Abstract
Objectives
Cold snare polypectomy (CSP) is a common, simple, and safe procedure; however, it has a high rate of unclear margins. We analyzed the risk factors for unclear margins of colorectal polyp.Methods
We retrospectively investigated colorectal polyps treated with CSP between July 2021 and July 2022, excluding those that could not be retrieved or pathologically nonneoplastic and hyperplastic polyps without margin evaluation. The clinicopathological features and risk factors for unclear margins were analyzed. Furthermore, the polyps were divided into two groups: those resected by experts and those resected by trainees. A 1 : 1 propensity score matching was performed. After matching, the risk factors for unclear margins in each group were analyzed as secondary outcomes.Results
We analyzed 237 patients with 572 polyps; the margins were negative in 58.6% (negative group) and unclear in 41.4% (unclear group). The unclear margin was significantly higher at straddling folds ( P = 0.0001), flexure points ( P = 0.005), and in the procedures performed by trainees ( P < 0.0001). Altogether, 198 propensity score matched pairs were explored for secondary outcomes. There were no significant differences in risk factors for unclear margins in the expert group, while in the trainee group, the unclear margin was significantly higher at the straddling folds ( P = 0.0004) and flexure points ( P = 0.005).Conclusions
We demonstrated that straddling folds, flexure points, and procedures performed by the trainees were significant risk factors for unclear margins, and we hypothesized that the rate of unclear margins will reduce as the trainees accumulate experience at difficult sites.Free full text

Risk factors for unclear margin in cold snare polypectomy for colorectal polyp
Abstract
Objectives
Cold snare polypectomy (CSP) is a common, simple, and safe procedure; however, it has a high rate of unclear margins. We analyzed the risk factors for unclear margins of colorectal polyp.
Methods
We retrospectively investigated colorectal polyps treated with CSP between July 2021 and July 2022, excluding those that could not be retrieved or pathologically nonneoplastic and hyperplastic polyps without margin evaluation. The clinicopathological features and risk factors for unclear margins were analyzed. Furthermore, the polyps were divided into two groups: those resected by experts and those resected by trainees. A 1 : 1 propensity score matching was performed. After matching, the risk factors for unclear margins in each group were analyzed as secondary outcomes.
Results
We analyzed 237 patients with 572 polyps; the margins were negative in 58.6% (negative group) and unclear in 41.4% (unclear group). The unclear margin was significantly higher at straddling folds (P = 0.0001), flexure points (P = 0.005), and in the procedures performed by trainees (P < 0.0001). Altogether, 198 propensity score matched pairs were explored for secondary outcomes. There were no significant differences in risk factors for unclear margins in the expert group, while in the trainee group, the unclear margin was significantly higher at the straddling folds (P = 0.0004) and flexure points (P = 0.005).
Conclusions
We demonstrated that straddling folds, flexure points, and procedures performed by the trainees were significant risk factors for unclear margins, and we hypothesized that the rate of unclear margins will reduce as the trainees accumulate experience at difficult sites.
Introduction
Colorectal cancer is one of the most common neoplasms worldwide. Its incidence is increasing; however, the mortality remains flat globally and is decreasing in Japan [1]. This is probably due to an increase in colorectal cancers detected by screening colonoscopy and endoscopic resection as early treatment. Cold snare polypectomy (CSP) has been widely used as a feasible treatment for a clean colon [2,3] because of its low risk of complications, such as delayed bleeding and perforation [4–9]. In addition, CSP can be safely performed even with antithrombotic therapy [10–13]. CSP is an essential treatment as antithrombotic medication has become more common in aging populations with lifestyle-related cardiovascular and cerebrovascular diseases. However, CSP that does not require a high-frequency power supply unit is not expected to have a burn effect and has problems such as the risk of residual tumor [14,15] and a high rate of unclear margins [16–21]. One report suggested that unclear margins of CSP are associated with residual tumor [16]. Furthermore, there are cases in which mucosal cancer is accidentally detected in polyps resected by CSP [16,17]. Hence, reducing unclear margins is important for the curability of polyps, and we investigated the risk factors for unclear margins of colorectal polyp.
Method
Study design and participants
This retrospective study was conducted at a single center. This study was approved by the Clinical Ethics Committee of Yokohama City University Graduate School of Medicine in accordance with the Declaration of Helsinki. We enrolled patients who performed CSP at our institution between July 2021 and July 2022. Inclusion criteria were patients >20 years of age with colorectal polyps of up to 10 mm endoscopically diagnosed as adenomas or sessile serrated lesions (SSL). The exclusion criteria were as follows: (i) polyps that could not be retrieved endoscopically, (ii) polyps that were pathologically nonneoplastic and hyperplastic polyps without margin evaluation, (iii) patients with infectious diseases that require systemic treatment, (iv) pregnant or potentially pregnant patients, (v) patients with anemia requiring blood transfusion (hemoglobin <7.0 g/dl) or bleeding tendency (Platelet <50 000), and (vi) other patients deemed unfit for this study.
Procedures
For bowel preparation, 1500–2000 ml of polyethylene glycol electrolyte was used to clean the bowel. Some patients consumed a low-residue diet and 24 mg of sennoside the previous day. Most examinations were performed under conscious sedation with midazolam, and patients who complained of pain were administered pentazocine. All patients underwent examinations using endoscopes that provide narrow-band imaging (NBI) with magnifying endoscopy (PCF-H290ZI, PCF-Q260AZI, channel size 3.2 mm; CF-HQ290I, CF-XZ1200I, CF-EZ1500DI, channel size 3.7 mm; Olympus Medical Systems Corporation, Tokyo, Japan) with a light source (EVIS X1 CV-1500, EVIS LUCERA ELITE CLV-290SL; Olympus) and carbon dioxide insufflation. The polyps were resected using a SnareMaster Plus 10 mm (Olympus) and Captivator II 10 mm (Boston Scientific, Natick, Massachusetts, USA). Cecal intubation was verified by identifying the appendiceal orifice and ileocecal valve, and the colorectum was sequentially observed. Polyps were diagnosed using white-light imaging, chromoendoscopy, and NBI with magnification. Regarding the magnifying endoscopy findings based on NBI, if the polyp had regular neoplastic vessels, a surface pattern (Japan NBI Expert Team classification type 2A), and enlarged crypt openings, or thick and branched vessels, it was diagnosed as an adenoma and SSL, respectively [22]. CSP was performed using conventional procedures. After positioning the polyp in the center of the snare loop, it was resected with the surrounding mucosa. We injected water into the resection site using a water jet to stop bleeding and checked for remnants. If immediate bleeding continued after resection, hemostasis was performed using clips. The removed polyps were retrieved through the working channel of the endoscope and were trapped in separate bottles attached to the suction tube. The retrieved polyps were examined histopathologically after fixation in 10% formalin. Histologically, the polyps were divided into positive, negative, and unclear margins. Positive margins were neoplastic lesions after definitive resection. Negative margins were neoplastic-free lesions after definitive resection. Otherwise, margins were unclear.
Study outcomes
The primary outcome was identifying the risk factors for unclear margins of colorectal polyps. To clarify the risk factors, we investigated the age, sex, location (right colon/left colon to the splenic flexure and rectum), polyp size, morphology (protruded type including 0-Is, 0-Isp; flat elevated type, including 0-IIa), pathological findings, endoscopist (expert/trainee), endoscope channel size, presence or absence of a straddling fold, and flexure point. Regarding secondary outcomes, we divided the polyps into an expert group, which included expert endoscopists, and a trainee group, which included trainee endoscopists. We adjusted for patient characteristics in both groups using propensity score matching and analyzed the risk factors for unclear margins in each group as a sub-analysis. The size was measured using a short-axis diameter (10 mm), with the snare device fully open. A straddling fold was defined as a polyp located on a semilunar fold. The flexure points include the hepatic flexure, middle transverse colon, splenic flexure, and sigmoid-descending junction with poor scope maneuverability. These endoscopic findings were analyzed retrospectively using medical records of the endoscopic images and schema. Experts were defined as ≥500 colonic polypectomies and ≥1000 colonoscopies, while two experts and five trainees performed endoscopy in this study.
Statistical analysis
We analyzed the clinicopathological features in both groups using the χ2 and Fisher exact tests for categorical data, and continuous variables were expressed as medians and interquartile ranges using the Wilcoxon rank-sum test. For the secondary outcomes, propensity score matching was used to compare the expert group with the trainee group. To balance bias, propensity scores were calculated using logistic regression with seven variables: three variables exhibiting a significant difference in group comparison (size, straddling fold, and flexure point) and four clinically important variables for unclear margins (age, sex, location, and morphology). P < 0.05 was considered to be statistically significant. All statistical analyses were performed using JMP Pro ver. 16.0 (SAS Institute, Inc., Cary, North Carolina, USA).
Result
Study flow
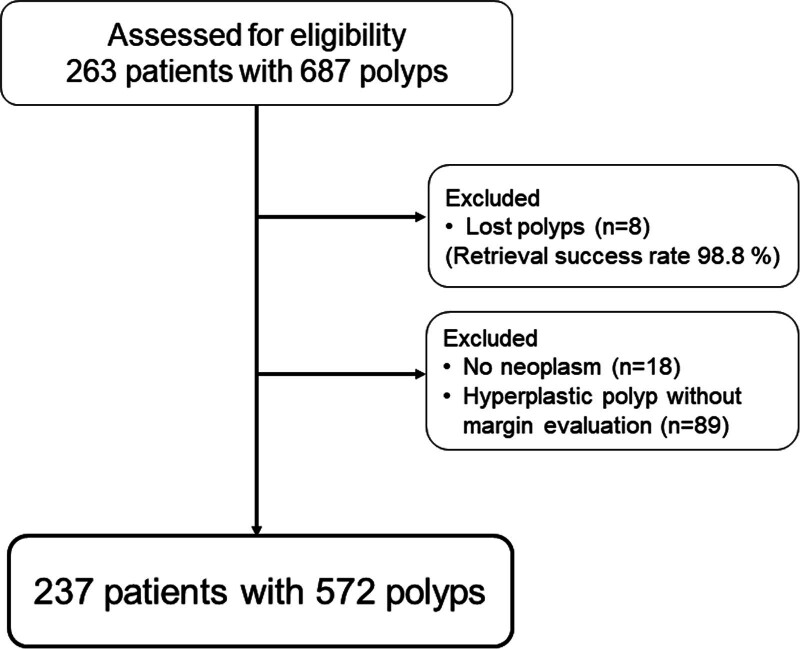
We performed CSP in 263 patients with 687 polyps at Yokohama City University Graduate School of Medicine between July 2021 and July 2022. Figure Figure11 illustrates the recruitment process of patients with polyps. Of the 687 polyps, we excluded those that could not be retrieved (n = 8), those that were pathologically nonneoplastic (n = 18), and those with hyperplastic polyps without margin evaluation (n = 89). Hence, 237 patients with 572 polyps were enrolled in this study, and the retrieval success rate for all lesions was 98.8%.
Clinicopathological features
Table Table11 shows the clinicopathological features of the patients with polyps. The participants included 157 (66.2%) men and 80 (33.8%) women, with a median age of 71.0 years old [interquartile range (IQR): 63.0–77.0]. Two polyps were resected per patient (IQR: 1.5–3.0). Regarding polyp location, 70.8% (405/572), 26.6% (152/572), and 2.6% (15/572) of the polyps were in the right colon, the left colon and rectum, respectively. The median polyp size was 3.0 mm (IQR: 3.0–5.0), and 85.3% of polyps were ≤5 mm. Regarding polyp morphology, 66.3% (379/572) of the polyps were protruding, while 33.7% (193/572) were flat elevated. Histologically, 98.4% (563/572) and 1.6% (9/572) of the polyps were adenomas and SSL, respectively, and no cases of carcinoma were observed in this study. The experts resected 64.3% (368/572) of the polyps, and the trainees resected 35.7% (204/572). Regarding the endoscope, 79.5% (455/572) of the polyps were resected using a 3.7 mm channel scope, and 20.5% (117/572) of the polyps were resected using a 3.2 mm channel scope. Regarding the characteristics of polyps, 26.2% (150/572) of the polyps were at straddling folds, and 14.5% (83/572) of the polyps were at flexure points. Negative and unclear margins occurred in 58.6% (335/572) and 41.4% (237/572) of polyps, respectively, and no cases of positive margins were observed in this study. No cases of delayed bleeding or perforation were observed.
Table 1.
Clinicopathological features of CSP
| Polyps (n = 572) | |
|---|---|
| Patients number | 237 |
| Median age (IQR), years | 71.0 (63.0–77.0) |
| Sex | |
 Male, % (n) Male, % (n) | 66.2 (157) |
 Female, % (n) Female, % (n) | 33.8 (80) |
| No. of polyps per patients (IQR) | 2.0 (1.5–3.0) |
| Location | |
 Right colon, % (n) Right colon, % (n) | 70.8 (405) |
 Left colon, % (n) Left colon, % (n) | 26.6 (152) |
 Rectal, % (n) Rectal, % (n) | 2.6 (15) |
| Median polyp size (IQR), mm | 3.0 (3.0–5.0) |
| Size | |
 1–5 mm, % (n) 1–5 mm, % (n) | 85.3 (488) |
 6–10 mm, % (n) 6–10 mm, % (n) | 14.7 (84) |
| Morphology | |
 Protruded, % (n) Protruded, % (n) | 66.3 (379) |
 Flat elevated, % (n) Flat elevated, % (n) | 33.7 (193) |
| Pathological finding | |
 Adenoma, % (n) Adenoma, % (n) | 98.4 (563) |
 Sessile serrated lesion, % (n) Sessile serrated lesion, % (n) | 1.6 (9) |
| Endoscopist | |
 Expert (n = 2), % (n) Expert (n = 2), % (n) | 64.3 (368) |
 Trainee (n = 5), % (n) Trainee (n = 5), % (n) | 35.7 (204) |
| Channel size | |
 3.7 mm, % (n) 3.7 mm, % (n) | 79.5 (455) |
 3.2 mm, % (n) 3.2 mm, % (n) | 20.5 (117) |
| Straddling folds | |
 (+), % (n) (+), % (n) | 26.2 (150) |
 (−), % (n) (−), % (n) | 73.8 (422) |
| Flexure points | |
 (+), % (n) (+), % (n) | 14.5 (83) |
 (−), % (n) (−), % (n) | 85.5 (489) |
| Margin | |
 Negative, % (n) Negative, % (n) | 58.6 (335) |
 Unclear, % (n) Unclear, % (n) | 41.4 (237) |
 Positive, % (n) Positive, % (n) | 0.0 (0) |
| Adverse event | |
 Delayed bleeding, % (n) Delayed bleeding, % (n) | 0.0 (0) |
 Perforation, % (n) Perforation, % (n) | 0.0 (0) |
CSP, cold snare polypectomy; IQR, interquartile range.
Primary outcome
Table Table22 shows the clinicopathological features of the negative and unclear margins groups. No significant differences were observed in age, sex, location, size, morphology, pathological findings, or channel size between the negative and unclear groups. The unclear margin was significantly higher at the straddling folds (P = 0.0001) and flexure points (P = 0.005), respectively. Additionally, the polyps resected by the trainee had significantly higher unclear margins than those resected by the experts (P < 0.0001).
Table 2.
Analysis for risk factors of unclear margin
| Negative (n = 335) | Unclear (n = 237) | P value | |
|---|---|---|---|
| Median age (IQR), years | 70.0 (63.0–78.0) | 71.0 (64.5–76.0) | 0.497 |
| Sex | 0.544 | ||
 Male, % (n) Male, % (n) | 68.7 (230) | 66.2 (157) | |
 Female, % (n) Female, % (n) | 31.3 (105) | 33.8 (80) | |
| Location | 0.394 | ||
 Right colon, % (n) Right colon, % (n) | 69.3 (232) | 73.0 (173) | |
 Left colon, % (n) Left colon, % (n) | 27.5 (92) | 25.3 (60) | |
 Rectal, % (n) Rectal, % (n) | 3.3 (11) | 1.7 (4) | |
| Median polyp size (IQR), mm | 3.0 (3.0–5.0) | 4.0 (3.0–5.0) | 0.206 |
| Size | 0.103 | ||
 1–5 mm, % (n) 1–5 mm, % (n) | 83.3 (279) | 88.2 (209) | |
 6–10 mm, % (n) 6–10 mm, % (n) | 16.7 (56) | 11.8 (28) | |
| Morphology | 0.476 | ||
 Protruded, % (n) Protruded, % (n) | 65.1 (218) | 67.9 (161) | |
 Flat elevated, % (n) Flat elevated, % (n) | 34.9 (117) | 32.1 (76) | |
| Pathological finding | 0.500 | ||
 Adenoma, % (n) Adenoma, % (n) | 98.8 (331) | 97.9 (232) | |
 Sessile serrated lesion, % (n) Sessile serrated lesion, % (n) | 1.2 (4) | 2.1 (5) | |
| Channel size | 0.596 | ||
 3.7 mm, % (n) 3.7 mm, % (n) | 80.3 (269) | 78.5 (186) | |
 3.2 mm, % (n) 3.2 mm, % (n) | 19.7 (66) | 21.5 (51) | |
| Straddling folds | 0.0001a | ||
 (+), % (n) (+), % (n) | 20.3 (68) | 34.6 (82) | |
 (−), % (n) (−), % (n) | 79.7 (267) | 65.4 (155) | |
| Flexure points | 0.005a | ||
 (+), % (n) (+), % (n) | 11.0 (37) | 19.4 (46) | |
 (−), % (n) (−), % (n) | 89.0 (298) | 80.6 (191) | |
| Endoscopist | <0.0001a | ||
 Expert, % (n) Expert, % (n) | 71.0 (238) | 54.9 (130) | |
 Trainee, % (n) Trainee, % (n) | 29.0 (97) | 45.2 (107) |
IQR, interquartile range.
Secondary outcome
Significant differences regarding size (P = 0.038), straddling folds (P = 0.047), and flexure points (P = 0.013) were identified from clinical features of the expert and trainee groups before propensity score matching (Table (Table3).3). Propensity score matching was, therefore, used to reduce these biases. Accordingly, 198 score matched pairs were generated, and no significant differences were identified in any of the factors across the groups. In the expert group (Table (Table4),4), there were no significant differences on any clinical features, and the straddling folds and flexure points were not risk factors for unclear margins. In the trainee group (Table (Table5),5), the median polyp size in the unclear group was significantly larger than that in the negative group (P = 0.005). Furthermore, the unclear margin was significantly higher at the straddling folds (P = 0.0004) and flexure points (P = 0.005).
Table 3.
Clinical features of the expert and trainee group and propensity score matching
| Before propensity score matching | After propensity score matching | |||||
|---|---|---|---|---|---|---|
| Expert(n = 368) | Trainee(n = 204) | P value | Expert(n = 198) | Trainee(n = 198) | P value | |
| Median age (IQR), years | 70.0 (64.0–77.8) | 72.0 (63.0–76.8) | 0.387 | 71.0 (64.0–78.0) | 72.0 (63.0–76.3) | 0.895 |
| Sex | 0.457 | 1.000 | ||||
 Male, % (n) Male, % (n) | 68.8 (253) | 65.7 (134) | 65.2 (129) | 64.7 (128) | ||
 Female, % (n) Female, % (n) | 31.3 (115) | 34.3 (70) | 34.9 (69) | 35.4 (70) | ||
| Location | 0.681 | 0.951 | ||||
 Right colon, % (n) Right colon, % (n) | 69.6 (256) | 73.0 (149) | 72.2 (143) | 72.2 (143) | ||
 Left colon, % (n) Left colon, % (n) | 27.7 (102) | 24.5 (50) | 24.8 (49) | 25.0 (50) | ||
 Rectal, % (n) Rectal, % (n) | 2.7 (10) | 2.5 (5) | 3.0 (6) | 2.5 (5) | ||
| Median polyp size (IQR), mm | 3.0 (3.0–5.0) | 3.0 (3.0–4.0) | 0.038b | 3.0 (3.0–4.0) | 4.0 (3.0–4.0) | 0.118 |
| Morphology | 0.927 | 0.915 | ||||
 Protruded, % (n) Protruded, % (n) | 66.0 (243) | 66.7 (136) | 67.2 (133) | 68.2 (135) | ||
 Flat elevated, % (n) Flat elevated, % (n) | 34.0 (125) | 33.3 (68) | 32.8 (65) | 31.8 (63) | ||
| Channel size | 0.829 | 0.532 | ||||
 3.7 mm, % (n) 3.7 mm, % (n) | 79.9 (294) | 78.9 (161) | 81.3 (161) | 78.3 (155) | ||
 3.2 mm, % (n) 3.2 mm, % (n) | 20.1 (74) | 21.1 (43) | 18.7 (37) | 21.7 (43) | ||
| Straddling fold | 0.047a | 0.827 | ||||
 (+), % (n) (+), % (n) | 23.4 (86) | 31.4 (64) | 29.3 (58) | 30.8 (61) | ||
 (−), % (n) (−), % (n) | 76.6 (282) | 68.6 (140) | 70.7 (140) | 69.2 (137) | ||
| Flexure point | 0.013a | 0.588 | ||||
 (+), % (n) (+), % (n) | 11.7 (43) | 19.6 (40) | 15.2 (30) | 17.7 (35) | ||
 (−), % (n) (−), % (n) | 88.3 (325) | 80.4 (164) | 84.9 (168) | 82.3 (163) | ||
IQR, interquartile range.
Table 4.
Sub-analysis for risk factors of unclear margin in the expert group
| Negative (n = 129) | Unclear (n = 69) | P value | |
|---|---|---|---|
| Median age (IQR), years | 70.0 (64.0–79.0) | 71.0 (63.0–76.0) | 0.646 |
| Sex | 0.757 | ||
 Male, % (n) Male, % (n) | 64.3 (83) | 66.7 (46) | |
 Female, % (n) Female, % (n) | 35.7 (46) | 33.3 (23) | |
| Location | 0.622 | ||
 Right colon, % (n) Right colon, % (n) | 72.1 (93) | 72.5 (50) | |
 Left colon, % (n) Left colon, % (n) | 24.0 (31) | 26.1 (18) | |
 Rectal, % (n) Rectal, % (n) | 3.9 (5) | 1.5 (1) | |
| Median polyp size (IQR), mm | 3.0 (3.0–4.0) | 3.0 (3.0–4.0) | 0.431 |
| Morphology | 0.155 | ||
 Protruded, % (n) Protruded, % (n) | 63.6 (82) | 73.9 (51) | |
 Flat elevated, % (n) Flat elevated, % (n) | 36.4 (47) | 26.1 (18) | |
| Channel size | 0.567 | ||
 3.7 mm, % (n) 3.7 mm, % (n) | 79.8 (103) | 84.1 (58) | |
 3.2 mm, % (n) 3.2 mm, % (n) | 20.2 (26) | 15.9 (11) | |
| Straddling fold | 0.413 | ||
 (+), % (n) (+), % (n) | 27.1 (35) | 33.3 (23) | |
 (−), % (n) (−), % (n) | 72.9 (94) | 66.7 (46) | |
| Flexure point | 0.538 | ||
 (+), % (n) (+), % (n) | 14.0 (18) | 17.4 (12) | |
 (−), % (n) (−), % (n) | 86.1 (111) | 82.6 (57) |
IQR: interquartile range.
Table 5.
Sub-analysis for risk factors of unclear margin in the trainee group
| Negative (n = 96) | Unclear (n = 102) | P value | |
|---|---|---|---|
| Median age (IQR), years | 72.0 (63.0–76.8) | 72.0 (64.5–76.3) | 0.861 |
| Sex | 0.053 | ||
 Male, % (n) Male, % (n) | 71.9 (69) | 57.8 (59) | |
 Female, % (n) Female, % (n) | 28.1 (27) | 42.2 (43) | |
| Location | 0.835 | ||
 Right colon, % (n) Right colon, % (n) | 70.8 (68) | 73.5 (75) | |
 Left colon, % (n) Left colon, % (n) | 26.0 (25) | 24.5 (25) | |
 Rectal, % (n) Rectal, % (n) | 3.1 (3) | 2.0 (2) | |
| Median polyp size (IQR), mm | 3.0 (3.0–4.0) | 4.0 (3.0–4.0) | 0.005b |
| Morphology | 0.450 | ||
 Protruded, % (n) Protruded, % (n) | 70.8 (68) | 65.7 (67) | |
 Flat elevated, % (n) Flat elevated, % (n) | 29.2 (28) | 34.3 (35) | |
| Channel size | 0.228 | ||
 3.7 mm, % (n) 3.7 mm, % (n) | 82.3 (79) | 74.5 (76) | |
 3.2 mm, % (n) 3.2 mm, % (n) | 17.7 (17) | 25.5 (26) | |
| Straddling fold | 0.0004a | ||
 (+), % (n) (+), % (n) | 18.8 (18) | 42.2 (43) | |
 (−), % (n) (−), % (n) | 81.3 (78) | 57.8 (59) | |
| Flexure point | 0.005a | ||
 (+), % (n) (+), % (n) | 9.4 (9) | 25.5 (26) | |
 (−), % (n) (−), % (n) | 90.6 (87) | 74.5 (76) |
IQR: interquartile range.
Discussion
This retrospective study revealed the risk factors for unclear margins in endoscopic procedure. CSP is a simple method that does not require a high-frequency power supply unit and was initially used extensively in Europe [4]; it is also used in East Asia, including Japan [5]. According to polyp studies [2,3], a clean colon is important, and the removal of colorectal polyps can reduce the risk of developing colorectal cancer. CSP is efficient for achieving a clean colon due to its short procedure time, simple methods, and low complication rate. The most significant advantage of CSP is the low rate of delayed bleeding. Polyp removal with a high-frequency power supply unit is associated with complications such as delayed bleeding and perforation due to thermal damage, and 8.6% of polyps removed by hot snare polypectomy cause delayed bleeding [23]. However, delayed bleeding is rare in CSP [4–9], and the rate is 0–1.2% even in high-risk patients receiving antithrombotic drugs [10–13]. Horiuchi et al. suggested that the reason for the lower delayed bleeding rate was that CSP causes less submucosal vascular injury than conventional treatment [10]. Therefore, CSP is favorable as a treatment for achieving clean colon in the current situation, where the number of patients receiving antithrombotic therapy is increasing.
However, CSP has problems, such as the risk of residual tumor [14,15] and a high rate of unclear margins [16–21]. Therefore, the European Society of Gastrointestinal Endoscopy clinical guidelines recommend the use of CSP as the preferred technique to remove diminutive polyps ≤5 mm and sessile polyps 6–9 mm in size [24]. In Japan, CSP is indicated for 10 mm non-pedunculated adenomas [25]. Shichijo et al. found residual tumors in 1% of patients after CSP, following a biopsy of the central part of the mucosal defect [14]. Regarding long-term results, Horiuchi et al. reported no recurrence within 3 years after CSP for polyps of 8–15 mm [15], whereas Yoshida et al. reported that the local recurrence rate of CSP was 6.3% for high-grade dysplasia or intramucosal cancer [16]. Regarding the evaluation of the margin in CSP, several studies reported that the complete resection rate for pathologically negative margins for polyps ≤10 mm is 32.4–91% [16–21]. Some studies reported that unclear margins are not necessarily associated with residual tumor [18,26], while another study reported that positive horizontal margins are a risk factor for local recurrence [16]. Therefore, this study aimed to identify the risk factors for unclear margins in endoscopic procedure.
Herein, we showed that the risk factors for unclear margins were the straddling folds, flexure points, and endoscopists. Regarding the reason for the high unclear margin rate, polyps at the straddling folds, protruding convexly on the semilunar folds, and at the flexure points where the operability of the scope is poor, are difficult to resect completely with the surrounding normal mucosa. Therefore, these endoscopic findings were considered as risk factors for unclear margins. There have been some reports on the usefulness of polyp resection for these problems. Abe et al. performed extended CSP, including that of the surrounding normal mucosa, on polyps with a >1 mm circumferential margin [27], and the complete resection rate was significantly higher than that of conventional CSP. Underwater CSP is also useful [28,29]. Under water immersion, the intestinal wall remains wrinkled, and tension is reduced compared with carbon dioxide insufflation, flattening the semilunar fold. Underwater CSP has a higher negative margin rate than conventional CSP, because it can be resected with adequate surrounding normal mucosa. In addition, an endoscopic technique using a dedicated cold snare can improve the negative margin rate. A dedicated snare increases the complete resection rate more than a conventional snare, and a thinner wire and shape may be more effective for sharp polyp resection [20]. However, endoscopic mucosal resection (EMR) with a high-frequency power supply unit is required for polyps >10 mm or suspected intramucosal cancers and other devices need to be prepared.
We found that the rate of unclear margins in the trainee group was significantly higher at difficult sites such as straddling folds and flexure points. However, no significant differences were observed with the expert group, and straddling folds and flexure points may not be associated with risk factors for unclear margins in the expert group. These results suggest the possibility of a learning curve, in which the negative margin rate improves with experience. Several studies have reported on the polyp treatment learning curve. Choi et al. reported that in cases in which EMR was performed by a trainee for colorectal polyps, the complete resection rate was lower than that performed by an expert, and accumulating experience improved the complete resection rate [30].
This study has several limitations. First, this retrospective study was conducted at a single facility without randomized controlled trials. Second, we cannot follow-up residual tumors and local recurrence in the long-term and consider a long-term prospective analysis. Third, retrieval problems and pathological evaluation may cause unclear margins. Small and thin specimens of CSP can sometimes be fragmented when suctioning, and accurate pathological evaluation is difficult.
In conclusion, straddling folds, flexure points, and endoscopists (trainees) were significant risk factors for unclear margins. Polyps at the straddling folds and flexure points are difficult to resect, including their surrounding normal mucosa, and accumulating experience at such difficult sites may improve the negative margin rate.
Acknowledgements
We would like to thank Editage (www.editage.com) for the English language editing.
S.M. and R.I. contributed to conception and designed the study. R.I., H.K., H.S., H.A., Y.S., A.I., Y.G., S.S., and K.I. contributed to acquisition of data. S.M. and R.I. contributed to the data analysis and interpretation. R.I. drafted the manuscript. S.M. contributed to critical revision of this article. S.M. and R.I. contributed to the statistical analyses. S.M. approved the final draft of this manuscript. All the authors listed have contributed substantially to the design, data collection, analysis, and editing of the manuscript.
The research ethics committee of our hospital approved this study (approval number: F230900003) on September 2023.
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
Conflicts of interest
There are no conflicts of interest.
References
 mm colorectal polyps: a multicentre randomised controlled trial (CRESCENT study).
Gut
2018; 67:1950–1957.
[Europe PMC free article] [Abstract] [Google Scholar]
mm colorectal polyps: a multicentre randomised controlled trial (CRESCENT study).
Gut
2018; 67:1950–1957.
[Europe PMC free article] [Abstract] [Google Scholar] mm in diameter.
Dig Endosc
2017; 29:594–601.
[Abstract] [Google Scholar]
mm in diameter.
Dig Endosc
2017; 29:594–601.
[Abstract] [Google Scholar] mm in diameter: a prospective randomized controlled trial.
Surg Endosc
2022; 36:6527–6534.
[Abstract] [Google Scholar]
mm in diameter: a prospective randomized controlled trial.
Surg Endosc
2022; 36:6527–6534.
[Abstract] [Google Scholar]Full text links
Read article at publisher's site: https://doi.org/10.1097/meg.0000000000002845
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Resection depth and layer of cold snare polypectomy versus endoscopic mucosal resection.
J Gastroenterol, 53(11):1171-1178, 07 Mar 2018
Cited by: 32 articles | PMID: 29516270
Histopathological analysis of cold snare polypectomy and its indication for colorectal polyps 10-14 mm in diameter.
Dig Endosc, 29(5):594-601, 17 May 2017
Cited by: 19 articles | PMID: 28160332
Efficacy and Safety of Cold Snare Polypectomy of Colorectal Polyps 10-15 mm with a Hybrid Snare: A Prospective Observational Pilot Study.
Digestion, 104(5):391-399, 16 Jun 2023
Cited by: 0 articles | PMID: 37331350
AGA Clinical Practice Update on Appropriate and Tailored Polypectomy: Expert Review.
Clin Gastroenterol Hepatol, 22(3):470-479.e5, 28 Nov 2023
Cited by: 1 article | PMID: 38032585
Review

 1
1