Abstract
Free full text

IgG1 antibodies to acetylcholine receptors in ‘seronegative’ myasthenia gravis†
Associated Data
Abstract
Only around 80% of patients with generalized myasthenia gravis (MG) have serum antibodies to acetylcholine receptor [AChR; acetylcholine receptor antibody positive myasthenia gravis (AChR-MG)] by the radioimmunoprecipitation assay used worldwide. Antibodies to muscle specific kinase [MuSK; MuSK antibody positive myasthenia gravis (MuSK-MG)] make up a variable proportion of the remaining 20%. The patients with neither AChR nor MuSK antibodies are often called seronegative (seronegative MG, SNMG). There is accumulating evidence that SNMG patients are similar to AChR-MG in clinical features and thymic pathology. We hypothesized that SNMG patients have low-affinity antibodies to AChR that cannot be detected in solution phase assays, but would be detected by binding to the AChRs on the cell membrane, particularly if they were clustered at the high density that is found at the neuromuscular junction. We expressed recombinant AChR subunits with the clustering protein, rapsyn, in human embryonic kidney cells and tested for binding of antibodies by immunofluorescence. To identify AChRs, we tagged either AChR or rapsyn with enhanced green fluorescence protein, and visualized human antibodies with Alexa Fluor-labelled secondary or tertiary antibodies, or by fluorescence-activated cell sorter (FACS). We correlated the results with the thymic pathology where available. We detected AChR antibodies to rapsyn-clustered AChR in 66% (25/38) of sera previously negative for binding to AChR in solution and confirmed the results with FACS. The antibodies were mainly IgG1 subclass and showed ability to activate complement. In addition, there was a correlation between serum binding to clustered AChR and complement deposition on myoid cells in patients’ thymus tissue. A similar approach was used to demonstrate that MuSK antibodies, although mainly IgG4, were partially IgG1 subclass and capable of activating complement when bound to MuSK on the cell surface. These observations throw new light on different forms of MG paving the way for improved diagnosis and management, and the approaches used have applicability to other antibody-mediated conditions.
Introduction
Myasthenia gravis (MG) is an antibody-mediated autoimmune disease of the neuromuscular junction. Over 80% of patients with generalized MG have serum antibodies to acetylcholine receptors (AChRs), which cause increased AChR degradation, complement-mediated damage to the post-synaptic membrane and reduced number of AChRs at the neuromuscular junction, leading to muscle weakness and fatigue (Drachman, 1994; Vincent, 2002). Antibodies to another neuromuscular junction protein, the muscle specific kinase (MuSK), were first identified in 70% of patients negative for AChR antibody (Hoch et al., 2001) and have been reported in a variable proportion of AChR antibody-negative MG patients worldwide, ranging from 0% to 49% (Vincent and Leite, 2005).
The remaining generalized MG patients are consistently negative for antibodies to the soluble native AChR or MuSK used in standard assays, and are often referred to as seronegative myasthenia gravis (SNMG). There is increasing evidence that SNMG is more similar to acetylcholine receptor antibody positive myasthenia gravis (AChR-MG) than MuSK antibody positive myasthenia gravis (MuSK-MG) in clinical features and response to immunosuppressive treatments (Evoli et al., 2003; Sanders et al., 2003; McConville et al., 2004; Zhou et al., 2004; Lavrnic et al., 2005; Romi et al., 2005) and thymic pathology (Lauriola et al., 2005; Leite et al., 2005). Moreover, muscle biopsies indicate loss of AChR (Shiraishi et al., 2005) and complement deposition (M. Motomura, personal communication) similar to that of AChR-MG, and contrasting with the normal AChR numbers and lack of complement deposition in MuSK-MG (Shiraishi et al., 2005).
There are several possible explanations for the apparent lack of AChR antibodies in SNMG. There could be antibodies to another neuromuscular junction protein, but given the clinical features summarized above, the most likely reason is the failure of current assays to detect the antibodies because of loss of antigenic determinants in the solubilized AChR used in the radioimmunoprecipitation assay, or because the AChR antibodies have only low affinity/avidity for the soluble AChR. Therefore, we asked whether SNMG patients’ IgG and/or IgM antibodies might bind to AChR expressed in its native conformation on the cell surface, and whether the density of AChRs was a factor in determining binding. In two independent serum collections, we detected AChR antibodies to clustered AChR in a high proportion of previously SNMG patients, and showed that these antibodies are potentially pathogenic since they are IgG1 subclass and activate complement. In addition, in those cases where thymic tissue was available, we show a relationship with thymic pathology.
Methods
Clinical material
We studied the earliest serum/plasma samples available from 65 patients with generalized MG, diagnosed by clinical and electromyographic criteria. We re-assayed all samples for AChR and MuSK antibodies using commercial antigens (RSR Ltd, Cardiff, UK), and stratified the cases on the basis of these results. There were 17 AChR-MG, 24 MuSK-MG and 24 SNMG patients identified. As positive controls, we selected 10 patients with a spectrum of AChR antibody titres. Negative controls included 14 healthy individuals (7M : 7F, aged 20–63) and 14 patients with other autoimmune neurological diseases including three with multiple sclerosis, four with paraneoplastic CNS syndromes, three with Lambert Eaton myasthenic syndrome, two with Miller Fisher syndrome and two each with neuromyotonia and voltage-gated potassium channel Abs (6M : 8F, aged 25–74). Supplementary Table 1 summarizes the MG patients’ demographic and clinical details. In addition, we subsequently studied a further 14 sera from patients whose thymuses were available for immunohistological studies (Leite et al., 2007).
Plasmid constructs
pcDNA3.1-hygro was purchased from Invitrogen Ltd, CA, USA and pEGFP-N1 was purchased from Clontech Laboratories, CA, USA. cDNAs encoding human AChR subunits α- (P3A isoform), β-, γ-, δ- and ε-, (Beeson et al., 1993) were cloned into pcDNA3.1-hygro. cDNA encoding full length human MuSK (McConville et al., 2004) was also cloned into pcDNA3.1-hygro. Human rapsyn cDNA, AChR α-subunit or MuSK were also cloned into pEGFP-N1 so that the expressed proteins were tagged intracellularly with enhanced green fluorescent protein (EGFP) (Cossins et al., 2006).
Maintenance and transfection of human embryonic kidney cells
The human embryonic kidney (HEK) 293 cell-line was grown in Dulbecco's modified eagle's medium (DMEM) supplemented with 10% FCS (TCS Cellworks Ltd, Buckingham, UK) and 100 units/ml each of penicillin G and streptomycin (Invitrogen) at 37°C in an atmosphere of 5% CO2 to 50% confluence on 13 mm glass coverslips placed in 6-well cell culture plates. Cells were transiently co-transfected using polyethylenimine (PEI) with adult or foetal AChR and rapsyn-EGFP (with the α-, β-, δ- and ε- or γ-subunits and rapsyn-EGFP in the ratio of 2 : 1 : 1 : 1 : 1). Rapsyn was used so that the AChRs formed dense clusters on the cell surface (Sanes and Lichtman, 2001), and the EGFP allowed these clusters to be visualized. For cells with unclustered AChRs, rapsyn was omitted and the α-subunit was tagged with EGFP. For mock transfections, cells were transfected only with pcDNA 3.1 hygro and EGFP. MuSK DNA tagged with EGFP was used instead of the AChR subunits and rapsyn for the MuSK transfection. Later, cells were also co-transfected with AChR subunits, rapsyn, MuSK and the recently identified MuSK interacting cytoplasmic docking protein 7 (Dok-7) (Beeson et al., 2006; Okada et al., 2006) (hereafter described as ARMD co-transfection). The expression of the EGFP-tagged proteins was monitored using an Axion 200 inverted Zeiss fluorescence microscope.
Immunofluorescence staining of the cells and microscopy
Immunofluorescence staining of transfected HEK 293 cells was performed 48 h after transfection. The coverslips were transferred into 24-well cell culture plates and rinsed gently with DMEM-N-(2-hydroxyethyl)piperazine-N′-(2-ethanesulphonic acid) (HEPES) buffer (DMEM containing 20 mM HEPES). Cells were incubated with anti-AChR monoclonal antibody [mAb; anti-β-subunit, B3; (Jacobson et al., 1999)], or with patient or control sera/plasmas (1 : 20), in 1% bovine serum albumin (BSA) DMEM-HEPES buffer, for 1 h at room temperature (RT). Cells were washed three times with DMEM-HEPES buffer and fixed immediately with 3% formaldehyde in phosphate buffered saline (PBS) for 15 min at RT. After rinsing with PBS, cells were labelled as follows: (i) for the detection of IgG or IgM antibodies, cells were incubated with anti-mouse IgG-, anti-human IgG- or anti-human IgM-Alexa Fluor 568-conjugated secondary antibody (Invitrogen-Molecular Probes, Paisley, UK) at 1 : 750 in DMEM-HEPES buffer +1%BSA, for 45 min at RT; (ii) for the identification of IgG subclasses, cells were incubated with mouse anti-human IgG1 or mouse anti-human IgG4 (Binding site, Birmingham, UK) at 1 : 50 in HEPES buffered DMEM with 1% BSA, for 1 h at RT; after three washes, cells were labelled with goat anti-mouse isotype specific IgG-Alexa Fluor 568-conjugated antibody (Invitrogen-Molecular Probes, Paisley, UK). After both stainings, cells were washed four times in PBS, nuclei counterstained with DAPI (4′,6′-diamidino-2-phenylindole dichloride) and the coverslips mounted on slides in fluorescence mounting medium (DakoCytomation, Cambridge, UK). They were stored at 4°C and protected from light for ~24 h before examination and imaging on a fluorescence microscope with a MacProbe v4.3 digital image system. All the photographs were taken under similar conditions.
FACS analysis
For flow cytometry the cells were cultured in 175 ml flasks and transfected as described above, but without EGFP. Approximately 48 h later, cells were detached from the flasks using Trypsin/EDTA, centrifuged, counted and resuspended in DMEM at 107cells/ml. Hundred microlitre of the cells (106) were added to 100 μl of plasma sample diluted at 1 : 5 in DMEM + HEPES + 1% FCS + 5 mM EDTA + 0.02% sodium azide. The cells were incubated for 1 h at RT rotating gently, and then washed three times in FACS buffer (PBS + HEPES + 1%FCS + 5 mM EDTA + 0.02% sodium azide). To detect the bound antibody, cells were rotated with 100 μl of goat anti-human IgG-Alexa Fluor 488 at 1 : 50 for 45 min at RT, protected from light. After three washes, cells were resuspended in 400 μl of PBS + 5 mM EDTA, transferred into FACS tubes and analysed immediately on a FACScan (Becton Dickinson, NJ, USA). Data were acquired and analysed using CellQuest software. A population of 104 cells was gated to exclude cell debris and to concentrate the analysis on a defined population of cells with similar characteristics. Cells stained only with the secondary antibody were used to test the fluorescence intensity of unstained cells, and cells stained with plasma samples with very high titre on radioimmunoprecipitation assay and high score on the cell staining were used as positive controls. The results are expressed as the percentage of gated cells positively labelled by IgG-Alexa Fluor 488.
Detection of C3b and C5b-9 deposition
HEK cells expressing AChR clusters, MuSK or ARMD were sensitized with heat-inactivated sera (30 min at 56°C) from the above patients at 1 : 20 dilution for 30 min at 37°C and then washed briefly. Freshly frozen serum from a healthy donor was used as a source of complement. This serum was thawed gradually and first incubated with HEK 293 cells on ice for 30–45 min to remove natural antibodies and reduce subsequent non-specific complement activation. The pre-adsorbed serum was applied to the sensitized cells at 1 : 20 dilution for 30 min at 37°C. The cells were then fixed in 3% formaldehyde and all further steps were carried out on ice to prevent the loss of C3 fragments from the cell surface. The cells were incubated with a polyclonal rabbit anti-human C3c antibody that detects C3b (DakoCytomation, 1 : 500, 30 min at 4°C) or polyclonal rabbit anti-human C5b-9 (membrane attack complex, MAC) or a rabbit isotype control (Zymed Laboratories, CA, USA, provided ready for use), followed by Alexa Fluor-568 goat anti-rabbit IgG antibody (Invitrogen; 1 : 750, for 45 min at 4°C). Coverslips were mounted using fluorescence mounting medium (DakoCytomation) containing DAPI (1 : 1000). HEK cells were found to express significant amounts of complement regulator proteins, CD55, CD59 and CD46 during the initial experiments. Therefore, non-complement-activating monoclonal antibodies against CD55 and CD59 were used to block the functional effects of these complement regulators (2–6 h, 37°C) prior to the initial serum sensitization (Bodian et al., 1997; Harris et al., 2000).
Immunofluorescence analysis
For simplicity, AChR-EGFP and AChR/Rapsyn-EGFP are designated ‘AChR’ and ‘AChR clusters’, respectively. Similarly, all the Alexa Fluor 568-labelled antibodies (anti-mouse IgG or anti-human IgG or IgM) will be identified as mouse IgG or human IgG or IgM. As expected, the EGFP and AChR expression levels varied noticeably between cells, so we checked all coverslips in their entirety. They were coded and scored for the frequencies and intensities of surface antibody binding and for the pattern of labelling of AChR (diffuse or in microaggregates) and AChR clusters (macroaggregates). We also recorded any co-localization of the red-labelled Igs with the EGFP-labelled AChR or AChR clusters. We used the following scoring system systematically for every coverslip: (0) = no labelling; (0.5) = very weak labelling of very few transfected cells with no obvious co-localization; (1) = weak labelling of some of the transfected cells, with co-localization; (2) = moderate labelling of some (~20–50%) of the transfected cells, with precise co-localization; (3) = moderate/strong labelling of ~50–80% of the transfected cells, with perfect co-localization; (4) = strong labelling of virtually all transfected cells, with perfect co-localization. The scores for each serum were usually identical, the same values were usually obtained by two independent observers, and none of them varied more than one point. The final score represents the median of these observations for three independent experiments for each of the sera. On the basis of the results of control samples, those scoring consistently 1 or more were classified as positive.
To test whether the antibodies were binding exclusively to the bungarotoxin binding site, we retested 34 MG sera that had given positive binding to clustered adult AChR after pre-incubating the cells with α-bungarotoxin (α-BuTx; 1 μg/ml; 15 min).
To test the specificity of the antibody binding, we retested 7 MG samples (6 SNMG and one AChR-MG) all positive on clustered AChR, after pre-absorbing the serum samples with recombinant proteins representing the α-subunit or δ-subunit of AChR (Jacobson et al., 1999) or the four extracellular domains of MuSK (McConville et al., 2004) (three cycles of pre-absorption of 30 min each, using a total amount of 3 μg of recombinant protein per 1 μl of serum in 250 μl of 1% BSA DMEM-HEPES buffer).
Immunofluorescence for complement activation in thymic tissue
Thymic sections were processed as published elsewhere (Leite et al., 2005). Briefly, 5 µm thymic sections from routine formalin-fixed, paraffin-embedded blocks were de-waxed and rehydrated and then either microwaved in Target Retrieval Solution (DakoCytomation, Glostrup, Denmark) for cytokeratin, CD3, CD1a, CD20 or desmin antibodies, or pre-treated with protease Type XXIV [Sigma, UK; 0.0125% solution (w/v) in PBS] for C3c and desmin antibodies.
Pre-treated sections were blocked with a peroxidase-blocking reagent (DakoCytomation) and incubated with antibodies to human Cytokeratin, CD3, CD1a, CD20 or Desmin. The binding was detected with the peroxidase-based Envision+ (DakoCytomation) method, counterstained with haematoxylin and mounted (Leite et al., 2005). For double immunofluorescence labelling, pre-treated sections were incubated with a mixture of the two primary antibody dilutions (anti-C3c and anti-cytokeratin, anti-C3c and anti-desmin or anti-desmin and anti-CD20) and then incubated with isotype-specific secondary antibodies conjugated to Alexa Fluor 488 or 568 (Molecular Probes, Leiden, The Netherlands); slides were mounted, and nuclei counterstained with DAPI in fluorescence mounting medium (DakoCytomation).
All slides were coded and analysed systematically by a single blinded observer (M.I.L.): entire sections labelled for cytokeratin or CD3 were used to measure areas of total thymic tissue and the extra-parenchymal infiltrates (Leite et al., 2005). Myoid cells were counted over two entire sections from each case (one double-stained for desmin/CD20 and the other for desmin/cytokeratin); counts were averaged to calculate the total numbers and frequencies of myoid cells. We also counted separately the myoid cells located either in the normal medulla or adjacent to or within the infiltrates (designated ‘exposed’ to infiltrates) in all the sections double labelled for desmin/cytokeratin, and calculated the percentage of myoid cells in those thymic compartments. In the staining for complement components C3c and C3b, we recorded and quantified the labelled myoid cells according to the thymic compartments in which they were found. More detailed staining methods, positive and negative controls used as well as microscope and imaging analysis are published elsewhere (Leite et al., 2005).
Statistical analysis
We used GraphPad PRISM 4 to graph and analyse the data by the one-way ANOVA test, followed by Bonferroni's post-test, χ2 with Yates’ correction, Fisher's exact test, the non-parametric Spearman's rank correlation coefficient or linear regression. In the Figures, the columns show the mean and standard error of the mean (SEM), and lines represent mean or median values.
Results
Clinical data
All sera were first assayed for AChR antibodies and MuSK antibodies. The results are shown in Fig. 1A where the patients are divided into those with clearly positive AChR antibodies (>1000 c.p.m./μl or 2.0 nM; AChR-MGhigh), those with lower or borderline values (250–1000 c.p.m./μl or 0.5–2.0 nM; AChR-MGlow) and those with values within the control range (<250 c.p.m./μl or <0.5 nM), the latter divided into MuSK antibody positive (MuSK-MG) and MuSK/AChR antibody seronegative (SNMG). All the patients were typical for their subgroup with respect to age at onset and sex ratio, and MGFA grades at maximum severity (Supplementary Table 1). The duration of MG at sampling showed a very wide range (1–384 months) and was longer than 2 years in around 50% of the patients in each subgroup. Many of the patients, therefore, were on treatments at the time of sampling (Supplementary Table 1) including steroids, azathioprine and other drugs. Some in each group had also received thymectomy.
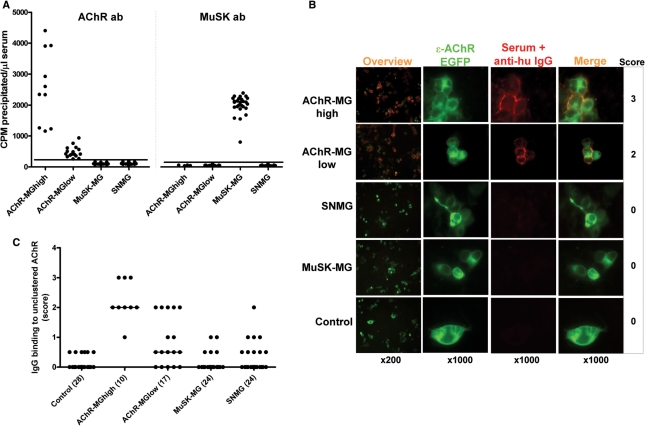
Detection of antibodies to AChR and MuSK and to unclustered AChRs on the transfected HEK cell surface. (A) AChR and MuSK antibodies were first measured by immunoprecipitation in all MG samples. Values <250 c.p.m./μl were within the control range and designated negative. Values of between 250 and 1000 c.p.m./μl were designated AChR-MGlow. (B) IgG antibody binding (red staining) of serum samples from MG groups and controls to unclustered adult AChR-EGFP expressed on HEK cells (green), with scores given on the right. (C) IgG binding scores in all patient and control samples. Lines show median values and number of samples tested are in brackets.
Binding of IgG to AChRs expressed on the HEK cell surface
We tested all sera for binding to AChR expressed on transfected HEK cells comparing with binding of control sera. The AChR in these experiments was tagged with EGFP so that the cells expressing AChR could be identified. Figure 1B shows examples of binding of IgG antibodies from each group. Binding of each serum was scored from 0 to 4 with the observers blind to the antibody status of the samples, except for the AChR-MGhigh sera used as positive controls. Binding of control sera to the AChR expressing cells scored 0–0.5 (mean + 3SD = 0.32), and values of 1 or greater were considered positive, therefore. As summarized in Table 1, all of the AChR-MGhigh samples and 7/17 of the AChR-MGlow sera were positive (both significantly different from control values). Interestingly, both the MuSK-MG and SNMG cohorts also contained a few sera that were weakly positive for binding to AChR, although the results in these two groups were not significantly different from controls (Fig. 1C).
Table 1
Frequency and characteristics of antibodies that bind to AChR or to MuSK expressed on HEK cells
| AChR-MG high (N = 10) | AChR-MG low (N = 17) | MuSK-MG (N = 24) | SNMG (N = 24) | Controls (N = 28) | |
|---|---|---|---|---|---|
| AChR-expressing HEK | |||||
| Binding to unclustered AChR | |||||
    IgG IgG | 10 | 7 | 2 | 4 | 0 |
    IgM IgM | 1 | 2 | 2 | 4 | 0 |
| Binding to clustered AChR | |||||
    IgG IgG | 10 | 17 | 8 | 14 | 0 |
    IgM IgM | 1 | 2 | 2 | 4 | 0 |
| Binding to clustered AChR in additional SNMG seraa | |||||
    (number tested) (number tested) | (14) | ||||
    IgG IgG | 11 | ||||
| IgG subclasses of antibodies to clustered AChR | |||||
    (number tested) (number tested) | (5) | (15) | (3) | (13) | (4) |
    IgG1 IgG1 | 5 | 13 | 3 | 11 | 0 |
    IgG4 IgG4 | 0 | 2 | 0 | 0 | 0 |
| Complement activation on clustered AChR-expressing cells | |||||
    (number tested) (number tested) | (5) | (13) | (13) | (5) | |
    C3b deposition C3b deposition | 5 | 8 | 3 | 0 | |
| MuSK-expressing HEK | |||||
| Binding to MuSK | |||||
    IgG IgG | 0 | 3 | 24 | 0 | 0 |
    IgM IgM | 0 | 3 | 0 | 1 | 0 |
| IgG subclasses of antibodies to MuSK | |||||
    (number tested) (number tested) | (21) | (2) | |||
    IgG1 IgG1 | 17 | 0 | |||
    IgG4 IgG4 | 21 | 0 | |||
    IgG4 > IgG1 IgG4 > IgG1 | 21 | – | |||
| Complement activation on MuSK-expressing cells | |||||
    (number tested) (number tested) | (21) | (4) | |||
    C3b deposition C3b deposition | 12 | 0 |
aAdditional SNMG sera from 14 patients with demographic and clinical characteristics similar to those of the 24 SNMG patients described in Supplementary Table 1.
Binding of IgG and IgM to clustered AChRs expressed on the HEK cell surface
For these experiments, we transfected the cells with AChR subunits and rapsyn-EGFP so that clusters of AChR/rapsyn could be identified by EGFP. Examples are shown in Fig. 2A and the scores in Fig. 2B. Again, control sera scored 0–0.5 (mean + 3SD = 0.95) and sera scoring 1 or more were considered positive. The AChR-MG sera all bound strongly to the clusters, which could easily be seen on the cell surface (Fig. 2A). In addition, many SNMG sera bound appreciably to the clustered AChRs (Fig. 2A and andB,B, Table 1). When the scores were compared with those for unclustered AChR (Fig. 2C), the results were significantly higher for binding to clustered AChR for AChR-MGhigh, AChR-MGlow and SNMG sera. Whereas, only 4/24 (17%) SNMG sera scored 1 or greater on unclustered AChR, 14/24 (58%) were positive on clustered AChR (P < 0.001 Fisher's exact test; Table 1).
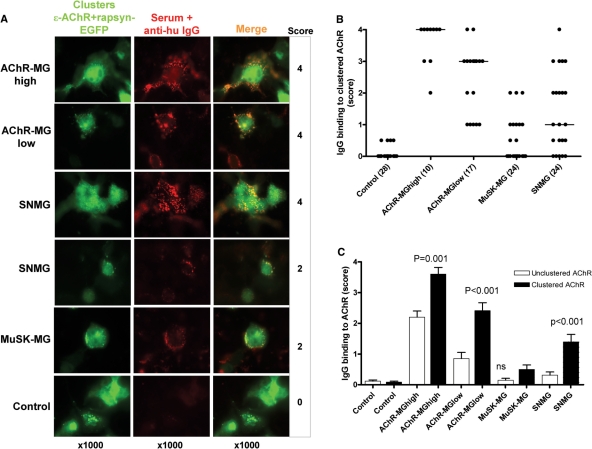
AChR clustering enhances detection of AChR antibodies. (A) Binding of IgG antibodies (red) to adult AChR clustered by rapsyn-EGFP on HEK cell surface (green) with scores shown on the right. (B) Scores for all samples tested. Median values are shown, and number of sera tested in brackets. (C) Comparison between IgG binding scores to unclustered and clustered AChR for all sera tested. Columns show means +SEM. There were differences between binding to clustered and unclustered AChR for AChR-MGhigh, AChR-MGlow and SNMG sera (all P < 0.001).
It was of interest to see if the high density of AChR in the clusters might detect binding of low-affinity IgM antibodies, whose presence in MG sera has been controversial. Very few MG sera had IgM antibodies (Table 1), and overall the scores were not different from those of the healthy controls (Supplementary Fig. 1 online).
To demonstrate the binding objectively, we performed FACS analysis on cells treated with selected sera (Fig. 3A). Again some of the SNMG sera bound weakly to the unclustered AChR but most SNMG sera showed significantly more (P < 0.01) binding to clustered AChR than to unclustered AChR (Fig. 3B). There was a strongly positive correlation between the visual scores for both clustered and unclustered AChR and the corresponding FACS results (Fig. 3C).

(A) FACS analysis of binding demonstrating the gating, based on untreated cells, and showing some example profiles. (B) Scatter plots of FACS analyses, comparing IgG binding (percentage of gated) to cells expressing unclustered or clustered AChR. The cut-off was based on the mean +2 SDs of results from seven healthy controls binding to clustered AChR. (C) Correlation between positive cells (percentage of gated) on FACS and binding scores obtained by visual inspection for the 39 sera tested.
Binding of IgG antibodies to adult or foetal AChR, and to the α-Butx binding site on the AChR
All of the experiments reported above used the adult form of the AChR, but it is possible that some sera are only reactive with foetal AChR. None of those samples that were negative on clustered adult AChR were positive for binding to clustered foetal AChR, and 36/39 of those that were positive bound equally to both forms. However, two AChR-MG and one SNMG sera showed strong binding only to adult AChR (e.g. Supplementary Fig. 2 online).
Since there have been concerns that the radioimmunoprecipitation assay fails to detect antibodies that bind to the ACh binding site on the AChR, which is occupied by 125I-α-bungarotoxin, we tested whether pre-incubation of the cells in α-Butx would prevent the binding of the SNMG antibodies. The only serum that did not bind in the presence of α-Butx (data not shown) was from a SNMG patient who had no IgG binding to unclustered AChR but scored four for IgG binding to clustered AChR, and recognized adult but not foetal AChR. The serum also scored 2 for IgM binding to either clustered or unclustered AChR. Interestingly, this serum was one whose IgM fraction strongly inhibited adult AChR currents in transfected HEK or CN21 cells (SNMG1 in Spreadbury et al., 2005).
Absorption of AChR antibodies with recombinant AChR subunits
To confirm the specificity of the immunostaining, and to see whether the antibodies in SNMG, like many in AChR-MG, bound the AChR α-subunit, we performed a limited number of absorption studies, using purified recombinant proteins representing the extracellular domains of the AChR α- and δ-subunits and MuSK as a control. Supplementary Table 2 online shows the results. There was limited absorption of the AChR-MGhigh serum with recombinant subunits, probably because there was an excess of AChR antibodies under the conditions that we tested and because typical AChR antibodies bind poorly to recombinant subunits. Binding of SNMG1, that was specific for the ε-containing adult form of the AChR, was not reduced by any of the three proteins, whereas the other SNMG sera, that bound to both adult and foetal AChR, showed reduced binding in the presence of α-subunit, but not in the presence of δ-subunit or MuSK, suggesting that they are mainly directed against the α-subunit and can recognize the recombinant α-subunit polypeptide.
IgG subclass and complement-fixation by SNMG antibodies
The cell-based assay described here offers a highly suitable substrate for determining whether the antibodies are able to activate complement when bound to their antigenic target in a native conformation. We found that the majority of SNMG antibodies were the complement-activating IgG1 rather than IgG4 (Fig. 4A, Table 1), similar to those of AChR-MG patients. Moreover, in the presence of fresh serum from a healthy control donor, 3 out of 13 SNMG samples (tested after heat-inactivation to remove any intrinsic complement activity) were capable of activating complement C3 and 6/13 SNMG sera activated the membrane attack complex C5-9 (MAC), demonstrating their potential to cause complement-mediated damage to the neuromuscular junction (Fig. 4B and C, Table 1), although, not unexpectedly, the scores for C3b or MAC deposition for SNMG sera were lower than those for AChR-MGlow or AChR-MGhigh sera (data not shown). Figure 4C shows the scores for the IgG subclasses, C3b and MAC deposition for nine SNMG sera tested in this manner.

Characterization of IgG antibodies to clustered adult AChR in AChR-MGlow and in SNMG samples. (A) Merged images show predominantly IgG1 (orange), and little IgG4 AChR antibody. (B) In the presence of fresh human serum and anti-human C3b or anti-human C5-9 (membrane attack complex, MAC), activated complement deposits were found in both AChR-MG and SNMG samples. Shown are C3b deposits for one AChR-MG serum, and C3b or MAC deposits for three SNMG sera. (C) Scores of IgG1 and IgG4 binding, and detection of C3b and MAC deposits in nine SNMG samples tested. Lines show median values and number of samples tested are in brackets. Sera from healthy individuals did not show any evidence of complement deposition.
Binding of sera to MuSK expressed on cell lines
We performed similar experiments with binding of the sera to MuSK-EGFP-transfected HEK cells. In this case we did not use any clustering protein, but MuSK expression was very high. All MuSK-MG sera were positive. None of the AChR-MGhigh sera showed detectable binding but three AChR-MGlow sera gave low positive results (Fig. 5A, Table 1). Interestingly, although the MuSK-MG antibodies were largely IgG4 (McConville et al., 2004), most sera also included IgG1 antibodies (Table 1, Fig. 5B and D) and complement activation was demonstrated by high scores for C3b deposition (Fig. 5C and D).
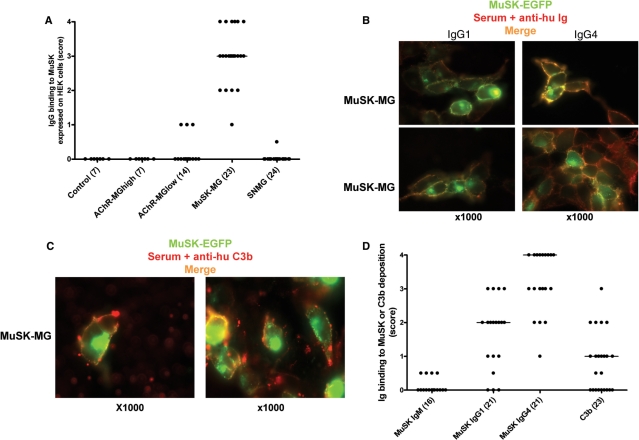
Detection of MuSK-MG antibodies binding to MuSK-EGFP transfected HEK cells and their characterization. (A) Scores of IgG binding to MuSK of all serum samples. (B) Merged pictures of IgG1 and IgG4 binding (yellow/orange) showing predominantly IgG4 antibodies but some IgG1. (C) C3b deposition (yellow/orange) in the presence of fresh human serum. (D) Scores of IgM, IgG1 and IgG4 antibody binding and scores of C3b deposition. Lines show median values and number of samples tested are in brackets.
Finally, we asked whether co-expressing ARMD would increase binding of SNMG sera. Four out of 10 SNMG samples that had not bound to rapysyn-clustered AChR showed weak binding (Score 1) when the cells were transfected to express the combination of proteins, suggesting that MuSK and Dok-7 might influence the AChR density or conformation. None of these samples showed detectable binding to cells transfected with MuSK or Dok-7 alone (data not shown).
Confirmation of AChR antibodies and evidence of thymic involvement
To confirm the results in the SNMG patients and to correlate these with thymic pathology, we then studied serum and thymus tissue from an additional 14 SNMG patients (Table 1), all of whom had been thymectomized, and thymic tissue of six of the AChR-MGlow patients. Firstly, we demonstrated that IgG antibodies to AChR clusters were present in 11/14 of the new SNMG patients, giving an overall rate of 25/38 (66%). As reported previously, many of the SNMG patients (Leite et al., 2005) and also six AChR-MGlow patients (not reported previously), had evidence of thymic involvement with typical lymphocytic infiltrates (Fig. 6A) and germinal centres (Fig. 6B and C) like those in AChR-MGhigh patients (Leite et al., 2005). Moreover, there were thymic myoid cells ‘exposed’ to the lymphocytic infiltrates in both the AChR-MGlow and SNMG cases (Fig. 6B–D), and these myoid cells frequently showed deposits of C3b (Fig. 6E and F). Overall, for the SNMG and AChR-MGlow samples together, there was a significant correlation between the binding to AChR clusters and the percentage of thymic tissue with infiltrates (r2 = 0.24; P = 0.016 data not shown) or the percentage of C3b positive myoid cells exposed to the infiltrates (r2 = 0.30; P = 0.006; not graphed in Fig. 6G). The correlation with C3b positive exposed myoid cells was also significant (r2 = 0.43; P = 0.04) for SNMG samples alone (graphed in Fig. 6G).

Thymic changes involved in AChR-MGlow and SNMG patients. (A) Size of infiltrates as a percentage of thymic tissue in all MG and control groups. (B and C) Distribution of myoid cells (stained for desmin, red) around or within the infiltrates/germinal centres (stained for CD20 on B cells, green) in AChR-MGlow (B) and in SNMG (C) thymi, and quantified in (D). (E and F) Signs of complement activation (deposits of C3b, red) on myoid cells (green) in AChR-MGlow (E) and SNMG thymus (F). (G) Correlation between the score of IgG binding to AChR clusters and the percentage of C3b positive myoid cells exposed to the infiltrates. In (A) and (D), column and symbols represents median and range, and lines show median values. In (B) and (C), bar = 100 μm. Data in (A) and (D) for MG patients, apart from AChR-MGlow patients, are derived from Leite et al., 2005, 2007. Data for AChR-MGlow are previously unpublished.
Discussion
Despite the lack of AChR antibodies detectable by routine immunoprecipitation assays, SNMG patients clearly have an antibody-mediated disease, responding to immunosuppressive treatment and plasma exchange in a similar manner to patients with AChR-MG and also frequently have thymic changes (Lauriola et al., 2005; Leite et al., 2005, 2007). Here we show that IgG from 66% of SNMG sera, taken from all stages of the disease course, binds to AChRs when they are clustered on the surface of a non-muscle cell line by co-transfecting with the cytoskeletal clustering protein rapsyn. Moreover, these antibodies are mainly complement-activating IgG1, and some were able to induce complement deposition on the AChR clusters, which must have been sufficiently dense to bind C1q and activate the classical complement pathway. In addition, the serum IgG antibody reactivity against AChR clusters in SNMG samples correlated with the deposits of complement on myoid cells around the infiltrates, implicating the thymic myoid cells in development of these antibodies. Overall our findings demonstrate convincingly for the first time that there is an immune response to the AChR in some of the patients whose serum antibodies are not detectable in routine immunoprecipitation assays, and provide evidence for immune mechanisms and thymic involvement similar to those in AChR antibody positive patients.
As expected, all of the AChR-MGhigh samples were positive on cells expressing either unclustered or clustered AChR; in addition, a significant proportion of AChR-MGlow sera bound to unclustered AChR and all of them to the clustered receptor. This latter finding is reassuring since it is always difficult to know whether sera with very low titres (e.g. 0.5–1.0 nM) are genuinely positive for AChR antibodies, and the results shown here suggest that they are. The most striking finding was, however, the significant binding of IgG to AChRs, when they had been clustered with rapsyn, in the majority of previously SNMG patients. These findings strongly imply that the SNMG antibodies are directed towards AChR, but bind appreciably only when the AChRs are packed densely in relatively immobile clusters. The density of AChR at the neuromuscular junction is extremely high due to clustering with rapsyn (Sanes and Lichtman, 2001); in animal models, rapsyn expression can determine the pathogenicity of AChR antibodies (Losen et al., 2005), which suggests that declustering may be associated with reduced antibody binding. In addition, our preliminary results, that suggest co-expression with MuSK and Dok-7 increases further the sensitivity of the test, leaves open the possibility that intracellular modifications of either AChR or MuSK (which both have intracellular as well as extracellular domains), or changes in the packing geometry of the clusters, can influence binding of these low affinity antibodies. Further modifications should allow us to optimize the assay and develop a technique more suitable for routine practice.
We have hypothesized (Leite et al., 2007) that the immune response to the AChR takes place in two stages in early-onset AChR-MG: first a helper T cell and antibody response to individual AChR subunits expressed in thymic epithelium, and second the spreading of this response to the native AChR conformation expressed by myoid cells. The ability of the α-subunit to compete for the SNMG antibodies, in absorption studies, is consistent with the possibility that they are initiated by unfolded α-subunits, rather than fully conformed AChR; and this might explain their low affinity for the native receptor, preventing them staying bound when the AChR is at low concentration in solution but allowing them to bind divalently to immobilized, densely clustered AChRs. Further work is needed to explore this. In support of the second stage, we have recently demonstrated complement deposition on myoid cells that are very close to, or even within, the lymphocytic infiltrates in typical AChR-MGhigh cases, including C1q, C3b and C9 (Leite et al., 2007). We also found similar but less intense deposits of C1q, C3c or C9 on myoid cells in thymus from 65% of SNMG patients. Here, we show that the antibodies binding to clustered AChR in SNMG and AChR-MGlow patients correlate with C3b deposition on myoid cells, supporting their involvement in the immune response in SNMG as well as AChR-MG patients.
Overall, these results support the growing appreciation that SNMG is similar in distribution of weakness, pathophysiology and treatment-responsiveness to AChR-MG. There is a positive response to acetylcholinesterase inhibitors, without major side effects, as well as to immunosuppressive therapy, particularly to corticosteroids. Moreover, not only are AChR numbers reduced in number at the endplates of muscle biopsies from SNMG patients (Shiraishi et al., 2005) but complement deposition is present (M. Motomura, unpublished data), and thymic changes are very similar to those in AChR-MG. Each of these findings contrast with those in MuSK-MG where the distribution of clinical weakness is more bulbar, the response to cholinesterases and immunosuppression is less good (Evoli et al., 2003) and there are minimal changes at the neuromuscular junction (Shiraishi et al., 2005) and in the thymus (Lauriola et al., 2005; Leite et al., 2005, 2007).
We found mainly IgG antibodies in the samples tested. However, IgM antibodies were found to bind equally to unclustered and clustered AChR in a few samples from both SNMG and MuSK-MG. This observation supports previous reports of IgM antibodies in some SNMG or MuSK-MG sera that inhibit AChR function in vitro (Yamamoto et al., 1991; Barrett-Jolley et al., 1994; Bufler et al., 1998; Blaes et al., 2000; Plested et al., 2002; Spreadbury et al., 2005). Some of those studies were carried out before the identification of MuSK antibodies in 2001, whereas later studies found similar effects in MuSK antibody negative and positive sera (Plested et al., 2002; Spreadbury et al., 2005). Thus, although IgG antibodies to both AChR and MuSK in individual sera have only extremely rarely been identified using radioimmunoprecipitation assays, our detection of antibodies to clustered AChR in a few MuSK-MG samples (Fig. 2), or to MuSK in a few AChR-MGlow or SNMG sera, raises the possibility that low affinity IgG and IgM antibodies to AChR may be found in both groups of patients.
Our results clearly show the specificity of the IgG antibodies in SNMG and in AChR-MGlow. Most of them bind equally to the adult or foetal AChR as long as it is clustered, thus representing the receptor in its native conformation. Only three samples, one of them of an SNMG patient, bound exclusively to the adult receptor. Interestingly, the binding of this SNMG plasma (SNMG-1 in Spreadbury et al., 2005) to the AChR clusters was inhibited by α-Butx, but not by the AChR α-subunit polypeptide itself, which suggests that it binds to the adult-specific ε-subunit close to the α-Butx binding site that is at the interface between the α- and ε-subunits. The remaining SNMG sera tested were inhibited by α-subunit, suggesting that they bind more conventionally to sites on the two α-subunits. The antibodies we detected to clustered AChR do not ‘block’ α-Butx binding or lead to substantial loss of surface AChR (Farrugia et al., 2007) or AChR clusters when the cells are incubated with the SNMG sera under a variety of conditions (S. Jacob, unpublished data), suggesting that these antibodies do not represent those ‘blocking’ or ‘modulating’ antibodies that are found in some sera negative by radioimmunoprecipitation (Chan et al., 2007). Overall, their IgG subclass and complement-activating abilities, but general lack of specificity for the α-Butx binding site and failure to affect surface AChR in culture, suggests that complement-mediated lysis rather than antigenic modulation or pharmacological block are the likely mechanisms by which these antibodies cause neuromuscular junction failure.
We were surprised to see that MuSK antibodies are also able to activate complement on the cell surface. This is likely to be due to the presence of complement-activating IgG1 in these samples, in addition to the predominant IgG4 subclass. This could have implications for the pathophysiology of the disease, although the high concentration of MuSK achieved in the transfected cells, possibly higher than that at the mature neuromuscular junction itself, may enhance the ability of the antibodies to activate complement.
We have tested samples of patients who visited our clinic not only early, but also later in the disease, partly because of the difficulty in diagnosis. As a consequence, some of patients were already on immunosuppressive treatment and a few had even been thymectomized before sampling. We focused on the detection of AChR antibodies in SNMG by clustering the receptor on the HEK cell surface, and our results show the presence of specific IgG antibodies that bind to AChR only, or preferentially, when it is expressed in its native conformation at a density similar to that at the neuromuscular junction. These findings strongly support their pathogenic role. Moreover, they have important clinical and technical implications since they should eventually help to provide the basis of a new assay for detection of AChR and other antibodies in MG, and the approaches we used have implications for diagnosis of the growing number of other antibody-mediated diseases of the peripheral and central nervous systems.
Supplementary material
Supplementary material is available at Brain online.
Acknowledgements
We are grateful to the Medical Research Council, the Muscular Dystrophy Campaign (J.C., D.B., N.W. and A.V.) and The Wellcome Trust (Programme no. 068590, BPM) for support. M.I.L. was supported by Fundação para a Ciência e a Tecnologia, Portugal. S.J. was supported by the Muscular Dystrophy Campaign and the Myasthenia Gravis Association, UK. S.V. was supported by the Patrick Berthoud Charitable Trust, UK. A.V. and the Department of Clinical Neurology in Oxford receive royalties and payments for antibody tests.
Glossary
Abbreviations:
| AChR | acetylcholine receptor |
| AChR-MG | acetylcholine receptor antibody positive myasthenia gravis |
| α-Butx | alpha-bungarotoxin |
| ARMD | acetylcholine receptor, rapsyn, muscle specific kinase and Dok-7 |
| BSA | bovine serum albumin |
| Dapi | 4′,6′-diamidino-2-phenylindole dihydrochloride |
| DMEM | Dulbecco's modified eagle's medium |
| Dok-7 | docking protein 7 |
| EGFP | enhanced green fluorescent protein |
| HEK | human embryonic kidney cells |
| HEPES | N-(2-hydroxyethyl)piperazine-N′-(2-ethanesulphonic acid) |
| MG | myasthenia gravis |
| MuSK | muscle specific kinase |
| MuSK-MG | MuSK antibody positive myasthenia gravis |
| PBS | phosphate buffered saline |
| PEI | polyethylenimine |
| SNMG | seronegative myasthenia gravis |
| TE671 | human rhabdomyosarcoma/medulloblastoma cell line |
References
- Barrett-Jolley R, Byrne N, Vincent A, Newsom-Davis J. Plasma from patients with seronegative myasthenia gravis inhibit nAChR responses in the TE671/RD cell line. Pflugers Arch. 1994;428:492–8. [Abstract] [Google Scholar]
- Beeson D, Brydson M, Betty M, Jeremiah S, Povey S, Vincent A, et al. Primary structure of the human muscle acetylcholine receptor. cDNA cloning of the gamma and epsilon subunits. Eur J Biochem. 1993;215:229–38. [Abstract] [Google Scholar]
- Beeson D, Higuchi O, Palace J, Cossins J, Spearman H, Maxwell S, et al. Dok-7 mutations underlie a neuromuscular junction synaptopathy. Science. 2006;313:1975–8. [Abstract] [Google Scholar]
- Blaes F, Beeson D, Plested P, Lang B, Vincent A. IgG from “seronegative” myasthenia gravis patients binds to a muscle cell line, TE671, but not to human acetylcholine receptor. Ann Neurol. 2000;47:504–10. [Abstract] [Google Scholar]
- Bodian DL, Davis SJ, Morgan BP, Rushmere NK. Mutational analysis of the active site and antibody epitopes of the complement-inhibitory glycoprotein, CD59. J Exp Med. 1997;185:507–16. [Europe PMC free article] [Abstract] [Google Scholar]
- Bufler J, Pitz R, Czep M, Wick M, Franke C. Purified IgG from seropositive and seronegative patients with mysasthenia gravis reversibly blocks currents through nicotinic acetylcholine receptor channels. Ann Neurol. 1998;43:458–64. [Abstract] [Google Scholar]
- Chan KH, Lachance DH, Harper CM, Lennon VA. Frequency of seronegativity in adult-acquired generalized myasthenia gravis. Muscle Nerve. 2007;36:651–8. [Abstract] [Google Scholar]
- Cossins J, Burke G, Maxwell S, Spearman H, Man S, Kuks J, et al. Diverse molecular mechanisms involved in AChR deficiency due to rapsyn mutations. Brain. 2006;129:2773–83. [Abstract] [Google Scholar]
- Drachman DB. Myasthenia gravis. N Engl J Med. 1994;330:1797–810. [Abstract] [Google Scholar]
- Evoli A, Tonali PA, Padua L, Monaco ML, Scuderi F, Batocchi AP, et al. Clinical correlates with anti-MuSK antibodies in generalized seronegative myasthenia gravis. Brain. 2003;126:2304–11. [Abstract] [Google Scholar]
- Farrugia ME, Bonifati DM, Clover L, Cossins J, Beeson D, Vincent A. Effect of sera from AChR-antibody negative myasthenia gravis patients on AChR and MuSK in cell cultures. J Neuroimmunol. 2007;185:136–44. [Abstract] [Google Scholar]
- Harris CL, Spiller OB, Morgan BP. Human and rodent decay-accelerating factors (CD55) are not species restricted in their complement-inhibiting activities. Immunology. 2000;100:462–70. [Abstract] [Google Scholar]
- Hoch W, McConville J, Helms S, Newsom-Davis J, Melms A, Vincent A. Auto-antibodies to the receptor tyrosine kinase MuSK in patients with myasthenia gravis without acetylcholine receptor antibodies. Nat Med. 2001;7:365–8. [Abstract] [Google Scholar]
- Jacobson L, Beeson D, Tzartos S, Vincent A. Monoclonal antibodies raised against human acetylcholine receptor bind to all five subunits of the fetal isoform. J Neuroimmunol. 1999;98:112–20. [Abstract] [Google Scholar]
- Lauriola L, Ranelletti F, Maggiano N, Guerriero M, Punzi C, Marsili F, et al. Thymus changes in anti-MuSK-positive and -negative myasthenia gravis. Neurology. 2005;64:536–8. [Abstract] [Google Scholar]
- Lavrnic D, Losen M, Vujic A, De Baets M, Hajdukovic LJ, Stojanovic V, et al. The features of myasthenia gravis with autoantibodies to MuSK. J Neurol Neurosurg Psychiatry. 2005;76:1099–102. [Europe PMC free article] [Abstract] [Google Scholar]
- Leite MI, Jones M, Strobel P, Marx A, Gold R, Niks E, et al. Myasthenia gravis thymus. Complement vulnerability of epithelial and myoid cells, complement attack on them, and correlations with autoantibody status. Am J Pathol. 2007;171:893–905. [Europe PMC free article] [Abstract] [Google Scholar]
- Leite MI, Strobel P, Jones M, Micklem K, Moritz R, Gold R, et al. Fewer thymic changes in MuSK antibody-positive than in MuSK antibody-negative MG. Ann Neurol. 2005;57:444–8. [Abstract] [Google Scholar]
- Losen M, Stassen MH, Martínez-Martínez P, Machiels BM, Duimel H, Frederik P, et al. Increased expression of rapsyn in muscles prevents acetylcholine receptor loss in experimental autoimmune myasthenia gravis. Brain. 2005;128:2327–37. [Abstract] [Google Scholar]
- McConville J, Farrugia ME, Beeson D, Kishore U, Metcalfe R, Newsom-Davis J, et al. Detection and characterization of MuSK antibodies in seronegative myasthenia gravis. Ann Neurol. 2004;55:580–4. [Abstract] [Google Scholar]
- Okada K, Inoue A, Okada M, Murata Y, Kakuta S, Jigami T, et al. The muscle protein Dok-7 is essential for neuromuscular synaptogenesis. Science. 2006;312:1802–5. [Abstract] [Google Scholar]
- Plested CP, Tang T, Spreadbury I, Littleton ET, Kishore U, Vincent A. AChR phosphorylation and indirect inhibition of AChR function in seronegative MG. Neurology. 2002;59:1682–8. [Abstract] [Google Scholar]
- Romi F, Aarli JA, Gilhus NE. Seronegative myasthenia gravis: disease severity and prognosis. Eur J Neurol. 2005;12:413–8. [Abstract] [Google Scholar]
- Sanders DB, El-Salem K, Massey JM, McConville J, Vincent A. Clinical aspects of MuSK antibody positive seronegative MG. Neurology. 2003;60:1978–80. [Abstract] [Google Scholar]
- Sanes JR, Lichtman JW. Induction, assembly, maturation and maintenance of a postsynaptic apparatus. Nat Rev Neurosci. 2001;2:791–805. [Abstract] [Google Scholar]
- Shiraishi H, Motomura M, Yoshimura T, Fukudome T, Fukuda T, Nakao Y, et al. Acetylcholine receptors loss and postsynaptic damage in MuSK antibody-positive myasthenia gravis. Ann Neurol. 2005;57:289–93. [Abstract] [Google Scholar]
- Spreadbury I, Kishore U, Beeson D, Vincent A. Inhibition of acetylcholine receptor function by seronegative myasthenia gravis non-IgG factor correlates with desensitisation. J Neuroimmunol. 2005;162:149–56. [Abstract] [Google Scholar]
- Vincent A. Unravelling the pathogenesis of myasthenia gravis. Nat Rev Immunol. 2002;2:797–804. [Abstract] [Google Scholar]
- Vincent A, Leite MI. Neuromuscular junction autoimmune disease: muscle specific kinase antibodies and treatments for myasthenia gravis. Curr Opin Neurol. 2005;18:519–25. [Abstract] [Google Scholar]
- Yamamoto T, Vincent A, Ciulla TA, Lang B, Johnston I, Newsom-Davis J. Seronegative myasthenia gravis: a plasma factor inhibiting agonist-induced acetylcholine receptor function copurifies with IgM. Ann Neurol. 1991;30:550–7. [Abstract] [Google Scholar]
- Zhou L, McConville J, Chaudhry V, Adams RN, Skolasky RL, Vincent A, et al. Clinical comparison of muscle-specific tyrosine kinase (MuSK) antibody-positive and -negative myasthenic patients. Muscle Nerve. 2004;30:55–60. [Abstract] [Google Scholar]
Articles from Brain are provided here courtesy of Oxford University Press
Full text links
Read article at publisher's site: https://doi.org/10.1093/brain/awn092
Read article for free, from open access legal sources, via Unpaywall:
https://academic.oup.com/brain/article-pdf/131/7/1940/13734185/awn092.pdf
Free to read at brain.oxfordjournals.org
http://brain.oxfordjournals.org/cgi/content/abstract/131/7/1940
Free after 24 months at brain.oxfordjournals.org
http://brain.oxfordjournals.org/cgi/content/full/131/7/1940
Free after 24 months at brain.oxfordjournals.org
http://brain.oxfordjournals.org/cgi/reprint/131/7/1940.pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/514886
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1093/brain/awn092
Article citations
[Peripheral neuroimmunological diseases - Neuropathological insights and clinical perspectives].
Nervenarzt, 95(10):920-931, 20 Sep 2024
Cited by: 0 articles | PMID: 39302417
Type 2 hypersensitivity disorders, including systemic lupus erythematosus, Sjögren's syndrome, Graves' disease, myasthenia gravis, immune thrombocytopenia, autoimmune hemolytic anemia, dermatomyositis, and graft-versus-host disease, are THαβ-dominant autoimmune diseases.
Virulence, 15(1):2404225, 16 Sep 2024
Cited by: 0 articles | PMID: 39267271 | PMCID: PMC11409508
Review Free full text in Europe PMC
Efficacy and safety of rozanolixizumab in patients with muscle-specific tyrosine kinase autoantibody-positive generalised myasthenia gravis: a subgroup analysis of the randomised, double-blind, placebo-controlled, adaptive phase III MycarinG study.
Ther Adv Neurol Disord, 17:17562864241273036, 12 Sep 2024
Cited by: 0 articles | PMID: 39297052 | PMCID: PMC11409299
Serological Markers of Clinical Improvement in MuSK Myasthenia Gravis.
Neurol Neuroimmunol Neuroinflamm, 11(6):e200313, 09 Sep 2024
Cited by: 0 articles | PMID: 39250722 | PMCID: PMC11385952
Overcoming therapeutic challenges: Successful management of a supposedly triple seronegative, refractory generalized myasthenia gravis patient with efgartigimod.
Eur J Neurol, 31(7):e16306, 08 May 2024
Cited by: 1 article | PMID: 38716750 | PMCID: PMC11236002
Go to all (236) article citations
Other citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Multiple antibody detection in 'seronegative' myasthenia gravis patients.
Eur J Neurol, 24(6):844-850, 04 May 2017
Cited by: 27 articles | PMID: 28470860
MuSK myasthenia gravis IgG4 disrupts the interaction of LRP4 with MuSK but both IgG4 and IgG1-3 can disperse preformed agrin-independent AChR clusters.
PLoS One, 8(11):e80695, 07 Nov 2013
Cited by: 89 articles | PMID: 24244707 | PMCID: PMC3820634
Effect of sera from AChR-antibody negative myasthenia gravis patients on AChR and MuSK in cell cultures.
J Neuroimmunol, 185(1-2):136-144, 01 Mar 2007
Cited by: 27 articles | PMID: 17335909
Neuromuscular junction autoimmune disease: muscle specific kinase antibodies and treatments for myasthenia gravis.
Curr Opin Neurol, 18(5):519-525, 01 Oct 2005
Cited by: 83 articles | PMID: 16155434
Review
Funding
Funders who supported this work.
Medical Research Council (1)
Disease mechanisms and RNA-based therapies for pathogenic mutations at the neuromuscular synapse
Professor David Beeson, University of Oxford
Grant ID: G117/490
Wellcome Trust (1)
Complement membrane attack and its control.
Prof Paul Morgan, Cardiff University
Grant ID: 068590

 1,2
1,2