excitatory messenger between neurons [11], its intracellular levels are finely regulated and an excessive load can lead to metabolic instabilities and in the worst case, cell death [12,13]. Finally, direct activation includes channel phosphorylation, mechanical stimuli and conformational coupling to IP3 receptors. Moreover, TRP channels can be indirectly activated by heating and cell swelling through second messengers or other unknown mechanisms [14].
The TRPM family is composed of TRPM1/M3, TRPM4/M5, TRPM6/M7, TRPM2 and TRPM8 [15]. These channels show a TRP segment at the C-terminal transmembrane domain that can be divided in two regions: a coiled-coils domain, which participates in the assembly of the channel into its tetrameric form and a second variable region. The coiled-coils domain is a structural motif in proteins, which allows the formation of α-helices that zip up together in a helical coil conformation [16]. Coiled-coils are identified in protein sequences by their characteristic repetition of aliphatic residues that alternates every few residues to form seven residue reappearances. Although the sequence patterns are an expression of three-dimensional coiled-coil structures, the resulting assembly cannot be accurately predicted [2]. TRPM proteins are implicated in a plethora of physiological mechanisms. In particular, TRPM2 is involved in the response to oxidative stress.
In the brain, TRPM2 is the most abundant TRP channel [17]. As a newly identified non-selective Ca2+-permeable cation channel and a sensor of reactive oxygen species (ROS), TRPM2 channel has recently been indicated to be involved in several physiological and pathological processes of the central nervous system (CNS) through the modulation of multiple signaling pathways.
In this review, after a brief insight in the main features of TRPM2, we focused on the role of this protein in aging and in common chronic and acute neurodegenerative diseases.
2. TRPM2 inside the Brain
TRPM2 channel was described for the first time in 1998 by Nagamine et al. [18]. This protein is a Ca2+-permeable non-selective cation channel without voltage-dependent behavior. The molecular basis for the permeability of TRPM2 to divalent cations, such as Ca2+ and Mg2+, is still not fully understood but it seems to be regulated by amino acid residues located between the pore helix and the selectivity filter in the channel pore [19,20]. It is widely expressed, especially in the CNS, heart, lung, liver and pancreas [17,21,22]. Recently, Zhang et al. [23] described the structure of TRPM2 channel from Nematostella vectersis, especially when bound to Ca2+, by electron cryo-microscopy (cryo-EM). They demonstrated that the structure of TRPM2 differs in local geometry and surface polarity from the other TRPM family channels, which may explain many of its unique functional features. In particular, the larger pore diameter and the negative surface charge of pores may contribute to determining its higher Ca2+ permeability compared to TRPM4 [23]. Within the brain, TRPM2 is expressed in microglia, astrocytes and neurons of the hippocampus, substantia nigra, striatum and cortex [15,24]. Due to its ubiquitous distribution, the channel may be involved in several physiological processes. The intracellular Ca2+ is a driving force for TRPM2 activity, which also depends on the presence of extracellular hydrogen peroxide (H2O2) [25,26]. TRPM2 is composed by a nudix hydrolase domain that is highly homologous to the ADP pyrophosphatase, NUDT9 [27,28] (Figure 1). Several extracellular stimuli, including ROS, H2O2, amyloid β-peptide (Aβ) and tumor necrosis factor-α (TNF-α), have been shown to induce TRPM2 activation via metabolic production of intracellular poly ADP-ribose (ADPR) polymerase, which is an enzyme that transfers multiple ADPR groups to proteins [25,29,30]. The inhibitors of polyADPR polymerase prevent TRPM2 activation without blocking the channel directly [30].

TRPM2 protein structure. TRPM2 channel includes 6 transmembrane domains, with a re-entry loop that forms a pore located between domains 5 and 6. Regulatory regions of the channel are contained within the cytoplasmic N- and C-termini.
TRPM2 is expressed in both neurons and glia and it contributes to hippocampal synaptic plasticity [31,32]. In 2011, Xie et al. [32] showed that the long term depression (LTD) is selectively impaired in TRPM2−/− mice because of the inhibition of the glycogen synthase kinase 3β (GSK3β) and decreased postsynaptic density protein 95 (PSD95). TRPM2 is also involved in neuronal development and plays an inhibitory role in neurite outgrowth. Indeed, the inhibition of TRPM2 increases the axonal growth [15,33]. In light of this, TRPM2−/− mice show longer neurites and more spines than wild type animals. Moreover, TRPM2 appears to be responsible for the physiological activation of the microglia mediated by ROS and (lipopolysaccharide) LPS signaling, while it is also involved in nitric oxide (NO) production [34,35]. Indeed, as suggested by studies involving TRPM2−/− mice, the channel’s sensitivity to H2O2 depends on the activation state of the microglia [36]. In 2014, Miyake et al. [37] demonstrated in cultured microglia that combined treatment with LPS and interferon-γ (IFNγ) can result in TRPM2-mediated extracellular Ca2+ influx. This signal is absent after pharmacological blockade or gene deletion of TRPM2 channel, while p38 mitogen-activated protein kinases (p38MAPK) and c-Jun N-terminal kinases (JNK) signaling contribute to the activation of microglia induced by LPS or IFNγ [37].
3. TRPM2 outside the Brain
In addition to CNS expression, TRPM2 is also localized in different cell types of the peripheral immune system, including neutrophils [38,39], macrophages [40,41], lymphocytes [42,43], monocytes [44] and dendritic cells [45]. Following an injury, Ca2+ influx through TRPM2 can directly mediate cytokine release, which contributes to the recruitment and activation of the inflammatory response [46]. Interestingly, in 2011, Knowles et al. [47] demonstrated that TRPM2-deficient mice showed low levels of cytokines, IL-12 and IFNγ and consequently, were more susceptible to infection. As shown by Di et al. [48], TRPM2 can also inhibit ROS production and prevent endotoxin-induced inflammation in phagocytic cells. Indeed, the depolarization of the plasma membrane causes a decrease in ROS levels mediated by (nicotinamide adenine dinucleotide phosphate) NADPH oxidase. As a result, TRPM2−/− mice that were exposed to endotoxins demonstrated augmented inflammatory responses and decreased survival compared to wild type animals. Moreover, Kashio et al. proved that TRPM2 may be involved in the phagocytic activity enhanced by fever in mice peritoneal macrophages [49]. To summarize, TRPM2 can be considered as a H2O2-activated cation channel that is involved in the host-defense system of the body.
Outside of the immune context, functional TRPM2 is also expressed in pancreatic β-cells where its activity contributes to the regulation of insulin secretion [49,50]. Insulin is secreted by pancreatic islets to control blood glucose levels and the downregulation of TRPM2 diminishes glucose-stimulated insulin secretion [51,52]. Recently, Uchida et al. [51] have shown both impaired glucose tolerance and decreased insulin secretion in TRPM2−/− mice. Although plasma insulin levels were similar in wild type and TRPM2−/− mice, the basal blood glucose levels were higher in transgenic animals than the others.
Moreover, the peculiarity of TRPM2 is its temperature sensitivity [49,50]. In recent years, Tan et al. demonstrated that TRPM2 is the ion channel responsible for the non-painful warmth sensitivity in somatosensory neurons, which they determined through a complex combination of electrophysiology, imaging, and RNA sequencing techniques [53,54]. In addition, as suggested in 2016 by Song et al., TRPM2 is also a hypothalamic temperature sensor. Indeed, the channel is able to limit the fever response and prevent overheating [55]. As Togashi et al. reported in 2006 [50], the activation of TRPM2 channel by heating was observed in HEK293T cells when the temperature ranges between 33 °C and 34 °C. However, the ion current that was evoked without any endogenous agonists was consistently lower than that in the presence of agonists, as confirmed by the same author in 2012 [49]. Although the temperature threshold for TRPM2 is around 47 °C, H2O2 treatment lowers the temperature needed for its activation to physiological temperature, showing that redox signals and temperature act as synergic stimuli [56,57]. In addition, TRPM2 is proposed to function in monocyte chemotaxis, which is known to be regulated by ADPR [58]. Nevertheless, TRPM2 channel is expressed throughout the body [17] and its role in Ca2+ homeostasis makes it an eligible candidate to mediate Ca2+-dependent physiological processes.
4. Role of TRPM2 in Brain Diseases
In developed countries, neurodegenerative diseases are considered as the most frequent cause of death after cancer and they have been predicted to surpass cancer as the most frequent cause before the year of 2040 [59]. Neurodegenerative diseases can be classified into two predominant types: chronic diseases (Alzheimer’s disease, AD; and Parkinson’s disease, PD) and acute diseases (cerebral ischemia, CI; and traumatic brain injury, TBI). They are all characterized by the presence of several common risk factors. In particular, oxidative stress, inflammation and aging play a crucial role in the evolution of these diseases [60]. It is important to understand that aging has numerous effects on the brain, including neurochemical changes, alterations in blood flow and reductions in white matter [61].
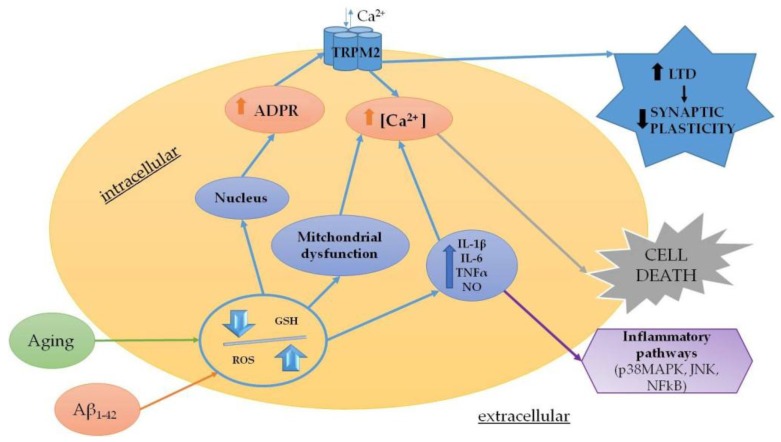
Oxidative stress results from an imbalance between the production of prooxidant and antioxidant agents, which are responsible for the rapid detoxification of the reactive intermediates on biological systems [62]. ROS production is not only physiological and occurs with respiration, but also increases in pathological transitory conditions (infections) or in more devastating diseases, such as chronic and acute neurodegenerative diseases, cancer, diabetes mellitus and autoimmune diseases [63,64]. Several studies suggested that the pathophysiology of neurodegenerative diseases is closely related to inflammatory responses and oxidative stress mediated by microglia [65,66,67]. Considering this, the role of oxidative stress may be considered as the most critical step in the treatment of these pathologies. It is worth mentioning that oxidative stress is also a feature of the physiological aging process. Furthermore, aging is responsible for neuronal Ca2+ dysregulation, reduction in antioxidant defense, increases in oxidative stress and perturbation of energy metabolism [68,69]. The involvement of oxidative stress during the aging process is widely accepted. Indeed, ROS increases with age and leads to functional alterations and pathological conditions [70]. Evidently, TRPM2 is thought to play a role in the aging process within the brain. Indeed, aging is related to decreased levels of glutathione (GSH) in vitro and in vivo [71]. GSH is the main intracellular reducing agent [72] and the first line of defense against ROS and reactive nitrogen species (RNS), which are generated as the by-products of aerobic metabolism. During aging, the cellular levels of GSH decrease and the resulting decline in antioxidant defense contributes to the increased susceptibility to age-related neurodegenerative diseases [73,74]. The depletion of GSH with age may also be associated with increased intracellular Ca2+ and its subsequent toxicity [75]. This process may involve TRPM2 as a recent study has demonstrated that the loss of inhibition by GSH enhances TRPM2 activity in hippocampal neurons [73]. As a consequence, the TRPM2 activity is associated with increased LTD [76] and these changes in synaptic plasticity produce an age-related decrease in synaptic strength, which may underlie the memory impairment associated with aging [20].
4.1. Chronic Neurodegenerative Diseases
Since TRPM2 is abundantly expressed in the CNS, it is not unexpected that this channel has been related to neurodegenerative diseases [77]. AD is the most frequent cause of senile dementia, which affects millions of people worldwide [78]. This disease is characterized by a progressive decline in cognitive function and in the final stage, patients are completely unable to carry out any daily activities. AD is characterized by a dramatic increase in oxidative stress and neuroinflammatory conditions, intracellular Ca2+ dysregulation and the presence of protein depositions, such as extracellular beta-amyloid (Aβ) plaques and intracellular tangles of tau protein [79]. Animals treated with Aβ show impaired learning and memory; activated astrocytes and microglia; and disturbed activation of c-JNK and GSK3β [80,81], which supports the role of glial cells in AD pathology. Activated astrocytes produce and release gamma-aminobutyric acid (GABA) by monoamine oxidase-B. By acting on presynaptic GABA receptors, the released GABA from astrocytes decreases the spike probability of granule cells in the dentate gyrus of AD model mice, which impairs the synaptic plasticity, such as learning and memory abilities [82]. One of the most accepted hypotheses implies that mitochondrial dysfunction and oxidative stress are the primary events in the onset of pathology [83]. Interestingly, different studies have observed that oxidative stress modifies Ca2+ homeostasis in AD patients and in animal models [84,85]. Indeed, accumulating evidence suggests that an overloaded intracellular free Ca2+ concentration increases mitochondrial membrane depolarization, oxidative stress and apoptotic cell death [86].
GSH has a physiological decrease in aging and a pathological decrease in AD [87]. To this end, primary neuronal cells may be cultured beyond 3 weeks to obtain characteristic cellular changes that have been associated with aging and neurodegeneration [88]. In these neurons, the increased current of TRPM2 channel can be decreased by GSH supplementation [73]. Furthermore, the depletion of GSH can induce oxidative stress, disturbance of Ca2+ homeostasis and apoptosis of hippocampal neurons through activation of TRPM2 channel [89].
Oxidative stress caused by the inhibition of GSH biosynthesis induces human microglia and astrocytes to secrete TNF-α, IL-6 and nitrite ions and to increase the concentration of intracellular Ca2+. These effects are correlated with the activation of inflammatory signaling of p38MAPK, JNK, and nuclear factor kappa-light-chain-enhancer of activated B cells (NFκB), which are reduced by the pharmacological blockade of TRPM2 channel or genetic ablation of its expression [21]. This confirms the contribution of TRPM2 channel to AD pathology [90].
Fonfria et al. exposed primary striatal cells to 20 μmol/L of monomeric Aβ to demonstrate an increase in intracellular Ca2+ and cell death, which were partially blocked when the cultures were transfected to inhibit TRPM2 functions [91]. More evidence of the contribution of TRPM2 to pathology and cognitive decline was recently provided in a transgenic mouse model of AD [92]. In this work, Ostapchenko et al. [93] showed that the knockout of TRPM2 reduces endoplasmic reticulum stress, age-dependent spatial memory deficit and microglia activation in APP/PS1 mice following Aβ treatment, although TRPM2−/−/APP/PS1 transgenic mice did not show any change in plaques formation. These results suggest that the deletion of TRPM2 channel is protective in AD, which may be achieved through the activation of microglia. Indeed, TRPM2 participates in the neuroinflammation induced by Aβ through microglia activation and generation of TNFα in a pathway, which involves ROS activation of poly (ADP-ribose) polymerase-1 (PARP-1) [94] (Figure 2).
Finally, TRPM2 has been proposed to contribute to cerebrovascular dysfunction in AD following the channel activation in vascular endothelial cells by Aβ [95]. These authors have demonstrated in different AD models that the cerebrovascular dysfunction requires oxidative stress-induced PARP-1 activity and even depends on TRPM2 activation in cerebral endothelial cells, which results in increased intracellular Ca2+. These findings highlight the possibility that endothelial TRPM2 channels may be a potential therapeutic target to counteract the cerebrovascular effects of Aβ.
TRPM2 has been also associated with bipolar disorder. Patients with Type I bipolar disorder show high basal Ca2+ levels and a susceptibility locus on chromosome 21q22.3., which is a region containing the TRPM2 gene [96,97]. Although TRPM2 variants with a single amino substitution (e.g., Asp543Glu) have been detected in patients with bipolar disorder, the role of these variants in the pathogenesis of the bipolar disorder remains unknown. Alterations in TRPM2 channel expression and function have also been demonstrated in other chronic diseases, such as Western Pacific amyotrophic lateral sclerosis (ALS) and PD. Intriguingly, Hermosura et al. have identified a mutation (P1018L) in ALS and PD patients, which is located in the pore loop of the TRPM2 channel and induces rapid channel inactivation [98].
4.2. Acute Neurodegenerative Diseases
Acute neuronal damage involves several processes, including excitotoxicity, inflammation, necrosis and apoptosis. In general, focal impairment of cerebral blood flow diminishes the delivery of substrates and impairs the maintenance of ionic gradients. As a consequence, dendritic and presynaptic voltage-dependent Ca2+ channels are activated and excitatory amino acids are released into the extracellular space. This starts a process called “excitotoxicity”, which represents the most important mechanism of cell death in stroke, CNS trauma and epilepsy [99]. Pharmacological blockade of glutamate receptors can prevent excitotoxicity and may have a neuroprotective effect, as demonstrated in animal models of stroke [100]. Although glutamate excitotoxicity is the main mechanism involved in neuronal death in CI, the significant contribution of Ca2+-permeable non-selective cation channels has also been demonstrated [20]. TRPM2 activation has been linked to cell death, suggesting that the channel can be considered as a key downstream player of several signaling pathways mediating cell death in response to CI and reperfusion injury [101]. Indeed, in a rat model of ischemia, the expression of TRPM2 mRNA increased from 1 to 4 weeks following stroke induction. The increased expression of TRPM2 was related to its transcriptional upregulation in glial cells in response to oxidative stress, which resulted in the promotion of cytokine release, exacerbation of inflammation and initiation of neuronal death [46]. Thus, following transient ischemia, the TRPM2 expressed in glia may be involved in the consequent injury [102].
Similarly, several studies have highlighted the link between ischemic neuronal death and TRPM2 activation [103,104]. A key source of ROS in the brain is produced by microglia and astrocytes. In this scenario, ROS may either directly or indirectly induce neuronal cell death or may increase glial proliferation to protect neurons. A wide range of stimuli activates microglia and the effects of activating factors are partially mediated by the modulation of intracellular Ca2+ [102]. In summary, TRPM2 may be considered as the connection point between Ca2+- and ROS-dependent signaling pathways [105] (Figure 3). In this context, Jia et al. [106] showed both in vitro and in vivo that TRPM2 inhibition or knockdown is neuroprotective against CI. Indeed, the pharmacological inhibitors and RNA interference targeting TRPM2 reduced the infarct volume in vivo and decreased neuronal cell death in vitro. In 2006, Fonfria et al. demonstrated that the up-regulation of TRPM2 correlates with microglial activation in a rat model of stroke [102]. Authors reported that the up-regulation of TRPM2 is associated over time with microglial activation. They also demonstrated that TRPM2 plays a key role in the stress-induced activation of these cells in rat primary microglia.
Interestingly, Shimizu et al. demonstrated the important contribution of TRPM2 in CI, especially in males, suggesting a sex difference in the role of TRPM2 in ischemic cell death [107]. To date, the patients with stroke are extremely challenging to treat, which is possibly due to the lack of knowledge about the sex difference in this disease [108,109]. The most important and non-modifiable risk factors for stroke are age and gender and hence, Shimizu et al. focused on a membrane-permeable selective inhibitor of TRPM2 that can be used to understand its role in neuronal injury following focal CI in aged mice of both sexes [61]. Moreover, Jia et al. showed that the TRPM2 inhibitor clotrimazole reduces hippocampal CA1 neuronal injury in male mice and may be a potential neuroprotective agent against ischemic damage [106]. Thus, the gender specific effect of TRPM2 inhibition in focal CI is of particular interest, considering the different vulnerability of sexes to CI in the human population [110].
5. Future Perspectives
In conclusion, we have shown multiple aspects of TRPM2 in the human body, especially in the brain. The versatile and intriguing nature of TRPM2 have made it one of the most fascinating ion channels in our body. The role of TRPM2 in health and disease is becoming increasingly relevant. We have to emphasize that the future use of drugs that are able to block TRPM2 function has raised some concerns about the potential side effects. As we discussed in this review, the role of TRPM2 in a plethora of physiological processes, such as insulin release, temperature sensation-regulation and immune function, inevitably needs to be considered in any study or development of new therapeutic strategies. Hopefully, extensive research on pharmacological tools will aid in characterizing TRPM2 and will be essential to develop selective neuroprotective strategies in order to counteract neurodegeneration and to slow down the aging process and improve the quality of life in the elderly population.
Acknowledgments
Authors would like to thank Gloria Munson for the language editing.
Funding
This research was funded by Ministero dell’Istruzione, dell’Università e della Ricerca (MIUR), PRIN 2015 (Prot. 20152HKF3Z).
Conflicts of Interest
The authors declare no conflict of interest.
References
Articles from Cells are provided here courtesy of Multidisciplinary Digital Publishing Institute (MDPI)
Full text links
Read article at publisher's site: https://doi.org/10.3390/cells7070082
Read article for free, from open access legal sources, via Unpaywall:
https://www.mdpi.com/2073-4409/7/7/82/pdf?version=1532242627
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/45461469
Article citations
[Construction of HEK293T cell line stably expressing TRPM2 channel based on PiggyBac transposition system and its application in drug screening for cerebral ischemia and other diseases].
Zhejiang Da Xue Xue Bao Yi Xue Ban, 53(5):604-614, 01 Oct 2024
Cited by: 0 articles | PMID: 39343750 | PMCID: PMC11528149
The odyssey of the TR(i)P journey to the cellular membrane.
Front Cell Dev Biol, 12:1414935, 23 Jul 2024
Cited by: 0 articles | PMID: 39108834 | PMCID: PMC11300232
Review Free full text in Europe PMC
Therapeutic potential of transient receptor potential (TRP) channels in psychiatric disorders.
J Neural Transm (Vienna), 131(9):1025-1037, 15 Jul 2024
Cited by: 0 articles | PMID: 39007920
Review
Roles of TRPM channels in glioma.
Cancer Biol Ther, 25(1):2338955, 29 Apr 2024
Cited by: 0 articles | PMID: 38680092 | PMCID: PMC11062369
Review Free full text in Europe PMC
Ion Channel Genes in Painful Neuropathies.
Biomedicines, 11(10):2680, 29 Sep 2023
Cited by: 4 articles | PMID: 37893054 | PMCID: PMC10604193
Review Free full text in Europe PMC
Go to all (19) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Glia and TRPM2 Channels in Plasticity of Central Nervous System and Alzheimer's Diseases.
Neural Plast, 2016:1680905, 28 Jan 2016
Cited by: 15 articles | PMID: 26942016 | PMCID: PMC4749827
Review Free full text in Europe PMC
Inhibitory effects of AG490 on H2O2-induced TRPM2-mediated Ca(2+) entry.
Eur J Pharmacol, 742:22-30, 30 Aug 2014
Cited by: 16 articles | PMID: 25179574
Effect of oxidative stress on TRPM2 and TRPC3 channels in B lymphoblast cells in bipolar disorder.
Bipolar Disord, 14(2):151-161, 01 Mar 2012
Cited by: 26 articles | PMID: 22420591
Funding
Funders who supported this work.
Ministero dell’Istruzione, dell’Università e della Ricerca (1)
Grant ID: 20152HKF3Z