Abstract
Background
Extracellular matrix (ECM) proteins are the major constituents of the muscle cell micro-environment, imparting instructive signalling, steering cell behaviour and controlling muscle regeneration. ECM remodelling is among the most affected signalling pathways in COPD and aged muscle. As a fraction of COPD patients present muscle atrophy, we questioned whether ECM composition would be altered in patients with peripheral muscle wasting (atrophic COPD) compared to those without muscle wasting (non-atrophic COPD).Methods
A set of ECM molecules with known impact on myogenesis were quantified in vastus lateralis muscle biopsies from 29 COPD patients (forced expiratory volume in 1 s 55±12% predicted) using ELISA and real-time PCR. COPD patients were grouped to atrophic or non-atrophic based on fat-free mass index (<17 or ≥17 kg·m-2).Results
Atrophic COPD patients presented a lower average vastus lateralis muscle fibre cross-sectional area (3872±258 μm2) compared to non-atrophic COPD (4509±198 μm2). Gene expression of ECM molecules was found significantly lower in atrophic COPD compared to non-atrophic COPD for collagen type I alpha 1 chain (COL1A1), fibronectin (FN1), tenascin C (TNC) and biglycan (BGN). In terms of protein levels, there were no significant differences between the two COPD cohorts for any of the ECM molecules tested.Conclusions
Although atrophic COPD presented decreased contractile muscle tissue, the differences in ECM mRNA expression between atrophic and non-atrophic COPD were not translated at the protein level, potentially indicating an accumulation of long-lived ECM proteins and dysregulated proteostasis, as is typically observed during deconditioning and ageing.Free full text

Expression of intramuscular extracellular matrix proteins in vastus lateralis muscle fibres between atrophic and non-atrophic COPD
Associated Data
Abstract
Background
Extracellular matrix (ECM) proteins are the major constituents of the muscle cell micro-environment, imparting instructive signalling, steering cell behaviour and controlling muscle regeneration. ECM remodelling is among the most affected signalling pathways in COPD and aged muscle. As a fraction of COPD patients present muscle atrophy, we questioned whether ECM composition would be altered in patients with peripheral muscle wasting (atrophic COPD) compared to those without muscle wasting (non-atrophic COPD).
Methods
A set of ECM molecules with known impact on myogenesis were quantified in vastus lateralis muscle biopsies from 29 COPD patients (forced expiratory volume in 1 s 55±12% predicted) using ELISA and real-time PCR. COPD patients were grouped to atrophic or non-atrophic based on fat-free mass index (<17 or ≥17 kg·m−2).
Results
Atrophic COPD patients presented a lower average vastus lateralis muscle fibre cross-sectional area (3872±258 μm2) compared to non-atrophic COPD (4509±198 μm2). Gene expression of ECM molecules was found significantly lower in atrophic COPD compared to non-atrophic COPD for collagen type I alpha 1 chain (COL1A1), fibronectin (FN1), tenascin C (TNC) and biglycan (BGN). In terms of protein levels, there were no significant differences between the two COPD cohorts for any of the ECM molecules tested.
Conclusions
Although atrophic COPD presented decreased contractile muscle tissue, the differences in ECM mRNA expression between atrophic and non-atrophic COPD were not translated at the protein level, potentially indicating an accumulation of long-lived ECM proteins and dysregulated proteostasis, as is typically observed during deconditioning and ageing.
Shareable abstract
Intramuscular mRNA expression of crucial extracellular matrix molecules affecting homeostasis is lower in the vastus lateralis muscle of atrophic compared to non-atrophic COPD, suggesting lower baseline transcriptional activity https://bit.ly/4a2Vdp2
Introduction
COPD is a progressive lung disease with patients experiencing disabling symptoms despite the use of approved therapies [1]. In COPD, loss of peripheral muscle mass is among the most prevalent extrapulmonary manifestations that constitutes a strong determinant of mortality and is associated with poor clinical outcomes, independent of the degree of airflow obstruction [1, 2]. Besides myofibrillar catabolic protein breakdown, the multinucleated muscle cells lose nuclei, whereas the tissue displays altered adaptive structural features including a diminished proportion of type I fibres, an increased proportion of type II fibres (in particular type IIx), a reduction in muscle fibre cross-sectional area (CSA), impaired oxidative capacity, and lowered mitochondria and capillary density [1]. Multiple aetiologies underlie the loss of peripheral muscle mass in COPD, including muscle disuse, oxidative stress, local inflammation, hypoxaemia, protein anabolism/catabolism imbalance and impaired regenerative capacity [1, 3, 4]. However, the exact mechanisms leading to the underlying peripheral muscle wasting in COPD are not fully understood.
The intramuscular extracellular matrix (ECM) is a highly malleable connective tissue embedding all cells in the muscle and creating the cell micro-environment. The ECM forms a molecular lattice of collagenous components, proteoglycans, matricellular proteins and adhesion receptors which is highly adaptable and responsive to external stimuli, including physical activity and muscle injury [5]. Appreciated for its essential role in force transmission and effective muscle contraction, the ECM also plays a critical role in maintaining muscle homeostasis and mediating muscle adaptation and regeneration [5]. Forming the satellite cell niche, the ECM integrates biophysical and biochemical signals via cell–cell and/or cell–matrix communication, essential for muscle regeneration. In ageing and myopathies (e.g. Duchenne muscular dystrophy), dysregulated ECM composition compromises muscle function and homeostasis [6]. The ECM degradation and remodelling are among the most affected signalling pathways in COPD and aged muscle [7, 8]. However, the composition of intramuscular ECM has not previously been studied in the peripheral muscles of patients with COPD.
The aim of this retrospective analysis was to investigate the expression of selected intramuscular ECM proteins that are considered to be crucial for muscle homeostasis [5, 9] in the vastus lateralis muscle of COPD patients. Considering that altered intramuscular ECM composition has been found in a number of pathological states and long-term conditions manifesting muscle wasting [6, 7, 10], it was anticipated that ECM expression would differ in the vastus lateralis muscle of COPD patients with muscle wasting (atrophic COPD) compared to those without muscle wasting (non-atrophic COPD).
Material and methods
Study population
In the present study, we analysed muscle specimens from 29 male clinically stable COPD patients [11]. COPD patients met the following entry criteria: 1) post-bronchodilator forced expiratory volume in 1 s (FEV1) <50% predicted and FEV1/forced vital capacity (FVC) <70% without significant post-bronchodilator reversibility (<10% FEV1 % pred); and 2) optimal medical therapy without regular use of systemic corticosteroids. A group of 14 young, healthy, sedentary male participants were included [12] to provide a reference for the normal ECM muscle protein composition, as literature is mostly restricted to relative gene expression [5, 9]. This allowed better understanding of changes at the translational level between COPD and healthy individuals. Muscle specimens were analysed at Northumbria University Newcastle (Newcastle upon Tyne, UK) in accordance with the Human Tissue Act 2004 and with approval from Northumbria University Newcastle Ethics Committee (HLSIV220916-V2).
Muscle biopsy phenotypic analyses
Vastus lateralis muscle percutaneous biopsies were obtained as previously described [11]. Biopsies were analysed blindly for fibre type classification, fibre CSA and capillary/fibre ratio as previously described [11]. The latter analysis refers to the number of capillaries identified in a certain area divided by the number of fibres found in the corresponding muscle section [11].
Quantitative real-time PCR analysis
Total RNA was extracted using an RNeasy Fibrous Tissue kit (Qiagen) according to the manufacturer's protocol. Quantified with a NanoDrop spectrophotometer (Thermo Fisher Scientific), up to 1 μg RNA was used for synthesising cDNA with SuperScript II reverse transcriptase and RNaseOUT (Invitrogen) [13]. Primers (Eurofins MWG) were designed using Primer3 software (https://primer3.ut.ee). Details on the primers used in this study can be found in the supplementary material. Quantitative real-time PCR data are presented as fold change relative to the housekeeping gene glyceraldehyde 3-phosphate dehydrogenase (GAPDH), estimated using the 2−ΔΔCt method. Gene expression of the following ECM molecules was analysed: collagen type I heterodimer chains (COL1A1 and COL1A2), collagen type IV (COL4A1), fibronectin (FN1), tenascin C (TNC), integrin β1 (ITGB1), osteopontin (SPP1), secreted protein acidic and rich in cysteine (SPARC), decorin (DCN) and biglycan (BGN). To gain insight as to whether the changes in ECM in COPD muscle are indicative of the myogenic potential, the expression of two myogenic regulatory factors (MRFs), paired box 7 (PAX7) and myogenic differentiation 1 (MYOD1), was examined [14].
Protein extraction and analyses
Muscle tissue was lysed in cOmplete Mini protease inhibitor cocktail (Roche). Total protein was quantified using the Pierce BCA protein assay kit (Thermo Fisher Scientific). Total protein loading onto ELISA was normalised as 1 μg to quantify: collagen I (ab285250; Abcam); collagen IV (orb562147) and integrin β1 (orb563558) (Biorbyt); osteopontin (DOST00), fibronectin (DFBN10) and SPARC (DSP00) (R&D Systems); and tenascin C (EH446RB), decorin (EHDCN) and biglycan (EH45RB) (Invitrogen) according to the manufacturer's instruction. The ELISA's sensitivity and detection range are shown in the supplementary material.
Statistical analyses
Data on participant demographics and muscle fibre morphology are presented as mean±sem. Differences across the three groups (healthy, atrophic and non-atrophic COPD) were assessed by one-way ANOVA, and between two groups (atrophic and non-atrophic COPD) were assessed by the unpaired t-test. Molecular data presenting a non-normalised distribution were analysed using an unpaired two-tailed Mann–Whitney test. Data are presented as median (25–75% percentiles (interquartile range)). Correlations between the expression level of target genes/proteins and phenotypic characteristics were explored using Spearman's correlation coefficient (rs); 95% confidence intervals and significance values (p-values) were calculated. All statistical tests were carried out using Prism version 9.02 (GraphPad, San Diego, CA, USA). The level of statistical significance was set at p<0.05.
Results
Study population
COPD patient demographic and lung function characteristics are detailed in table 1. COPD patients were grouped according to their fat-free mass index (FFMI) as non-atrophic (FFMI >17 kg·m−2, n=19) and atrophic (FFMI <17 kg·m−2, n=10) [4]. The two groups of COPD patients (atrophic and non-atrophic) did not differ in terms of age and severity of airflow obstruction (table 1). Demographics of healthy participants are presented in supplementary table S1.
TABLE 1
Demographic and lung function characteristics of non-atrophic and atrophic COPD patients
| COPD | ||
|---|---|---|
| Non-atrophic (n=19) | Atrophic (n=10) | |
| Age (years) | 67.10±1.89 | 63.00±2.13 |
| Weight (kg) | 77.35±3.09 | 62.94±2.29* |
| BMI (kg·m−2) | 28.81±1.17 | 21.50±0.66* |
| FFMI (kg·m−2) | 19.04±0.40 | 15.64±0.38* |
| FEV1 (L) | 1.18±0.12 | 0.98±0.14 |
| FEV1 (% pred) | 44.07±4.84 | 37.52±6.13 |
| FVC (L) | 2.84±0.19 | 2.49±0.17 |
| FVC (% pred) | 80.55±5.04 | 72.86±5.94 |
Data are presented as mean±sem. BMI: body mass index; FFMI: fat-free mass index; FEV1: forced expiratory volume in 1 s; FVC: forced vital capacity. *: p<0.05 significance level between non-atrophic and atrophic COPD groups.
Atrophic COPD patients presented a lower average muscle fibre CSA consistent with lower type IIa and type IIx muscle fibre CSA compared to non-atrophic COPD (table 2). Furthermore, COPD patients presented smaller mean fibre CSA, higher distribution of fibre type IIa and lower capillary/fibre ratio compared to healthy individuals (supplementary table S2).
TABLE 2
Muscle fibre morphological characteristics of non-atrophic and atrophic COPD patients
| COPD | ||
|---|---|---|
| Non-atrophic (n=19) | Atrophic (n=10) | |
| Fibre type distribution (%) | ||
 Type I Type I | 32.0±3.2 | 33.6±2.9 |
 Type II Type II | 67.5±3.3 | 65.9±3.0 |
 Type IIa Type IIa | 52.3±3.7 | 50.4±6.5 |
 Type IIx Type IIx | 15.2±2.2 | 15.5±3.6 |
| Cross-sectional area (μm2) | ||
 Mean Mean | 4509±198 | 3872±258* |
 Type I Type I | 4716±271 | 4717±215 |
 Type IIa Type IIa | 4507±247 | 3695±337* |
 Type IIx Type IIx | 3649±208 | 2872±250* |
| Capillary/fibre ratio | 1.41±0.13 | 1.44±0.09 |
Data are presented as mean±sem. *: p<0.05 significance level between non-atrophic and atrophic COPD groups.
Differences in ECM composition between atrophic and non-atrophic COPD
There were only a few differences in the composition of ECM between atrophic and non-atrophic COPD cohorts. These were limited to changes in mRNA expression levels for COL1A1, FN1, TNC and BGN, which were found to be significantly greater in non-atrophic COPD compared to atrophic COPD (figure 1). However, no other differences in mRNA expression were observed between atrophic and non-atrophic COPD patients. In terms of protein levels, there were no significant differences between the two COPD cohorts for all biomarkers tested (table 3).
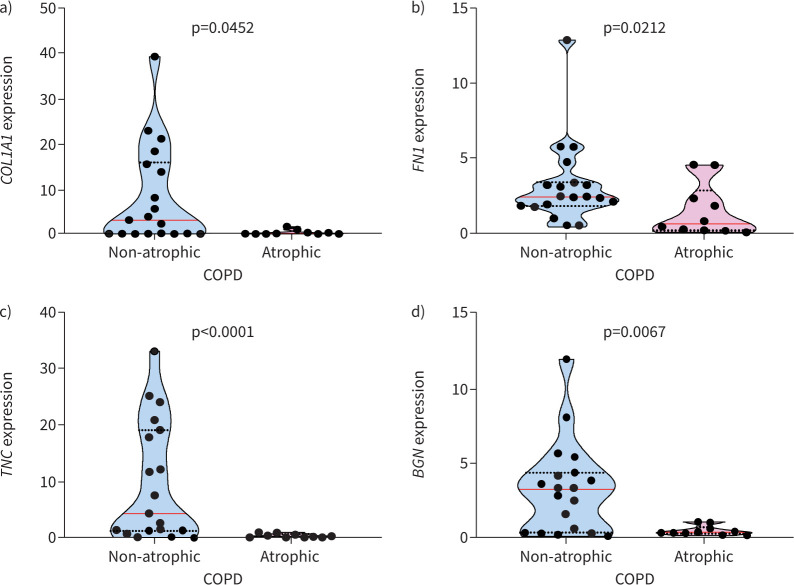
Muscle from atrophic COPD patients shows decreased gene transcription for extracellular matrix proteins compared to non-atrophic. Muscle from atrophic and non-atrophic COPD mRNA expression was quantified for a) collagen type I alpha 1 (COL1A1), b) fibronectin (FN1), c) tenascin C (TNC) and d) biglycan (BGN). Violin graphs show the median (red line) and lower and upper quartiles (black dotted lines); individual participant values are represented as black data points. Data are presented as fold change relative to the housekeeping gene glyceraldehyde 3-phosphate dehydrogenase (GAPDH).
TABLE 3
Protein expression# of extracellular matrix molecules in the vastus lateralis muscle of non-atrophic and atrophic COPD patients
| COPD | ||
|---|---|---|
| Non-atrophic | Atrophic | |
| Collagen type I (pg·mL−1) | 293.3 (185.3–443.1) | 382.5 (311.9–532.7) |
| Collagen type IV (pg·mL−1) | 366.3 (247.4–515.3) | 467.9 (289.9–586.9) |
| Fibronectin (pg·mL−1) | 11 950 (6213–17 950 (6213–17 380) 380) | 8847 (7425–9856) |
| Integrin β1 (pg·mL−1) | 48.4 (35.2–99.9) | 62.9 (50.2–94.4) |
| Osteopontin (pg·mL−1) | 75.68 (44.68–106.6) | 62.32 (28.46–315.5) |
| Tenascin C (pg·mL−1) | 11.6 (5.1–15.2) | 9.9 (5.7–12.7) |
| SPARC (pg·mL−1) | 1504.0 (1128.0–1732.0) | 1726.0 (1404.0–1926.0) |
| Decorin (pg·mL−1) | 44.4 (38.6–51.6) | 42.8 (39.5–47.1) |
| Biglycan (pg·mL−1) | 230.8 (125.7–325.0) | 241.3 (170.9–401.9) |
Data are presented as median (interquartile range). SPARC: secreted protein acidic and rich in cysteine. #: protein expression was measured with ELISA.
Myogenic potential is associated with ECM expression
Muscle mRNA expression of PAX7 was significantly greater in non-atrophic COPD compared to atrophic COPD (figure 2a). Similarly, MYOD1 mRNA was significantly increased in non-atrophic COPD compared to atrophic COPD (figure 2b). Among the ECMs analysed, SPARC mRNA expression was positively correlated and associated with the mRNA expression of PAX7 (rs=0.57; p=0.005) and MYOD1 (rs=0.47; p=0.0002) in all COPD patients.
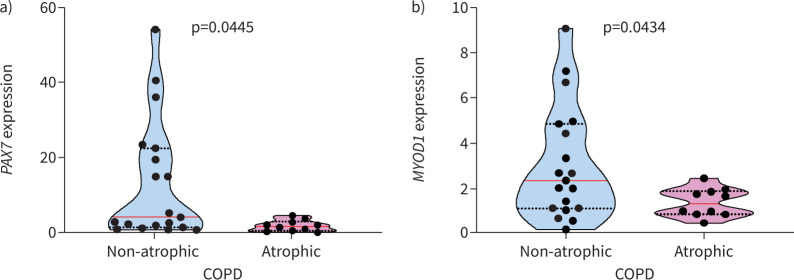
Atrophic COPD patients demonstrate lower paired box 7 (PAX7) and myogenic differentiation 1 (MYOD1) muscle mRNA expression. a) PAX7 and b) MYOD1 mRNA gene expression was analysed in non-atrophic and atrophic COPD patients. Violin graphs show the median (red line) and lower and upper quartiles (black dotted lines); individual participant values are represented as black data points. Data are presented as fold change relative to the housekeeping gene glyceraldehyde 3-phosphate dehydrogenase (GAPDH).
Structural collagen is decreased in COPD muscle
Differences in muscle ECM composition were observed between COPD and healthy individuals. Specifically, collagen type I protein was significantly lower in individuals with COPD compared to healthy individuals (supplementary table S3). This is consistent with the mRNA expression results, which showed that the collagen type I limiting assembling chain COL1A2 [15] was significantly lower in COPD patients compared to healthy individuals (supplementary figure S1a). However, no differences were observed in the non-fibrillar collagen type IV protein level (supplementary table S3).
Adhesion molecules are affected in COPD muscle
Fibronectin is a pro-adhesive glycoprotein, a regulator of myogenesis and a pro-fibrotic marker [5, 16]. In patients with COPD, fibronectin was more abundant compared to healthy controls and was the most abundant protein among the biomarkers measured (table 3 and supplementary table S3). However, at the mRNA level, there was no difference in FN1 expression between COPD and healthy individuals (supplementary figure S1B). Tenascin C, which inhibits fibronectin-induced cell adhesion migration and ECM remodelling [17], was the least abundant ECM among the biomarkers tested, and it was less abundant in COPD muscle compared to healthy individuals (table 3 and supplementary table S3).
Fibronectin mediates integrin–ECM attachment transmitting signals between ECM and intracellular domains [7, 18, 19]. Among the integrin receptors, integrin β1 is the main receptor for fibronectin. To understand if the increased expression of fibronectin protein in COPD patients leads to an increase in molecular signalling between ECM and intracellular domains, we studied the expression of integrin β1 [18, 19]. However, we found no significant difference in protein and mRNA expression of integrin β1 between COPD and healthy participants (supplementary table S3 and supplementary figure S1c).
Matricellular ECM proteins accumulate in muscle of COPD patients
COPD is characterised by systemic and local muscle inflammation [1, 20]. The pro-inflammatory osteopontin is a glycoprotein encoded by the SPP1 gene known to inhibit muscle regeneration in dystrophic and ageing muscle [21, 22]. In COPD patients, osteopontin protein levels were found to be 2.6 times greater compared to healthy individuals (supplementary table S3). However, SPP1 mRNA expression was found to be downregulated in COPD patients compared to healthy individuals (supplementary figure S1d), indicating that osteopontin protein turnover may be affected.
SPARC, also known as osteonectin, is an exercise-induced myokine which is expressed in various tissues during remodelling/repair and is related to muscle regeneration [23]. SPARC at the protein level was observed to be higher in COPD compared to healthy individuals (supplementary table S3). However, SPARC mRNA expression was significantly downregulated in COPD patients compared to healthy individuals (supplementary figure S2a).
Expression of proteoglycans is altered in COPD
Decorin and biglycan proteoglycans play a significant role in the arrangement of collagen fibrils and myogenesis [9]. The lower level of decorin protein in COPD patients compared to healthy controls (supplementary table S3) may have a negative impact on COPD muscle mass, as decorin binds to myostatin, neutralising its function, and also induces the expression of MYOD1 and follistatin [5, 24]. Although the mRNA expression of DCN was also lower in COPD patients compared to healthy individuals (supplementary figure S2b), the difference was not significant. On the other hand, biglycan, which shares similar glycosaminoglycan chain composition and possibly some of decorin functions [5, 16], was not significantly different between COPD and healthy controls (supplementary table S3 and supplementary figure S2c).
Discussion
This study presents novel data on the intramuscular expression of crucial ECM molecules affecting homeostasis of the vastus lateralis muscle in COPD patients. The differences in ECM composition between atrophic and non-atrophic COPD were limited to a decrease in mRNA expression of COL1A1, FN1, TNC and BGN in atrophic COPD. This suggests that atrophic COPD muscle has lower baseline transcriptional activity, which could be due to severe muscle deconditioning [10, 25]. While atrophic muscle presented decreased contractile muscle tissue, the differences in ECM mRNA expression between atrophic and not atrophic COPD were not translated at the protein level, potentially indicating an accumulation of long-lived ECM proteins and dysregulated proteostasis, as is typically observed during deconditioning and ageing [10, 25]. Accumulation of long-lived ECM proteins in muscle increases the possibility of adverse post-translational modifications known to have adverse effects on aged muscle tissue [26]. According to findings in muscle wasting [10], mRNA expression of ECM molecules was downregulated in COPD muscle, indicating wider gene expression changes. These changes provide insight not only into the effect of COPD on muscle ECM but also into the accelerated ageing process in COPD [27].
The ECM composition in muscles from COPD patients is significantly different from that of healthy young individuals. In COPD muscle, there is an increased presence of glycoproteins such as fibronectin, osteopontin and SPARC, while the abundance of collagen type I, tenascin C and decorin is reduced. These findings are consistent with previous studies showing increased deposition and accumulation of intramuscular ECM in COPD muscle leading to greater levels of fibrosis and replacement of muscle cells with adipose tissue [8, 28].
Type I collagen is the most prevalent type of collagen. Reduced expression of collagen type I in COPD muscle can affect muscle tissue structure, remodelling and force transmission [5, 16]. This reduction in collagen mRNA level is consistent with other studies on ageing and a model of muscle wasting [10]. However, when there are long periods of bed rest or immobilisation, the proportion of collagen and connective tissue in the muscle is reported to be increased, suggesting that inactivity leads to a decline in muscle mass accompanied by accumulation of collagen [29]. Recent studies demonstrated that age-related decline in proteostasis leads to heterogeneity in collagen content and less dense organisation, influencing muscle capacity of adaptation and homeostasis [25]. Additionally, the slowed turnover rate and high half-life of collagens make these proteins subject to the accumulation of deleterious advanced glycation end-products [26].
Matricellular glycoproteins are multifunctional proteins that have the ability to interact with other ECM proteins or cells through integrin receptors [22]. While osteopontin is typically undetectable in normal muscle fibres and increases after muscle injury [30], our research shows that the level of osteopontin protein was 2.6-fold greater in COPD patients compared to healthy individuals. Similarly, in old mice, muscle osteopontin was found to be upregulated by 8-fold in macrophages, 1.5-fold in satellite cells and 2-fold in senescent fibro-adipogenic cells, which was associated with inhibited myogenesis [22, 31]. Although no other studies have explored the regulation of osteopontin in the peripheral muscles of COPD patients, osteopontin plays a critical role in muscle pathology, specifically in Duchenne muscular dystrophy [21]. In fact, inhibition of osteopontin reduces fibrosis and improves muscle function in mdx mice [21]. Given that chronic osteopontin overexpression may lead to chronic damage, fibrosis and functional impairment of the damaged muscle [21, 22], the increase in osteopontin levels in COPD muscle compared to the muscle of healthy individuals may be linked with the severity of muscle wasting in COPD.
Fibronectin is an essential glycoprotein for cell adhesion and migration, playing a critical role in muscle regeneration and adaptation [18]. It has been found to increase in muscle from COPD patients and rats after muscle immobilisation [32]. However, its accumulation in the interstitial space can increase the distance for diffusion, making it harder for substrates and hormones to be delivered [18, 19]. Our finding is also in line with a study demonstrating that enhanced abundance in fibronectin levels in the aged gastrocnemius muscle of mice was significantly associated with reduced muscular strength and accompanied by reduced expression of myogenin, a marker of myogenic regeneration and differentiation [33]. Along with fibronectin, tenascin C is critical for ECM remodelling initiation following unloading because it promotes satellite cell expansion during muscle regeneration [9, 16]. However, tenascin C protein levels were found to be lower in COPD patients compared to healthy individuals, which is in agreement with the low levels of tenascin C seen in non-actively remodelling muscle [16, 34]. However, at the mRNA level, TNC expression was not homogeneously increased in COPD muscle compared to healthy individuals, confirming earlier findings [8]. Low levels of tenascin C in COPD muscle may affect particularly glycolytic-type muscle fibres as its deficiency in mice leads to fast-twitch muscle fibre mass loss and atrophy [34]. Thus, the dysregulated expression of tenascin C in COPD muscle could mediate loss of muscle mass in these patients. Tenascin C is part of a pleiotropic pathway that protects type II fibre mass from impaired damage-repair cycles in the muscle [35].
SPARC is a protein that has several functions, including protecting and stabilising the cytoskeleton during myogenesis. It is also important for maintaining muscular function [23]. Normally, SPARC facilitates collagen trafficking from areas where it is produced to areas where it is not. However, when SPARC is overexpressed this process is disrupted, and collagen deposition may decrease [23]. In people with COPD, SPARC levels are increased and SPARC accumulates in the serum [36]. To compensate for this, the mRNA expression of SPARC decreases in COPD muscle. Calcium influx, which occurs during satellite cell differentiation, controls SPARC mRNA expression [37]. This process relies on expression of MRFs PAX7 and MYOD1. Accordingly, the mRNA expression of MRFs PAX7 and MYOD1 was associated with SPARC [38].
The matrisome, which is a network of proteins that provide structural support to cells, is maintained by interactions of proteoglycans with collagens. In COPD patients, decreased levels of the proteoglycan decorin, along with increased expression of myostatin expression, could negatively impact normal muscle regeneration and growth, potentially by dysregulating the interplay between decorin/myostatin [11, 39]. Myostatin is a well-established inhibitor of muscle mass accretion and a negative regulator of myogenesis, and was found to be greater in COPD patients with muscle wasting [11]. Decorin, on the other hand, can suppress myostatin's activity either by directly binding to the molecule or by indirectly affecting the activity of transforming growth factor-β1 and follistatin. The dysregulated expression of these molecules in the vastus lateralis muscle may partly be responsible for the differences in mean fibre CSA between patients with COPD and healthy individuals.
Considering that most of the studies in muscle ECM are performed in mice tissue, this is a unique study reporting gene expression and protein profiling entirely in human samples and relevant to COPD patients. Certainly, the use of muscle samples from young instead of age-matched healthy individuals is a limiting factor in this study. While the selected ECM molecules studied were not exhaustive, our findings provide a novel conceptual framework for the ECM contribution to muscle adaptation during disuse and muscle wasting in COPD patients. The ECM undergoes remodelling during homeostatic conditions and muscle injury to ensure regular cellular renewal and to support muscle repair and maintenance [5].
As myogenic benefits of regular physical activity are dependent on the ECM micro-environment affecting how cells respond to mechanostimulus [9], our findings may reflect the lower habitual physical activity levels previously reported in patients with COPD compared to healthy individuals [40]. Future studies should investigate whether the reported differences in the ECM profile between atrophic and non-atrophic COPD patients could be altered after an exercise-based rehabilitation programme.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary methods 00857-2023.SUPPLEMENT
Figure S1 00857-2023.SUPPLEMENT
Figure S2 00857-2023.SUPPLEMENT2
Footnotes
Provenance: Submitted article, peer reviewed.
Author contributions: E. Kritikaki performed experiments, analysed data and wrote the original draft. G. Terzis performed muscle fibre phenotypical characterisation and data analysis. M. Soundararajan performed data analysis. I. Vogiatzis was responsible for the study design and wrote the original draft. D.C.M. Simoes was responsible for conceptualisation, study design and supervision, performed experiments and data analysis, and wrote the manuscript.
Conflict of interest: I. Vogiatzis and D.C.M. Simoes are associate editors of this journal. The authors declare no competing financial interests. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Support statement: D.C.M. Simoes received funding from Northumbria University Newcastle and Animal Free Research UK (grant numbers 176-Simoes-NORTHUMBRIA and 184-Simoes-NORTHUMBRIA). The funders had no role in the study design, data collection and analysis or preparation of the manuscript. E. Kritikaki was funded by a Northumbria University RDF PhD scholarship.
Ethics statement: Muscle specimens were analysed at Northumbria University Newcastle (Newcastle upon Tyne, UK) in accordance with the Human Tissue Act 2004 and with approval from Northumbria University Newcastle Ethics Committee (HLSIV220916-V2).
References
Articles from ERJ Open Research are provided here courtesy of European Respiratory Society
Full text links
Read article at publisher's site: https://doi.org/10.1183/23120541.00857-2023
Citations & impact
This article has not been cited yet.
Impact metrics
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/163987694
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Genome-wide mRNA expression profiling in vastus lateralis of COPD patients with low and normal fat free mass index and healthy controls.
Respir Res, 16:1, 08 Jan 2015
Cited by: 28 articles | PMID: 25567521 | PMCID: PMC4333166
Differences in micro-RNA expression profile between vastus lateralis samples and myotubes in COPD cachexia.
J Appl Physiol (1985), 126(2):403-412, 13 Dec 2018
Cited by: 4 articles | PMID: 30543501
2D-DIGE proteomic analysis of vastus lateralis from COPD patients with low and normal fat free mass index and healthy controls.
Respir Res, 18(1):81, 03 May 2017
Cited by: 8 articles | PMID: 28468631 | PMCID: PMC5415759
Muscle fibre type shifting in the vastus lateralis of patients with COPD is associated with disease severity: a systematic review and meta-analysis.
Thorax, 62(11):944-949, 25 May 2007
Cited by: 147 articles | PMID: 17526675 | PMCID: PMC2117111
Review Free full text in Europe PMC

 1
1