Abstract
Free full text

Upregulation of L-Type Ca2+ Channels in Reactive Astrocytes after Brain Injury, Hypomyelination, and Ischemia
Abstract
Anti-peptide antibodies that specifically recognize the α1 subunit of class A–D voltage-gated Ca2+ channels and a monoclonal antibody (MANC-1) to the α2 subunit of L-type Ca2+ channels were used to investigate the distribution of these Ca2+ channel subtypes in neurons and glia in models of brain injury, including kainic acid-induced epilepsy in the hippocampus, mechanical and thermal lesions in the forebrain, hypomyelination in white matter, and ischemia. Immunostaining of the α2 subunit of L-type Ca2+ channels by the MANC-1 antibody was increased in reactive astrocytes in each of these forms of brain injury. The α1C subunits of class C L-type Ca2+ channels were upregulated in reactive astrocytes located in the affected regions in each of these models of brain injury, although staining for the α1 subunits of class D L-type, class A P/Q-type, and class B N-type Ca2+ channels did not change from patterns normally observed in control animals. In all of these models of brain injury, there was no apparent redistribution or upregulation of the voltage-gated Ca2+ channels in neurons. The upregulation of L-type Ca2+ channels in reactive astrocytes may contribute to the maintenance of ionic homeostasis in injured brain regions, enhance the release of neurotrophic agents to promote neuronal survival and differentiation, and/or enhance signaling in astrocytic networks in response to injury.
Multiple isoforms of the principal α1 subunit of voltage-gated Ca2+channels, designated class A through E, have been cloned from rat brain (Snutch et al., 1990; Snutch and Reiner, 1992; Soong et al., 1993;Zhang et al., 1993). The rat brain class C and D genes encode L-type Ca2+ channel α1 subunits (Hui et al., 1991; Snutch et al., 1991; Chin et al., 1992; Dubel et al., 1992; Seino et al., 1992; Williams et al., 1992; Tomlinson et al., 1993), which have high affinity for dihydropyridine Ca2+ channel antagonists and have been shown to be localized predominantly in the soma and proximal dendrites of neurons throughout the brain (Hell et al., 1993). A similar pattern of distribution in the cell body and proximal dendrites was observed by using a monoclonal antibody (MANC-1), which recognizes the α2 subunit of L-type Ca2+ channels (Ahlijanian et al., 1990; Westenbroek et al., 1990). The MANC-1 antibody also was shown to label the processes of astrocytes. The class B α1 subunit forms an N-type, high-voltage-activated Ca2+ channel having high affinity for ω-conotoxin GVIA (Dubel et al., 1992; Williams et al., 1992; Fujita et al., 1993; Stea et al., 1993) and is localized predominantly in dendritic shafts and synaptic terminals (Westenbroek et al., 1992a). The class A α1 subunit forms high-voltage-activated Ca2+ channels which are blocked by ω-agatoxin IVA and ω-conotoxin MVIIC. Their functional properties closely resemble Q-type Ca2+ channels that have been described in cerebellar granule cells (Zhang et al., 1993; Randall and Tsien, 1995). These channels are localized predominantly in presynaptic terminals and dendritic shafts (Westenbroek et al., 1995). Ca2+ channels containing α1E subunits have some features of a low-voltage-activated Ca2+ channel (Soong et al., 1993; Williams et al., 1994) and are localized mainly in cell bodies and less frequently in dendrites of neurons in the CNS (Yokoyama et al., 1995). Localization of these non-L-type calcium channels in glia has not been studied in detail to date.
Voltage-gated calcium channels consist of multiple subunits. The L-type calcium channel is a complex of five different protein subunits: α1, α2δ, β, and γ (Takahashi et al., 1987). The α1 and α2 subunits copurify with equimolar stoichiometry (Sharp et al., 1987; Takahashi et al., 1987). The α2δ subunit was first cloned and sequenced from rabbit skeletal muscle (Ellis et al., 1988) and later from rat brain (Kim et al., 1992) and human brain (Williams et al., 1992). The α2/δ subunits are derived from a single gene (Ellis et al., 1988). Alternative splicing mechanisms have been proposed to give rise to variants of the α2 subunit (Kim et al., 1992).
Disruption of Ca2+ regulation accompanies numerous neurological dysfunctions, including epilepsy (Heinemann et al., 1977;Fletcher et al., 1996; Burgess et al., 1997) and ischemia (Cheung et al., 1986; Choi, 1988). Gliosis, which is commonly associated with brain injury also, induces the transformation of normal astrocytes into reactive astrocytes (Hatten et al., 1991; Norton et al., 1992). One of the many functions of astrocytes is to buffer extracellular ion levels so that neurons can maintain their excitability (Lux et al., 1986;Walz, 1989). Voltage-gated Ca2+ channels are expressed in astrocytes (MacVicar, 1984) and may serve as one route by which these cells respond to and regulate extracellular levels of ions or trigger intracellular calcium release (Dani et al., 1992).
In light of the importance of Ca2+ homeostasis in neurological dysfunction, we have examined the expression of voltage-gated Ca2+ channels in brain astrocytes in animal models of epilepsy, hypomyelination, mechanical and thermal lesions, and ischemia, using antibodies specific for the five α1 subunit subtypes of brain Ca2+channels. Our results indicate that class C L-type Ca2+ channels are upregulated in reactive astrocytes produced in response to each of these diverse forms of brain injury.
MATERIALS AND METHODS
Antibodies
Production and purification of antibodies against peptides CNA1, CNB2, CNC1, and CND1, which recognize the α1 subunit of rat brain class A, B, C, or D neuronal Ca2+channels, respectively, have been described along with their normal patterns of staining in rat brain (Westenbroek et al., 1992a, 1995;Hell et al., 1993). Production and purification of the monoclonal antibody MANC-1, which recognizes the α2 subunit of skeletal muscle L-type Ca2+ channels, have been reported previously (Ahlijanian et al., 1990; Westenbroek et al., 1990). The polyclonal antibody against glial fibrillary acidic protein (GFAP) was purchased from Dako (Carpinteria, CA). Goat anti-rabbit IgG, goat anti-mouse IgG, mouse PAP, rabbit PAP, normal mouse serum, and normal rabbit serum were obtained from Zymed (San Francisco, CA) The FITC-, RITC-, and Texas Red-conjugated secondaries, along with the avidin–biotin complex, were purchased from Vector (Burlingame, CA).
Animal models
Five rodent models of neuronal cell damage or dysfunction were studied.
Shiverer mouse. Fifteen young adultshiverer or wild-type mice were obtained from the breeding colony at Baylor College of Medicine (Houston, TX).
Kainate-lesioned rat. Twelve adult rats were anesthetized with ketamine–Rompun, and kainic acid (KA; 0.6 μg in 1 μl of saline per hemisphere, pH 7.4) was injected bilaterally into the ventricles according to the atlas of Paxinos and Watson (1986). The coordinates were 0.92 mm posterior to bregma, 1.4 mm lateral to the midline, and 3.6 mm ventral to the dural surface. Sham-operated animals were injected with saline. These animals were killed 2–3 weeks after KA injection for processing for immunocytochemistry.
Ischemic gerbil. Eight male Mongolian gerbils (70–100 gm; Tumblebrook Farms, West Brookfield, MA) were subjected to a 5 min uni- or bilateral common carotid artery occlusion, as described previously (Lin et al., 1990).
Mechanical and thermal lesions in gerbils. Six gerbils were anesthetized with sodium pentobarbital (50 mg/kg), and the somatosensory cortex was injured by aspiration or by thermal lesioning of the superficial layers of the neocortex. A vertical stab injury also was made in the neocortex and the underlying striatum with a 1 ml Hamilton syringe.
Immunocytochemistry
Studies involving shiverer mice and kainate-lesioned rats were performed via the following procedures. All animals were anesthetized with sodium pentobarbitol and intracardially perfused with a solution of 4% paraformaldehyde in PB (0.1 m sodium phosphate, pH 7.4) containing 0.34% l-lysine and 0.05% sodium m-periodate (McLean and Nakane, 1974). The brains were removed immediately from the cranium and post-fixed either overnight (for tissue incubated in MANC-1 or GFAP) or for 2 hr (for tissue incubated in anti-CNA1, anti-CNB2, anti-CNC1, or anti-CND1 antibodies). Then the tissue was sunk successively in 10% (8 hr), 20% (12 hr), and 30% (48 hr) solutions (w/v) of sucrose in 0.1m PB. Sagittal sections (35 μm) were cut on a sliding microtome and placed in 0.1 m PB for 16–24 hr.
Free-floating sections were processed for immunocytochemistry by the indirect peroxidase–antiperoxidase (PAP) technique, as reported previously (Westenbroek et al., 1989). Briefly, the sections were pretreated with the following alcohol series to quench endogenous peroxidase activity: 70% ethanol for 5 min, 100% methanol with 0.3% H2O2 for 10 min, PB for 5 min, and finally PBS (0.05 m sodium phosphate, 150 mm NaCl, pH 7.4) for 2 hr. Then the tissue was incubated for 36 hr at 4°C in the following antisera: PAS-purified MANC-1 antiserum (diluted 1:20), anti-GFAP (diluted 1:1200), affinity-purified anti-CNA1 (diluted 1:15), affinity-purified anti-CNB2 (diluted 1:15), affinity-purified anti-CNC1 (diluted 1:15), or affinity-purified anti-CND1 (diluted 1:15). All antisera were diluted in PBS containing 0.1% Triton X-100 and 1% normal goat serum. The sections were treated as follows at room temperature, unless other temperatures are specified: rinsed for 1 hr in PBS containing 0.1% Triton X-100, incubated in goat anti-mouse IgG (only the tissue incubated in MANC-1) or in goat anti-rabbit IgG (all other tissue), diluted 1:30 for 1 hr at 37°C, rinsed for 1 hr in PBS containing 0.1% Triton X-100, incubated in mouse PAP (only tissue incubated in MANC-1) or rabbit PAP (all other tissue) diluted 1:100 for 1 hr at 37°C, rinsed in PBS for 15 min, rinsed for 5 min in PB, rinsed in TB (0.1 m Tris-HCl, pH 7.4), treated with 0.04% of 3,3′ diaminobenzidine and 0.003% H2O2 in TB for 10 min, and finally rinsed in TB for 10 min to stop the reaction. The tissue sections were mounted on subbed glass slides, dehydrated, cleared in xylene, and coverslipped. The sections were viewed and photographed with a Leitz Dialux (Wetzlar, Germany) research microscope. Immunocytochemical controls included replacing the primary antisera with normal rabbit serum, normal mouse serum, or no serum at all. The MANC-1 antibody was preabsorbed with purified calcium channels, as described previously (Ahlijanian et al., 1990). Peptide block of anti-CNA1, anti-CNB2, anti-CNC1, and anti-CND1 was performed as described previously (Westenbroek et al., 1992a, 1995; Hell et al., 1993). Control sections showed no discrete staining of cells, fibers, or terminals. Several dilutions were tested to determine the optimum concentration of the primary antisera.
The gerbils used in the ischemia studies were anesthetized and perfused with 3.5% paraformaldehyde in 0.1 m PB at pH 7.4 for 2 d (n = 1), 4 d (n = 2), 7 d (n = 2), 2 weeks (n = 1), 3 weeks (n = 1), and 4 weeks (n = 1) after surgery. Animals with mechanical lesions were anesthetized deeply and perfused transcardially with 3.0% paraformaldehyde in 0.1m PB at 1 week (n = 2), 2 weeks (n = 2), or 3 weeks (n = 2) after lesioning. In both cases, brains were cut in 40 μm sections with an American Optical microtome. Brain sections from the damaged areas were stained for single and double immunofluorescence staining with a panel of antibodies. These antibodies included polyclonal antibody to GFAP (dilution 1:20–200), monoclonal MANC-1 antibody against the α2 subunits of L-type Ca2+ channels (dilution 1:50–100), and polyclonal antibody CNB2 against N-type Ca2+ channels (dilution 1:20–100). After incubation in the primary antibody for 12–18 hr at 4°C, antigens were visualized with FITC-, RITC-, or Texas Red-conjugated secondary antibodies or an avidin–biotin complex. Double immunofluorescence analysis was performed by mixed or sequential incubation in the primary antibodies, as described previously (Lin et al., 1993). Controls included the omission of the primary or secondary antibodies and the use of interspecific secondary antibodies. Sections were analyzed with a Nikon Labophot (Tokyo, Japan) epifluorescent microscope with appropriate filters.
RESULTS
Kainate model of epilepsy
Intraventricular injection of KA causes a selective loss of neurons in the CA3 subfield of the hippocampus. This loss of neurons results in chronic hyperexcitability in both the CA1 pyramidal cell layer (Franck and Schwartzkroin, 1985; Franck et al., 1988; Cornish and Wheal, 1989) and in the dentate gyrus (Tauck and Nadler, 1985). Because the KA lesion model is often used as a model for epilepsy, we investigated whether changes in the distribution of voltage-gated Ca2+ channels may accompany the lesion-induced hyperexcitability. Using the MANC-1 monoclonal antibody against the α2 subunit of L-type Ca2+ channels, we observed no detectable changes at the light microscopic level in the staining pattern of neurons in tissue sections from animals treated with KA; however, reactive astrocytes present in the CA3 region surrounding the KA lesion became more immunoreactive for the α2 subunit of L-type Ca2+ channels. In control animals (Fig.(Fig.11A) MANC-1 labels the somata and proximal dendrites of CA3 pyramidal neurons as well as the astrocytic processes (see also Fig. Fig.22A,D). In KA-lesioned animals (Fig. (Fig.11B) there is extensive MANC-1 staining of reactive astrocytes in the damaged area, indicating an upregulation of L-type Ca2+ channels in astrocytes. Because the PAP technique is a nonlinear enzymatic amplification method, it is not possible to quantify the number of Ca2+ channels in the soma and proximal dendrites of neurons precisely. However, the pattern of MANC-1 distribution remains unaltered in surviving neurons, suggesting that there are no major changes in L-type Ca2+ channel expression.
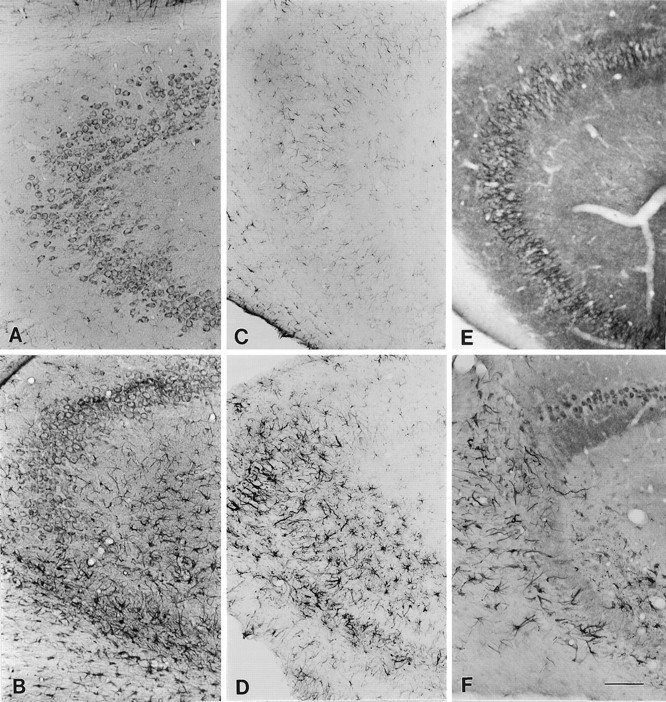
Distribution of L-type Ca2+channels and GFAP in the CA3 region of the hippocampus of kainate acid-injected or in uninjected rats. A, Section stained with MANC-1 illustrating the distribution of the α2subunit of L-type Ca2+ channels in the cell soma, proximal dendrites, and astrocytic processes of a control rat.B, Expression of MANC-1 in reactive astrocytes and remaining neurons after kainate acid injection. C, Control section stained with anti-GFAP. D, Section stained with anti-GFAP illustrating the presence of reactive astrocytes after kainate acid injection. E, Section stained with anti-CNC1 illustrating the pattern of distribution of the α1 subunit of class C L-type Ca2+channels in control rats. F, Section from animal injected with kainate acid illustrating the presence of anti-CNC1 staining in reactive astrocytes and the remaining neurons. Scale bar, 200 μm.
Distribution of L-type Ca2+channels and GFAP in the CA1 region of the hippocampus of kainate acid-injected and uninjected rats. A, Section stained with MANC-1 illustrating the distribution of the α2subunit of L-type Ca2+ channels along neurons and astrocytic processes of a control rat. B, Section from a control animal stained with anti-GFAP. C, Section labeled with anti-CNC1 illustrating the pattern of distribution of the α1 subunit of class C L-type Ca2+channels in control rats. D, Section from kainate acid-injected animal stained with MANC-1 to illustrate that no changes occurred in the distribution of the α2 subunit in neurons or astrocytes. E, Section stained with GFAP demonstrating that the pattern of distribution of this protein is unaltered in the CA1 region after kainate acid injection.F, Section from the same experimental animal stained with anti-CNC1 illustrating that the distribution of the α1 subunit of L-type Ca2+ channels remains unaltered in the CA1 region. G, Control section demonstrating the lack of staining observed when MANC-1 is preabsorbed with purified Ca2+ channels. H, Control section in which the primary antiserum was replaced by normal mouse IgG. I, Control section in which the primary antibody was replaced by normal rabbit serum. Arrowheadspoint to the pyramidal cell body layer. Scale bar, 100 μm.
To confirm the presence of reactive astrocytes, we stained tissue sections from KA-lesioned animals and control animals by using an antibody to GFAP, a marker for reactive astrocytes. In normal or sham-operated animals (Fig. (Fig.11C) there is staining with GFAP of some astrocytic processes throughout the hippocampus. In sections from KA-lesioned animals (Fig. (Fig.11D) there is much stronger GFAP immunoreactivity in reactive astrocytes, which are found mainly in the CA3 region of the experimental tissue where the pyramidal neurons are absent as a result of cell death caused by KA. These reactive astrocytes are characterized by hypertrophic cell bodies and large thick processes (Fig. (Fig.11B,D).
Antibodies that recognize two different isoforms of the α1 subunits of rat brain neuronal L-type Ca2+ channels also were used in this study. In KA-treated tissue there is labeling of class C L-type Ca2+ channels in reactive astrocytes located in the CA3 region of the hippocampus (Fig. (Fig.11F). In control tissue there is essentially no labeling of astrocytes in the hippocampus with the anti-CNC1 antibody (Fig. (Fig.11E), and the pattern of neuronal staining is similar to previously published findings (Hell et al., 1993). The detection of the α2subunit in astrocytes in these sections by using MANC-1 as compared with the lack of detection of the α1 subunit by using anti-CNC1 may reflect the fact that the MANC-1 is a monoclonal antibody and is more sensitive than the polyclonal anti-CNC1 antibodies, which are anti-peptide antibodies.
In contrast to the CA3 region, the pattern of MANC-1 staining in neurons and astrocytic processes located in the CA1 region remains unaltered in animals treated with KA, as compared with controls (Fig.(Fig.22A,D). Likewise, in tissue sections from animals treated with KA, the pattern of GFAP (Fig.(Fig.22B,E) or anti-CNC1 (Fig. (Fig.22C,F) labeling remained unchanged in the CA1 region, as compared with normal animals. No detectable changes were observed in the dentate gyrus between normal animals and animals treated with KA when staining was done with the MANC-1, anti-GFAP, or anti-CNC1 antibodies (data not shown). Figure Figure22G represents a control section illustrating the lack of staining observed when the MANC-1 antibody is preabsorbed with purified calcium channels. Figure Figure2,2, H andI, shows control sections taken from normal animals in which the primary antibody is replaced by mouse serum or rabbit serum, respectively. The lack of staining in these sections supports the specificity of detection of Ca2+ channels by the antibodies used.
Unlike class C L-type Ca2+ channels, class D L-type Ca2+ channels are not expressed in reactive astrocytes under these conditions. There is staining only of neuronal cell bodies in experimental sections stained with CND1 antibody, and no reactive astrocytes in the hippocampus were immunopositive (Fig.(Fig.33A). In the CA3 region, where there is loss of neurons after KA injection, we observed a lack of cell body staining, but the pattern of staining of neurons by anti-CND1 was similar to control animals in the CA1 and CA2 regions and in the dentate gyrus.
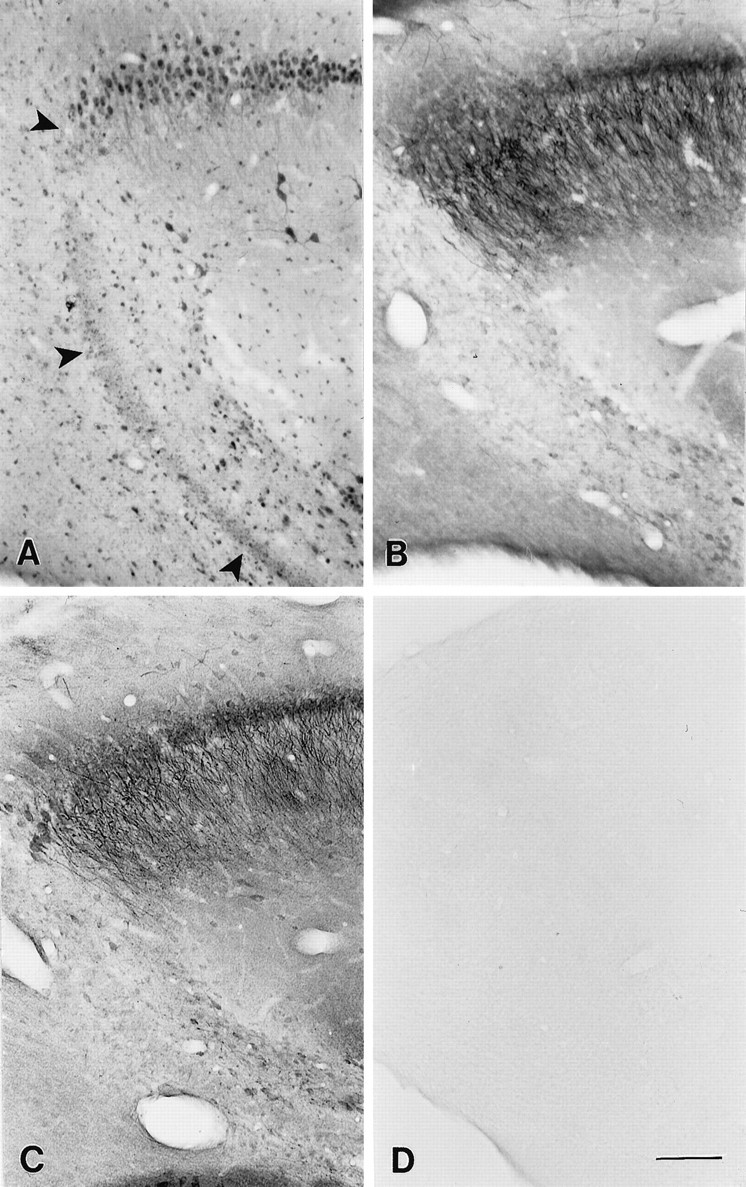
Distribution of class A, B, and D Ca2+ channels in the CA3 region of the hippocampus after kainic acid injection. A, Section stained with anti-CND1 antibodies illustrating the localization of class D channels in cell bodies of the remaining neurons and not in the pyramidal neurons affected by the kainic acid injection (arrows) or in reactive astrocytes. B, Section incubated with anti-CNA1 antibodies showing the lack of staining in the pyramidal neurons affected by the kainic acid injection, as compared with the normal pattern of staining along the length of the remaining pyramidal cells. C, Section stained with anti-CNB2 antibody illustrating the normal pattern of class B distribution in the remaining neurons after kainic acid injection. D, Control section in which the primary antibody was omitted. Scale bar, 200 μm.
We also observed no changes in the pattern of neuronal staining in KA-treated sections with anti-CNA1, which recognizes the α1 subunit of class A rat brain Ca2+channels, except for the loss of neurons in CA3 (Fig. (Fig.33B). In addition, there was no staining of reactive astrocytes in control or treated tissue with the anti-CNA1 antibody (Fig. (Fig.33B) or the anti-CNB2 antibody against the α1 subunit of class B N-type Ca2+ channels (Fig. (Fig.33C). FigureFigure33D presents a control section illustrating the lack of staining observed when the primary antibody is left out.
The lack of detectable changes in the distribution or level of expression of any of the classes of Ca2+ channels in neurons must be interpreted carefully. It is possible that there is a small but significant up- or downregulation of Ca2+channels in these neurons, but not to an extent that is detectable with the immunochemical methods used in this study. Only large changes are detected easily with immunocytochemistry, especially when the PAP method is used. Because individual astrocytes increase their volume and surface area with gliosis, it is possible that the number of L-type Ca2+ channels is upregulated to a similar extent as GFAP and other proteins that increase with activation of the astrocyte. However, when the processes are viewed under high magnification, it appears that the density of staining along the process clearly has increased, as compared with staining along a control astrocytic process, suggesting an increase in channel density and physiological function.
Shiverer mouse model of hypomyelination
The autosomal recessive mutant mouse shiverer exhibits a severe lack of myelination within its CNS, which results in a hyperexcitable phenotype with tremor and seizures but normal motor strength (Readhead and Hood, 1990). Previous studies have demonstrated upregulation and redistribution of Type II sodium channels (Noebels et al., 1991; Westenbroek et al., 1992b) and voltage-gated K+ channels (Wang et al., 1995) in axons in major fiber tracts such as the corpus callosum, internal capsule, fimbria, fornix, and corpus medullare of the cerebellum in theshiverer mouse. We observed no changes in the distribution of Class A through Class D Ca2+ channels inshiverer neurons when compared with wild-type animals, suggesting that there is no major alteration in expression patterns of these channels and no major redistribution of Ca2+channels within individual neurons. The changes that we observed in Ca2+ channel expression and distribution occurred instead in the reactive astrocytes present in the affected fiber tracts. In wild-type mice, L-type Ca2+ channels recognized by MANC-1 are localized to the soma and proximal dendrites of neurons in the gray matter and to the fine processes of astrocytes located throughout the corpus callosum (Fig.(Fig.44A). In young adultshiverer mice there is extensive gliosis extending throughout the corpus callosum, and these reactive astrocytes are immunopositive for MANC-1 (Fig. (Fig.44B). GFAP staining in wild-type (Fig. (Fig.44C) and mutant mice (Fig.(Fig.44D) confirms that the massive gliosis observed in the corpus callosum of shiverer mice is attributable to the appearance of reactive astrocytes. At higher magnification, GFAP-positive reactive astrocytes located in the corpus callosum ofshiverer mice (Fig. (Fig.44F) have enlarged somas and thick processes, as compared with astrocytes found in wild-type mice (Fig. (Fig.44E).
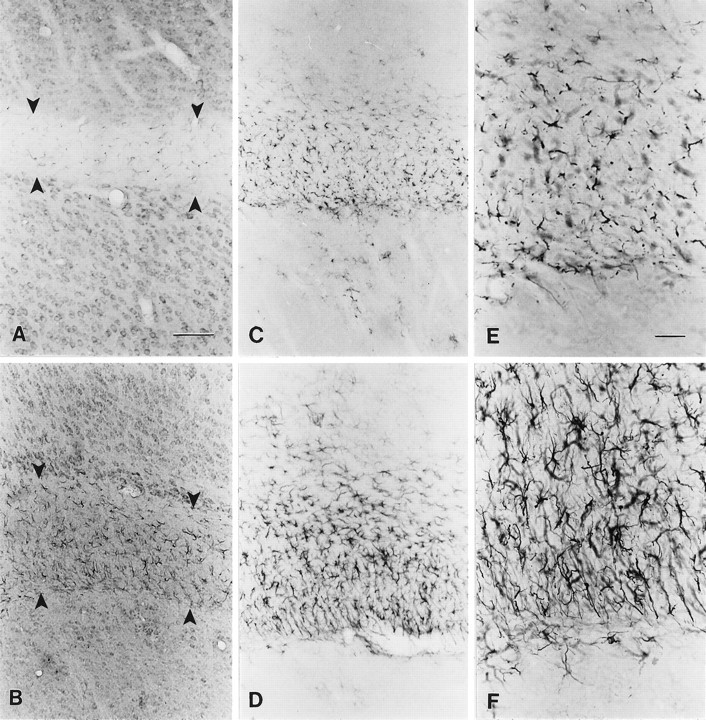
Localization of MANC-1, GFAP, and anti-CNC1 in wild-type and shiverer mice. A, Section incubated with MANC-1 demonstrating the presence of L-type Ca2+ channels in the neuronal cell bodies and in the processes of astrocytes found in the corpus callosum of wild-type mice (between arrows). B, Section stained with MANC-1 illustrating the presence of L-type Ca2+channels in reactive astrocytes found in the corpus callosum ofshiverer mice. C, Tissue from a wild-type mouse stained with anti-GFAP showing the pattern of astrocyte staining.D, Tissue section from a shiverer mouse stained with anti-GFAP illustrating numerous reactive astrocytes in the corpus callosum. E, Higher magnification of anti-GFAP staining in wild-type mice. F, Higher magnification of anti-GFAP staining in shiverer mice illustrating the enlarged somas and large, thick processes of reactive astrocytes, as compared with wild-type mice. Scale bars: 200 μm inA–D; 100 μm in E, F.
Similar gliosis occurred in other regions containing hypomyelinated fiber tracts. MANC-1 staining in the internal capsule was increased in reactive astrocytes (Fig. (Fig.55C), as compared with wild-type mice (Fig. (Fig.55A). In regions surrounding the internal capsule the pattern of MANC-1 staining in neurons was unaltered. The reactive astrocyte staining was confirmed with GFAP staining (Fig. (Fig.55B,D). This pattern of increased density of Class C Ca2+ channels in reactive astrocytes was observed to a lesser extent in the fimbria, fornix, and corticofugal and corticopedal bundles of myelinated fibers that traverse the caudate putamen (data not shown) and to a moderate extent in the corpus medullare, the underlying white matter that contains myelinated afferent and efferent inputs to the cerebellum (Fig.(Fig.66A,B,E,F). In the corpus medullare, reactive astrocytes are MANC-1-, GFAP-, and CNC1-positive (Fig. (Fig.66B,D,F), as compared with wild-type mice (Fig. (Fig.66A,C,E). The distribution of MANC-1 and CNC1 immunoreactivity in neurons remained the same in both sets of animals. In addition, the anti-CNA1, anti-CNB2, and anti-CND1 antibodies were tested in shiverer mice, and we observed no identifiable changes in the pattern of distribution of these Ca2+ channels in neurons or glia (data not shown). Thus, the upregulation of Class C L-type Ca2+channels in reactive astrocytes in white matter is specific.
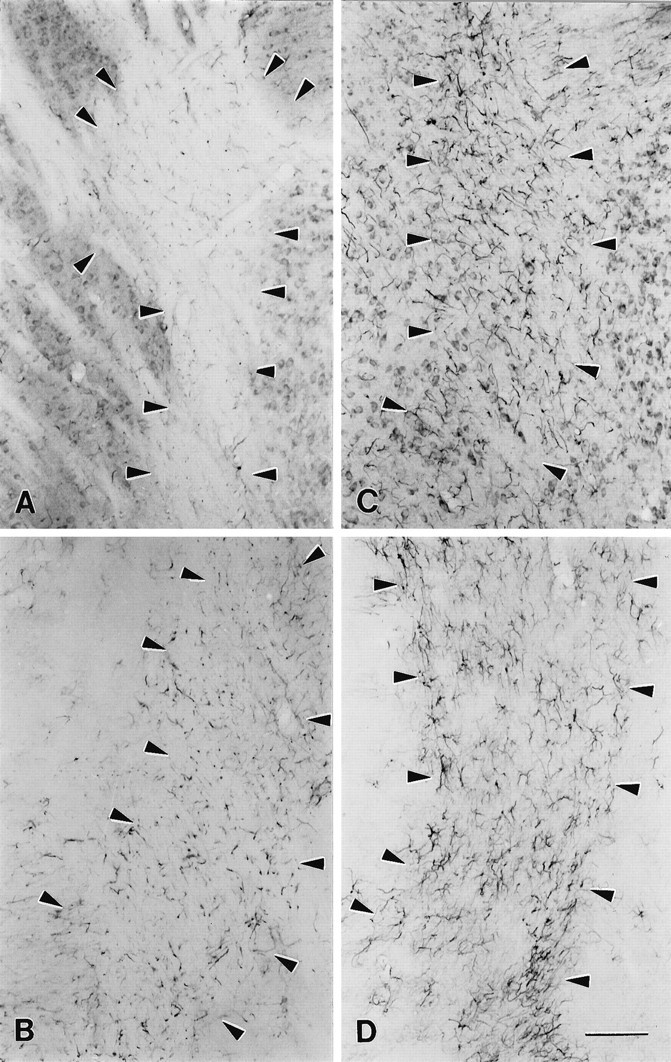
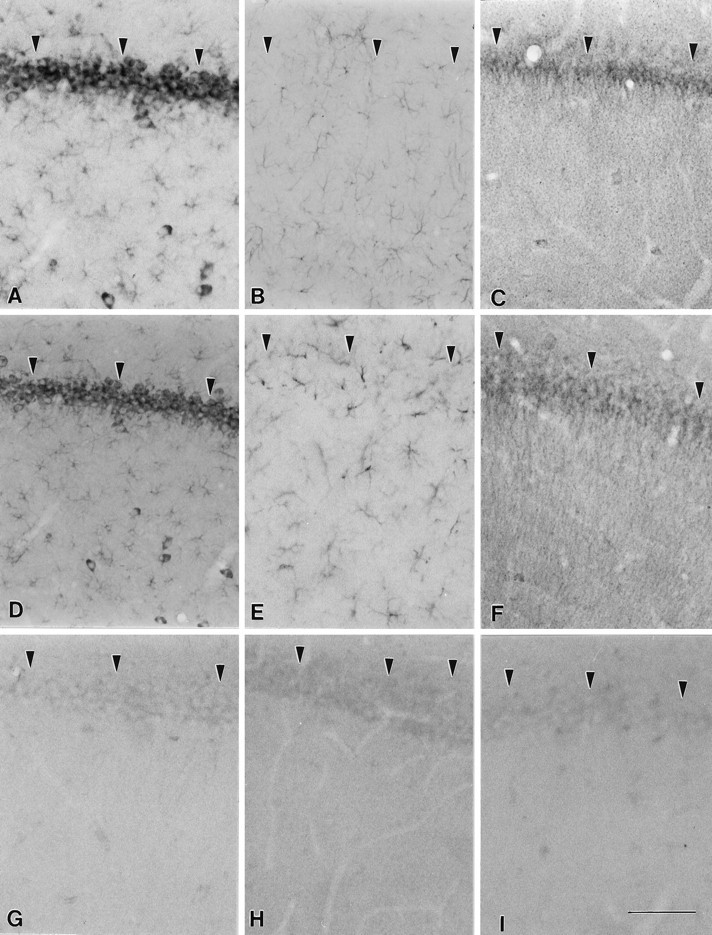
Localization of MANC-1 and GFAP in the internal capsule of wild-type and shiverer mice.A, Tissue section from wild-type mouse stained with MANC-1 illustrating low levels of expression of L-type Ca2+ channels in astrocytes located in the internal capsule. B, Tissue section stained with anti-GFAP that shows the distribution L-type Ca2+ channels in wild-type mice. C, Micrograph illustrating the staining of MANC-1 in reactive astrocytes located in the internal capsule of ashiverer mouse. D, Section from ashiverer mouse illustrating the increased staining observed in the internal capsule with anti-GFAP antibodies.Arrowheads outline the region of the internal capsule in wild-type and mutant mice. Scale bar, 200 μm.
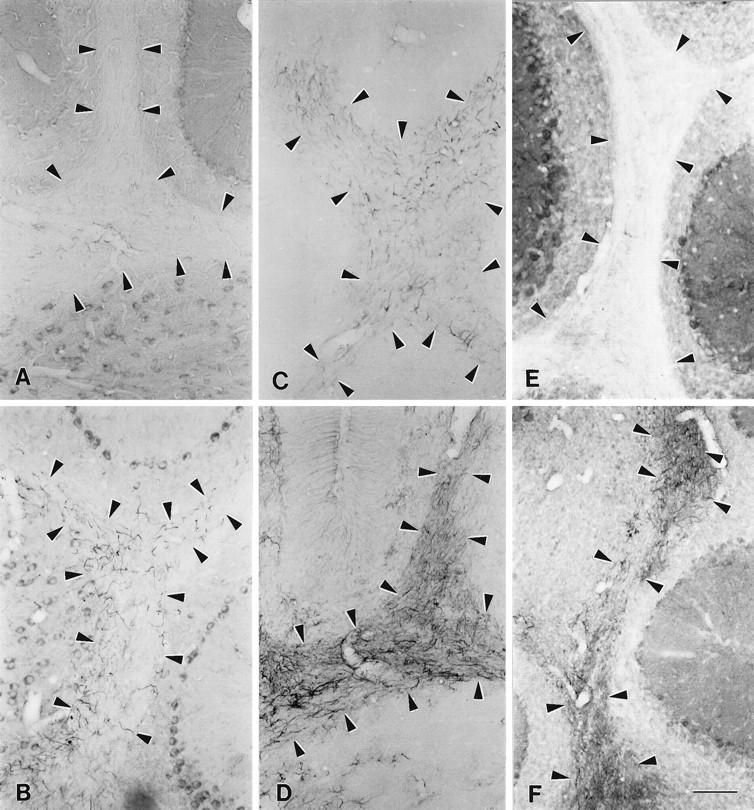
Distribution of L-type Ca2+channels and GFAP in the cerebellum of wild-type andshiverer mice. A, MANC-1 staining in a section from the cerebellum of a wild-type mouse. B, MANC-1 staining in a section from the cerebellum of ashiverer mouse illustrating staining in astrocytic processes in the corpus medullare region. C, Section from a wild-type mouse stained with anti-GFAP antibodies.D, Section stained with anti-GFAP antibodies illustrating the presence of reactive astrocytes in the white matter of the cerebellum of a shiverer mouse. E, Section stained with anti-CNC1 antibodies showing the pattern of class C Ca2+ channel staining in the corpus medullare of the cerebellum of a wild-type mouse. F, Section from ashiverer mouse demonstrating the staining of reactive astrocytes located in the corpus medullare of the cerebellum with anti-CNC1 antibodies. Arrowheads outline the region of the corpus medullare region in wild-type and mutant mice. Scale bar, 100 μm.
Mechanical and thermal lesions
Using the MANC-1 antibody, we detected L-type Ca2+ channel expression in reactive astrocytes in areas damaged by mechanical or thermal lesions. An example of MANC-1 expression in reactive astrocytes from a stab wound in the striatum is shown in Figure Figure77A. Reactive astrocytes expressing MANC-1 immunoreactivity were found in the retrogradely and anterogradely degenerating areas, such as the ventroposterior thalamic nucleus, after aspiration of the somatosensory cortex (Fig. (Fig.77B). Abundant elongated reactive astrocytes were found around the superficial cortical layers after thermal lesions of the neocortex by using double immunofluorescence (Fig.(Fig.77C,D). With the exception of tanycytes near the third ventricles of the median eminence (Fig.(Fig.8)8) and a diffuse staining in the fimbria–fornix, no MANC-1 immunoreactivity was noted in astrocytes in nondamaged areas or unlesioned animals. Similar results were observed along the needle tracks in the KA-treated animals where there was extensive gliosis, and the reactive astrocytes stained positive for only MANC-1 and anti-CNC1 (data not shown). In contrast, expression of N-type Ca2+ channels was not observed in any reactive astrocytes in these lesioned animals (data not shown), indicating a specific upregulation of L-type Ca2+channels.
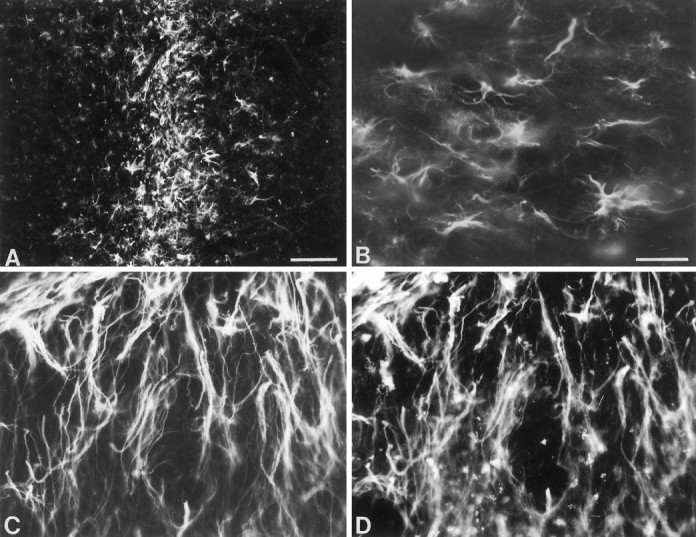
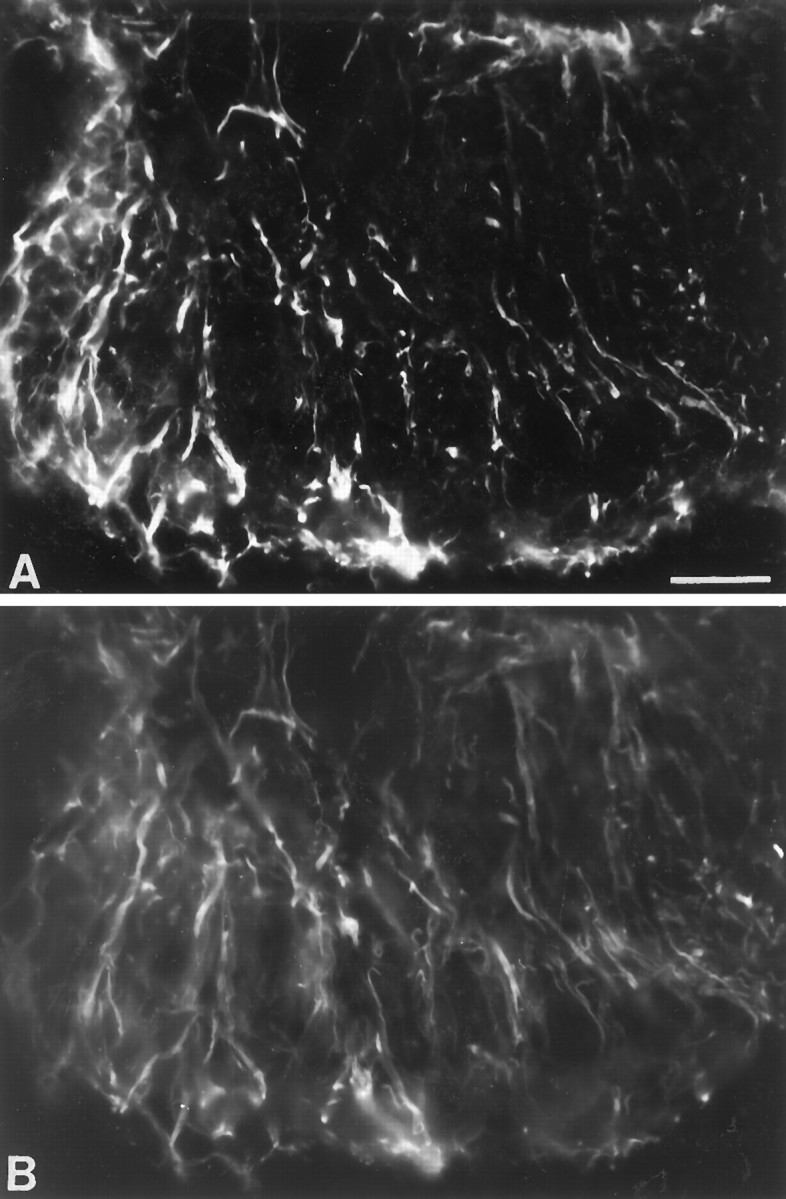
Expression of L-type Ca2+channels in reactive astrocytes after mechanical lesions.A, Photomicrographs showing abundant reactive astrocytes stained by MANC-1 in the striatum 3 weeks after a stab wound.B, Photomicrographs showing abundant reactive astrocytes stained by MANC-1 in the ventro-posterior nucleus of the thalamus 3 weeks after aspiration of the somatosensory cortex. C, Double immunofluorescent staining of MANC-1 (Texas Red) and GFAP (D, FITC) of reactive astrocytes in the superficial layers of the neocortex 3 weeks after thermal lesions of the adjacent neocortex. Scale bars: 200 μm in A; 50 μm inB.
Ischemic model
We also examined the expression of L-type Ca2+channels in reactive astrocytes that appear in ischemic brain regions. Expression of L-type Ca2+ channels recognized by MANC-1 in reactive astrocytes was not obvious in the forebrain 2–4 d after ischemic insults. However, increased expression of MANC-1-immunoreactive Ca2+ channels was clearly detectable in reactive astrocytes in the forebrain nuclei 7 d after ischemic injury in areas known to be vulnerable to ischemia. For example, reactive astrocytes labeled by both MANC-1 and GFAP in double immunofluorescence were noted in the CA1 sector of the hippocampus (Fig. (Fig.99A,B) and the dorsolateral sector of the striatum (Fig. (Fig.99C,D). In addition, reactive astrocytes in white matter that surround the injured fiber bundles in ischemically damaged areas also were found to express MANC-1 immunoreactivity. Thus, expression of L-type Ca2+ channels is upregulated in response to ischemic brain injury as well.
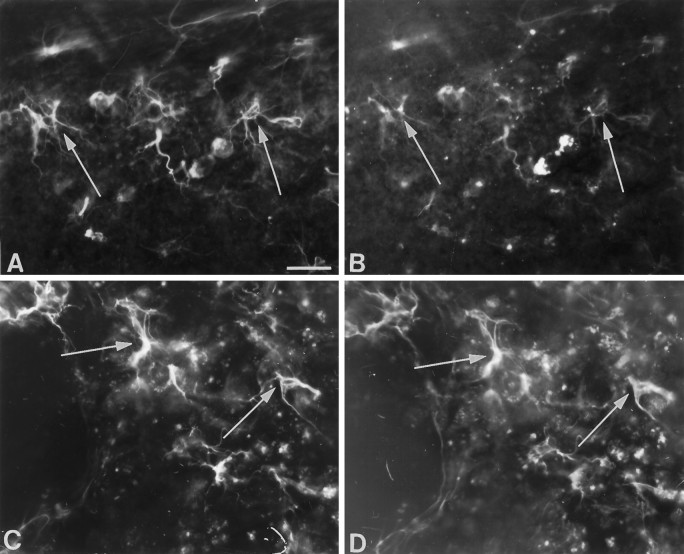
Expression of MANC-1 immunoreactivity in astrocytes in forebrain nuclei after ischemic injury. A, B, Double immunofluorescent staining of MANC-1 (A, Texas Red) and GFAP (B, FITC) in reactive astrocytes in the CA1 sector of the hippocampus 7 d after experimental ischemia, as described in Materials and Methods. Thearrows point to examples of reactive astrocytes double-stained for MANC-1 and GFAP. C, D, Reactive astrocytes expressing L-type Ca2+ channels stained by MANC-1 (C, Texas Red) and GFAP (D, FITC) in the dorsolateral sector of the striatum 7 d after ischemia. The arrows point to two representative examples. Scale bar, 30 μm.
DISCUSSION
Increased expression of L-type Ca2+ channels in reactive glia is a common response to injury
Our results show that specific upregulation of the level of α1 and α2 subunits of L-type Ca2+ channels is a common feature of the activation of reactive astrocytes in response to diverse forms of injury and that increased expression of the α1C subunit of Class C L-type Ca2+ channels is likely to be responsible for this upregulation. There are thought to be common elements in the response of astrocytes to brain injury, although many experiments suggest that reactive gliosis is not a stereotypic response and varies widely in duration, degree of hyperplasia, and time course of expression of GFAP mRNA and immunostaining (for review, see Norton et al., 1992). It is not clear which aspect of the different injurious treatments used in these studies may represent the common stimulus for the activation of reactive astrocytes. Possible common elements of these diverse forms of neuronal injury include increased extracellular K+caused by hyperexcitability and/or cell death and possibly increased release of neurotransmitters and neuromodulators in response to the depolarization caused by increased extracellular K+.
In the mammalian CNS, extracellular [K+]o has a resting value of 3 mm and may increase to 5–15 mm during intense neuronal activity or epileptiform bursting (Somjen, 1979). During pathological conditions such as hypoxia and anoxia (Blank and Kirshner, 1977), hypoglycemia (Astrup and Norberg, 1976), and spreading depression (Nicholson et al., 1978; Kraig et al., 1991), the value of [K+]o may reach 30–80 mm. One possible mechanism by which elevations in [K+]o may influence astroglial function is via voltage-gated Ca2+ channels. Studies that used cultured astrocytes (MacVicar et al., 1991) or acutely isolated astrocytes (Duffy and MacVicar, 1994) have demonstrated that elevations in [K+]o evoke increases in [Ca2+]i by promoting Ca2+ influx via voltage-gated Ca2+ channels. In cultured astrocytes these increases in [Ca2+]i are dihydropyridine-sensitive, suggesting the involvement of L-type Ca2+ channels (MacVicar et al., 1991), whereas in acutely isolated astrocytes these increases are not sensitive to dihydropyridines (Duffy and MacVicar, 1994).
After brain injury there is an increase in the expression and/or release of various growth factors and cytokines in both neurons and glia. Some examples include astrocyte production and release of basic fibroblast growth factor (bFGF) after injury (Finklestein et al., 1988;Walicke and Baird, 1988), an increase in bFGF levels after forebrain ischemia (Kiyota et al., 1991), an increase in nerve growth factor (NGF) mRNA levels in the rat hippocampus after limbic seizures (Gall and Isackson, 1989), increased NGF and brain-derived neurotrophic factor (BDNF) mRNA levels in adult rat brain after KA administration (Ballarin et al., 1991), ciliary neurotrophic factor (CNTF) upregulation in astrocytes after traumatic brain injury (Ip et al., 1993), and BDNF increases in astrocytes (Rudge et al., 1995). Reactive astrocytes express GABA (Lin et al., 1993; Ochi et al., 1993; Anderson et al., 1994), the adhesion embryonic form of neuronal cell molecule (N-CAM) (Salle et al., 1992), neuron-specific enolase and microtubule-associated protein (MAP-2) (Lin and Matesic, 1994), nestin (Lin et al., 1995), and calbindin-D28K (Freund et al., 1990; Liu and Graybiel, 1992). In addition, reactive astrocytes express both NGF and p75 NGF receptor after ischemia (Lee et al., 1995). These various substances are not observed in quiescent astrocytes.
Astrocytes may react to signals to restore the natural extracellular ionic conditions and provide growth factor support to prevent further neuronal damage. The increased density of calcium channels in glia may allow enhanced uptake of extracellular Ca2+ to clear the extracellular space of excess Ca2+ and to initiate the release of cytokines and growth factors that support neuronal survival. Release of neurotrophic factors from astroglia can be blocked by the inhibition of L-type Ca2+ channels (Vaca and Wendt, 1992). This suggests that the upregulation of L-type Ca2+ channels in reactive astrocytes may be involved in a cascade of signals that increases the release of neurotrophic factors from astrocytes.
The expression of L-type Ca2+ channels in cultured astrocytes is modulated by other factors also. Corvalan and colleagues (1990) have demonstrated that cocultivation of neurons and astrocytes modulates the expression of two types of Ca2+channels, including an L-type channel. Several groups also have shown that Ca2+ currents similar to the neuronal L-type can be detected in astrocytes only when they are treated with substances known to increase intracellular levels of cAMP (MacVicar and Tse, 1988; Barres et al., 1989). These studies suggest that expression of L-type Ca2+ channels is regulated by many different second messenger pathways.
Epilepsy and voltage-gated Ca2+ channels
Numerous observations have shown that Ca2+ is involved in the generation of epileptic activity in the CNS (for review, see Dichter, 1989). Extracellular Ca2+concentration decreases at the site of the seizure focus during interictal discharge, while the concentration of K+increases (Heinemann et al., 1977). It has been suggested that the elevation of K+ and the decrease of Ca2+ in the extracellular space brings neurons closer to threshold and enhances their synchronization (Dichter, 1989). Work in hippocampal slice preparations has demonstrated that the lowering of the extracellular Ca2+ concentration results in seizure-like tonic depolarization and rapid discharge of neurons (Yaari et al., 1986). Subsequently, epileptic depolarizations of single neurons and populations of neurons have been shown to be depressed by organic Ca2+ channel blockers (Bingmann et al., 1988; Bingmann and Speckmann, 1989; Aicardi and Schwartzkroin, 1990; Pohl et al., 1992). The decrease in the extracellular concentration of Ca2+ that occurs during seizures (Heinemann et al., 1977) has long been presumed to reflect an influx of Ca2+ into discharging neurons via voltage-gated Ca2+ channels. However, astrocytes have been shown to express both receptor-operated and voltage-gated Ca2+ channels (MacVicar, 1984; Pearce et al., 1986;MacVicar and Tse, 1988; Barres et al., 1989, 1990; Hertz et al., 1989;Salm and McCarthy, 1990). This suggests that the decreases in extracellular Ca2+ concentration observed during seizure activity may be attributable to the influx of Ca2+ into astrocytes as well as neurons. Electrophysiological studies in cultured astrocytes suggest that astrocytes possess physiologically functional L-, N-, and T-type Ca2+ channels and that phenytoin can modulate significantly the depolarization-induced Ca2+ influx transient of astrocytes at least via L- and T- type Ca2+ channels (Greenberg et al., 1984; Twombly et al., 1988). Our immunocytochemical findings of the upregulation of the α1 and α2 subunits of L-type Ca2+ channels in reactive astrocytes in epileptic tissue show that these in vitro studies are relevant for astrocytes in sites of injury in vivo.
Hypomyelination and voltage-gated Ca2+ channels
In the shiverer mouse model, reduced myelination causes a hyperexcitable phenotype. 3H-saxitoxin binding studies have demonstrated that the number of sodium channels is increased in the large-caliber fiber pathways in the brain of theshiverer mouse (Noebels et al., 1991). Immunocytochemical studies have shown that there is an increased expression of type II sodium channels along these large fibers (Westenbroek et al., 1992b). In addition, Wang and colleagues (1995) have demonstrated that the mKv1.1 and mKv1.2 potassium channels are concentrated at nodes of Ranvier in wild-type mice, but in shiverer mice these K+ channels spread along the length of the axon and are not concentrated at the node. It appears that impulse conduction in central axons is retained and that loss of myelination causes hypomyelinated axons to serve as ectopic foci for abnormal activity. Our findings suggest that the α1 and α2subunits of L-type Ca2+ channels are upregulated in astrocytes within hypomyelinated fiber tracts. This increase in the astroglial sink for calcium ions may lower local extracellular divalent cation levels during glial depolarization, thereby raising axonal excitability and favoring ectopic impulse activity in adjacent nerve fibers. Increased calcium influx may lead to release of neuromodulators which provide for the hypomyelinated axons.
Ischemia and voltage-gated Ca2+ channels
Numerous studies have demonstrated that excess influx of Ca2+ contributes to neuronal injury and death during cerebral hypoxia or ischemia (Siesjo and Bengtsson, 1989; Haddad and Jiang, 1993). Elevation of neuronal cytosolic free Ca2+ concentration may occur via voltage-gated Ca2+ channels, binding of excitatory neurotransmitters to one of several classes of glutamate receptors, and reversal of sodium/Ca2+ exchange stimulated by cell depolarization. In ischemia, the NMDA subtype of glutamate receptor, which has substantial Ca2+ permeability, has been hypothesized as the chief cause of cytotoxic elevations in Ca2+ concentrations (Choi, 1988). Several recent studies using rat brain slices suggest that changes in intracellular Ca2+ during ischemia are attributable to multiple mechanisms, are inhibited incompletely by combined ion channel blockade, and are associated with the disruption of the cell membrane integrity (Kral et al., 1993; Bickler and Hansen, 1994). Our results indicate that there is no change in the apparent distribution or level of expression of L- or N-type Ca2+ channels in neurons during the first 4 d of ischemia but that there is a substantial upregulation of class C L-type Ca2+channels in reactive astrocytes. These results agree with the studies of Choi (1988), which show that voltage-gated Ca2+channels may not have a significant role in cell death that occurs after ischemia. However, studies in which astrocytes were grown in culture and exposed to glucose/oxygen deprivation in the presence and absence of extracellular Ca2+ suggest that influx of extracellular Ca2+ via L-type voltage-gated Ca2+ channels may contribute to astroglial injury during cerebral ischemia (Haun et al., 1992). Release of cytokines and growth factors from reactive astrocytes may be increased because of their increased density of L-type Ca2+ channels and may provide support for neuronal survival or regrowth during and after ischemic injury.
Footnotes
Research at the University of Washington was supported by National Institutes of Health Grants NS25155 to J.E.F. and NS22625 to W.A.C. plus a grant from the Human Frontiers Research Program to W.A.C. Research at Allegheny University was supported by the Alzheimer’s Foundation. Research at Baylor College of Medicine was funded by National Institutes of Health Grant NS29709 to J.L.N.
Correspondence should be addressed to Dr. William A. Catterall, Department of Pharmacology, Box 357280, University of Washington, Seattle, WA 98195.
Dr. Bausch’s present address: Duke University Medical Center, Durham, NC 27710.
REFERENCES
Articles from The Journal of Neuroscience are provided here courtesy of Society for Neuroscience
Full text links
Read article at publisher's site: https://doi.org/10.1523/jneurosci.18-07-02321.1998
Read article for free, from open access legal sources, via Unpaywall:
http://www.jneurosci.org/content/jneuro/18/7/2321.full.pdf
Free after 6 months at www.jneurosci.org
http://www.jneurosci.org/cgi/content/full/18/7/2321
Free after 6 months at www.jneurosci.org
http://www.jneurosci.org/cgi/reprint/18/7/2321.pdf
Free to read at www.jneurosci.org
http://www.jneurosci.org/cgi/content/abstract/18/7/2321
Citations & impact
Impact metrics
Citations of article over time
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1523/jneurosci.18-07-02321.1998
Article citations
Nimodipine attenuates neuroinflammation and delayed apoptotic neuronal death induced by trimethyltin in the dentate gyrus of mice.
J Mol Histol, 55(5):721-740, 31 Jul 2024
Cited by: 0 articles | PMID: 39083161
Cav3.2 channel regulates cerebral ischemia/reperfusion injury: a promising target for intervention.
Neural Regen Res, 19(11):2480-2487, 15 Dec 2023
Cited by: 1 article | PMID: 38526284 | PMCID: PMC11090426
Deletion of voltage-gated calcium channels in astrocytes decreases neuroinflammation and demyelination in a murine model of multiple sclerosis.
J Neuroinflammation, 20(1):263, 14 Nov 2023
Cited by: 3 articles | PMID: 37964385 | PMCID: PMC10644533
Calpain as a Therapeutic Target for Hypoxic-Ischemic Encephalopathy.
Mol Neurobiol, 61(1):533-540, 29 Aug 2023
Cited by: 2 articles | PMID: 37642934
Review
The Role of Voltage-Gated Calcium Channels in Basal Ganglia Neurodegenerative Disorders.
Curr Neuropharmacol, 21(2):183-201, 01 Jan 2023
Cited by: 8 articles | PMID: 35339179 | PMCID: PMC10190140
Review Free full text in Europe PMC
Go to all (94) article citations
Data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Enhanced expression of L-type Ca2+ channels in reactive astrocytes after ischemic injury in rats.
Neurosci Lett, 302(2-3):93-96, 01 Apr 2001
Cited by: 24 articles | PMID: 11290395
Intracellular calcium and cell death during ischemia in neonatal rat white matter astrocytes in situ.
J Neurosci, 18(18):7232-7243, 01 Sep 1998
Cited by: 62 articles | PMID: 9736645 | PMCID: PMC6793242
Activated astrocytes in areas of kainate-induced neuronal injury upregulate the expression of the metabotropic glutamate receptors 2/3 and 5.
Exp Brain Res, 137(1):1-11, 01 Mar 2001
Cited by: 89 articles | PMID: 11310162
Altered Homeostatic Functions in Reactive Astrocytes and Their Potential as a Therapeutic Target After Brain Ischemic Injury.
Curr Pharm Des, 23(33):5056-5074, 01 Jan 2017
Cited by: 13 articles | PMID: 28699523
Review
Funding
Funders who supported this work.
NINDS NIH HHS (5)
Grant ID: NS22625
Grant ID: R01 NS029709
Grant ID: NS29709
Grant ID: NS25155
Grant ID: R01 NS022625