Abstract
Free full text

Field and laboratory perspectives on fentanyl and carfentanil decontamination
Abstract
Abuse of the highly toxic compound fentanyl and its analogues is increasing, raising serious public health concerns due to their potency and availability. Therefore, there is a need for decontamination methodologies to safely remove fentanyl to avoid harmful exposure. In this study, the efficacy of commercial and in-house synthesized decontamination agents (Dahlgren Decon, RSDL (Reactive Skin Decontamination Lotion), FAST-ACT (First applied sorbent treatment against chemical threats), GDS2000, alldecont MED, bleach, Domestos Spray Bleach, Effekt Klor, MgO, TiO2-nanodiamond, and CeO2) were evaluated for the degradation of fentanyl and carfentanil under controlled laboratory conditions and on wooden floor surfaces. Liquid chromatography/mass spectrometry analysis showed that oxidative decontamination agents were the most effective, with N-oxides identified as major degradation products. The physiological effects of these N-oxides were also investigated regarding their ability to activate the µ-opioid receptor and their metabolism in human liver microsomes. The results provide empirical evidence that complements prior research findings on the degradation of fentanyl and carfentanil using a variety of decontamination agents.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-74594-z.
Introduction
Fentanyl and structural analogues thereof are potent synthetic opioids that have been successfully used as clinical drugs for acute pain management and anesthetics since their discovery in the 1960s1. However, besides important clinical applications, fentanyl and various fentanyl analogues are frequently abused and play a critical role in the ongoing opioid epidemic and opioid overdose deaths2–5. The abundant abuse of fentanyl during the last decade has provoked an increase in the availability of illicitly manufactured fentanyl and fentanyl analogues as well as the amount of diverted pharmaceutical products, especially in North America but also to some extent in Europe6,7. Fentanyl is between 50 and 100 times more potent than morphine, and the potency of various fentanyl analogues can be even higher2. One of the most potent opioids known is carfentanil which is 100 times more potent than fentanyl8. Carfentanil has emerged as a known additive to other drugs of abuse and has been involved in several overdose-related illnesses and deaths in the United States during the latest decade9. Owing to their very high potency, fentanyl and various analogues can be considered as hazardous materials that pose a serious risk not only to drug abusers but also to people who encounter these substances under other circumstances, such as first responders, crime scene investigators and clinical laboratory personnel10. The accidental hazard of fentanyl is mainly due to inhalation exposure whereas the risk of physiological effects upon dermal exposure has been assessed to be low. However, under certain circumstances such as high exposure or skin penetration enhancing conditions, skin penetration of fentanyl may also pose a hazardous risk11–13. In addition to occupational safety and health risks, the possibility of an antagonistic deliberate release of fentanyl or fentanyl analogues in public places could endanger public health. These compounds are widely available, highly potent, fast acting and easy to disperse. Therefore, although unlikely, the consequences of such an incident could be severe14.
From a hazardous material perspective, it is important to develop appropriate protocols for the efficient decontamination of fentanyl-contaminated areas to enable the rapid removal of fentanyl and fentanyl analogues to a safe level and eliminate the risk of further contamination and unintentional exposure to emergency personnel and public. Decontamination strategies based on the chemical degradation of fentanyl are preferred over those based on physical removal alone due to its toxicity. Even low-level contamination, due to insufficient decontamination, can possess a risk for unprotected individuals. However, to date, scientific studies on the effective decontamination of fentanyl and fentanyl analogues are limited. There is especially a lack of studies targeting effective decontamination methods that can minimize the risk of occupational exposure during operationally relevant scenarios15. In a comprehensive review from 2020, Bazley et al.. summarized the focus and progress of research performed on the decontamination of fentanyl and fentanyl analogues in operational and laboratory settings. Remaining gaps and challenges were highlighted, such as a lack of information on the degradation kinetics, applicability of decontamination methods to fentanyl analogues and chemical neutralization of fentanyl and its analogues in operationally relevant situations16. Most studies have focused on the forced degradation of fentanyl in controlled laboratory settings including acidic, basic, thermal, photon-assisted and oxidative degradation17–20. Oxidative methods, based on aqueous solutions of hypochlorite, hydrogen peroxide, percarbonate or peracetic acid, are currently regarded as the most efficient methods for fentanyl degradation. The most comprehensive published study on the degradation of fentanyl in aqueous oxidative solutions under laboratory settings was performed by Qi et al.17. Their research was followed by more recent studies examining the efficacy of commercially available oxidative decontamination and cleaning solutions for the decontamination of fentanyl and fentanyl analogues.
For example, Froelich et al. examined the efficacy of OxiClean, a commercial stain remover containing sodium percarbonate, for the removal of fentanyl and acetylfentanyl from benchtop surfaces21. They showed that OxiClean was more effective than water alone for cleaning surfaces, as long as the surface was scrubbed and rinsed while the decontaminant was still wet. A commercial decontaminant manufacturer (First Line Technology) presented a report showing that their product Dahlgren Decon, based on peracetic acid as an oxidizing agent, effectively degrades fentanyl and carfentanil to non-hazardous degradation products in controlled laboratory settings22,23. Sisco et al. compared the effectiveness of Dahlgren Decon and OxiClean for decontamination of fentanyl and analogues from laboratory benchtop surfaces and non-porous ceramic floor surfaces and detected less than 5% fentanyl in the resulting surface samples24. Fentanyl decontamination under operational settings was recently published by Oudejans et al.., who investigated cleaning of indoor surfaces using several commercially available decontamination and cleaning products25. All studied decontamination solutions contained oxidizing agent, including the common oxidants percarbonate, hypochlorite, hydrogen peroxide and peracetic acid. Their results showed that spray application of decontaminants containing peracetic acid or acidified bleach (hypochlorite) as oxidizing agents were very effective for the decontamination of fentanyl, whereas other oxidants had a noticeably lower decontamination efficacy. Except for the targeted analysis of norfentanyl in sample wipes in the study of Oudejans et al.., all these studies relied on detection of the remaining amount fentanyl as a measure of decontamination efficiency.
At present, the results from the studies presented above form the basis for operational guidelines devised by the National Institute for Occupational Safety and Health (NIOSH), InterAgency Board for Emergency Preparedness and Response (IAB) and U.S. Environmental Protection Agency (EPA)15,26.
However, many of the gaps and challenges pointed out by Bazely et al. in 2020 remain16. There is still a need to develop optimized decontamination procedures for fentanyl and its analogues that are operationally applicable, and such methodologies must be confirmed to result in non-hazardous degradation products. It is in general difficult, and an apparent risk for confounding factors, to compare results from scientific studies performed under different experimental conditions and analyzed by different methodologies. This becomes particularly evident when considering results from previous studies on fentanyl decontamination, as some of the studies not only differ in their experimental conditions but also in their areas of focus. In addition, they used different analysis methods and definitions to determine the decontamination efficacy. In this work, we present experimental data on fentanyl and carfentanil degradation by various decontamination agents in solutions that are comparable and complementary to previously performed studies. To start to address the above mentioned gaps, our study compared the efficacy of various decontamination agents to degrade fentanyl and carfentanil both under laboratory conditions and on wooden floor surfaces for field application. The decontamination solutions included acidic, basic, oxidative and adsorbing agents. Degradation products were analyzed by liquid chromatography/mass spectrometry (LC/MS) to determine the degradation efficiency. Identification of formed degradation products were confirmed by synthesized and commercial references. In addition, potential physiological effects of major degradation products were investigated regarding their activation of the µ-opioid receptor and metabolism in human liver microsomes. The study also explored Direct Analysis in Real Time with thermal desorption and high-resolution mass spectrometry (DART-TD-HRMS) as rapid analysis technique for verifying decontamination effectiveness and as an alternative to LC/MS for detecting precipitated fentanyl.
Results and discussion
Degradation of fentanyl and carfentanil in decontamination solutions
The degradation efficacy of different decontamination agents for chemical degradation of fentanyl and carfentanil was examined under controlled laboratory settings and was defined as high, medium or low depending on the remaining amount of intact fentanyl and carfentanil (Table 1). High =
= no intact fentanyl and carfentanil detected after 10 min, medium
no intact fentanyl and carfentanil detected after 10 min, medium =
= less than 50% of intact fentanyl and carfentanil detected after 1 h and/or full degradation observed within 24 h and low
less than 50% of intact fentanyl and carfentanil detected after 1 h and/or full degradation observed within 24 h and low =
= more than 50% of fentanyl and carfentanil detected after 1 h and/or detectable amounts still remains after 24 h. If no degradation was evident within 24 h, the decontamination agent was considered to have a negligible degradation efficacy. In Fig. 1, the time-dependent degradation of fentanyl and carfentanil in aqueous solutions is shown for hydrogen peroxide (H2O2), bleach, sodium hydroxide (NaOH), alldecont MED and Dahlgren Decon. The results agree with previous studies demonstrating that oxidative decontamination solutions containing hypochlorite, hydrogen peroxide or peroxy acids are effective for the degradation of fentanyl and carfentanil17. The commercial product Dahlgren Decon, which releases peracetic acid upon reaction of peracetyl borate with water, was the most effective, degrading fentanyl and carfentanil completely within 10 min i.e. no signals of fentanyl or carfentanil were detected by LC/MS analysis. Decontamination solutions with hypochlorite as the active ingredient were less effective but completely degraded both fentanyl and carfentanil within 24 h (1% fentanyl remained in alldecont MED after 26 h). The degradation efficacy of hydrogen peroxide was lower for both fentanyl and carfentanil compared with results by Qi et al.17, who reported 53% degradation of fentanyl after 60 min of stirring in a 0.2 M hydrogen peroxide solution. Our experiments were performed without stirring since this is not an applicable action in practical field use. The results showed that 48% of intact fentanyl remained after 19 h in hydrogen peroxide. The degradation of carfentanil in hydrogen peroxide was more effective but still, 23% of intact carfentanil remained after 18 h. To investigate the impact of stirring on the degradation efficacy of hydrogen peroxide, the fentanyl degradation experiments were repeated with stirring (800 rpm). However, although the degradation efficacy increased, it was still considerably lower than the results obtained by Qi et al. (Table 1). The low degradation efficacy of hydrogen peroxide was also demonstrated in a study by Langmaier et al., who detected no degradation of fentanyl in a solution of 44 mM hydrogen peroxide during 5 h27.
more than 50% of fentanyl and carfentanil detected after 1 h and/or detectable amounts still remains after 24 h. If no degradation was evident within 24 h, the decontamination agent was considered to have a negligible degradation efficacy. In Fig. 1, the time-dependent degradation of fentanyl and carfentanil in aqueous solutions is shown for hydrogen peroxide (H2O2), bleach, sodium hydroxide (NaOH), alldecont MED and Dahlgren Decon. The results agree with previous studies demonstrating that oxidative decontamination solutions containing hypochlorite, hydrogen peroxide or peroxy acids are effective for the degradation of fentanyl and carfentanil17. The commercial product Dahlgren Decon, which releases peracetic acid upon reaction of peracetyl borate with water, was the most effective, degrading fentanyl and carfentanil completely within 10 min i.e. no signals of fentanyl or carfentanil were detected by LC/MS analysis. Decontamination solutions with hypochlorite as the active ingredient were less effective but completely degraded both fentanyl and carfentanil within 24 h (1% fentanyl remained in alldecont MED after 26 h). The degradation efficacy of hydrogen peroxide was lower for both fentanyl and carfentanil compared with results by Qi et al.17, who reported 53% degradation of fentanyl after 60 min of stirring in a 0.2 M hydrogen peroxide solution. Our experiments were performed without stirring since this is not an applicable action in practical field use. The results showed that 48% of intact fentanyl remained after 19 h in hydrogen peroxide. The degradation of carfentanil in hydrogen peroxide was more effective but still, 23% of intact carfentanil remained after 18 h. To investigate the impact of stirring on the degradation efficacy of hydrogen peroxide, the fentanyl degradation experiments were repeated with stirring (800 rpm). However, although the degradation efficacy increased, it was still considerably lower than the results obtained by Qi et al. (Table 1). The low degradation efficacy of hydrogen peroxide was also demonstrated in a study by Langmaier et al., who detected no degradation of fentanyl in a solution of 44 mM hydrogen peroxide during 5 h27.
Table 1
Degradation efficacy of carfentanil and fentanyl by different degradation agents.
| Class of decontamination agent | Decontamination agent | Carfentanil | Fentanyl |
|---|---|---|---|
| Laboratory prepared decontaminants | H2O2 10% | Low | Low |
| H2O2 10%, stirring at 800 rpm | Low | Low | |
| Ca(ClO)2 0.2 M | Mid | Mid | |
| HCl 6 M | None | None | |
| NaOH 5% | Low | None | |
| CeO2 | Low | Low | |
| MgO | Low | Low | |
| TiO2-ND | Mid/High* | Mid/High* | |
| Commercial decontaminants | Bleach | Mid | Mid |
| Acidified bleach (pH 5) | High | High | |
| alldecont MED | Mid | Mid | |
| Dahlgren Decon | High | High | |
| GDS2000 | None | None | |
| RSDL | None | None | |
| FAST-ACT | Mid/High* | Mid/High* |
High =
= no fentanyl and carfentanil detected after 10 min, Medium
no fentanyl and carfentanil detected after 10 min, Medium =
= less than 50% of fentanyl and carfentanil detected after one hour and/or full degradation observed within 24 h and Low
less than 50% of fentanyl and carfentanil detected after one hour and/or full degradation observed within 24 h and Low =
= more than 50% of fentanyl and carfentanil remaining after one hour and/or detectable amounts still remaining after 24 h. None
more than 50% of fentanyl and carfentanil remaining after one hour and/or detectable amounts still remaining after 24 h. None =
= negligible degradation observed after 24 h.
negligible degradation observed after 24 h.
*Only low amounts of fentanyl and carfentanil were observed after 10 min. However, no degradation products were detected, only the disappearance of fentanyl and carfentanil. Therefore, there was no evidence of the degradation efficacy of these decontamination agents. Instead, the removal of fentanyl and carfentanil was most likely due to adsorption.
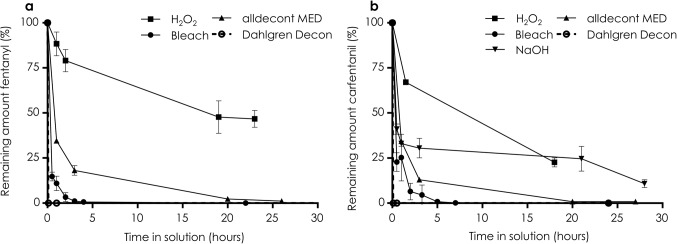
Time-dependent degradation of (a) fentanyl and (b) carfentanil in oxidative decontamination solutions during the first 30 h. Error bars represent the standard deviation of three replicate measurements.
Alkaline decontamination solutions have previously been shown to have a very poor fentanyl degradation efficacy18,28. We investigated three different alkaline decontamination solutions (Table 1): two commercial products (Reactive Skin Decontamination Lotion (RSDL) and GDS2000) and aqueous sodium hydroxide (5%). RSDL and GDS2000 were used as received from the manufacturers, undiluted. As shown in previous studies, sodium hydroxide exhibited a poor fentanyl degradation efficacy. However, carfentanil was degraded by slow hydrolysis of the methyl ester. Neither fentanyl nor carfentanil were degraded by RSDL or GDS2000. This was expected since the degrading effect of these decontaminants works through nucleophilic substitution, which has no effect on fentanyl or carfentanil. Although no chemical degradation of fentanyl in RSDL was observed in our study, Verheij et al. showed that RSDL was an efficient decontaminant of fentanyl from metal substrates29 and Dalton et al. demonstrated that RSDL effectively removed carfentanil from skin11. However, no degradation of fentanyl was observed during skin decontamination with RSDL12.
Dry decontamination using a solid absorptive material, such as earth minerals, activated charcoal or talcum powder, offers an alternative to solution-based decontamination. In this study, we examined the decontamination efficiency of the commercial dry decontaminant FAST-ACT (First applied sorbent treatment against chemical threats) and three in-house synthesized metal oxide powders (Table 1). These materials have been shown to adsorb chemical warfare agents and related compounds30. FAST-ACT consists of mixed aggregates of magnesium oxide (MgO) and titanium dioxide (TiO2) nanoparticles, whereas the in-house produced powders contained aggregates of pure phase metal oxide nanoparticles (MgO and CeO2) or a two-phase composite (TiO2-ND). These studies were performed with a 1:50 weight ratio of fentanyl or carfentanil to the solid decontaminant. The experiments were performed in deuterated chloroform to monitor the reaction by nuclear magnetic resonance (NMR) analysis. The efficacy of MgO and CeO2 in decreasing the amount fentanyl and carfentanil was found to be modest. However, in the case of TiO2-ND, less than 20% of fentanyl and carfentanil remained after 10 min. In FAST-ACT, less than 5% of the compounds remained after 10 min. These results were based on the remaining amount fentanyl and carfentanil compared to control samples where no decontaminant was added. Analyses were performed by LC/MS and 1H-NMR, but no degradation products were detected. Therefore, the observed effect was assumed to be due to adsorption of fentanyl and carfentanil on the decontaminant rather than degradation.
Effect of solubility on the fentanyl degradation efficacy in bleach
Despite its high degradation efficacy, Dahlgren Decon has several disadvantages such as high cost, the viscosity of the solution, accessibility and ease of use (the three components have to be mixed prior to use in the field). A readily available alternative is commercial chlorine-based bleach (pH >
> 11). However, a major drawback hampering its use is fentanyl in basic solutions mainly exists in the free base form, which has poor aqueous solubility. Qi et al. previously observed precipitation of solid fentanyl in an aqueous calcium hypochlorite solution. In addition, we visually observed formation of precipitated fentanyl that dissolved as the degradation reaction progressed. Furthermore, a 10-fold increase of fentanyl concentration resulted in the formation of insoluble aggregates, which remained insoluble for several months despite stirring and addition of more bleach.
11). However, a major drawback hampering its use is fentanyl in basic solutions mainly exists in the free base form, which has poor aqueous solubility. Qi et al. previously observed precipitation of solid fentanyl in an aqueous calcium hypochlorite solution. In addition, we visually observed formation of precipitated fentanyl that dissolved as the degradation reaction progressed. Furthermore, a 10-fold increase of fentanyl concentration resulted in the formation of insoluble aggregates, which remained insoluble for several months despite stirring and addition of more bleach.
Acidification of bleach, until fentanyl exists mainly in its salt form, has been shown to increase the degradation efficacy25. Thus, we tested a bleach solution acidified to pH 5 and found it effectively degraded fentanyl and carfentanil within five minutes, based on no detection of fentanyl or carfentanil by LC/MS analysis (Table 1). In addition to increasing the solubility, lowering the pH of bleach increases the concentration of freely available chlorine in the solution thereby increasing the oxidative effect. Nevertheless, acidified bleach is highly corrosive and therefore not practical to use as a decontaminant in operational situations17,24,31.
In an attempt to increase the solubility of fentanyl in bleach, addition of ethanol, tetrahydrofuran (THF) and the commercial detergent TWEEN® 20 were investigated. Unfortunately, none of these additives were successful in preventing the formation of aggregates in solutions with high fentanyl concentration. Addition of ethanol (50%) to the bleach solution decreased the degradation efficiency independently of the fentanyl concentration.
Analysis of degradation products formed in decontamination solutions
Detection of degradation products strengthens the evaluation of decontamination efficiency. Identification of degradation products demonstrates that the hazardous compound has been rendered harmless. In addition, identification of the products formed during decontamination adds important information to rule out the formation of possible harmful degradation products. Hence, we performed a non-target analysis of fentanyl and carfentanil degradation products in the most effective oxidative decontamination solutions. Chemical structures of the major degradation products identified after degradation of fentanyl and carfentanil in Dahlgren Decon, bleach, alldecont MED and hydrogen peroxide are presented in Fig. 2. The N-oxides of fentanyl and carfentanil respectively were found to be the dominating degradation products. In the hypochlorite solutions bleach and alldecont MED, the second largest signals were identified as monochlorinated nor-products. A second monochlorinated degradation product was also detected for both fentanyl and carfentanil, but its structure could not be determined. In hydrogen peroxide, primarily oxidized degradation products were observed. For both carfentanil and fentanyl, N-oxides were the major degradation products. For fentanyl, both major degradation products eluted after fentanyl, implying they were both N-oxide isomers32.

Structures of fentanyl, carfentanil and their main degradation products identified in the decontamination solutions.
Nearly all the observed degradation products were detected by LC/HRMS analysis (see Supplementary Data Fig. S1-S9 and Table S1-S9, for spectra, molecular weight, retention times and proposed structural formulas of degradation products not structurally identified). Only trace amounts of a few nonpolar degradation products were detected solely by GC/HRMS (see Supplementary Data Table S10 and Fig. S10 for structures of degradation products identified by GC/HRMS).
When comparing the amounts of intact fentanyl and carfentanil remaining in the decontamination solutions to the formed amounts of degradation products, it should be recognized that their response factor (sensitivity) might differ depending on the method and instrumentation used. Construction of calibration curves of reference standards of N-oxides and nor-products of fentanyl and carfentanil showed that the nor-products had equal response factors to those of fentanyl and carfentanil. However, the N-oxides exhibited response factors that were about 50% lower than those of fentanyl and carfentanil (Table 2). Complex matrix solutions effects may also influence the response factors. Therefore, it is important to bear in mind that an absence of fentanyl and carfentanil should not be interpreted as evidence of degradation. Instead, detection of degradation products provides more conclusive evidence of successful degradation.
Table 2
Peak area response factors of extracted molecular ion trace of fentanyl, carfentanil and two of their degradation products analyzed by UHPLC-HRMS.
| Carfentanil | Fentanyl | |
|---|---|---|
| Original compound | 1.94 E + + 05 05 | 1.40 E + + 05 05 |
| Nor-product | 2.01 E + + 05 05 | 1.38 E + + 05 05 |
| N-oxide product | 8.21 E + + 04 04 | 7.42 E + + 04 04 |
Degradation of fentanyl and carfentanil upon surface decontamination
Degradation efficiency of three different decontamination solutions
To further investigate the proposed effectiveness of commercial cleaning sprays used in other studies, we tested the degradation effectiveness of two cleaning sprays (Domestos Spray Bleach and Effekt Klor, henceforth referred to as cleaning spray 1 and spray 2, respectively)33,34 containing comparable amounts of sodium hypochlorite17,24. The sprays’ efficiency in removing and degrading fentanyl and carfentanil spills on a wooden floor surface where compared to that of Dahlgren Decon, for which we knew the degradation products. Photographs of the steps involved in the sampling procedure for a possible decontamination scenario are shown in Fig. 3. Fentanyl and carfentanil contaminated surfaces were treated with the three different decontamination solutions for 30 min, followed by wiping, extraction of the wipes and LC/MS analysis of the extracts for identification of the formed degradation products and remaining amounts of intact fentanyl and carfentanil.

Photographs showing the main steps in the sampling procedure for decontaminating carfentanil from a wooden surface. (a) contaminated surface, (b) decontamination using cleaning spray, (c) wiping of the surface, d-e) extraction and f) decontaminated surface.
First, we investigated whether the decontamination efficiency differed depending on whether the contaminant was applied to the surface as a powder or as a solution that was allowed to evaporate before decontamination was performed. No difference in decontamination efficiency between the two application methods was observed for both fentanyl and carfentanil. Since the solution-based approach allowed more precise and accurate application of the compounds onto the surface, it was used in the following studies. Extracts of the wipes (Fig. 3E) were analyzed for known degradation products detected by LC/MS. To ensure the recovery of intact fentanyl and carfentanil from the extracts, positive control experiments were performed using water instead of the decontamination solution. The intensity obtained for the positive control was then used to normalize the results of the decontaminated samples. Negative controls were also used to assess background signals in the decontamination data. A broad search of degradation products was performed. In the negative controls of Dahlgren Decon, an ion with the same mass as a degradation product of fentanyl was detected. Additionally, both Dahlgren Decon and the cleaning sprays caused considerable ion suppression, resulting in the N-oxides of fentanyl and carfentanil being the only degradation products that could be confidently determined.
As shown in Fig. 4, no traces of intact fentanyl and carfentanil were detected by LC/MS analysis in extracts from wipes used to sample contaminated surfaces after treatment with Dahlgren Decon, consistent with the results described above and previously published studies24,25. Figure 4 also shows that high amounts of the N-oxides of fentanyl and carfentanil were detected, demonstrating that Dahlgren Decon effectively degraded fentanyl and carfentanil and that the absence of intact agents was not due to alternative causes. An additional analysis, including fentanyl only, was performed on extracted wipe samples from surfaces treated with the two commercial cleaning sprays 1 and 2. The results show that only low amounts of intact fentanyl and N-oxide were detected after treatment with these sprays, Fig. 4. Although the amount of intact fentanyl was considerably lower than in the positive control, this was most likely due to poor recovery of fentanyl from the wipes caused by agglomeration in the cleaning sprays rather than successful degradation of fentanyl. To confirm effective degradation, higher amounts of detected N-oxide, or other degradation product, would have been necessary. Experimental data demonstrated that the N-oxide of fentanyl did not undergo agglomeration. It should be noted that the fentanyl control at these concentrations saturated the MS detector, making it impossible to quantify and compare to the N-oxide. In further studies, the possibility of using the 13C-isotope of fentanyl to avoid saturation, will be elucidated.
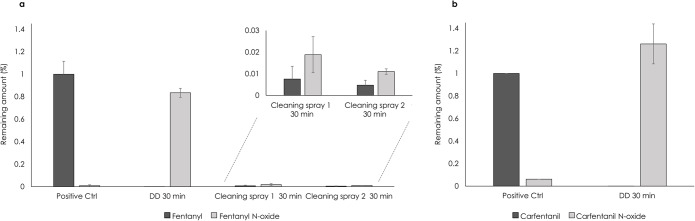
Surface sampling of (a) fentanyl and (b) carfentanil and their corresponding N-oxides after 30 min decontamination. Dahlgren Decon (DD) was used for decontamination of both fentanyl and carfentanil (n =
= 6). Commercial cleaning spray 1 and 2 were only used for decontamination of fentanyl (n
6). Commercial cleaning spray 1 and 2 were only used for decontamination of fentanyl (n =
= 5). Extracts from sample wipes were analyzed using LC/MS and the N-oxide amounts were recalculated using a response factor determined on the same LC/MS instrument as used in the experiment. Data were further normalized to the values obtained for the positive control (100%). Error bars represent the standard deviation.
5). Extracts from sample wipes were analyzed using LC/MS and the N-oxide amounts were recalculated using a response factor determined on the same LC/MS instrument as used in the experiment. Data were further normalized to the values obtained for the positive control (100%). Error bars represent the standard deviation.
By utilizing the cleaning sprays, the application of the decontamination solution became highly efficient. However, if the surface was contaminated with visible amounts of powder, we noticed a risk that the powder could be dispersed during the spraying process. Cleaning spray 2 also exhibited a tendency to dry rapidly, which could slow down the decontamination process. Dahlgren Decon exhibited a higher viscosity and remained wet throughout the decontamination periods tested, lasting up to 2 h. The primary drawback of Dahlgren Decon was the extended preparation time.
DART-TD-HRMS analysis to confirm decontamination efficiency
Direct Analysis in Real Time with a thermal desorption unit coupled to high-resolution mass spectrometry (DART-TD-HRMS) is a method capable of the rapid analysis of samples suspected to contain harmful compounds in cases where a quick identification and response are vital and/or when there are many samples to screen35–38. In this study, the technique was evaluated as a rapid analysis method for confirmation of the decontamination efficiency. In addition, DART-TD-HRMS was explored as an alternative method to address the above-discussed challenge of detecting precipitated fentanyl by LC/MS. Fentanyl and carfentanil contaminated wooden floor surfaces were treated with a decontamination solution, following the same procedure as described above. Since both cleaning sprays showed comparable results, cleaning spray 1 was replaced by tap water to obtain a measure of the decontamination achievable by mechanical cleaning and water alone. The pH of the cleaning spray was 9.5. Hence, the solubility of fentanyl and carfentanil, despite the aid of surfactants, was expected to be poor. Surface sampling by wiping was carried out directly after cleaning and one day later. In addition, to investigate the stability of the surface sample on the sample trap, an additional sample was collected on day 1, which was stored in a capped vial and analyzed with the day 2 sample the next day.
As expected, no difference (p-value >
> 0.05) was observed in the decontamination efficiency between tap water and the cleaning spray (Fig. 5). Therefore, the results suggested that the lack of detected fentanyl and carfentanil was probably due to agglomeration rather than degradation. These results raise questions regarding the findings of previous studies that relied solely on the absence of fentanyl when concluding cleaning solutions to be effective21,24. However, Froelich et al.. also noted that the fentanyl probably was transferred from the contaminated surface to the paper towel21. The surfaces treated with Dahlgren Decon showed no trace of remaining active substance. Although, DART-TD-MS is not a quantitative method, it was clear that the contaminants were detectable on the surfaces at the same levels on day 2 as they were on day 1. Furthermore, the stored sample traps from day 1 analyzed the following day yielded similar results. This demonstrates that a sample could be taken at one place and transported to another for analysis the following day without any loss of information. If a DART-TD-MS instrument can be accessed nearby, it would be possible to evaluate the decontamination efficacy of a contaminated surface within minutes of surface sampling. These results imply that the direct analysis of surface samples has a great potential for rapidly screening many samples to locate a possible contaminant or to confirm that a surface has been properly decontaminated.
0.05) was observed in the decontamination efficiency between tap water and the cleaning spray (Fig. 5). Therefore, the results suggested that the lack of detected fentanyl and carfentanil was probably due to agglomeration rather than degradation. These results raise questions regarding the findings of previous studies that relied solely on the absence of fentanyl when concluding cleaning solutions to be effective21,24. However, Froelich et al.. also noted that the fentanyl probably was transferred from the contaminated surface to the paper towel21. The surfaces treated with Dahlgren Decon showed no trace of remaining active substance. Although, DART-TD-MS is not a quantitative method, it was clear that the contaminants were detectable on the surfaces at the same levels on day 2 as they were on day 1. Furthermore, the stored sample traps from day 1 analyzed the following day yielded similar results. This demonstrates that a sample could be taken at one place and transported to another for analysis the following day without any loss of information. If a DART-TD-MS instrument can be accessed nearby, it would be possible to evaluate the decontamination efficacy of a contaminated surface within minutes of surface sampling. These results imply that the direct analysis of surface samples has a great potential for rapidly screening many samples to locate a possible contaminant or to confirm that a surface has been properly decontaminated.

Surface analysis of fentanyl and carfentanil by DART-TD-HRMS after mechanical cleaning with water as a reference (Ref, 100%), cleaning spray 2 and Dahlgren Decon (DD). Sampling was conducted and the strip was analyzed immediately after cleaning (Day 1) as well as on the following day (Day 2). An additional sampling was performed immediately after cleaning and the sample strip was stored for later analysis (Stored). Error bars represent the standard deviation of three replicate measurements.
Potential efficacy of N-oxides on the µ-opioid receptor and their metabolism in human liver microsomes
Fentanyl and carfentanil produce their physiological effect through stimulation of µ-opioid receptors. Agonists to the µ-opioid receptor are widely used as therapeutics for analgesia and sedation. However, activation of µ-opioid receptor signalling also mediates numerous other central nervous system and gastrointestinal effects of which central respiratory depression is the most life-threatening opioid-related side effect39. Primarily, drugs such as fentanyl and carfentanil are converted from the active compounds to the inactive form through hepatic metabolism. The ability of fentanyl, carfentanil and their N-oxide degradation products (fentanyl N-oxide and carfentanil N-oxide) to bind to and subsequently activate the µ-opioid receptor was compared using a cell-based receptor-binding assay. In addition, the in vitro metabolic stability of the compounds was investigated using a human liver microsome (HLM) assay.
Fentanyl (EC50 12.5 nM) and carfentanil (EC50 3.1 nM) exhibited high potency to activate the µ-opioid receptor (Table 3). The relatively higher agonist activity of carfentanil compared to fentanyl corresponds well to the difference in clinical potency described for both compounds and in previous receptor-binding studies40,41. The ability of fentanyl N-oxide (EC50 1521 nM) and carfentanil N-oxide (EC50 360 nM) to activate the µ-receptor was approximately 120-fold lower compared to the parent compound (dose-response curves in Supplementary Data Fig. S11). These data further strengthens the importance of efficient agent degradation during decontamination to reduce potential exposure risks.
Table 3
Activation of the µ-opioid receptor and metabolic fate of N-oxides. Calculated median effective concentration (EC50) values, relative potency and half-life.
| Compound | EC50 (nM) | Relative potency | HLM t1/2 (min) |
|---|---|---|---|
| Carfentanil | 3.1 (2.3–4.3) | n.a. | 4.5 (4.1–4.9) |
| Carfentanil N-oxide | 360 (271–475) | 116 | 42 (35–53) |
| Fentanyl | 12.5 (10.8–14.6) | n.a. | 20 (19–22) |
| Fentanyl N-oxide | 1521 (1267–1823) | 121 | 78 (55–131) |
| Morphine | 89 (69–114) | n.a. | - |
95% confidence interval values are shown in parentheses. The relative potency is the potency compared to the parent compound. Morphine is included for comparison.
Analysis of the stability of fentanyl and carfentanil in phase I metabolism (Supplementary Data Fig. S12) displayed a HLM half-life of 20 min and 4.5 min, respectively (Table 3). In a previous study using a comparable assay, the HLM half-life of carfentanil was reported to be 7.8 min, which corresponds well to the present study32. Fentanyl was developed to possess clinical short-acting properties but the offset of action involves both rapid compound redistribution to fat and muscle depots in addition to efficient hepatic metabolism42. The main degradation products of fentanyl and carfentanil following phase I-metabolism is N-dealkylated metabolites and the nor-metabolites were the primary degradation products detected during sample analysis also in the present study. In addition to rapid metabolism, a major nor-carfentanil metabolite has displayed comparable µ-receptor activity as the parent compound, which may contribute to prolonged toxidromic effects observed for carfentanil in comparison to fentanyl41. The two N-oxides displayed a HLM half-life of 78 min and 42 min for fentanyl N-oxide and carfentanil N-oxide, respectively. The main metabolite identified was oxidized N-oxides but small quantities of corresponding nor-products were also detected. No reformation of fentanyl or carfentanil was identified. Altogether, the low µ-receptor potency for the N-oxides and no reformation to the parent compound suggests low risk for physical effects following accidental exposure. However, other potential targets and the elimination half-life for N-oxides should be considered due to the relative long-acting properties observed.
Conclusions
Degradation of fentanyl and carfentanil was studied under controlled laboratory conditions in various decontamination solutions and on wooden floor surfaces to simulate field conditions. The experimental data were found to be comparable and complementary to previously performed studies on fentanyl degradation and verified that oxidative decontamination solutions were the most effective. The results showed that the commercial decontamination product Dahlgren Decon is an efficient decontamination solution for fentanyl and carfentanil, as evidenced by the formation of N-oxides of fentanyl and carfentanil and that no intact substances could be detected by LC/MS analysis after 30 min decontamination with Dahlgren Decon. Initially, commercial bleach-based cleaning sprays also seemed to perform well at surface decontamination, lowering the detected levels of fentanyl and carfentanil considerably after 30 min. Nevertheless, contrary to Dahlgren Decon, only low levels of N-oxide and no other degradation products were detected. Further investigation led to the conclusion that the cleaning sprays did not perform any better than tap water for fentanyl and carfentanil degradation. The reason for the difficulty in detecting fentanyl in the cleaning sprays was not fully established, but agglomeration due to poor solubility in aqueous solutions seems plausible. These results highlight that identification of degradation products adds important information when evaluating the decontamination efficacy, rather than solely relying on an absence of fentanyl and carfentanil. The results from this study also demonstrates the importance of analysis method development for accurate quantification of degradation products.
In addition to solution-based decontamination of fentanyl and carfentanil, this study adds new information on the decontamination of fentanyl and carfentanil by dry decontaminants. Commercially available FAST-ACT and the in-house synthesized TiO2-ND absorbent were shown to be effective for removal of fentanyl and carfentanil from solution. To the best of our knowledge, there has been no previously published data on metal oxide based decontamination of fentanyl related substances. Our results indicate that metal oxides could have potential as dry decontaminants and further studies, under operational relevant conditions, would be of interest.
Fentanyl and carfentanil are highly toxic compounds and small residual amounts following decontamination could still cause poisoning. In humans, the intravenous median toxic dose (TD50) has been determined to be 2 µg/kg and the greater toxicity of carfentanil is commonly acknowledged, which are agent amounts well above the detection limits for chemical analysis in the present studies40,43. However, both the exposure route and agent toxicity must be considered when defining potential exposure risks. Following decontamination using agent degrading solutions, the percutaneous route is the most likely exposure risk. However, inhalation is considered as the major concern for fentanyl and analogues as the skin contact risk has been evaluated to be rather low in previous studies13,44. Decontamination performed using dry particulates would, on the other hand, increase the risk for inhalation exposure.
This study identified that N-oxides of fentanyl and carfentanil were the major degradation products in oxidative decontamination solutions. Investigation of their physiological effects showed that they were significantly less potent in eliciting a response at the µ-opioid receptor level than fentanyl and carfentanil and that they could be metabolized by liver enzymes. In addition we showed that no reformation of fentanyl or carfentanil from the N-oxides occurred. These results indicate that the risk of developing adverse effects after exposure to the degradations products is less than that from fentanyl and carfentanil.
Methods and materials
Chemicals
Fentanyl•HCl and carfentanil•HCl were synthesized in ≥ 95% purity according to previously published procedures45. Norfentanyl (CAS 1609-66-1) and norcarfentanil (CAS 72996-78-2) were commercially available as oxalate solutions, 1 mg/mL and 0.1 mg/mL analytical standards in methanol, respectively. Milli-Q water (MQ) was used for preparing all aqueous solutions and eluents for LC/MS analysis. The following chemicals were used as received from the manufacturers to prepare the decontamination solutions: ethanol (96%), hydrochloric acid (HCl, 37%), hydrogen peroxide (H2O2, 30%, stabilized for synthesis), sodium hydroxide (NaOH), TWEEN® 20 (for synthesis), calcium hypochlorite (Ca(ClO)2, for synthesis), tetrahydrofuran (THF, extra pure), Deuterated chloroform (CDCl3, 99.8%, stabilized with Ag), acetonitrile (MeCN, hypergrade LC/MS) and methanol (UHPLC/MS).
95% purity according to previously published procedures45. Norfentanyl (CAS 1609-66-1) and norcarfentanil (CAS 72996-78-2) were commercially available as oxalate solutions, 1 mg/mL and 0.1 mg/mL analytical standards in methanol, respectively. Milli-Q water (MQ) was used for preparing all aqueous solutions and eluents for LC/MS analysis. The following chemicals were used as received from the manufacturers to prepare the decontamination solutions: ethanol (96%), hydrochloric acid (HCl, 37%), hydrogen peroxide (H2O2, 30%, stabilized for synthesis), sodium hydroxide (NaOH), TWEEN® 20 (for synthesis), calcium hypochlorite (Ca(ClO)2, for synthesis), tetrahydrofuran (THF, extra pure), Deuterated chloroform (CDCl3, 99.8%, stabilized with Ag), acetonitrile (MeCN, hypergrade LC/MS) and methanol (UHPLC/MS).
Synthesis of chemicals
General method for preparing of N-oxides of fentanyl and carfentanil;
m-chloroperoxybenzoic acid (m-CPBA) was purified prior to use by NaOH/buffer wash46. A solution of m-CPBA (1.05 equiv.) in 2 mL CHCl3 was added dropwise to a 0 °C stirred solution of fentanyl or carfentanil in 3 mL CHCl3. The reaction mixture was allowed to reach room temperature (rt) overnight. Analysis by thin layer chromatography (MeOH/CHCl3 1:9) showed full consumption of the starting material (Rf fentanyl: 0.64, fentanyl N-oxide 0.54, Rf carfentanil: 0.57, carfentanil N-oxide 0.51).
The reaction mixture was washed two times with 10 mL of 10% NaHCO3, followed by 10 mL of brine, then dried over NaSO4, filtered and concentrated. The resulting oil was purified by preparative HPLC on a Gilson PLC2050 system using a Macherey-Nagel VP NUCLEODUR C18 ec column (150 ×
× 21 mm, 5 μm, 110 Å) with a flow rate of 20 mL/min, detection at 215 nm and eluent system: A - aq 0.1% CF3CO2H, and B −
21 mm, 5 μm, 110 Å) with a flow rate of 20 mL/min, detection at 215 nm and eluent system: A - aq 0.1% CF3CO2H, and B − 0.1% CF3CO2H in MeCN.
0.1% CF3CO2H in MeCN.
Fentanyl N-oxide
100 mg of fentanyl (0.29 mmol) afforded 59 mg of fentanyl N-oxide in a yield of 56%.
1H NMR (500 MHz, CDCl3): δ =
= 1.02 (t, J
1.02 (t, J =
= 7.4 Hz, 3 H), 1.77–1.83 (m, 2 H), 1.95 (q, J
7.4 Hz, 3 H), 1.77–1.83 (m, 2 H), 1.95 (q, J =
= 7.4 Hz, 2 H), 2.22–2.33 (m, 2 H), 3.14–3.25 (m, 4 H), 3.60–3.67 (m, 2 H), 3.67–3.75 (m, 2 H), 4.78–4.87 (m, 1H), 7.08–7.13 (m, 2 H), 7.20–7.25 (m, 3 H), 7.28–7.33 (m, 2 H), 7.40–7.47 (m, 3 H); 13C NMR (125 MHz, CDCl3): δ
7.4 Hz, 2 H), 2.22–2.33 (m, 2 H), 3.14–3.25 (m, 4 H), 3.60–3.67 (m, 2 H), 3.67–3.75 (m, 2 H), 4.78–4.87 (m, 1H), 7.08–7.13 (m, 2 H), 7.20–7.25 (m, 3 H), 7.28–7.33 (m, 2 H), 7.40–7.47 (m, 3 H); 13C NMR (125 MHz, CDCl3): δ =
= 9.5, 25.0, 28.4, 28.9, 49.7, 63.8, 71.3, 127.1, 128.91, 128.94, 129.8, 130.0, 136.5, 137.9, 174.1; HRMS (ESI) m/z calcd. for [M
9.5, 25.0, 28.4, 28.9, 49.7, 63.8, 71.3, 127.1, 128.91, 128.94, 129.8, 130.0, 136.5, 137.9, 174.1; HRMS (ESI) m/z calcd. for [M +
+ H]+: 353.2224, found 353.2224.
H]+: 353.2224, found 353.2224.
Carfentanil N-oxide
77.5 mg of carfentanil (0.18 mmol) afforded 42 mg of carfentanil N-oxide in a yield of 52%.
1H NMR (125 MHz, CDCl3): δ =
= 0.98 (t, J
0.98 (t, J =
= 7.4 Hz, 3 H), 1.92 (q, J
7.4 Hz, 3 H), 1.92 (q, J =
= 7.4 Hz, 2 H), 2.25–2.32 (m, 2 H), 2.42–2.51 (m, 2 H), 3.17–3.23 (m, 2 H), 3.52–3.66 (m, 6 H), 3.81 (s, 3 H), 7.19–7.25 (m, 3 H), 7.28–7.34 (m, 4 H), 7.42–7.49 (m, 3 H); 13C NMR (125 MHz, CDCl3): δ
7.4 Hz, 2 H), 2.25–2.32 (m, 2 H), 2.42–2.51 (m, 2 H), 3.17–3.23 (m, 2 H), 3.52–3.66 (m, 6 H), 3.81 (s, 3 H), 7.19–7.25 (m, 3 H), 7.28–7.34 (m, 4 H), 7.42–7.49 (m, 3 H); 13C NMR (125 MHz, CDCl3): δ =
= 9.1, 27.8, 28.8, 28.9, 52.8, 60.2, 61.1, 71.0, 127.1, 128.92, 128.94, 129.4, 129.9, 130.1, 136.3, 138.4, 173.3, 174.5; HRMS (ESI) m/z calcd. for [M
9.1, 27.8, 28.8, 28.9, 52.8, 60.2, 61.1, 71.0, 127.1, 128.92, 128.94, 129.4, 129.9, 130.1, 136.3, 138.4, 173.3, 174.5; HRMS (ESI) m/z calcd. for [M +
+ H]+: 411.2278, found 411.2276.
H]+: 411.2278, found 411.2276.
Decontamination agents
Commercial products
Commercial decontamination agents were purchased from the following manufacturers: Dahlgren Decon (First Line Technology, Fredericksburg, VA, USA), RSDL (Reactive Skin Decontamination Lotion Kit, Emergent BioSolutions Inc., Gaithersburg, MD, USA), GDS2000 (Kärcher Futuretech GmbH, Schwaikheim, Germany) and alldecont MED (OWR GmbH, Elztal-Rittersbach, Germany). Bleach (Klorin, Colgate-Palmolive, Lynby, DK), Domestos Spray Bleach (cleaning spray 1, Unilever UK Limited, Kingston Upon Thames, UK) and Effekt Klor (cleaning spray 2, Europris, AS, Norway) were purchased from a local grocery store. FAST-ACT (First applied sorbent treatment against chemical threats) was generously provided by FAST-ACT (Bonita Springs, FL, USA). All commercial products were applied as recommended by the manufacturers.
Titanium dioxide-nanodiamond (TiO2-ND)
1 g of TiOSO4 was dissolved in 200 mL of distilled warm water (35 °C) acidified with 1 mL of H2SO4, and then 10 g of urea was dissolved in the solution. 0.0125 g of nanodiamond (ND) powder with primary particles of 5 nm (New Metals and Chemicals Corporation, Ltd., Tokyo, Japan) was dispersed in 1 L of distilled water by bath sonication (1 h, 300 W) and then slowly mixed with TiOSO4/urea solution. The obtained mixture was heated at 90 °C for 6 h under vigorous stirring. The obtained grayish precipitate was washed three times with water by decantation, filtered, air-dried and annealed at 300 °C for 2 h under ambient air. Characterization of TiO2-ND was performed by previously published methods revealing a crystallite size of about 10 nm, BET surface area of 232 m2/g and total pore volume of 0.28 cm3/g30. A representative SEM image of the TiO2-ND sample is shown in Supplementary Data Fig. S13.
Cerium dioxide (CeO2)
One liter of an aqueous solution of Ce(NO3)3·6H2O (0.2 mol/L) was precipitated with an excess of NH4HCO3 (0.5 mol/L) under stirring (to reach basic pH) to form a white precipitate of cerium carbonate. The suspension was further agitated for 1 h and left to age overnight. Afterwards, the precipitate was separated by filtration, washed with deionized water and dried in an oven at 105 °C. The resulting CeO2 powder was then calcined at 500 °C for 2 h. Characterization was performed by previously published methods revealing a crystallite size of about 28 nm, BET surface area of 85 m2/g and total pore volume of 0.07 cm3/g47. A representative SEM image of the CeO2 sample is shown in Supplementary Data Fig. S14.
Magnesium oxide (MgO)
200 mL of NaOH solution (0.5 mol/L) was added under constant stirring to 100 mL of 0.5 mol/L MgSO4 aqueous solution forming a white precipitate. The resulting suspension was heated at 80 °C for 6 h with constant stirring, then cooled to room temperature, washed by decantation (three times) and dried in an oven at 105 °C. The resulting MgO powder was calcined at 300 °C for 2 h. Characterization was performed by previously published methods, revealing a BET surface area of about 125 m2/g47. A representative SEM image of the MgO sample is shown in Supplementary Data Fig. S15.
LC/MS analysis
Degradation of fentanyl and carfentanil in H2O2, HCl, NaOH, CaClO2, alldecont MED, bleach, RSDL and GDS2000
LC/MS analysis was conducted using a Waters LC system equipped with a Waters 2998 PDA detector and Waters Acquity QDa detector. A 20 µL volume of sample was injected into a Waters XBridge C18 column (3.5 μm, 4.6 ×
× 75 mm) for sample separation. Solvent A (MQ
75 mm) for sample separation. Solvent A (MQ +
+ 0.1% formic acid) and solvent B (MeCN
0.1% formic acid) and solvent B (MeCN +
+ 0.1% formic acid) were used with the following gradient: A (%) 100; 100; 0; 0; 100; 100 at time (min) 0; 0.1; 7; 9; 9.5; 10. The eluent flow rate was set to 1.5 mL/min. Mass detection was performed in the mass range 100 to 700 Da, with electro spray ionization in both the positive (ES +) and negative (ES -) mode, a cone voltage of 20 V and scan frequency of 2 Hz.
0.1% formic acid) were used with the following gradient: A (%) 100; 100; 0; 0; 100; 100 at time (min) 0; 0.1; 7; 9; 9.5; 10. The eluent flow rate was set to 1.5 mL/min. Mass detection was performed in the mass range 100 to 700 Da, with electro spray ionization in both the positive (ES +) and negative (ES -) mode, a cone voltage of 20 V and scan frequency of 2 Hz.
Degradation of fentanyl and carfentanil in Dahlgren Decon, FAST-ACT, MgO, TiO2-ND and CeO2Degradation of fentanyl and carfentanil upon surface decontamination
LC/MS analysis was conducted using a Waters Acquity UPLC classic system equipped with a Waters Micromass Quatromicro detector. A 2 µL volume of sample was injected into a waters Acquity UPLC BEH C18 1.7 μm, 2.1 ×
× 50 mm column for sample separation. Solvent A (MQ: MeCN [90:10]
50 mm column for sample separation. Solvent A (MQ: MeCN [90:10] +
+ 0.1% formic acid) and solvent B (MeCN
0.1% formic acid) and solvent B (MeCN +
+ 0.1% formic acid) were used with the following gradient: A (%) 100; 100; 0; 0; 100; 100 at time (min) 0; 0.1; 4; 5; 5.1; 6. The eluent flow rate was set to 0.5 mL/min. Mass detection was performed over the mass range m/z of 220–600, with electro spray ionization in the positive mode (ESI +), a cone voltage of 17 V and scan time of 1 s. The detection limits for fentanyl and carfentanil was 0.4 ng and 0.8 ng respectively (signal-to-noise of three). These detection limits correspond to 0.4% of the initial fentanyl concentration and 0.8% carfentanil concentration in the time dependent degradation studies when 2 µL diluted degradation mixture was injected and analysed.
0.1% formic acid) were used with the following gradient: A (%) 100; 100; 0; 0; 100; 100 at time (min) 0; 0.1; 4; 5; 5.1; 6. The eluent flow rate was set to 0.5 mL/min. Mass detection was performed over the mass range m/z of 220–600, with electro spray ionization in the positive mode (ESI +), a cone voltage of 17 V and scan time of 1 s. The detection limits for fentanyl and carfentanil was 0.4 ng and 0.8 ng respectively (signal-to-noise of three). These detection limits correspond to 0.4% of the initial fentanyl concentration and 0.8% carfentanil concentration in the time dependent degradation studies when 2 µL diluted degradation mixture was injected and analysed.
Time-dependent degradation study and microsome analysis
LC/MS analysis was conducted using a ThermoScientific Dionex Ultimate 3000 RS UHPLC instrument coupled to a Bruker Daltonik Impact HD mass spectrometer to quantify the amounts of fentanyl and carfentanil remaining and degradation products and metabolites formed. A sample volume of between 2 and 10 µL was injected into a Waters Acquity UPLC 2.1 ×
× 100 mm, 1.8 μm, HSS T3 column. The column temperature was set to 40 °C, and the flow rate was 500 µl/min. Solvent A (MQ
100 mm, 1.8 μm, HSS T3 column. The column temperature was set to 40 °C, and the flow rate was 500 µl/min. Solvent A (MQ +
+ 0.1% formic acid) and solvent B: (MeCN
0.1% formic acid) and solvent B: (MeCN +
+ 0.1% formic acid) were used with the following gradient: A (%) 80; 80; 2; 2; 80; 80 at time (min) 0; 1; 2.9; 3.4; 3.5; 4. The mass spectrometer was set to monitor m/z of 50–800, with the electro spray operated in the positive mode (ES +) at a frequency of 5 Hz. A second full scan function, so called bbCID (broad band Collision Induced Detection) with a collision cell set to 25 eV energy for fragmentation of ions was included in all analyses. Calibration of the mass scale for each analysis file was accomplished by considering sodium formate clusters at the start of each analysis. Data analysis was performed with Bruker Daltonics Compass QuantAnalysis 2.2. The detection limits for both fentanyl and carfentanil was 0.03 ng (signal-to-noise of three). This detection limit corresponds to 0.3% of the initial opioid concentration in the time dependent degradation studies when 2 µL diluted degradation mixture was injected and analysed.
0.1% formic acid) were used with the following gradient: A (%) 80; 80; 2; 2; 80; 80 at time (min) 0; 1; 2.9; 3.4; 3.5; 4. The mass spectrometer was set to monitor m/z of 50–800, with the electro spray operated in the positive mode (ES +) at a frequency of 5 Hz. A second full scan function, so called bbCID (broad band Collision Induced Detection) with a collision cell set to 25 eV energy for fragmentation of ions was included in all analyses. Calibration of the mass scale for each analysis file was accomplished by considering sodium formate clusters at the start of each analysis. Data analysis was performed with Bruker Daltonics Compass QuantAnalysis 2.2. The detection limits for both fentanyl and carfentanil was 0.03 ng (signal-to-noise of three). This detection limit corresponds to 0.3% of the initial opioid concentration in the time dependent degradation studies when 2 µL diluted degradation mixture was injected and analysed.
DART-TD-HRMS analysis
A direct analysis in real time-simplified voltage and pressure (DART-SVP) ion source (IonSense Inc., Saugus, MA, USA) was used in combination with a Vapur®-interface (SI-410-GIST, IonSense Inc., Saugus, MA, USA), thermal desorption (TD) unit (Bruker-IonSense Inc., Saugus, MA, USA) and Bruker Daltonics Maxis Impact time-of-flight high-resolution mass spectrometer. Nitrogen was used as the ionization gas. Samples were introduced to the TD unit using DSA Detection sample traps (part no: ST1318P). The DART source temperature was 500 ºC and the temperature in the TD unit was set to 300 ºC. The DART ion source was run in the positive mode, with a grid voltage of 350 V. The mass spectrometer was run in the positive mode using full scan and broadband collision-induced detection (bbCID, collision voltage of 25 eV) over the mass range m/z of 50–650 at 5 Hz. The detection limit of carfentanil and fentanyl on DART-HRMS without the TD-unit was 50 ng.
NMR spectroscopy
The purity of the synthesized compounds and adsorption of fentanyl and carfentanil on the adsorbents FAST-ACT, MgO, TiO2-ND and CeO2 were analyzed using a Bruker AvanceIII Ultrashield 500 MHz NMR spectrometer equipped with a Cryoprobe 5 mm BBFO head.
Degradation of fentanyl and carfentanil in aqueous decontamination solutions
Stock standard aqueous solutions of fentanyl•HCl and carfentanil•HCl were prepared with concentrations of 10 mg/mL. Aqueous solutions of dispersed decontamination agents were prepared in MQ water prior to use with the following concentrations: H2O2 (10%), HCl (6 M), Ca(ClO)2 (0.2 M), NaOH (5%). Commercial bleach was diluted to a concentration of 0.18 M sodium hypochlorite. RSDL, alldecont MED, GDS2000 and Dahlgren Decon were used as received and applied according to the manufacturers’ instructions. Degradation studies were performed by adding 50 µL of stock solution to 450 µL of decontamination solution in a 2 mL glass vial to achieve a concentration of 1 mg/mL of fentanyl•HCl and carfentanil•HCl. The degradation reactions were initially mixed by a vortex mixer and then left without stirring at room temperature. For degradation studies of the solid decontaminants FAST-ACT, MgO, CeO2 and TiO2-ND, stock standard solutions of fentanyl•HCl and carfentanil•HCl were prepared in CDCl3 to give a 10 mM concentration. To a 2 mL glass vial with 50 mg of solid decontaminant in 250 µL CDCl3, 250 µL of the stock solution was added to achieve a 1:50 ratio of fentanyl/carfentanil to the solid decontaminant. All degradation reactions were followed by LC/MS at several time-points. For LC/MS analysis, 10 µL of the degradation mixture was extracted from the degradation reaction and diluted in 190 µl of MQ. LC/MS-analysis was performed immediately upon dilution without any delays or storage of the extracted samples. No detected fentanyl in these experiments corresponded to less than 0.4% of the original amount and due to the higher detection limit of carfentanil, less than 0.8% of the original amount was below the detection limit. The degradation efficacy was classed as high, medium or low depending on the amount of intact fentanyl and carfentanil remaining in the decontamination solution at specific time points: high =
= no intact fentanyl and carfentanil remaining after 10 min, medium
no intact fentanyl and carfentanil remaining after 10 min, medium =
= less than 50% of intact fentanyl and carfentanil remaining after 1 h and/or full degradation observed within 24 h and low
less than 50% of intact fentanyl and carfentanil remaining after 1 h and/or full degradation observed within 24 h and low =
= more than 50% of fentanyl and carfentanil remaining after 1 h and/or detectable amounts still remains after 24 h. If no degradation was evident within 24 h, the decontamination solution was assessed to have a negligible degradation efficacy. Degradation of fentanyl and carfentanil by the solid decontamination agents was also monitored by 1H NMR spectroscopy. In the case of FAST-ACT and TiO2-ND, both the NMR and LC/MS signals of fentanyl and carfentanil disappeared within 10 min without any new signals appearing. This indicated that no fentanyl or carfentanil remained in the solution. However, the lack of signal from any degradation products indicated that the disappearance of fentanyl and carfentanil was due to absorption onto the solid decontamination agent rather than degradation. All degradation experiments were performed in duplicate.
more than 50% of fentanyl and carfentanil remaining after 1 h and/or detectable amounts still remains after 24 h. If no degradation was evident within 24 h, the decontamination solution was assessed to have a negligible degradation efficacy. Degradation of fentanyl and carfentanil by the solid decontamination agents was also monitored by 1H NMR spectroscopy. In the case of FAST-ACT and TiO2-ND, both the NMR and LC/MS signals of fentanyl and carfentanil disappeared within 10 min without any new signals appearing. This indicated that no fentanyl or carfentanil remained in the solution. However, the lack of signal from any degradation products indicated that the disappearance of fentanyl and carfentanil was due to absorption onto the solid decontamination agent rather than degradation. All degradation experiments were performed in duplicate.
Time-dependent degradation studies
The most effective aqueous decontamination solutions were further examined in a time-dependent degradation study, from which the half-lives of fentanyl and carfentanil were estimated. Stock standard solutions of the free base of fentanyl and carfentanil were prepared with concentrations of 20 mg/mL in ethanol. Decontamination solutions of H2O2 (10%), Dahlgren Decon, alldecont MED and bleach were prepared as described above for the relative degradation studies. 25 µL of stock solution was added to a 2 mL vial with 475 µL of decontamination solution. The reaction mixtures were initially mixed by a vortex mixer and then left without stirring at room-temperature. Degradation was continuously measured by LC/HRMS. For the analysis, 5 µL of the degradation mixture was extracted from the degradation reaction and diluted to 1000 µL in MQ. LC/MS-analysis was started within 5 min after dilution without any delays or storage of the extracted samples. Time-dependent degradation curves in Fig. 1 were constructed in GraphPad Prism 6.07 for windows (www.graphpad.com). All time-dependent degradation experiments were performed in triplicate. Stability tests of fentanyl, carfentanil and fentanyl N-oxide in water and acetonitrile, showed that all compounds are stable in both solutions, see Supplementary Fig. S16 and S17.
Degradation of fentanyl and carfentanil upon surface decontamination
Degradation of fentanyl and carfentanil on a typical surface was investigated using a wooden floor surface divided into 10 ×
× 10 cm sections. Stock standard solutions of fentanyl•HCl and carfentanil•HCl dissolved in methanol were prepared with a concentration of 10 mg/mL. 2 mg of fentanyl or carfentanil was applied onto the surfaces by adding 50 µL aliquots of the substance solution repeatedly, with drying between each addition. The purpose of employing multiple addition rounds was to mitigate the potential risk of fentanyl or carfentanil solution spreading beyond the intended area.
10 cm sections. Stock standard solutions of fentanyl•HCl and carfentanil•HCl dissolved in methanol were prepared with a concentration of 10 mg/mL. 2 mg of fentanyl or carfentanil was applied onto the surfaces by adding 50 µL aliquots of the substance solution repeatedly, with drying between each addition. The purpose of employing multiple addition rounds was to mitigate the potential risk of fentanyl or carfentanil solution spreading beyond the intended area.
Four decontamination solutions (Dahlgren Decon, MQ, Domestos Spray Bleach (cleaning spray 1) and Effekt Klor (cleaning spray 2)) were applied to the surface by repeated addition (4 ×
× 150 µl), covering the whole sample, and left for 30 min prior to sampling. A positive control was conducted using active substance and MQ instead of the cleaning agent, alongside a negative control that exclusively utilized the cleaning agent.
150 µl), covering the whole sample, and left for 30 min prior to sampling. A positive control was conducted using active substance and MQ instead of the cleaning agent, alongside a negative control that exclusively utilized the cleaning agent.
The surfaces applied with Dahlgren Decon were still wet after 30 min, whereas those applied with cleaning spray were dried out. Hence, different methods of sampling were conducted to enable uptake from all surfaces. Surfaces decontaminated with Dahlgren Decon were swabbed using two dry cotton wipes, whereas surfaces decontaminated with the cleaning sprays were swabbed using two moist wipes applied with 300 µL of MQ. The positive and negative controls were swabbed with cotton wipes moistened with 600 µL of MQ. The wipes were place in vials containing 2 mL MeCN and sonicated for 10 min, then the extracts were transferred to new vials. The samples were diluted to a concentration of 5 µg/mL for the cleaning sprays, 4.35 µg/mL for Dahlgren Decon, 3.85 µg/mL for the negative control, and 1.93 µg/mL for the positive controls. The dilution factor was compensated for in the calculations. LC/MS-analysis of the samples were performed immediately after extraction and dilution without any delays or storage of the samples. Data analysis was performed by manual integration of the peak area using MassLynx (v4.2 SCN 1007) for carfentanil EIC 395, carfentanil N-oxide EIC 411, fentanyl EIC 337 and fentanyl N-oxide EIC 353. To investigate potential N-oxide agglomeration, the procedure was repeated using a fentanyl N-oxide standard (2 mg/surface) and the two cleaning sprays, each in triplicate, together with four positive control experiments.
To ensure comprehensive coverage of the entire sample area the surfaces were swabbed according to the standard procedures of the Swedish Chemical, Biological, Radiological, and Nuclear (CBRN) defence teams using an ‘S’ pattern in three directions: first horizontally, then vertically, and finally diagonally. Two chemists performed the swabbing process, with their assignments randomized based on the decontamination solution used, to minimize bias in the results. Before the experiments, various types of swabs and swabbing techniques were optimized to ensure accuracy and efficiency.
Decontamination efficiency evaluated by DART analysis
Contaminated surfaces were prepared by application of 100 µL of the 10 mg/mL stock solution of fentanyl or carfentanil in methanol. The solution was left to dry, leaving 1 mg of active substance on the surface, before application of 1.5 mL of cleaning agent (MQ, Dahlgren Decon or cleaning spray 2). Next, each decontamination solution was placed on three test squares. After 30 min, the surface was wiped with water-soaked cotton swabs until visually clean. The surface was sampled by wiping a sample trap over the surface, which was analyzed directly by DART-TD-HRMS. The next day, a second sampling was performed, following the same procedure.
Effects of fentanyl N-oxide and carfentanil N-oxide on the µ-opioid receptor
Effects of fentanyl N-oxide and carfentanil N-oxide on activation of the human µ-opioid receptor were measured using an intracellular calcium flux assay (FLIPR Ca 6 assay kit, Molecular Devices). CHO-K1 cells expressing the human µ-opioid receptor (hMOR-CHO-K1-Gαqi5, Multispan, Inc.) were cultured in DMEM/F12 medium, supplemented with 10% (v/v) fetal bovine serum, 250 µg/ml hygromycin and 10 µg/ml puromycin, and maintained at 37 °C in a humidified environment of 95% air, 5% CO2. Cells were plated at 45000 cells per well into a 96-well clear bottom black CellBIND microplate (Corning® 3340) and incubated overnight to achieve 90–100% confluence at the time point for the assay. On the day of assay, the growth medium was decanted and the wells were washed twice with 100 µl of assay buffer (Hank´s Balanced Salt Solution) with 20 mM HEPES, pH 7.4) to remove any excess growth medium. Cells were incubated with 100 µl assay buffer and 100 µl Calcium 6 assay dye solution containing 2.5 mM probenecid for 2 h at 37 °C. Calcium flux was monitored upon addition of the compounds in a FlexStation®3 microplate reader. Fluorescence (λex =
= 485 nm, λem
485 nm, λem =
= 525 nm) was initially measured for 17 s to establish a baseline. Afterwards, varying concentrations of the test compound in assay buffer were added and changes in fluorescence units were recorded for another 58 s. The background fluorescence of cells without dye or dye without cells was negligible. The fluorescence response during a total of 75 s of continuous measurement at 30 °C was analyzed by calculating the area under the curve (AUC) and plotting it against the compound concentration. The concentration-response curves were fitted in GraphPad Prism version 10.1.2 for windows (www.graphpad.com) using nonlinear regression with a four-parameter logistic equation to determine the EC50 values. Fentanyl, carfentanil and morphine were used as references. All data are expressed as mean values of triplicate measurements with the 95% confidence interval given in parentheses from two separate experiments.
525 nm) was initially measured for 17 s to establish a baseline. Afterwards, varying concentrations of the test compound in assay buffer were added and changes in fluorescence units were recorded for another 58 s. The background fluorescence of cells without dye or dye without cells was negligible. The fluorescence response during a total of 75 s of continuous measurement at 30 °C was analyzed by calculating the area under the curve (AUC) and plotting it against the compound concentration. The concentration-response curves were fitted in GraphPad Prism version 10.1.2 for windows (www.graphpad.com) using nonlinear regression with a four-parameter logistic equation to determine the EC50 values. Fentanyl, carfentanil and morphine were used as references. All data are expressed as mean values of triplicate measurements with the 95% confidence interval given in parentheses from two separate experiments.
Metabolic stability of fentanyl N-oxide and carfentanil N-oxide
Human liver microsomes (HLMs) (pooled from 50 mixed gender donors; XenoTech, ) were used for measurements of phase I metabolism of fentanyl N-oxide and carfentanil N-oxide. For the HLM incubations, compounds were incubated in 0.25 mL of HLM suspension for 1 h at 37°C under constant shaking. HLM suspensions containing 1 mg/ml HLM, 10 mM potassium phosphate buffer (pH 7.4) and 3 mM magnesium chloride were prepared in triplicate for each compound. After 5 min preincubation of the compounds (1 µM) at 37°C, 2 mM β-nicotinamide adenine dinucleotide 2’-phosphate reduced tetrasodium salt hydrate (NADPH) was added to initiate the reaction. Samples were collected at 0, 1, 2.5, 5, 10, 15, 30 and 60 min and immediately quenched in ice-cold acetonitrile. Fentanyl and carfentanil were included for reference. Negative control reactions were performed without NADPH. Samples were stored at -80 °C prior to analysis. Data were measured in triplicate in two separate experiments and analyzed by determining the HLM half-life from a plot of the natural logarithm of the percentage of compound remaining (remaining compound peak area/compound peak area at t =
= 0) against incubation time48,49. The half-life is expressed as mean values of triplicate measurements with the 95% confidence interval given in parentheses.
0) against incubation time48,49. The half-life is expressed as mean values of triplicate measurements with the 95% confidence interval given in parentheses.
Safety notes
As fentanyl and carfentanil are highly toxic substances, synthesis and sample preparation were performed in a fume hood. Analyses were performed in dedicated laboratories for this purpose with no personnel present in the same room as the instrument during the analyses due to a separate control room.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This work was financed by the Swedish Ministry of Defence. LÖ and JH would like to thank the EU and the Swedish Research Agency Formas for funding, in the frame of the collaborative international consortium GreenWaterTech financed under the ERA-NET AquaticPollutants Joint Transnational Call (GA no. 869178). ERA-NET is an integral part of the activities developed by the Water, Oceans and AMR Joint Programming Initiatives.We thank Štepán Stehlík, Jakub Ederer and Martin Šťastný for their help with the synthesis of the samples TiO2-ND, CeO2 and MgO, respectively, and Sandra Lindberg Lindberg for the synthesis of fentanyl•HCl.
Author contributions
All authors have contributed to planning, performing and/or reviewing significant parts of the studies included in the manuscript.
Data availability
All relevant data is provided within the manuscript or supplementary information files. Additional data will be provided by corresponding author on reasonable request.
Declarations
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Pernilla Lindén and Lina Mörén contributed equally to this work.
References
 +
+ exposure. J. Transp. Secur.12, 73–82 (2019). [Google Scholar]
exposure. J. Transp. Secur.12, 73–82 (2019). [Google Scholar]Articles from Scientific Reports are provided here courtesy of Nature Publishing Group
Full text links
Read article at publisher's site: https://doi.org/10.1038/s41598-024-74594-z
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Degradation of pesticides with RSDL® (reactive skin decontamination lotion kit) lotion: LC-MS investigation.
Toxicol Lett, 293:241-248, 08 Nov 2017
Cited by: 4 articles | PMID: 29128639
Carfentanil: a narrative review of its pharmacology and public health concerns.
Can J Anaesth, 66(4):414-421, 21 Jan 2019
Cited by: 21 articles | PMID: 30666589
Review
Detection of fentanyl and fentanyl analogues in biological samples using liquid chromatography-high resolution mass spectrometry.
Forensic Sci Int, 300:13-18, 17 Apr 2019
Cited by: 9 articles | PMID: 31063883
Decontamination of Toxic Industrial Chemicals and Fentanyl by Application of the RSDL® Kit.
J Spec Oper Med, 20(1):55-59, 01 Jan 2020
Cited by: 1 article | PMID: 32203607
Funding
Funders who supported this work.
Swedish Ministry of Defence
The collaborative international consortium GreenWaterTech financed under the ERA-NET AquaticPollutants Joint Transnational Call (1)
Grant ID: 869178

 1
1