Abstract
Objective
The investigation regarding the clinical significance of quantitative hepatitis B core antibody (anti-HBc) during chronic hepatitis B (CHB) treatment is limited. The aim of this study was to determine the performance of anti-HBc as a predictor for hepatitis B e antigen (HBeAg) seroconversion in HBeAg-positive CHB patients treated with peginterferon (Peg-IFN) or nucleos(t)ide analogues (NUCs), respectively.Design
This was a retrospective cohort study consisting of 231 and 560 patients enrolled in two phase IV, multicentre, randomised, controlled trials treated with Peg-IFN or NUC-based therapy for up to 2 years, respectively. Quantitative anti-HBc evaluation was conducted for all the available samples in the two trials by using a newly developed double-sandwich anti-HBc immunoassay.Results
At the end of trials, 99 (42.9%) and 137 (24.5%) patients achieved HBeAg seroconversion in the Peg-IFN and NUC cohorts, respectively. We defined 4.4 log10 IU/mL, with a maximum sum of sensitivity and specificity, as the optimal cut-off value of baseline anti-HBc level to predict HBeAg seroconversion for both Peg-IFN and NUC. Patients with baseline anti-HBc ≥4.4 log10 IU/mL and baseline HBV DNA <9 log10 copies/mL had 65.8% (50/76) and 37.1% (52/140) rates of HBeAg seroconversion in the Peg-IFN and NUC cohorts, respectively. In pooled analysis, other than treatment strategy, the baseline anti-HBc level was the best independent predictor for HBeAg seroconversion (OR 2.178; 95% CI 1.577 to 3.009; p<0.001).Conclusions
Baseline anti-HBc titre is a useful predictor of Peg-IFN and NUC therapy efficacy in HBeAg-positive CHB patients, which could be used for optimising the antiviral therapy of CHB.Free full text
Original article
Baseline quantitative hepatitis B core antibody titre alone strongly predicts HBeAg seroconversion across chronic hepatitis B patients treated with peginterferon or nucleos(t)ide analogues
Abstract
Objective
The investigation regarding the clinical significance of quantitative hepatitis B core antibody (anti-HBc) during chronic hepatitis B (CHB) treatment is limited. The aim of this study was to determine the performance of anti-HBc as a predictor for hepatitis B e antigen (HBeAg) seroconversion in HBeAg-positive CHB patients treated with peginterferon (Peg-IFN) or nucleos(t)ide analogues (NUCs), respectively.
Design
This was a retrospective cohort study consisting of 231 and 560 patients enrolled in two phase IV, multicentre, randomised, controlled trials treated with Peg-IFN or NUC-based therapy for up to 2 years, respectively. Quantitative anti-HBc evaluation was conducted for all the available samples in the two trials by using a newly developed double-sandwich anti-HBc immunoassay.
Results
At the end of trials, 99 (42.9%) and 137 (24.5%) patients achieved HBeAg seroconversion in the Peg-IFN and NUC cohorts, respectively. We defined 4.4 log10 IU/mL, with a maximum sum of sensitivity and specificity, as the optimal cut-off value of baseline anti-HBc level to predict HBeAg seroconversion for both Peg-IFN and NUC. Patients with baseline anti-HBc ≥4.4 log10 IU/mL and baseline HBV DNA <9 log10 copies/mL had 65.8% (50/76) and 37.1% (52/140) rates of HBeAg seroconversion in the Peg-IFN and NUC cohorts, respectively. In pooled analysis, other than treatment strategy, the baseline anti-HBc level was the best independent predictor for HBeAg seroconversion (OR 2.178; 95% CI 1.577 to 3.009; p<0.001).
Conclusions
Baseline anti-HBc titre is a useful predictor of Peg-IFN and NUC therapy efficacy in HBeAg-positive CHB patients, which could be used for optimising the antiviral therapy of CHB.
Introduction
Chronic HBV infection remains a major health burden and the main risk factor for the development of hepatocellular carcinoma worldwide. Profound and sustained suppression of HBV replication has been identified as the key determinant for achieving the goals of therapy to reduce liver damage and prevent development of endstage of liver diseases.1–3 In patients with hepatitis B e antigen (HBeAg)-positive chronic hepatitis B (CHB), HBeAg seroconversion has been established as a key surrogate marker of treatment response, usually associated with clinical remission and a lifelong inactive state with an excellent outcome.1
Over the last decades, seven drugs (two interferons (IFNs) and five nucleos(t)ide analogues (NUCs)) have been approved for the treatment of CHB. However, the efficacy of the current available drugs is still unsatisfactory. A 1-year course of IFNs and NUCs only results in HBeAg seroconversion in 30%–40% and around 20%,4–11 respectively. Hence, many efforts have been made to explore more valuable predictors of efficacy aiming to realise the individualised treatment of CHB and optimise the efficacy of current drugs.
Previous studies have identified pretreatment HBV DNA and alanine aminotransferase (ALT) levels as well as early on-treatment HBV DNA level, quantitative hepatitis B surface antigen (HBsAg) and HBeAg as the predictors related to the outcome of IFN or NUC treatment.12–15 Most of the above predictors are virus-related factors. However, the treatment efficacy of CHB, which is a virus–host interaction disease, is also associated with the immunology status of host. Historically, clinicians relied on elevated ALT as a surrogate marker for host anti-HBV activities. It is a very useful and convenient one and thus widely adopted. However, the exact relationship between ALT elevation and anti-HBV immune responses is not clearly defined so far. Thus, it is reasonable to explore other immunology factors related to antiviral efficacy. The classic HBV-specific CD4 or CD8 cells assay is the gold standard, but such assays are difficult to conduct in patients because of both host human leucocyte antigen and viral polymorphisms. In this study, we investigated the value of one HBV-specific adaptive immunity, namely, the level of hepatitis B core antibody (anti-HBc). It is one of the most classical serological markers for HBV infection and has been widely used in screening of chronic HBV infection combined with HBsAg.16 However, the clinical significance of quantitative anti-HBc during CHB treatment is still unknown. Recently, Yuan et al proposed that higher anti-HBc levels may reflect a stronger host-adaptive anti-HBV immune activity, and thus might predict the response of patients receiving anti-HBV therapies. This hypothesis has been demonstrated in two small sample size cohorts, the results of which showed that pretreatment anti-HBc could be an additional predictor for HBeAg seroconversion both in the IFN and NUC treated cohorts.17 Due to limited sample size and insufficient control of the cohorts, these new findings warranted a more rigorous validation.
Therefore, we aimed to determine the performance of anti-HBc titre as a predictor for HBeAg seroconversion in two large well-controlled cohorts of HBeAg-positive CHB patients receiving peginterferon (Peg-IFN) or NUC-based therapy, respectively.
Patients and methods
Patients
This was a retrospective cohort study consisting of patients enrolled in two phase IV, multicentre, randomised, controlled trials of Peg-IFN- or NUC-based therapy for up to 2 years, respectively (the Peg-IFN and NUC cohorts).18 19 All the patients enrolled in the two trials had the same inclusion and exclusion criteria: HBsAg-positive for at least 6 months, HBeAg-positive, and hepatitis B e antibody-negative, HBV DNA >5 log10 copies/mL, ALT ≥2 and <10×upper limit of normal, without any antiviral treatment within 6 or 12 months. The main findings and other eligibility criteria of these studies are reported elsewhere.18 19 Allocation and treatment strategy in the two trials are shown in figure 1.
To overcome some of drawbacks of retrospective studies (eg, missing data and risk of selection bias), all the patients who completed the trials were included in the analyses.
The study was approved by the Ethics Committee of Nanfang Hospital. Written informed consent was obtained from all patients.
Clinical and laboratory evaluation
In the two trials, clinical and laboratory assessments were done every 12 or 16 weeks from baseline to the end of study. HBV DNA level and HBV serological markers were measured with the platform of Roche COBAS Taqman (with the lower limit of detection of 12 IU/mL or 69.84 copies/mL) and Elecsys (Peg-IFN cohort) or ARCHITECT i2000SR (NUC cohort) in the central laboratory, respectively. Serum ALT levels were assessed at local laboratories according to standard procedures. HBeAg seroconversion at the end of trials was defined as the treatment endpoint.
Quantitative anti-HBc evaluation
Quantitative anti-HBc evaluation was conducted in a blinded fashion, relative to HBV treatment status and other characteristics, for all the available samples in the two trials by using a newly developed double-sandwich anti-HBc (both immunoglobulin (Ig)M and IgG) immunoassay validated by WHO anti-HBc standards.20 The double-sandwich anti-HBc assay used in the study has good reproducibility and reliability. For details, please see the online supplementary figure S1.
Statistical analysis
Data were expressed as counts and percentages for categorical variables and as mean and SD for continuous variables. Qualitative and quantitative differences between subgroups were analysed using χ2 or Fisher's exact tests for categorical parameters and the Student's t test or Mann–Whitney test for continuous parameters, as appropriate. For analyses of performance of quantitative anti-HBc level and change at specific timepoints in predicting treatment outcome, areas under the receiver operator characteristic curve (AUROC) of two parameters were calculated. The AUROCs were compared by Delong test. Sensitivity, specificity, positive predictive value, negative predictive value, positive likelihood ratio (LR+) and negative likelihood ratio (LR−) of several cut-off values of anti-HBc levels were calculated to explore the best cut-off value in predicting treatment outcome in both the Peg-IFN and NUC cohorts. Univariable and multivariable analyses were used to determine predictors of treatment outcome. All statistical tests were two-sided. Statistical significance was taken as p<0.05. All analyses were done with SPSS V.18.0.
Results
Patient characteristics
In all, 231 and 560 patients were enrolled in the analysis of the Peg-IFN and NUC cohorts, respectively. The demographic, virological and clinical characteristics of the patients are summarised in table 1. The mean age was 29.8±8.4 years, predominantly men (80.8%) and 61.7% of patients were infected with HBV genotype C in patients. The mean of baseline ALT, HBV DNA, HBsAg and HBeAg levels was 194.6±172.7 IU/mL, 8.5±1.1 log10 copies/mL, 4.1±0.7 log10 IU/mL and 2.5±0.9 log10 PEIU/mL, respectively. At the end of studies, 99 (42.9%) and 137 (24.5%) patients achieved HBeAg seroconversion in the Peg-IFN and NUC cohorts, respectively (table 1).
Table 1
Clinical characteristics of patients in Peg-IFN and NUC cohorts
| Peg-IFN cohort (N=231) | NUC cohort (N=560) | p Value | |
|---|---|---|---|
| Male gender (%) | 184 (79.7) | 455 (81.3) | 0.604 |
| Age, years | 29.1±6.8 | 30.1±8.9 | 0.084 |
| HBV genotype (%) | 0.557 | ||
 B B | 81 (35.1) | 217 (38.8) | |
 C C | 148 (64.1) | 340 (60.7) | |
 Others Others | 2 (0.9) | 3 (0.5) | |
| Baseline ALT level, IU/mL | 200.0±172.7 | 192.4±172.7 | 0.005 |
 ≥2×ULN (%) ≥2×ULN (%) | 189 (81.8) | 448 (80.0) | 0.557 |
| Baseline HBV DNA level, log10 copies/mL | 8.4±1.3 | 8.5±1.1 | 0.646 |
 ≥ 9 log10 copies/mL (%) ≥ 9 log10 copies/mL (%) | 87 (37.7) | 218 (39.4) | 0.739 |
| Baseline HBsAg level, log10 IU/mL | 4.0±0.7 | 4.2±0.7 | <0.001 |
| Baseline HBeAg level, log10 PEIU/mL | 2.4±1.0 | 2.6±0.9 | 0.005 |
| Baseline anti-HBc level, log10 IU/mL | 4.3±0.5 | 4.2±0.5 | 0.038 |
| HBeAg seroconversion at end of study (%) | 99 (42.9) | 137 (24.5) | <0.001 |
ALT, alanine aminotransferase; anti-HBc, hepatitis B core antibody; HBeAg, hepatitis B e antigen; HBsAg, hepatitis B surface antigen; NUC, nucleos(t)ide analogue; Peg-IFN, peginterferon; ULN, upper limit of normal.
Kinetics of quantitative anti-HBc during antiviral treatments
At baseline, the mean quantitative anti-HBc levels were 4.3±0.5 and 4.2±0.5 log10 IU/mL in the Peg-IFN and NUC cohorts, respectively. During Peg-IFN treatment, the mean anti-HBc level decreased to 3.6 log10 IU/mL at week 48 while, during NUC therapy, the mean anti-HBc level declined to 3.2 log10 IU/mL at week 52, and subsequently stabilised at 3.0 log10 IU/mL from week 52 to week 104 (figure 2A C). Patients treated with NUC showed significantly greater decline in anti-HBc levels than those treated with Peg-IFN at weeks 24, 36 and 48/52, respectively (p<0.001).

Kinetics of anti-HBc at different timepoints in Peg-IFN (A) and NUC (C) cohorts; anti-HBc levels at different timepoints according to treatment response in Peg-IFN (B) and NUC (D) cohorts. Peg-IFN, peginterferon; NUC, nucleos(t)ide analogue; anti-HBc, hepatitis B core antibody.
Moreover, anti-HBc levels in patients stratified by the treatment endpoint were further analysed as shown in figure 2B, D. In the Peg-IFN cohort, patients with HBeAg seroconversion had higher anti-HBc level than those without HBeAg seroconversion at the baseline and during the first 24-week treatment period with significant difference. In the NUC cohort, patients with HBeAg seroconversion also had higher anti-HBc level than those without HBeAg seroconversion from baseline to week 104 (p<0.05).
Performance of anti-HBc level for HBeAg seroconversion
To evaluate the quantitative anti-HBc levels and changes during early period of treatment, we further study the anti-HBc level and change at baseline, week 12 and week 24 by using the receiver operating characteristic curves. The AUROC of anti-HBc level (Peg-IFN cohort 0.640; NUC cohort 0.646) was highest at baseline and also higher than anti-HBc change from baseline in the Peg-IFN and NUC cohorts (figure 3).
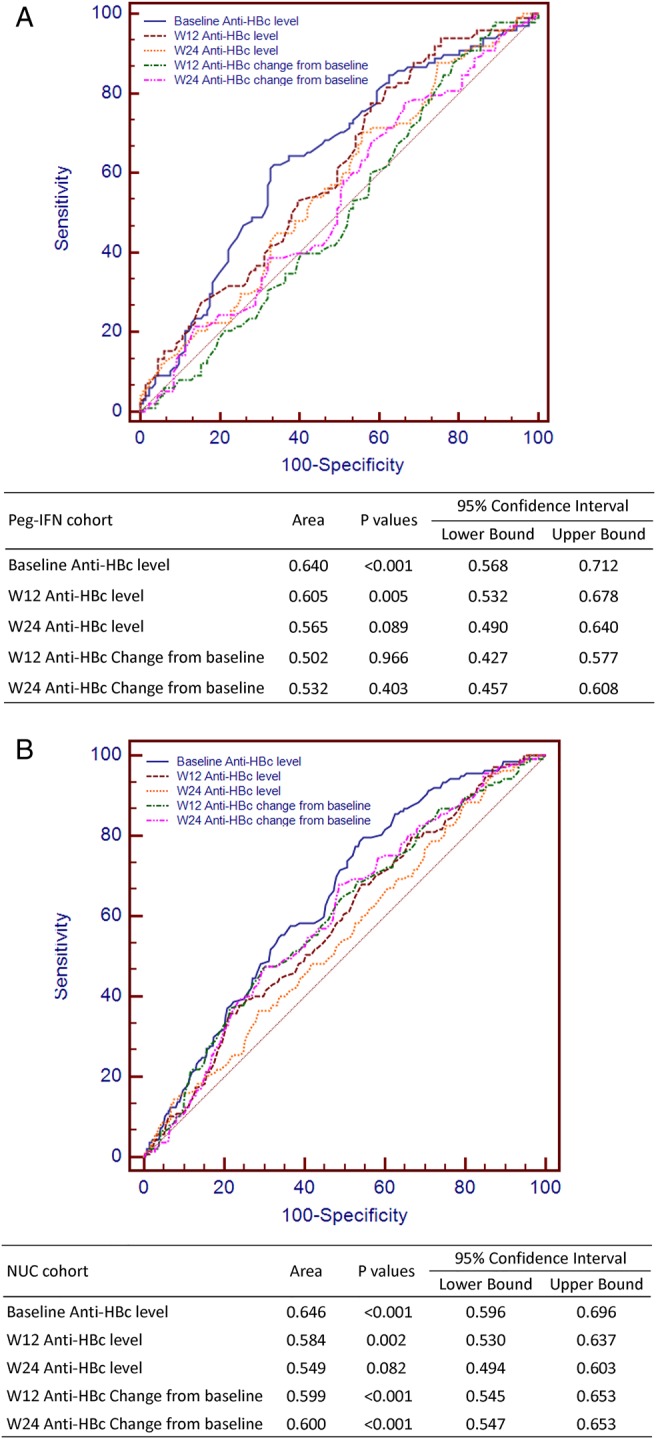
AUROCs of anti-HBc at different timepoints in Peg-IFN (A) and NUC (B) cohorts. AUROC, areas under the receiver operator characteristic curve; Peg-IFN, peginterferon; NUC, nucleos(t)ide analogue; anti-HBc, hepatitis B core antibody.
Table 2 shows the sensitivity and specificity of baseline anti-HBc level in predicting HBeAg seroconversion during IFN and NUC treatment. Six cut-off values were chosen because the sum of sensitivity and specificity was relatively high both in the Peg-IFN and NUC cohorts. Using the lowest cut-off value, the sensitivity in predicting HBeAg seroconversion was 87.9% and 90.5%, and the specificity was 26.5% and 30.7% in the Peg-IFN and NUC cohorts, respectively. Adopting the highest cut-off value, the specificity was increased to 74.2% and 79.0%, whereas the sensitivity was decreased to 46.5% and 37.2% in the Peg-IFN and NUC cohorts, respectively. If the two cohorts are combined together, the sum of sensitivity and specificity would achieve the highest when the cut-off value is 4.4 log10 IU/mL. Therefore, we adopted 4.4 log10 IU/mL as the optimal cut-off value of baseline anti-HBc level in the following analyses.
Table 2
Performance of baseline anti-HBc level in predicting HBeAg seroconversion in Peg-IFN and NUC cohorts
| Cohorts | Cut-off values | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | LR+ | LR– |
|---|---|---|---|---|---|---|---|
| Peg-IFN | ≥4.0 | 87.9 | 26.5 | 47.3 | 74.5 | 1.20 | 0.46 |
| ≥4.1 | 83.8 | 37.1 | 50.0 | 75.4 | 1.33 | 0.44 | |
| ≥4.2 | 72.7 | 46.2 | 50.3 | 69.3 | 1.35 | 0.59 | |
| ≥4.3 | 68.7 | 53.8 | 52.7 | 69.6 | 1.49 | 0.58 | |
| ≥4.4 | 60.6 | 66.7 | 57.7 | 69.3 | 1.82 | 0.59 | |
| ≥4.5 | 46.5 | 74.2 | 57.5 | 64.9 | 1.80 | 0.72 | |
| NUC | ≥4.0 | 90.5 | 30.7 | 29.7 | 90.9 | 1.31 | 0.31 |
| ≥4.1 | 84.7 | 37.8 | 30.6 | 88.4 | 1.36 | 0.41 | |
| ≥4.2 | 73.0 | 49.4 | 31.8 | 85.0 | 1.44 | 0.55 | |
| ≥4.3 | 58.4 | 59.3 | 31.7 | 81.5 | 1.44 | 0.70 | |
| ≥4.4 | 48.9 | 68.8 | 33.7 | 80.6 | 1.57 | 0.74 | |
| ≥4.5 | 37.2 | 79.0 | 36.4 | 79.5 | 1.77 | 0.80 | |
| Overall | ≥4.0 | 89.4 | 29.7 | 35.1 | 86.8 | 1.27 | 0.36 |
| ≥4.1 | 84.3 | 37.7 | 36.5 | 85.0 | 1.35 | 0.42 | |
| ≥4.2 | 72.9 | 48.6 | 37.6 | 80.8 | 1.42 | 0.56 | |
| ≥4.3 | 62.7 | 58.0 | 38.8 | 78.5 | 1.49 | 0.64 | |
| ≥4.4 | 53.8 | 68.3 | 41.9 | 77.7 | 1.70 | 0.68 | |
| ≥4.5 | 41.1 | 77.8 | 44.1 | 75.7 | 1.85 | 0.76 |
anti-HBc, hepatitis B core antibody; HBeAg, hepatitis B e antigen; LR−, negative likelihood ratio; LR+, positive likelihood ratio; NPV, negative predictive value; NUC, nucleos(t)ide analogue; Peg-IFN, peginterferon; PPV, positive predictive value.
Correlation between baseline characteristics and treatment endpoint
In order to further evaluate baseline characteristics in predicting HBeAg seroconversion, a multivariate analysis was conducted with inclusion of age, gender, HBV genotypes, baseline ALT level, baseline HBV DNA level, baseline quantitative HBsAg/HBeAg and anti-HBc levels in the model. The regression analysis showed that baseline anti-HBc level was a strong predictor for HBeAg seroconversion either in the Peg-IFN or NUC cohort (Peg-IFN: OR 2.658, 95% CI 1.519 to 4.651, p=0.001; NUC: OR 1.994, 95% CI 1.336 to 2.975, p=0.001, respectively). Besides baseline anti-HBc level, HBV DNA and ALT were the independent predictors in the Peg-IFN cohort (HBV DNA: OR 2.448, 95% CI 1.344 to 4.458, p=0.003; ALT: OR 2.378, 95% CI 1.096 to 5.159, p=0.028); HBV DNA and age were the independent predictors in the NUC cohort (HBV DNA: OR 1.762, 95% CI 1.148 to 2.706, p=0.010; age: OR 1.964, 95% CI 1.061 to 3.636, p=0.032) (table 3).
Table 3
Baseline variables associated with HBeAg seroconversion in Peg-IFN and NUC cohorts
| Factors | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p Value | OR | 95% CI | p Value | |
| Peg-IFN cohort | ||||||
 Age Age | 0.932 | 0.354 to 2.456 | 0.887 | |||
 Gender Gender | 1.223 | 0.643 to 2.327 | 0.540 | |||
 Genotype Genotype | 1.403 | 0.816 to 2.413 | 0.220 | |||
 Baseline ALT level Baseline ALT level | 2.132 | 1.030 to 4.416 | 0.041 | 2.378 | 1.096 to 5.159 | 0.028 |
 Baseline HBV DNA level Baseline HBV DNA level | 2.622 | 1.486 to 4.626 | 0.001 | 2.448 | 1.344 to 4.458 | 0.003 |
 Baseline HBsAg level Baseline HBsAg level | 2.320 | 1.362 to 3.952 | 0.002 | |||
 Baseline HBeAg level Baseline HBeAg level | 2.435 | 1.363 to 4.350 | 0.003 | |||
 Baseline Anti-HBc level Baseline Anti-HBc level | 3.077 | 1.790 to 5.289 | <0.001 | 2.658 | 1.519 to 4.651 | 0.001 |
| NUC cohort | ||||||
 Age Age | 1.924 | 1.050 to 3.527 | 0.034 | 1.964 | 1.061 to 3.636 | 0.032 |
 Gender Gender | 1.637 | 1.029 to 2.603 | 0.037 | |||
 Genotype Genotype | 1.137 | 0.768 to 1.682 | 0.522 | |||
 Baseline ALT level Baseline ALT level | 1.514 | 0.901 to 2.545 | 0.118 | |||
 Baseline HBV DNA level Baseline HBV DNA level | 1.930 | 1.267 to 2.939 | 0.002 | 1.762 | 1.148 to 2.706 | 0.010 |
 Baseline HBsAg level Baseline HBsAg level | 1.737 | 1.170 to 2.579 | 0.006 | |||
 Baseline HBeAg level Baseline HBeAg level | 1.251 | 0.846 to 1.851 | 0.262 | |||
 Baseline Anti-HBc level Baseline Anti-HBc level | 2.110 | 1.424 to 3.126 | <0.001 | 1.994 | 1.336 to 2.975 | 0.001 |
| Overall | ||||||
 Treatment strategy Treatment strategy | 2.316 | 1.675 to 3.202 | <0.001 | 2.237 | 1.598 to 3.132 | <0.001 |
 Age Age | 1.784 | 1.087 to 2.927 | 0.022 | |||
 Gender Gender | 1.487 | 1.025 to 2.158 | 0.036 | |||
 Genotype Genotype | 1.182 | 0.866 to 1.614 | 0.292 | |||
 Baseline ALT level Baseline ALT level | 1.715 | 1.128 to 2.609 | 0.012 | 1.707 | 1.100 to 2.647 | 0.017 |
 Baseline HBV DNA level Baseline HBV DNA level | 2.123 | 1.521 to 2.964 | <0.001 | 1.964 | 1.387 to 2.781 | <0.001 |
 Baseline HBsAg level Baseline HBsAg level | 2.066 | 1.514 to 2.820 | <0.001 | |||
 Baseline HBeAg level Baseline HBeAg level | 1.648 | 1.199 to 2.267 | 0.002 | |||
 Baseline Anti-HBc level Baseline Anti-HBc level | 2.509 | 1.836 to 3.428 | <0.001 | 2.178 | 1.577 to 3.009 | <0.001 |
The variables enrolled in logistic regression analysis were age (≤40 vs >40 years), gender (female vs male), genotype (non-C vs C), baseline ALT level (≥2 vs <2 ULN), baseline HBV DNA level (<9 vs ≥9 log10 copies/mL), baseline HBsAg level (<4 vs ≥4 log10 IU/mL), baseline HBeAg level (<3 vs ≥3 log10 PEIU/mL), baseline anti-HBc level (≥4.4 vs <4.4 log10 IU/mL) and treatment strategy (Peg-IFN vs NUC).
ALT, alanine aminotransferase; anti-HBc, hepatitis B core antibody; HBeAg, hepatitis B e antigen; HBsAg, hepatitis B surface antigen; NUC, nucleos(t)ide analogue; Peg-IFN, peginterferon; ULN, upper limit of normal.
Then, we conducted the multivariate analysis among the overall patients, and the results of pooled analysis showed that other than treatment strategies, baseline anti-HBc level was the best independent predictor for HBeAg seroconversion (OR 2.178; 95% CI 1.577 to 3.009; p<0.001). HBV DNA (OR 1.964; 95% CI 1.387 to 2.781; p<0.001) and ALT (OR 1.707; 95% CI 1.100 to 2.647; p=0.017) were the next predictors for HBeAg seroconversion among the overall population (table 3).
Rates of HBeAg seroconversion among patients with favourable baseline characteristics or early on-treatment response
In the Peg-IFN and NUC cohorts, 104 (45.0%) and 199 (35.5%) patients had baseline anti-HBc ≥4.4 log10 IU/mL among which 57.7% (60/104) and 33.7% (67/199) achieved HBeAg seroconversion at the end of trials. If baseline anti-HBc and HBV DNA (cut-off value 9 log10 copies/mL) were combined together, patients with baseline anti-HBc ≥4.4 log10 IU/mL and baseline HBV DNA <9 log10 copies/mL had 65.8% (50/76) and 37.1% (52/140) of HBeAg seroconversion in the Peg-IFN and NUC cohorts, respectively. Conversely, only 25.4% (15/59) and 14.5% (23/159) patients achieved HBeAg seroconversion among patients with anti-HBc <4.4 log10 IU/mL and baseline HBV DNA ≥9 log10 copies/mL in the Peg-IFN and NUC cohorts, respectively (figure 4).
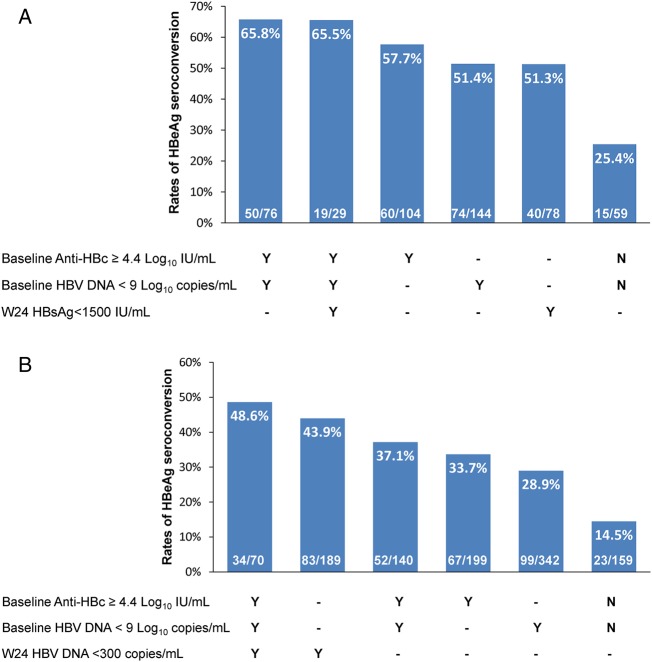
Treatment response among subgroups of patients stratified by parameters at baseline and week 24 in Peg-IFN (A) and NUC (B) cohorts. Peg-IFN, peginterferon; NUC, nucleos(t)ide analogue; anti-HBc, hepatitis B core antibody; HBsAg, hepatitis B surface antigen.
Previous studies had identified quantitative HBsAg and HBV DNA levels as the on-treatment predictors during Peg-IFN and NUC treatment, respectively. In the current study, 51.3% (40/78) of patients with week 24 HBsAg <1500 IU/mL and 43.9% (83/189) of patients with week 24 HBV DNA <300 copies/mL achieved HBeAg seroconversion in the Peg-IFN and NUC cohorts, respectively. Moreover, we further examined the rates of HBeAg seroconversion among patients with baseline anti-HBc ≥4.4 log10 IU/mL and baseline HBV DNA <9 log10 copies/mL, and taking into account their early on-treatment response, the results showed that, among the above subgroups, 65.5% (19/29) and 48.6% (34/70) could achieve HBeAg seroconversion in the Peg-IFN and NUC cohorts, respectively (figure 4).
Discussion
To our knowledge, this is the first comprehensive and definitive analysis to assess the performance of quantitative anti-HBc level, a novel immunological biomarker, in patients with CHB treated with anti-HBV agents. The robust results of these analyses are supported by the large, well-controlled cohorts comprised of patients treated with Peg-IFN- or NUC-based therapy and the relatively complete data collection. Our results demonstrated that a baseline anti-HBc level ≥4.4 log10 IU/mL is associated with higher rates of HBeAg seroconversion in CHB patients treated with both Peg-IFN and NUC.
Currently, a variety of parameters have been identified for the prediction of antiviral treatment efficacy in patients CHB. HBV DNA and ALT levels have been widely accepted as the traditional universal biomarkers in both IFN and NUC treated patients. However, many other predictors are only applicable for one kind of treatment strategy. For example, quantitative HBsAg is mainly applied in predicting efficacy of Peg-IFN, and its value in predicting efficacy of NUC is controversial;13 the genetic predictors (eg, interleukin (IL)-28 polymorphisms) were also predominantly investigated among patients treated with Peg-IFN.21 In this study, we demonstrated the general applicability of quantitative baseline anti-HBc level in predicting the efficacy of antiviral treatment with Peg-IFN or NUC. Furthermore, we also defined a unified optimal cut-off value of 4.4 log10 IU/mL with a maximum sum of sensitivity and specificity for both Peg-IFN and NUC treatment, which will be convenient for its application in real-life clinical practice.
In order to compare the baseline quantitative anti-HBc with other known baseline predictors, we conducted a multivariate regression analysis in Peg-IFN treated patients, NUC treated patients and overall population, respectively. The results indicated that baseline anti-HBc level could predict HBeAg seroconversion independently with the highest OR value among kinds of baseline parameters both in the Peg-IFN and NUC cohorts. Interestingly, after pooling the two cohorts together, other than treatment strategy, the baseline anti-HBc level was also the independent predictor with the highest OR value (2.178). In addition, baseline HBV DNA and ALT levels were also independently related to the treatment outcome as expected, which confirmed previous studies in patients with CHB and clearly indicated that our study cohort has limited issue of bias. Accordingly, we identified a subgroup of patients with baseline anti-HBc ≥4.4 log10 IU/mL and baseline HBV DNA <9 log10 copies/mL, which could achieve 65.8% and 37.1% of HBeAg seroconversion in the Peg-IFN and NUC cohorts, respectively, whereas the rates of HBeAg seroconversion among patients with unfavourable baseline characteristics were only 25.4% and 14.5% in the Peg-IFN and NUC cohorts, respectively.
Although baseline anti-HBc level as well as baseline HBV DNA and ALT levels proved to be independently associated with HBeAg seroconversion in the current study, the AUROC values of them were all less than 0.65 (see online supplementary figure S2), indicating that the overall predictability of them were not satisfactory. Moreover, previous studies had demonstrated that early on-treatment response was associated with the efficacy of antiviral treatment. Zeuzem et al15 proved that non-detectable serum HBV DNA at week 24 was the strongest predictor for better outcomes in a cohort treated with telbivudine. Liaw et al demonstrated that patients with HBsAg <1500 IU/mL at week 24 could achieve the highest rate of HBeAg seroconversion in a cohort treated with Peg-IFNα-2a.13 Thus, we further evaluated the treatment efficacy among subgroups of patients stratified by parameters at baseline and week 24. The results showed that among patients with baseline anti-HBc ≥4.4 log10 IU/mL and baseline HBV DNA <9 log10 copies/mL, the rate of HBeAg seroconversion had almost no change after taking into account the on-treatment response (ie, 24-week HBsAg <1500 IU/mL) in the Peg-IFN cohort (65.8% vs 65.5%); and increased from 37.1% to 48.6% after combining the on-treatment response (ie, 24-week HBV DNA <300 copies/mL) in the NUC cohort. The above results indicated that baseline parameters combined with on-treatment response could further improve the predictive value to some extent in the NUC cohort, but not in the Peg-IFN cohort. Accordingly, we concluded that the evaluation of baseline parameters was important during antiviral treatment, especially during Peg-IFN treatment because baseline parameters have been shown to be strongly related to the treatment efficacy, and they could allow physicians to optimise treatment before initiating antiviral treatment.
Until now, the investigation on the predictive value of anti-HBc in antiviral treatment is limited. Yuan et al17 had retrospectively investigated the usefulness of the baseline anti-HBc level in predicting post-treatment response in two cohorts of small sample sizes (NUC cohort, n=49; Peg-IFN cohort, n=48); the results also suggested that the baseline anti-HBc level may be an additional predictor for post-treatment response both in IFN and NUC, although cut-off values applied for IFN and NUC are different from the current study, which is possibly related to the sample size and different population studied.
The mechanism underlying the predictive value of anti-HBc titre is still unknown. Many studies have shown that cellular immune response against HBV virus is important in controlling the infection with this virus. Specifically, CD4 and CD8 T cell responses have been shown to play a central role in the outcome of HBV infection. Also, Oliviero et al22 examined the role of B cells in chronic HBV infection by assessing B cell phenotype and function. They concluded that B lymphocytes played a crucial role in mediating immune response against HBV in CHB patient. Anti-HBc IgM and IgG were produced by HBcAg-specific B lymphocytes. Besides the ability of B cells in producing neutralising antibodies against HBV, they could produce several cytokines, like IFNγ or IL-6, to inhibit viral replication in hepatocytes and modulate the activity of CD4 and CD8T cells responses. In addition, Zgair et al23 demonstrated that anti-HBc had an important role in the severity of CHB through inhibition or clearance of HBV through the hepatocytotoxic effect of anti-HBc-secreting B cells. Therefore, the high level of anti-HBc at baseline may reflect the higher adaptive immune status of the patients which correlated with a better outcome after antiviral therapy.
The study also indicated that the patients treated with NUC showed significantly greater decline in anti-HBc levels than those treated with Peg-IFN. As we all know, the antiviral mechanisms of Peg-IFN and NUC are different. The former suppresses HBV replication by enhancing host immune system to mount a defence against HBV; the latter works mainly by inhibiting HBV DNA synthesis and interfering with the reverse-transcriptase activity of HBV. Therefore, Peg-IFN treatment could induce greater host immune activation compared with NUC treatment, which could support the slower decline of anti-HBc titre in the Peg-IFN cohort. Another explanation for this phenomenon was that NUC or Peg-IFN therapy might have a different impact on the frequency or counting of anti-HBc-secreting B cells. However, this hypothesis needs be verified by examining the dynamic change of B cell phenotypes within these cohorts in the future.
There are several implications concerning the clinical application of anti-HBc titre in the optimisation of antiviral treatment for CHB patients. This biomarker should be applicable to all NUC therapy because previous studies had demonstrated comparable effect of current available NUC treatment for CHB on HBeAg seroconversion; in addition, various NUCs have similar mechanism in suppressing HBV replication.7–11 Furthermore, we defined a uniform cut-off value of baseline anti-HBc for IFN and NUC treatment, which would be convenient for its application in clinical practice. Because baseline anti-HBc level had the highest OR value by using the cut-off value, anti-HBc should be tested as one of the valuable baseline predictors before initiating antiviral treatment in the clinical practice in order to optimise the antiviral treatment.
Our study has the strength of two well-controlled cohorts and large sample size, which increased the statistical power and reliability of the results. Nonetheless, our study also has a few limitations. First, the treatment outcome evaluated in our study was HBeAg seroconversion, which is the surrogate endpoint. However, we believe that achieving serological response is also an important goal of anti-HBV treatment, especially for young patients in the Asia-Pacific region.1 Second, patients in the NUC cohort were treated with telbivudine with/without adefovir, which are no longer the first-line antiviral drug for CHB; however, we propose that anti-HBc titre could also be applied in other NUCs due to their similar mechanism of action and comparable effects on HBeAg seroconversion. Third, we only evaluated the predictive value of anti-HBc in two cohorts, but did not yet examine it in another independent cohort, which will undermine the credibility of the results to some extent. Fourth, the double-sandwich anti-HBc assay used in the study has not been widely validated and been commercialised, which will influence the application of anti-HBc in clinical practice, although this new assay had been validated by WHO anti-HBc standards.
In conclusion, baseline anti-HBc titre is a reliable predictor of Peg-IFN and NUC therapy efficacy in HBeAg-positive CHB patients, which might be used for pretreatment stratification aimed at optimising the treatment of CHB.
Acknowledgments
We thank the study investigators, coordinators, nurses, patients and their families for their contributions. We also wish to thank Professor Chunquan Ou from Department of Biostatistics, Southern Medical University, for her helpful assistance on the statistical analysis.
Footnotes
Collaborators: In addition to the authors, Chronic Hepatitis B Study Consortium includes the following persons: Xiaoguang Dou (Department of Infectious Diseases, Shengjing Hospital of China Medical University, Shenyang), Junping Shi (6th People’s Hospital, Hangzhou), Hong Ren (Department of Infectious Diseases, The second Affiliated Hospital, Chongqing Medical University, Chongqing), Maorong Wang (Department of Infectious Diseases, 81st PLA Hospital, Nanjing), Hong Ma (Liver Research Center, Beijing Friendship Hospital, Capital Medical University, Beijing), Zhiliang Gao (Department of Infectious Diseases, Sun Yat-Sen University 3rd Affiliated Hospital, Guangzhou), Hongfei Zhang (302nd PLA Hospital, Beijing) and Chengwei Chen (Department of Infectious Diseases, 85th PLA Hospital, Shanghai).
Contributors: JLH, NSX, HZ, JDJ, FML, JS and RF were involved in the study design. RF, JS, QY, QX, XFB, QN, JC, YYY, JQN, GFS, HW, DMT, MBW, SJC, MX, XYC, HT, JFS and Chronic Hepatitis B Study Consortium collected data. JLH, JS and RF analysed and interpreted the data and wrote the manuscript. JLH and NSX approved the final manuscript. All authors had full access to the final version of the report and agreed to the submission.
Funding: This study was funded by National Science and Technology Major Project (2012ZX10002003) and Key Clinical Specialty Discipline Construction Program.
Competing interests: QN has been a member of advisory committees or review panels, received consulting fees from Roche, Novartis, GlaxoSmithKline and Bristol-Myers Squibb and has received grant/research support from Roche, Novartis and Bristol-Myers Squibb. JDJ has acted as a consultant for Novartis, Bristol-Myers Squibb, GSK, Roche and Merck Sharp & Dohme. JLH has received consulting fees from Roche, Novartis, GSK and Bristol-Myers Squibb and has received grant/research support from Roche, Novartis and GSK.
Ethics approval: Ethics Committee of Nanfang Hospital.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
Full text links
Read article at publisher's site: https://doi.org/10.1136/gutjnl-2014-308546
Read article for free, from open access legal sources, via Unpaywall:
https://gut.bmj.com/content/gutjnl/65/2/313.full.pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/3063751
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1136/gutjnl-2014-308546
Article citations
Predicting clinical outcomes in chronic hepatitis B patients receiving nucleoside analogues and pegylated interferon alpha: a hematochemical and clinical analysis.
BMC Infect Dis, 24(1):1149, 13 Oct 2024
Cited by: 0 articles | PMID: 39396949 | PMCID: PMC11472561
Circulating HBsAg-specific B cells are partially rescued in chronically HBV-infected patients with functional cure.
Emerg Microbes Infect, 13(1):2409350, 29 Oct 2024
Cited by: 0 articles | PMID: 39470771 | PMCID: PMC11523254
Quantitative HBV Core Antibodies as a Prognostic Marker for HBeAg Seroclearance: A Systematic Review with Meta-Analysis.
Viruses, 16(7):1121, 12 Jul 2024
Cited by: 0 articles | PMID: 39066283 | PMCID: PMC11281513
Review Free full text in Europe PMC
Comparison of Autolumo A2000 Plus and Architect i2000 for detection of hepatitis B virus serological markers.
Heliyon, 10(14):e32698, 08 Jun 2024
Cited by: 0 articles | PMID: 39113996 | PMCID: PMC11304000
Quantitative hepatitis B core antibody and quantitative hepatitis B surface antigen: Novel viral biomarkers for chronic hepatitis B management.
World J Hepatol, 16(4):550-565, 01 Apr 2024
Cited by: 0 articles | PMID: 38689745 | PMCID: PMC11056893
Review Free full text in Europe PMC
Go to all (70) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Baseline hepatitis B core antibody predicts treatment response in chronic hepatitis B patients receiving long-term entecavir.
J Viral Hepat, 24(2):148-154, 28 Nov 2016
Cited by: 18 articles | PMID: 27891715
Association of hepatitis B core antibody level and hepatitis B surface antigen clearance in HBeAg-negative patients with chronic hepatitis B.
Virulence, 15(1):2404965, 24 Sep 2024
Cited by: 0 articles | PMID: 39317345 | PMCID: PMC11423664
Prediction model for sustained hepatitis B e antigen seroconversion to peginterferon alfa-2a in chronic hepatitis B.
J Gastroenterol Hepatol, 31(12):1963-1970, 01 Dec 2016
Cited by: 10 articles | PMID: 27075693
Adefovir dipivoxil and pegylated interferon alfa-2a for the treatment of chronic hepatitis B: a systematic review and economic evaluation.
Health Technol Assess, 10(28):iii-iv, xi-xiv, 1-183, 01 Jan 2006
Cited by: 30 articles | PMID: 16904047
ReviewBooks & documents Free full text in Europe PMC