Abstract
Background
Patients with hematologic malignancies exhibit persistent severe acute respiratory syndrome coronavirus 2 positivity over long periods after coronavirus disease 2019 (COVID-19) diagnosis. However, the frequency of, risk factors for, and prognosis of prolonged COVID-19 in immunocompromised patients remain unclear. Therefore, we investigated the long-term outcomes of COVID-19 in lymphoma patients and identified the associated factors and impact of prolonged COVID-19 on mortality.Methods
A multicenter retrospective cohort study of 583 lymphoma patients was conducted in 3 tertiary hospitals in South Korea. Patients receiving lymphoma treatment who were quarantined after obtaining a diagnosis of COVID-19 by polymerase chain reaction (PCR) or antigen test from August 2021 to September 2022 were examined.Results
Overall, 115 patients (19.7%) were diagnosed with COVID-19. Among 77 patients with clinical data, 24 had prolonged COVID-19. Patients in the prolonged COVID-19 group showed higher rates of receiving rituximab maintenance therapy following bendamustine and rituximab (BR) treatment for follicular lymphoma. This group did not show significant differences in clinical presentation within 30 days of COVID-19 diagnosis; however, it showed higher rates of re-admission due to COVID-19 pneumonia compared with the non-prolonged COVID-19 group. BR treatment followed by rituximab maintenance therapy is one of the risk factors for persistent PCR positivity, delayed or persistent pneumonia, and COVID-19 related admission after quarantine period. Prolonged COVID-19 was an independent risk factor for 1-year mortality.Conclusion
Prolonged COVID-19 was more frequent in lymphoma patients who received BR treatment followed by rituximab maintenance therapy and associated with unfavorable long-term outcomes and higher 1-year mortality.Free full text

Long-Term Outcomes of COVID-19 and Risk Factors for Prolonged or Persistent COVID-19 in Lymphoma Patients: A Multicenter, Retrospective Cohort Study
Abstract
Background
Patients with hematologic malignancies exhibit persistent severe acute respiratory syndrome coronavirus 2 positivity over long periods after coronavirus disease 2019 (COVID-19) diagnosis. However, the frequency of, risk factors for, and prognosis of prolonged COVID-19 in immunocompromised patients remain unclear. Therefore, we investigated the long-term outcomes of COVID-19 in lymphoma patients and identified the associated factors and impact of prolonged COVID-19 on mortality.
Methods
A multicenter retrospective cohort study of 583 lymphoma patients was conducted in 3 tertiary hospitals in South Korea. Patients receiving lymphoma treatment who were quarantined after obtaining a diagnosis of COVID-19 by polymerase chain reaction (PCR) or antigen test from August 2021 to September 2022 were examined.
Results
Overall, 115 patients (19.7%) were diagnosed with COVID-19. Among 77 patients with clinical data, 24 had prolonged COVID-19. Patients in the prolonged COVID-19 group showed higher rates of receiving rituximab maintenance therapy following bendamustine and rituximab (BR) treatment for follicular lymphoma. This group did not show significant differences in clinical presentation within 30 days of COVID-19 diagnosis; however, it showed higher rates of re-admission due to COVID-19 pneumonia compared with the non-prolonged COVID-19 group. BR treatment followed by rituximab maintenance therapy is one of the risk factors for persistent PCR positivity, delayed or persistent pneumonia, and COVID-19 related admission after quarantine period. Prolonged COVID-19 was an independent risk factor for 1-year mortality.
Conclusion
Prolonged COVID-19 was more frequent in lymphoma patients who received BR treatment followed by rituximab maintenance therapy and associated with unfavorable long-term outcomes and higher 1-year mortality.
INTRODUCTION
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in patients with hematologic malignancies, including lymphoma, is associated with a worse prognosis.1,2,3 This may be attributed to immunosuppression caused by the disease itself and its treatment.4 Moreover, these patients often experience persistent virus detection even after a long period since the initial diagnosis of coronavirus disease 2019 (COVID-19).5,6,7,8,9 According to previous studies, hematologic malignancy patients with lymphopenia or those who received B-cell depletion therapy, such as anti-cluster of differentiate (CD) 20 antibody therapy, or underwent hematopoietic stem cell transplantation within 1 year at an increased risk for prolonged or persistent COVID-19 infection.8,10
Prolonged COVID-19 is associated with subsequent episodes of clinical decompensation due to delayed clearance of SARS-CoV-2.8 Several studies have reported relapsing or progressively symptomatic COVID-19 with persistent SARS-CoV-2 positivity lasting for several months or longer in lymphoma patients receiving anti-CD20-antibody treatment.11,12 However, not all patients receiving B-cell depletion treatment will develop prolonged COVID-19. Some previous studies have also suggested that a history of anti-CD20 antibody treatment does not significantly affect the prognosis of COVID-19 patients, such as COVID-19 severity, hospitalization, intensive care unit (ICU) care, and mortality, despite the non-production of neutralizing antibodies against SARS-CoV-2 associated with the treatment.13
Most studies reporting persistent or prolonged COVID-19 have been published as single case reports or case series, and the analysis is usually limited to patients who received B-cell depletion treatment. Ichikawa et al. conducted a prospective study to determine the kinetics and characteristics of viral shedding and showed that anti-CD20 antibodies and bendamustine may be risk factors for prolonged viral shedding.14 However, the frequency of, risk factors for, and prognosis of prolonged COVID-19 in immunocompromised patients remain poorly understood. Identifying these pieces of information is essential for the appropriate COVID-19 management and cancer treatment, especially in lymphoma patients in whom prolonged COVID-19 is more frequently reported than other type of cancers. Therefore, this study aimed to investigate the clinical characteristics and long-term outcomes of COVID-19 in patients with lymphoma and identify the risk factors for prolonged COVID-19 infection, especially those related to lymphoma chemotherapy, and its impact on clinical outcomes.
METHODS
Study design and population
This multicenter retrospective cohort study was conducted in Sinchon, Gangnam, and Yongin Severance Hospital, South Korea. Patients diagnosed with lymphoma between January 2020 and August 2022, pathologic diagnosis was based on World Health Organization (WHO) classification15; patients diagnosed with follicular lymphoma from January 2018 were also included as they received a 2-year course of rituximab maintenance therapy.16 Lymphoma patients diagnosed with COVID-19 were finally included in the analysis. Patients aged < 18 years or with insufficient clinical data were excluded.
During the study period, patients visited the hematology outpatient clinic for routine follow-up of their lymphoma at least every 3 months or more frequently if they were undergoing lymphoma treatment as determined by the assigned hematologic physicians. At each visit, the patients were assessed for a history of COVID-19 diagnosis by polymerase chain reaction (PCR) or antigen test. Data regarding the severity of COVID-19, hospitalization status, and treatment details were obtained through history taking and documented in the medical records. For all patients admitted during the study period for any reason, a history of COVID-19 diagnosis was obtained; if COVID-19 was not confirmed within 3 months, a SARS-CoV-2 PCR test was conducted. A routine PCR follow-up test was not conducted after COVID-19 isolation clearance. However, if symptoms persisted or worsened, or if hospitalization was required within 3 months after the initial COVID-19 diagnosis, PCR testing was performed as part of the admission screening process. Patients diagnosed with COVID-19 were subjected to a mandatory 7-day quarantine according to the national guidelines. During the study period, patients undergoing lymphoma chemotherapy at the time of COVID-19 confirmation were considered for hospitalization regardless of the presence or absence of symptoms in South Korea, with remote treatment being made available if preferred by the individuals.
Definition and data collection
Data on age; sex; height; body weight; underlying diseases; lymphoma diagnosis and treatment; SARS-CoV-2 vaccination status; COVID-19 diagnosis, severity, and treatment; blood tests; and imaging results were collected by reviewing the electronic medical records. If the exact date of COVID-19 confirmation was not available, the confirmation date was considered as the 8th or 22nd day of each month, depending on whether it was early or late in the month, based on the information documented in the medical records. If only the month of confirmation was provided, the 15th day of that month was used as the approximate confirmation date.
Prolonged COVID-19 was defined as persistent positive results on PCR tests even after 30 days of COVID-19 diagnosis or persistence or worsening of COVID-19 pneumonia after 30 days of COVID-19 confirmation. The definition of COVID-19 pneumonia was based on two criteria: the presence of imaging findings consistent with a viral etiology, and the absence of evidence of a non-viral etiology, as determined by microbiological testing (e.g., bacterial culture, PCR, serological tests, etc.). Delayed pneumonia was defined as COVID-19 pneumonia that occurred after 30 days from the initial COVID-19 confirmation. “Prolonged neutropenia” was defined as an absolute neutrophil count of < 500/µL for 7 days or more. Similarly, “prolonged lymphopenia” was defined as an absolute lymphocyte count of < 500/µL for 7 days or more. The time points of COVID-19 diagnosis were divided into three categories based on the dominant variant of SARS-CoV-2 that accounted for over half of the cases detected in South Korea.
In this study, the severity of COVID-19 was divided into four categories according to the WHO guidelines: mild, moderate, severe, and critically-ill.17 Lymphoma treatments included those received within 1 year of COVID-19 diagnosis; for patients with follicular lymphoma, all chemotherapies administered during the study period were considered, including maintenance therapy.
Study endpoint
The primary endpoint of the current study was to evaluate the long-term outcomes of COVID-19 in lymphoma patients such as the presence of prolonged COVID-19, delayed pneumonia, re-admission, and the 90-, 180-, and 360-day mortality rates. For the secondary endpoints, we analyzed the factors associated with each of the long-term outcomes of COVID-19 in lymphoma patients, including prolonged COVID-19 and 360-day mortality.
Statistical analysis
Statistical analysis was performed using SPSS 26.0 (IBM; Armonk, NY, USA) and R 4.3.0. To compare the clinical characteristics and outcomes between patients with prolonged COVID-19 and those without prolonged COVID-19, the Mann–Whitney U test was used for continuous variables and the chi-square test or Fisher’s exact test for categorical variables. A multivariable logistic regression analysis was performed to compare variables that showed significant differences in the univariable analysis to identify the factors associated with the unfavorable long-term outcomes of COVID-19 in lymphoma patients. To analyze the impact of prolonged COVID-19 on mortality, the Kaplan–Meier curve and Cox regression analyses were utilized. A P value of < 0.05 was considered significant.
Ethics statement
The study was approved by the Institutional Review Board (IRB) of the Severance Hospital and conducted in accordance with the tenets of the Declaration of Helsinki. Since the study was retrospective in nature and the study participants were anonymized, the requirement for written consent was waived (IRB authorization number: 4-2023-0753).
RESULTS
Study population
During the study period, 583 lymphoma patients were screened. Of them, 115 patients (19.7%) were diagnosed with COVID-19 between August 2021 and September 2022; however, only 77 patients were finally included in the study after excluding those with insufficient data. Within 30 days after the diagnosis of COVID-19, 22.1% (17/77) of the patients continued to show a positive PCR result. In 23.4% (18/77) of the patients, pneumonia occurred or worsened after 30 days. During follow-up, 24 patients (31.0%) experienced prolonged COVID-19 (prolonged COVID-19 group), while 53 did not experience prolonged COVID-19 (non-prolonged COVID-19 group) (Supplementary Fig. 1).
Baseline characteristics and risk factors for prolonged COVID-19
The baseline characteristics of the study population at COVID-19 diagnosis are summarized in Table 1. No significant differences were observed in age, gender, laboratory data and underlying conditions other than the hematologic malignancies, between the prolonged- and non-prolonged COVID-19 groups. The prolonged COVID-19 group showed a higher incidence of follicular lymphoma (54.2% vs. 26.4%, P = 0.018) with no differences in terms of stage or disease status. When examining the anticancer regimens administered within 1 year, the proportion of patients receiving R-CHOP was lower in the prolonged- than in the non-prolonged COVID-19 group (25.0% vs. 49.1%, P = 0.049). Although the statistical significance was not evident, a higher proportion of patients in the prolonged COVID-19 group received bendamustine + B-cell depletion therapy (33.3% vs. 15.1%, P = 0.078).
Table 1
| Variables | Total (N = 77) | Non-prolonged COVID-19 (n = 53) | Prolonged COVID-19 (n = 24) | P value | ||
|---|---|---|---|---|---|---|
| Age, median [IQR] | 57 [45–66] | 58 [45–69] | 51 [43–66] | 0.222 | ||
| Age, yr ≥ 65 | 23 (29.9) | 16 (30.2) | 7 (29.2) | 0.928 | ||
| Female | 25 (32.5) | 17 (32.1) | 8 (33.3) | 0.913 | ||
| BMI, median [IQR] | 23.5 [21.8–25.0] | 23.1 [21.6–25.3] | 23.5 [21.5–24.6] | 0.947 | ||
| Baseline comorbidities | ||||||
| Hypertension | 14 (18.2) | 11 (20.8) | 3 (12.5) | 0.529 | ||
| Diabetes mellitus | 11 (14.3) | 6 (11.3) | 5 (20.8) | 0.303 | ||
| Cardiovascular disease | 3 (3.9) | 2 (3.8) | 1 (4.2) | 1.000 | ||
| Chronic kidney disease | 2 (2.6) | 2 (3.8) | 0 (0) | 1.000 | ||
| Chronic lung disease | 6 (7.8) | 3 (5.7) | 3 (12.5) | 0.369 | ||
| Chronic liver disease | 12 (15.6) | 7 (13.2) | 5 (20.8) | 0.500 | ||
| Othersa | 5 (6.5) | 5 (9.4) | 0 (0) | 0.317 | ||
| Type of hematologic malignancy | 0.149 | |||||
| Diffuse large B cell lymphoma | 45 (58.4) | 34 (64.2) | 11 (45.8) | 0.131 | ||
| Follicular lymphoma | 27 (35.1) | 14 (26.4) | 13 (54.2) | 0.018 | ||
| Mantle cell lymphoma | 2 (2.6) | 2 (3.8) | 0 (0) | |||
| Marginal zone B-cell lymphoma | 2 (2.6) | 2 (3.8) | 0 (0) | |||
| Primary central nervous system lymphoma | 1 (1.3) | 1 (1.9) | 0 (0) | |||
| Stage of hematologic malignancies | 0.218 | |||||
| 1 | 6 (7.8) | 4 (7.5) | 2 (8.3) | |||
| 2 | 12 (15.6) | 10 (18.9) | 2 (8.3) | |||
| 3 | 14 (18.2) | 12 (22.6) | 2 (8.3) | |||
| 4 | 45 (58.4) | 27 (50.9) | 18 (75.0) | |||
| Disease status | 0.165 | |||||
| Complete remission | 32 (41.6) | 25 (47.2) | 7 (29.2) | |||
| Partial remission | 17 (22.1) | 13 (24.5) | 4 (16.7) | |||
| Stable disease | 1 (1.3) | 0 (0) | 1 (4.2) | |||
| Progressive disease | 15 (19.5) | 8 (15.1) | 7 (29.2) | |||
| Not evaluated | 12 (15.6) | 7 (13.2) | 5 (20.8) | |||
| Treatment of hematologic malignancies | ||||||
| Hematopoietic stem cell transplantation | 5 (6.5) | 5 (9.4) | 0 (0) | 0.317 | ||
| Chemotherapy within 1 year before COVID-19 Dx | 69 (89.6) | 49 (92.5) | 20 (83.3) | 0.247 | ||
| Days from last chemotherapy to COVID-19 Dx, median [IQR] | 23 [13–58] | 21 [12–40] | 29.5 [14.75–82.5] | 0.119 | ||
| 1) Cytotoxic chemotherapy containing regimen | 59 (76.6) | 44 (83.0) | 15 (62.5) | 0.049 | ||
| R-CHOP (Rituximab + Cyclophosphamide + Doxorubicin + Prednisolone + Vincristine) | 32 (41.6) | 26 (49.1) | 6 (25.0) | 0.047 | ||
| Bendamustine + (Rituximab or Obinutuzumab) | 16 (20.8) | 8 (15.1) | 8 (33.3) | 0.078 | ||
| BR since diagnosis of lymphoma | 24 (31.2) | 12 (22.6) | 12 (50.0) | 0.016 | ||
| BR followed by rituximab maintenance | 15 (19.5) | 6 (11.3) | 9 (37.5) | 0.012 | ||
| BR without R-maintenance | 9 (11.7) | 6 (11.3) | 3 (12.5) | 1.000 | ||
| Dexamethasone + High-dose Cytarabine + Cisplatin | 6 (7.8) | 2 (3.8) | 4 (16.7) | 0.072 | ||
| Other regimensb | 11 (14.3) | 9 (17.0) | 2 (8.3) | 0.486 | ||
| 2) B cell depletion therapy | 64 (83.1) | 44 (83.0) | 20 (83.3) | 1.000 | ||
| B cell depletion therapy since diagnosis of lymphoma | 68 (88.3) | 48 (90.6) | 20 (83.3) | 0.448 | ||
| B cell depletion therapy within 6 month | 57 (74.0) | 39 (73.6) | 18 (75.0) | 1.000 | ||
| Rituximab (anti-CD20 antibody) | 64 (83.1) | 44 (83.0) | 20 (83.3) | 1.000 | ||
| Rituximab within 6 mon | 57 (74.0) | 39 (73.6) | 18 (75.0) | 1.000 | ||
| Days from last Rituximab to COVID-19, median [IQR] | 42 [18–107] | 43.5 [17–109.25] | 40 [21–119] | 0.909 | ||
| Othersc | 4 (5.2) | 2 (3.8) | 2 (8.3) | 0.585 | ||
| Baseline laboratory findingsd | ||||||
| White blood cell count, /μL, median [IQR] | 4,695 [3,428–6,633] | 4,700 [3,490–6,785] | 4,320 [2,770–6,570] | 0.582 | ||
| Absolute neutrophil count, /μL, median [IQR] | 2,785 [1,660–4,125] | 2,930 [1,955–4,180] | 2,190 [1,360–3,955] | 0.276 | ||
| Absolute lymphocyte count, /μL, median [IQR] | 960 [603–1,245] | 900 [620–1250] | 1,080 [485–1,260] | 0.879 | ||
| Prolonged neutropenia | 6 (7.8) | 3 (5.7) | 3 (12.5) | 0.346 | ||
| Prolonged lymphopenia | 6 (7.8) | 3 (5.7) | 3 (12.5) | 0.346 | ||
Values are presented as number (%) unless otherwise indicated.
COVID-19 = coronavirus disease 2019, IQR = interquartile range, BMI = body mass index, Dx = diagnosis, BR = bendamustine + rituximab.
aContains solid cancer (1 patient), autoimmune/connective tissue disease (1 patient), solid organ transplantation (3 patients).
bRegimen (number of patients): cyclophosphamide + vincristine + prednisolone (3), gemcitabine + oxaliplatin (2), ifosfamide + etoposide+ cytarabine + methotrexate (2), methotrexate + vincristine + dexamethasone (2), ifosfamide + carboplatin + etoposide (2), etoposide + vincristine + doxorubicin + cyclophosphamide + prednisolone (1), bortezomib + rituximab + cyclophosphamide + doxorubicin + prednisolone (1), and methotrexate + cytarabine + thiotepa (1).
cContains obinutuzumab (anti-CD20 antibody, 1 patient), epcoritamab (bispecific CD20-directed CD3 T-cell engager, 1 patient), tafasitamab (anti-CD19 antibody, 1 patient), and acalabrutinib (Bruton’s tyrosine kinase inhibitors, 1 patient).
dBaseline laboratory test results were collected from the time of the initial diagnosis of COVID-19, or in the absence of such results, the most recent test result prior to the initial diagnosis of COVID-19.
As the treatment for follicular lymphoma with bendamustine plus B-cell depletion therapy is followed by a two-year course of rituximab treatment, we included the history of bendamustine and rituximab (BR) chemotherapy from lymphoma diagnosis to COVID-19 diagnosis. We observed a significantly higher proportion of patients receiving BR chemotherapy in the prolonged- than in the non-prolonged COVID-19 group (50.0% vs. 22.6%, P = 0.016). Particularly, the rate of rituximab maintenance therapy following BR chemotherapy was significantly higher in this group (37.5% vs. 11.3%, P = 0.012).
Multivariable analysis showed that previous BR chemotherapy followed by rituximab maintenance (odds ratio [OR], 4.700; 95% confidence interval [CI], 1.437–15.377; P = 0.010) was an independent risk factor for prolonged COVID-19 (Supplementary Table 1).
Clinical features at the time of COVID-19 diagnosis
Among 77 lymphoma patients with COVID-19, 67 (87.0%) were diagnosed during the “Omicron period” (January–September 2022). Approximately 81.8% of the patients had mild to moderate COVID-19 at the time of diagnosis, thus not requiring oxygen therapy. Meanwhile, 23.4% (18/77) of the patients developed COVID-19 pneumonia within 2 weeks of diagnosis, and 3.9% (3) patients died within 1 month. No significant difference was found in the severity of COVID-19 at diagnosis and the short-term outcomes of COVID-19 including COVID-19 pneumonia development within 2 weeks and 28-day mortality between the two groups (Table 2).
Table 2
| Variables | Total (N = 77) | Non-prolonged COVID-19 (n = 53) | Prolonged COVID-19 (n = 24) | P value | |
|---|---|---|---|---|---|
| Vaccination status before COVID-19 diagnosis | 1.000 | ||||
| Not vaccinated | 21 (27.3) | 14 (26.4) | 7 (29.2) | ||
| 1st dose of primary series done | 5 (6.5) | 4 (7.5) | 1 (4.2) | ||
| Primary series completed | 21 (27.3) | 14 (26.4) | 7 (29.2) | ||
| Booter vaccination completed | 30 (39.0) | 21 (39.6) | 9 (37.5) | ||
| Period of diagnosis with COVID-19 | 0.381 | ||||
| Jan 2020–Jul 2021 (Wuhan) | 3 (3.9) | 1 (1.9) | 2 (8.3) | ||
| Aug 2021–Dec 2021 (delta) | 7 (9.1) | 5 (9.4) | 2 (8.3) | ||
| Jan 2022–Sep 2022 (omicron) | 67 (87.0) | 47 (88.7) | 20 (83.3) | ||
| Severity of COVID-19 (worst within the first 14 days of diagnosis) | 0.175 | ||||
| Mild to moderate (without oxygen demand) | 63 (81.8) | 43 (81.1) | 20 (83.3) | ||
| Severe | 8 (10.4) | 4 (7.5) | 4 (16.7) | ||
| Critically-ill | 6 (7.8) | 6 (11.3) | 0 (0) | ||
| COVID-19 treatment at COVID-19 diagnosis | |||||
| Regdanvimab | 1/56 (1.8) | 0 (0) | 1 (4.2) | 0.179 | |
| Nirmatrelvir/Ritonavir | 4/56 (7.1) | 3 (5.7) | 1 (4.2) | 0.556 | |
| Remdesivir | 15/24 (62.5) | 12 (22.6) | 3 (12.5) | 0.356 | |
| Steroid | 7/24 (29.2) | 5 (9.4) | 2 (8.3) | 1.000 | |
| Tocilizumab or baricitinib | 0/24 (0) | 0 | 0 | 1.000 | |
| Unknown (among hospitalized patients) | 8/32 (33.3) | 4 (7.5) | 4 (16.7) | ||
| Short-term outcomes of COVID-19 | |||||
| COVID-19 pneumonia within 2 weeks | 18 (23.4) | 14 (26.4) | 4 (16.7) | 0.349 | |
| Hospitalization at quarantine period | 32 (41.6) | 21 (39.6) | 11 (45.8) | 0.609 | |
| 28-day mortality | 3 (3.9) | 3 (5.7) | 0 (0) | 0.548 | |
COVID-19 = coronavirus disease 2019.
Long-term outcomes of COVID-19 and risk factors for unfavorable outcomes
Overall, 36 patients (46.8%) experienced COVID-19-associated pneumonia. The occurrence of COVID-19 pneumonia was significantly higher in the prolonged COVID-19 group (79.2% vs. 32.1%, P < 0.001); among them, 18 of 19 (94.7%) patients were accompanied by delayed/persistent pneumonia, and 12 of 18 (66.7%) presented with severe to critical illness.
Of the 77 patients, 15 (19.5%) required readmission due to COVID-19 exacerbation after the isolation period, and the readmission rate was significantly higher in the prolonged COVID-19 group (54.2% vs. 3.8%, P < 0.001). The mortality rates at 90 days, 180 days, and 1 year from COVID-19 diagnosis were 5.6%, 13.9%, and 22.2%, respectively. The prolonged COVID-19 group showed higher mortality rates at 180 days (25.0% vs. 8.3%, P = 0.074) and at 1 year (37.5% vs. 16.7%, P = 0.027). In terms of the outcomes related to hematologic malignancy, the prolonged COVID-19 group has caused more delays in chemotherapy (68.4% vs. 20.8%, P = 0.030) (Table 3).
Table 3
| Variables | Total (N = 77) | Non-Prolonged COVID-19 (n = 53) | Prolonged COVID-19 (n = 24) | P value | ||
|---|---|---|---|---|---|---|
| Prolonged infection | ||||||
| Total COVID-19 pneumonia | 36 (46.8) | 17 (32.1) | 19 (79.2) | < 0.001 | ||
| Delayed/persistent pneumonia | 18 (23.4) | 0 (0) | 18 (75.0) | < 0.001 | ||
| Mild to moderate (without oxygen demand) | 6 (7.8) | 0 (0) | 6 (25.0) | |||
| Severe | 5 (6.5) | 0 (0) | 5 (20.8) | |||
| Critically-ill | 7 (9.1) | 0 (0) | 7 (29.2) | |||
| Admission due to COVID-19 after quarantine period | 15 (19.5) | 2 (3.8) | 13 (54.2) | < 0.001 | ||
| More than 30 days after COVID-19 diagnosis | 10 (13.0) | 0 (0) | 10 (41.7) | |||
| Period from COVID-19 diagnosis to (re)admission date, median [IQR] | 35 [25.5–69] | |||||
| Mortalitya | Total (N = 72) | |||||
| 90-day mortality | 4 (5.6) | 3 (6.3) | 1 (4.2) | 1.000 | ||
| 180-day mortality | 10 (13.9) | 4 (8.3) | 6 (25.0) | 0.074 | ||
| 1-year mortality | 16 (22.2) | 7 (14.6) | 9 (37.5) | 0.027 | ||
| Hematologic malignancy-related outcomes | ||||||
| Delay in cancer treatment (except death) | 24 (31.2) | 11 (20.8) | 13 (68.4) | 0.030 | ||
| Disease progression | 14 (18.2) | 11 (20.8) | 3 (12.5) | 0.529 | ||
| Cancer-related mortalitya | 9/72 (12.5) | 5 (10.4) | 4 (16.7) | 0.469 | ||
Values are presented as number (%) unless otherwise indicated.
COVID-19 = coronavirus disease 2019, IQR = interquartile range.
aTwo patients with unclear survival status and three patients who died within 30 days after obtaining a COVID-19 diagnosis were excluded from the analysis.
The univariable and multivariable analyses of factors associated with each of the long-term outcomes are shown in Supplementary Table 2 and Table 4. BR treatment followed by rituximab maintenance therapy was not associated with COVID-19 pneumonia onset within 30 days, but associated with persistent PCR positivity 30 days after COVID-19 diagnosis (OR, 3.375; 95% CI, 1.104–10.317; P = 0.033), delayed or persistent pneumonia (OR, 8.833; 95% CI, 2.527–30.879; P = 0.001), total COVID-19 pneumonia (OR, 4.011; 95% CI, 1.046–15.382; P = 0.043), and COVID-19 related admission after the quarantine period (OR, 11.1; 95% CI, 2.758–44.678; P = 0.001) (Table 4).
Table 4
| Variables | (+) | (−) | P valuea | Adjusted odds ratio | 95% CI | P valueb | |
|---|---|---|---|---|---|---|---|
| PCR positive (more than 30 days after diagnosis) | n = 17 | n = 60 | |||||
| Age ≥ 65 | 5 (29.4) | 18 (30.0) | 0.963 | ||||
| BR chemotherapy | 9 (52.9) | 15 (25.0) | 0.028 | 3.375 | 1.104–10.317 | 0.033 | |
| Total COVID-19 pneumonia | n = 36 | n = 41 | |||||
| Age ≥ 65 | 15 (41.7) | 8 (19.5) | 0.034 | 3.326 | 1.097–10.081 | 0.034 | |
| BR followed by rituximab maintenance | 11 (30.6) | 4 (9.8) | 0.021 | 4.011 | 1.046–15.382 | 0.043 | |
| COVID-19 pneumonia within 30 days | n = 18 | n = 59 | |||||
| Age ≥ 65 | 11 (61.1) | 12 (20.3) | 0.001 | 6.155 | 1.968–19.247 | 0.002 | |
| BR followed by rituximab maintenance | 3 (16.7) | 12 (20.3) | 1.000 | ||||
| Delayed/persistent COVID-19 pneumonia after 30 days | n = 18 | n = 59 | |||||
| Age ≥ 65 | 5 (27.8) | 18 (30.5) | 0.825 | ||||
| BR followed by rituximab maintenance | 9 (50.0) | 6 (10.2) | < 0.001 | 8.833 | 2.527–30.879 | 0.001 | |
| Admission due to COVID-19 after quarantine period | n = 15 | n = 62 | |||||
| Age ≥ 65 | 4 (26.7) | 19 (30.6) | 1.000 | ||||
| BR followed by rituximab maintenance | 9 (50.0) | 6 (10.2) | < 0.001 | 11.100 | 2.758–44.678 | 0.001 | |
Values are presented as number (%).
CI = confidential interval, PCR = polymerase chain reaction, BR = bendamustine + rituximab, COVID-19 = coronavirus disease 2019.
aP value from the χ2 test.
bP value from the multivariate logistic regression analysis.
Impact of prolonged COVID-19 on long-term mortality
A multivariable analysis showed that underlying chronic lung disease (hazard ratio [HR], 4.5243; 95% CI, 1.201–17.035; P = 0.026) and prolonged COVID-19 (HR, 2.8618; 95% CI, 1.014–8.074; P = 0.047) were independently correlated with 1-year mortality (Table 5).
Table 5
| Variables | Dead (n = 16) | Alive (n = 56) | P valuea | Adjusted hazards ratio | 95% CI | P valueb |
|---|---|---|---|---|---|---|
| Age ≥ 65 | 6 (37.5) | 13 (23.2) | 0.335 | 1.0134 | 0.972–1.056 | 0.527 |
| Gender, female | 1 (6.3) | 23 (41.1) | 0.009 | 0.1424 | 0.018–1.108 | 0.063 |
| Chronic lung disease | 4 (25.0) | 1 (1.8) | 0.008 | 4.5243 | 1.201–17.035 | 0.026 |
| Prolonged COVID-19 | 9 (56.3) | 15 (26.8) | 0.027 | 2.8618 | 1.014–8.074 | 0.047 |
Values are presented as number (%).
Two patients with unclear survival status and three patients who died within 30 days after obtaining a COVID-19 diagnosis were excluded from the analysis.
CI = confidential interval, COVID-19 = coronavirus disease 2019.
aP value from the χ2 test.
bP value from the multivariate Cox regression analysis.
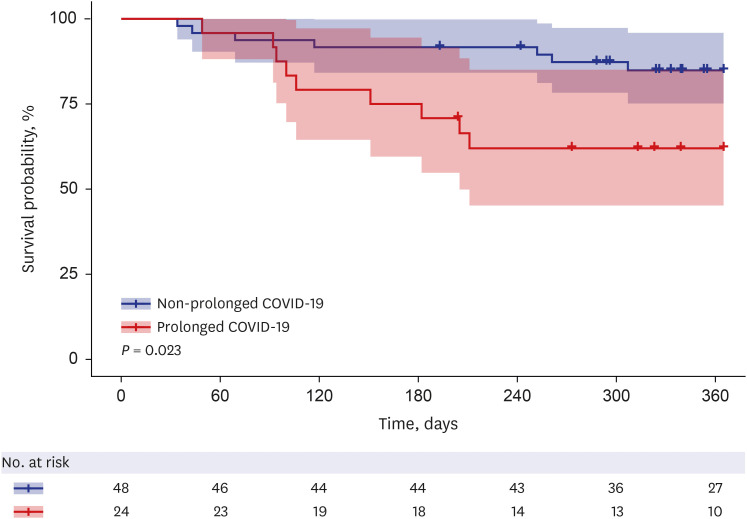
The prolonged COVID-19 group had a significantly higher mortality rate up to 1 year than the non-prolonged COVID-19 group (P = 0.023, log-rank test) (Fig. 1). Three patients who died within 30 days after COVID-19 diagnosis and two patients with uncertain mortality status were excluded from the analysis.
DISCUSSION
In this study, prolonged COVID-19 occurred in 22% of all lymphoma patients diagnosed with COVID-19. Receiving rituximab maintenance therapy following BR chemotherapy was an independent risk factor for developing prolonged COVID-19 in lymphoma patients. The readmission and 1-year mortality rates were significantly higher in the prolonged than in the non-prolonged COVID-19 group, and prolonged COVID-19 was an independent risk factor for 1-year mortality in the multivariable analysis.
Many studies have reported that B-cell depletion treatments such as rituximab increases the risk of persistent viral shedding and prolonged COVID-19 due to the impairment of antibody response8,18,19,20; some studies have reported that rituximab administration is associated with poor acute-phase outcomes such as severe COVID-19 and in-hospital mortality.1,20,21 In a study conducted by Levavi et al, the timing of rituximab treatment and the presence of anti-SARS-COV-2 antibody production had no significant impact on the outcomes such as hospitalization, ICU treatment, and death from COVID-19.13
Here, no difference was found in the status of rituximab treatment and the timing of rituximab administration between the prolonged- and non-prolonged COVID-19 groups. In a previous study of patients with X-linked agammaglobulinemia by Soresina et al.,22 antibodies are not required to overcome COVID-19, even in the absence of B-cell activity. A previous study has shown that recovery and survival from acute-phase COVID-19 are highly dependent on maintaining an adequate CD8 T-cell count than on achieving humoral immunity23; however, even with a strong CD8 T-cell response, poor viral clearance is associated with a higher risk of prolonged COVID-19. Lyudovyk et al.24 reported that patients who achieved viral clearance in the absence of a B-cell response also exhibited a marked CD4 T-cell response, suggesting that the combination of an impaired humoral response and a minimal or impaired CD4 T-cell response may be associated with a greater risk of prolonged/persistent COVID-19 compared with an impaired humoral response alone.
A previous BR chemotherapy followed by rituximab maintenance therapy was an independent risk factor for prolonged/persistent COVID-19 in this study. Bendamustine is an alkylating agent that binds to the deoxyribonucleic acid and causes damage, leading to cell apoptosis.25 It has a synergistic effect with rituximab and has been widely used as frontline chemotherapy for follicular lymphoma and marginal zone B-cell lymphoma.26 At least 75% of the patients were CD4 lymphopenic at the end of induction, and nearly 25% remained so after 3 years.27 Bendamustine treatment induces CD4 lymphopenia more potently than other chemotherapy regimens such as CHOP (Cyclophosphamide + Doxorubicin + Vincristine + Prednisolone) or CVP (Cyclophosphamide + Vincristine + Prednisolone) for lymphoma.27 Considering the impact of bendamustine, BR chemotherapy followed by rituximab maintenance may strongly inhibit the B-cell and CD4 T-cell responses, which may increase the risk of prolonged COVID-19 compared with rituximab treatment alone.
A significantly higher proportion of patients in the prolonged COVID-19 group had follicular lymphoma. Considering that 21 (77.8%) of the 27 follicular lymphoma patients in this study received BR chemotherapy and that follicular lymphoma was regarded as an indolent type of lymphoma, prolonged COVID-19 was more likely associated with BR treatment than with the malignancy itself.
In the risk factor analysis of long-term outcomes, only old age was the identified risk factor for acute phase pneumonia that occurred within 30 days of COVID-19 diagnosis; for delayed/persistent pneumonia that occurred or worsened after 30 days of COVID-19 diagnosis, rituximab maintenance therapy following BR chemotherapy was a risk factor, regardless of old age. Therefore, early pneumonia development is influenced by the classic COVID-19 pneumonia risk factors such as old age, but prolonged/persistent pneumonia is more influenced by treatment history such as BR chemotherapy.
The overall mortality up to 1 year was also significantly higher in the prolonged than in the non-prolonged group. In this study, rituximab maintenance therapy following BR chemotherapy was a risk factor for prolonged COVID-19, and the prolonged COVID-19 group showed higher mortality. These findings were consistent with a retrospective study conducted by Lee et al.28 The study included patients with B-cell lymphoma who received B-cell depleting therapy, and showed that prior bendamustine therapy was an independent risk factor for COVID-19–related mortality. Therefore, it is necessary to consider whether BR or rituximab maintenance should still be utilized for treating indolent lymphoma in the era of COVID-19.
This study is subject to a number of limitations. Primarily, due to the retrospective nature of this study, patients with lymphoma who self-reported having COVID-19 were included; hence, asymptomatic COVID-19 patients or untested COVID-19 patients were excluded. Furthermore, SARS-CoV-2 PCR testing was not conducted routinely on asymptomatic patients following a diagnosis of COVID-19. Consequently, some patients in the non-prolonged COVID-19 group may have experienced a persistent positive SARS-CoV-2 PCR test result even after 30 days. Furthermore, the definition of COVID-19 pneumonia as chest imaging consistent with viral pneumonia and no evidence of other etiology may have resulted in the inclusion of cases that were not actually COVID-19 pneumonia. This could have led to an overestimation of the prevalence of COVID-19-associated pneumonia.
Second, we were unable to collect detailed information on the COVID-19 treatment. For patients who were isolated at home or treated in other hospitals at the time of COVID-19 diagnosis, it was difficult to obtain accurate information about their treatment; hence, we were unable to analyze the impact of COVID-19 treatment on prolonged COVID-19 and long-term outcomes.
Third, the small sample size made it difficult to draw definitive conclusions based on the results of this study. In addition, the majority of lymphoma subtypes were DLBCL and follicular lymphoma, while other subtypes were only 5 patients in the non-prolonged COVID-19 group; laboratory studies investigating the immunity status of lymphoma patients and clinical studies analyzing more patients with other lymphoma treatments that may be associated with prolonged COVID-19 are warranted. Nevertheless, this study is significant because it is the first to analyze the prevalence and characteristics of symptomatic prolonged COVID-19 in patients with lymphoma and to report on the risk factors and long-term outcomes of prolonged COVID-19.
In conclusion, the risk of prolonged COVID-19 and mortality may be increased in patients with lymphoid malignancies receiving certain chemotherapy regimens, including but not limited to B-cell depletion treatment. Even after the introduction of the COVID-19 vaccine and the prevalence of the omicron subvariant with low severity and mortality, prolonged COVID-19 in immunocompromised individuals still has no specific treatment and can be associated with increased mortality and delayed treatment of the underlying disease. Therefore, further studies are needed to identify the risk factors and outcomes of prolonged infection and to analyze the immune responses in these patients.
ACKNOWLEDGMENTS
We would like to thank Editage (www.editage.co.kr) for English language editing.
Footnotes
Funding: This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2021R1I1A1A01055124), a faculty research grant from the Yonsei University College of Medicine (6-2021-0066) and a new faculty research seed money grant of Yonsei University College of Medicine for 2023 (2023-32-0045).
Disclosure: The authors have no potential conflicts of interest to disclose.
Author Contributions:
Conceptualization: Kim YR, Ahn JY.
Data curation: Lee JA, Ahn SM.
Formal analysis: Lee JA, Han M.
Investigation: Lee JA, Kim JS, Kim YR, Chung H, Cho H, Ku NS.
Methodology: Kim YR, Ahn JY, Lee JA, Jeong SJ.
Software: Lee JA, Lee YS.
Supervision: Yeom JS, Choi JY, Kim JS.
Visualization: Lee JA, Kim JH.
Writing - original draft: Lee JA, Ahn JY.
Writing - review & editing: Lee JA, Ahn JY, Kim YR.
SUPPLEMENTARY MATERIALS
Multivariable analysis of risk factors for prolonged COVID-19
Univariable analysis of the risk factors for long-term outcomes
Flow chart showing the participant selection process and analysis of the factors associated with COVID-19 in lymphoma patients.
References
Articles from Journal of Korean Medical Science are provided here courtesy of Korean Academy of Medical Sciences
Full text links
Read article at publisher's site: https://doi.org/10.3346/jkms.2024.39.e263
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
The evaluation of risk factors for prolonged viral shedding during anti-SARS-CoV-2 monoclonal antibodies and long-term administration of antivirals in COVID-19 patients with B-cell lymphoma treated by anti-CD20 antibody.
BMC Infect Dis, 24(1):715, 22 Jul 2024
Cited by: 1 article | PMID: 39039440 | PMCID: PMC11265166
Persistent SARS-CoV-2 infection with multiple clinical relapses in two patients with follicular lymphoma treated with bendamustine and obinutuzumab or rituximab.
Infection, 51(5):1577-1581, 19 Apr 2023
Cited by: 8 articles | PMID: 37076752 | PMCID: PMC10115373
Persistent viral shedding of severe acute respiratory syndrome coronavirus 2 after treatment with bendamustine and rituximab: A case report.
J Infect Chemother, 28(6):810-813, 31 Jan 2022
Cited by: 7 articles | PMID: 35115239 | PMCID: PMC8801906
Resolution of One-Year Persisting COVID-19 Pneumonia and Development of Immune Thrombocytopenia in a Follicular Lymphoma Patient With Preceding Rituximab Maintenance Therapy: A follow-up Report and Literature Review of Cases With Prolonged Infections.
Clin Lymphoma Myeloma Leuk, 21(10):e810-e816, 18 Jul 2021
Cited by: 22 articles | PMID: 34393077 | PMCID: PMC8286809
Review Free full text in Europe PMC
Funding
Funders who supported this work.
National Research Foundation of Korea (1)
Grant ID: NRF-2021R1I1A1A01055124
Yonsei University College of Medicine (2)
Grant ID: 6-2021-0066
Grant ID: 2023-32-0045

 3,* and
3,* and