Abstract
Free full text

Ectomycorrhiza and ethylenediurea reduced the impact of high nitrogen and ozone stresses and increased the growth of Cedrus deodara
Abstract
Cedrus deodara is the central conifer plant affected by ozone and nitrogen pollutants among forest species worldwide. The growth of C. deodara depends upon the ectomycorrhizal (ECM) association, which is usually disturbed by these factors. This study aims to understand how these factors affect plants at physiological and biochemical levels. Three fungal strain consortiums were inoculated with two-year-old C. deodara seedlings. The stresses of 100 kg N h−1and 100 ppb O3 were applied for six months to study their impact on chlorophyll and antioxidant enzymes (SOD, CAT, and APX). The results showed that C2 (Consortium of Cedrus deodara) positively impacted the growth of selected plant species. The high photosynthesis rate was determined by enhanced chlorophyll content, and C2-treated plants showed high chlorophyll content. Relatively, chlorophyll a and b contents increased significantly in the seedlings treated with Ethylenediurea (EDU) alone and with ozone stress. In addition, a significant difference was observed between EDU and O3-treated plants (14% EDU400-O3 and 23% EDU600-O3) and the control. Overall, antioxidant activities were higher in the treated samples than in the control. The order of SOD activity was C2 (448 U/gFW) and lowest (354.7 U/gFW) in control. APX also showed higher activity in treated plants in C1 ≥ C2 ≥ C3+O3, whereas CAT activity was the highest in C2 treatments. Ozone and nitrogen-stressed plants showed higher activities than EDU-treated plants compared to non-treated ones. Our findings highlight the importance of understanding the signaling effects of numerous precursors. Moreover, an extended investigation of seedlings developing into trees must be conducted to verify the potential of ectomycorrhizal strains associated with C. deodara and comprehend EDU's role as a direct molecular scavenger of reactive toxicants.
1. Introduction
Any adverse circumstance or chemical that interferes with the plant's metabolism, growth, or development is called plant stress [1]. Plants experience stress due to alterations in their surroundings. Abiotic stress is brought on by factors such as temperature extremes, water scarcity, nutrients deficiency, wind, and radiation. Many plant responses are triggered by stress, including changes in gene expression, metabolic activity, growth rates, crop yield, etc. [2]. Among air pollutants, tropospheric or “ground level” ozone (O3) is a secondary air pollutant widely regarded as the most harmful to vegetation. The concentration of ground-level ozone over the majority of the land surface has more than doubled since preindustrial times [3]. The tropospheric O3 concentration is 25–30 parts per billion by volume, which is close to 100 parts per billion in polluted areas. In the middle latitudes of the Northern Hemisphere, the surface O3 levels have risen since the beginning of industrialization to a current average per year of 35–50 ppb [4,5]. From 32 Mt yr−1 in 1860 to between 112 and 116 Mt yr−1 today, reactive N is being deposited worldwide. N deposition is still projected to slightly increase globally [6] according to current projections, whereas O3 projections are more complicated and rely on changing climate and antecedent emission conditions [5,7].
Ozone being highly oxidative can cause visual burns to plants' tissues, which reduce a plant's survival. Plants all around the world, particularly crops and plants in natural habitats, suffer considerable harm from ozone [8]. A wide range of O3 effects on forests, trees, and ecosystems have been observed through combined empirical and experimental investigations. These analyses demonstrate observable damage symptoms including chlorophyll loss, chlorotic mottling, and early senescence. O3 also has an impact on physiology, which can result in decreased photosynthesis as well as increased leaf senescence, foliar loss of macro- and micro-nutrients, and changes in resource allocation to increase above-ground biomass [9,10]. O3 influences reproductive structures such as floral sites, stigma and style surfaces, anthers, pollen, seeds, and fruits [11]. Additionally, it has been discovered that ozone makes forests more vulnerable to wind, dryness, insect, and pest invasions. (e.g. bark beetle, wood borer, fungal infection) [12]. Due to its powerful oxidative properties, ozone penetrates plants mostly via leaf stomata, where it destroys tissues and prevents photosynthesis [13]. A meta-analysis of 128 research papers found that high O3 concentrations of 409 ppb and 339 ppb on average reduced pollen germination by 30% and pollen tube development by 54%, respectively [14]. With respect to exposure- or dose-response correlations developed from field tests, numerous studies have also demonstrated that O3 can reduce crop yields at regional levels [15]. Due to human activity and East Asia's rapidly growing economy, the level of ozone is still rising, therefore it is possible to anticipate that O3 will continue to lower crop yields in significant agricultural regions in the future [16]. According to estimates from 2010 to 2012, the key food crops maize, wheat, rice, and soybean saw global yield reductions of 6.1, 4.4, 7.1, and 12.4%, respectively, due to O3 [13]. In a modeling study conducted for Asia, yield losses for wheat, rice, and legumes ranged between 5 and 48 percent, 3–47 percent, and 10–65 percent, respectively, at ambient O3 concentrations were in the ranges of 35–75 ppb (4–8 h growing season mean) [17].
Nitrogen is a limiting nutrient for the plant's development, growth, and metabolism because it is a component of several cellular parts, e.g., proteins, amino acids, chlorophyll, enzymes, DNA, and nitrogenous bases [18]. However, a deficiency of N leads to chlorosis, wilting, and death [19]. The concentration of N, lower or higher than required, leads to growth retardation or inhibition [20]. Rising reactive nitrogen (Nr) deposition has an impact on the formation and functioning of food webs in forest ecosystems. Most northern temperate and boreal forests' primary growth is typically constrained by the element nitrogen [21]. Anthropogenic soil acidification is mostly caused by an increase in N deposition, such as NOx-derived deposition or N fertilization. This process harms ecosystems by suppressing microbial activity, reducing fine root biomass, and reducing biodiversity [22].
The current NO emission rate is eight times higher than previous decade and is estimated to be around 200 Tg N yr−1 by the year 2050 [23]. A detrimental impact on Asian forests is likely due to the wet and dry average rate deposition of nitrogen (N) from the atmosphere, which is predicted to be 22 kg N ha−1 yr−1. This value exceeds the maximum value of approximately 50 kg N ha−1 yr−1 for East Asia, especially Japan [24]. The availability of nitrogen (N) for microbial growth and the modification of soil environmental factors (such as pH) can all have an impact on the activity of soil populations of microbes, the makeup of microbial communities, the synthesis of soil enzymes, and ultimately soil N transformations [25]. The pH and accessibility of soil N can both affect the overall composition of the microbial community [26]. The ratio of fungi to bacteria may change because of excessive N deposition, which may also reduce fungal biomass [27]. N addition considerably reduced the fungus to bacteria ratio and the microbial carbon to nitrogen ratio on average by 10 and 8.2%, respectively, according to a meta-analysis conducted on N-added experiments [28]. Globally, N fixation has increased by more than thrice due to human activity compared to natural rates; a large portion of this increase comes from burning fossil fuels and agricultural practices [29]. While forests have considerable aerodynamic resistance and very short atmospheric N residence durations, atmospheric deposition of NHx, NOy, or biological N does not fall uniformly over the surface of the Earth. As a result, localized atmospheric N deposition may be over ten-fold greater than its pre-industrial levels. Eastern North America and Europe had the highest levels of nitrogen deposition during the 20th century, but as a result of dips in Europe and upsurges in Asia, the latter now possesses the highest levels of nitrogen deposition [30]. Therefore, anthropogenic N has been deposited in forests all across the world in a variety of spatio-temporal patterns [31].
Fungi and trees form symbiotic associations with tree roots and roots inhabiting fungi known as mycorrhizal association. This association benefits both tree and fungi in providing C to fungi and in return fungi provide nutrients to the tree including nitrogen (N) and phosphorus (P) along with protection from pathogens and nutrient uptake [32]. Increased N availability and often acidified ecosystems caused by nitrogen deposition change how mycorrhizal fungi interact with their hosts and their abiotic surroundings [33]. Due to the enormous range of fungal species and the possible continuity between mutualistic relationship and parasitism in the operation of mycorrhizal associations, it is difficult to identify the impacts of individual fungal species on plants' growth rates and efficiency [34]. Several ECM fungi can form symbiotic relationships with the widely distributed greening tree species cedar (Cedrus deodara) and Scot pine (Pinus sylvestris L.) [35]. Rising atmospheric carbon dioxide levels prompted researchers to examine the symbiotic link between plants and ectomycorrhizal fungus and how it affects host plant photosynthesis and the carbon budget [36]. Cedrus deodara, Himalayan cedar, deodar, devdaru, bhadradaru, and amaradaru are some of the common names for Deodara in English, Hindi, and Sanskrit, respectively [37]. Due to its fragrant, awe-inspiring height, and majestic nature, deodar has been regarded as the home of gods since ancient times. It is widespread in the Western Himalayan regions of North-Central India, Northern Pakistan, Western Nepal, Eastern Afghanistan, and Southwestern Tibet at an altitude of 1200–3000 m, and needs acidic, well-drained soil to flourish. A well-known ornamental tree with a nice aroma, C. deodara, also Pakistan's national tree is typically grown in regions with winter temperatures below 10 °C because deodar trees cannot tolerate temperatures above 25 °C [38].
The species that can withstand the coldest conditions are found in Kashmir and Pakistan's northwest province. Numerous ECM fungi are known to be hosts of C. deodara, and it has been demonstrated that these fungi form unique ECM associations with Boletus edulis, B. hoarkii, and Octaviania densa [39].
Sun-lit plant growth chambers, open-top chambers (OTC), and free air concentration enrichment are the three types of experimental setups that are currently being utilized to quantify the effects of ozone on plants. Heagle et al. first launched OTC in the early 1970s; these are extensively adopted monitored-environment systems that use artificially generated air to push ozone into the chambers to maintain ozone concentrations at specified levels [40,41]. In this way, the atmospheric O3 serves as a control and the concentrations inside the chamber can be changed accordingly [42]. This technique helps forecast future O3 impacts. Numerous chemical substances have been tested to shield plants from damage brought on by ambient O3 to prevent chamber effects [43]. These include insecticides, fungicides, growth regulators, inert dust, foliar or soil-applied antioxidants, anti-senescence agents, and fungicides. These materials yielded varying degrees of success, but the majority did not receive further investigation [44].
Many agricultural and tree species have had their ozone sensitivity revealed and evaluated through the use of ethylenediurea (EDU), a common research method [45]. At Du Pont de Nemours and Company in Wilmington, Delaware, Carnahan et al. reported in 1978 that a newly synthesized chemical compound (ethylenediurea, abbreviated as EDU), that consists of phenylurea, N-[2-(2-(2-oxo-1-imidizolidinyl)-N′-phenylurea), protected bean plants (cv Pinto 111) from O3 injury once utilized as a foliar spray. EDU was absorbed by the roots and transported to the shoots. EDU that was given on leaves was absorbed by the leaves but was not transferred to more recent, untreated leaves [40,46]. Phaseolus vulgaris L. bean leaves were less susceptible to O3 damage when phenyl urea was applied topically, according to Tomlinson and Rich's 1974 report. Repeated treatments were therefore required to ensure ongoing defense against O3 damage when new leaves appeared and developed O3 susceptibility. A single foliar application of 500 ppm EDU was the ideal dosage needed to stop O3 damage induced by plant exposure to 80 ppb O3 for 150 min in a chamber following rigorous dose/response tests [47]. The protective capability of EDU against reactive oxygen species (ROS) was observed in wheat [48], carrot [49], olive [50], and spinach [51]. Using EDU in field trials may allow researchers to analyze the effect of CO2 without O3 effects, which would help answer the question of whether O3 wipes out CO2's beneficial effects on plants. Continued crop tests will also allow researchers to determine how ambient levels of O3 impact food sources. EDU is also valuable for assessing and validating O3 harm on plants in-situ when O3 air quality monitoring is unavailable [52].
The aim of this study was to assess the potential impact of combined O3 and N stress on seedling-stage Cedrus deodara, using EDU treatment. Although Cedrus deodara is known to benefit from mycorrhizal associations, particularly with ectomycorrhizal fungi, changes in environmental conditions such as abiotic stresses can adversely affect the ecology of these fungi and ultimately the plants that rely on them. This has led to a decline in forest ecosystems globally, including in Pakistan. The parameters examined in this study encompassed stem height and diameter, as well as the activity of antioxidant enzymes, including superoxide dismutase (SOD), catalase (CAT), and ascorbate peroxidase (APX). Additionally, the extent of visible ozone-induced damage on leaves and the abundance of total proteins were evaluated in response to O3, nitrogen stress, and ectomycorrhizal fungal strains isolated from the rhizosphere of Picea smithiana, C. deodara, and Pinus roxburgii. EDU has the potential to be a useful research tool for analyzing the effects of low- and high-level O3 on crops and forest trees, as well as the combined effect of O3 and N.
2. Materials and methods
2.1. Plant sampling and soil analysis
Two-year-old Cedrus deodara seedlings were collected from the Khyber Pakhtunkhwa Forest Department Abbottabad through the billion-tree afforestation project (Green Growth Initiative). These seedlings were maintained in Biotic and Abiotic Stress, transcriptomic and Proteomics Lab, COMSATS University Islamabad, Abbottabad Campus for one month before experimentation. Seedlings were irrigated regularly with tap water, and phenotypic data collection against the diameter and height of each seedling were measured before and after the experiment. The height of the plants was determined using a 30 cm-stainless steel scale, while the stem diameter was measured using a screw gauge.
2.2. Determination of physiological parameters
The height and diameter of 60 seedlings of each C. deodara were measured. A measuring tape was used to determine the height in centimeters (cm) from the seedling's cotyledon scar to the end of the shoot. The diameter was measured from the cotyledon scar using a micrometer screw gauge (zero error of −0.1 mm corrected) in millimeters (mm).
The temperature was measured by data loggers installed inside chambers, and humidity was measured with the same data logger. The average temperature was determined using the 5-h daily treatment temperature, which was obtained every hour. Prior treatment, the highest average temperature in both the control and treatment chambers was 34.8 °C. After treatment, the highest average 3 h temperature was noticed as 34.27 °C, while in the control chamber, the highest average 3 h temperature was 30.6 °C. Before the treatment, the highest average % relative humidity (RH) in both the control and treatment chamber was 89.7%. After treatment, the highest average of 3 h % of RH was noticed as 90.6%, while in the control chamber, the highest average of 3 % RH was 89.6%.
2.3. Isolation and re-culturing of fungal strains
Rhizosphere fungal strains were isolated from the soil collected from Pinus roxburgii, Cedrus deodara, and Picea smithiana using the serial dilution method, as explained by Asemoloye et al., 2017. Morphological characteristics of isolated fungal strains, such as a colony of isolated fungal strain color, elevation, margin, form, margin, and diameter on PDA, were determined. Molecular identification was made through the amplification of the rDNA of each fungal strain by using NS1 and NS8 primers [39]. Amplified PCR products of each strain were sent to Macrogen, Inc. Seoul, Republic of Korea for sequencing, and sequences of each strain were compared with available sequences at the National Center of Biotechnology Information (NCBI) database using the Basic Local Alignment Search tool (BLAST). Their phylogenetic relationship was studied by constructing a phylogenetic dendrogram. The already isolated fungal strains preserved in −80 °C refrigerator were used for inoculation. These strains were Anthracocystis flocculosa, Trichoderma reesei, Pseudozyma hubeiensis, Trichoderma koningiopsis, and Clonostachys rosea from the rhizosphere of P. roxburgii. Emmialate marginata, Purpureocillium lilacinum, and A. fumigate from the rhizosphere of C. deodara.
2.4. Fungal treatments
For fungal treatment, broth culture was prepared and filtered through a double layer of sterilized gauze. The soil in which seedlings were already grown was removed, and the root systems of seedlings were washed with tap water subsequently and adequately dipped into 3% H2O2 for 30 min for complete sterilization. Afterward, the root system of the seedlings was immersed in broth culture and NaCl-containing broth culture and transferred into the pot filled with autoclaved soil [53]. 10 mL of broth culture was also injected into the soil with injection. Treatments applied as the fungal consortium C1, C2, C3 from the rhizosphere of P. roxburgii, C. deodara, and Picea smithiana respectively.
2.5. Ozone and nitrogen treatment
Nitrogen (N) stress in the form of Ammonium Nitrate (NH4NO3) was given to the seedlings, and stress treatments were applied to each seedling inoculated with the fungal consortium: 0 kg N ha−1 (C) and 100 kg N ha−1 (T) equivalent to 0 mg l−1, and 145 mg l−1 respectively [54]. Plants were grown in triplicates for each stress, and the fumigation of O3 was for 5 h (10:00 a.m.–3 p.m.) daily in open-top chambers (OTCs) of 1.525 m in diameter and 3.048 m tall. Two pipes were connected to the ozone generator (one is a centrifugal air blower, and another is an ozone pipe) and OTCs. The ozone generator was set to 4-sec ozone generation with a time delay of 7 s for attaining 100 ppb while air blowing was continuous. OTCs was designed with a rectangular frame of aluminum with a transparent film cover [55]. The ozone is distributed through a pipe 80 cm above the canopy that releases air and ozone upwardly with some pressure, driven by a centrifugal blower. In the OTCs, O3 concentrations were analyzed by an O3 analyzer (ZA-XM-E-O3 Pump Priming Ozone Monitor). The O3 concentration was measured thrice each hour using an O3 analyzer. The following combinations were used as treatments: Control, C1+N, C2+N, C3+N, C1+O3, C2+O3, C3+O3, C1+N + O3, C2+N + O3, C3+N + O3.
2.6. EDU (ethylenediurea) treatment
Ethylenediurea (EDU) is an antiozonant that functions as an ozone protectant in different studies conducted on crops and model plants. C. deodara seedlings were treated with two dilutions of EDU, i.e., 400 and 600 ppm, along with control. It was applied to the plant as a foliar spray and fumigated with 100 ppb O3 [56].
2.7. Biochemical analysis
2.7.1. Extraction and quantification of chlorophyll contents
For chlorophyll extraction, the acetone method was used. Freeze-dried samples were used for the extraction of chlorophyll. 1 ml of acetone was added to the ground samples (100 mg), followed by shaking for 2 min using a vortex meter. The samples were centrifugated for 2 min, and the supernatant was transferred to a new microcentrifuge tube. The step was repeated by adding 1 ml methanol to a re-extracted pellet. Then the supernatant was mixed with the first supernatant and used for measurements. Absorbance was measured using a UV-110 spectrophotometer at two different wavelengths, i.e., 663 nm and 645 nm. Measurements were done in triplicates. Calculations were done using the method of [57] as follows:
2.7.2. Antioxidant enzyme assay
Leaf samples (150 mg) were mixed and homogenized with 1.25 mL of 100 mM phosphate buffer (pH 7.0) supplemented with 1 mM phenylmethylsulfonyl fluoride (PMSF) and 1% (w:v) polyvinyl-polypyrrolidone (PVPP) to calculate the significant antioxidant enzymatic activities, such as superoxide dismutase (SOD), and catalase (CAT). In the presence of 1 mM ascorbate, ascorbate peroxidase activity (APX) was measured (AsA). The samples were then centrifuged for 20 min at 12,000 g and 4 °C, and the resulting supernatant was used to measure the activity of each antioxidant enzyme at 560, 240, and 470 nm on a spectrophotometer (KONTRON, Milan, Italy).
SOD activity is a photochemical indicator of the enzyme's capacity to prevent the degradation of NBT in the presence of riboflavin. The amount of SOD enzyme needed to suppress NBT oxidation by 50% when light is present is referred to as one unit of the enzyme. The CAT activity was monitored to quantify the H2O2 intake over 3 min at 24 °C. The enzyme concentration required to break down 1 mol of H2O2 in 1-min equals one CAT unit. The quantity of enzyme required to oxidize 1 mol min−1AsA equals one unit of APX activity. The unit of measurement for all enzymatic activity was U/g FW [58].
2.8. Total protein extraction, quantification, and SDS-PAGE
The plant sample was ground finely (100 mg), and then homogenate was put in an ice-cold extraction buffer containing HEPES (100 mM, pH 6.8), NaCl (150 mM), CHAPS (0.1 % W/V), glycerol (10 % V/V), DTT (10 mM) and CaCl2 (50 mM). Centrifugation of plant samples was done for 10 min at 16,000
2.9. Statistical analysis
The experiment was conducted with at least three repetitions of each treatment. Every result was displayed as a mean with standard error bars. Utilizing the statistical analysis program Sigma Plot 11.0 (SPSS Science Software, Erkrath, Germany). By using Dun-multiple Can's comparison and analysis of variance (ANOVA), it was possible to compare the differences between the various treatments. If P
3. Results
3.1. Isolation and characterization of fungal strains
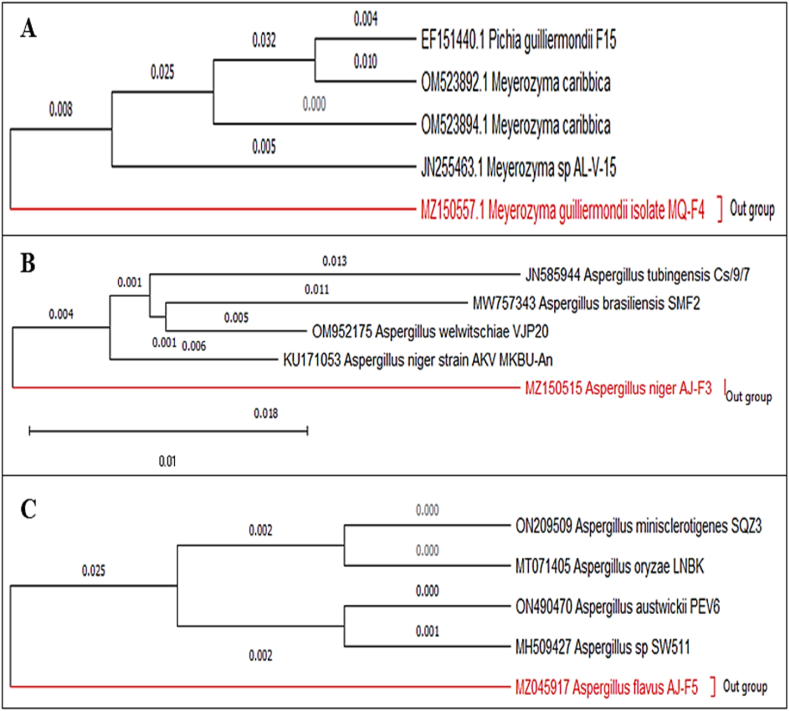
The morphological, microscopic, and molecular characteristics were used to identify the fungal strains that were isolated from Picea smithiana's rhizosphere. The colony form, colour, size, and height on PDA media were used to identify the strains morphologically. Each strain's microscopic properties, including the hyphae type, hyphal aggregates, fruiting bodies, and spores, were observed (Fig. 1). The strains were then identified using the morphological and microscopic characteristics in response to a sequence query on the NCBI database with the highest % similarity (Table 1). The query with the highest query cover and the highest degree of sequence and morphological similarity was chosen. All the new strains were then registered on GenBank (NCBI) and coded as AJ-F2 (Penicillium Cantabricum), AJ-F3 (Aspergillus Niger), MQ-F4 (Meyerozyma Guilliermondii), and AJ-F6 (Aspergillus flavus).

Morphological and microscopic identification of fungal strains isolated from the rhizosphere of Picea smithiana.
Table 1
Characters of fungal strains isolated from Picea smthiana rhizosphere.
| S. No | Strain Code | Strain color (front) | Strain color (back) | Form | Elevation | Diameter |
|---|---|---|---|---|---|---|
| 1 | AJ-F2 | Green | White | Irregular | Raised | 90-mm |
| 2 | AJ-F3 | Green and white | White | Irregular | Raised | 90-mm |
| 3 | MQ-F4 | Grey | Pale Yellow | Irregular | Raised | 30–35 mm |
| 4 | AJ-F6 | Light green | Yellow | Irregular | Flat | 45-mm |
The colony of AJ-F2 after seven days of incubation on PDA was 90-mm in diameter with abundant growth and high density. The form of the colony was circular, margin entire, greenish edges, center green, conidia abundant, reverse white color. For AJ-F3, the diameter was 30–35 mm in diameter, and the color of the colony was blackish with black edges. It was a cotton-like circular colony with entire circled margins.
The fungal strain MQ-F4 had a diameter of 25 mm. It grew as a flat colony with irregular margins. The colony color was grey from the front and yellow from the back. AJ-F5 was 90-mm in diameter, with abundant growth and high density. The form of the colony was circular, margin entire, white edges, center brown, conidia abundant, reverse white color.
3.2. Phylogenetic analysis
Phylogenetic trees were constructed to estimate the relationships among different species and the relationships of the sequences among themselves regardless of the host species. The phylogenetic tree showed a closed relationship between A. niger and A. tubingenesis, Meyerozyma Guilliermondii, Pichia Guilliermondii, and A. flavus to A. Oryza (Fig. 2).
3.3. Physiological parameters (height and diameter)
3.3.1. Effect of treatments on height and diameter
Seedlings’ height and diameter was measured before and after three months of treatment, as shown in Fig. 3 A-B. In the case of plant height, the least significant result was shown by EDU-400 and C3+O3. The highest percent increase in height was shown by C3 seedlings. This indicates that C3 had a positive effect on the growth of C. deodara. The highest significant difference was also shown by C3 seedling.

(A) Represents stem height using different ozone and nitrogen treatments and (B) represents diameter measurement using different ozone and nitrogen treatments.
According to the results below, a significant change in diameter was observed among all treated plants. The diameter of the seedlings treated with EDU-400 and C3+O3 showed the percent change of 1.653% and 0.66% with least significance. This means that EDU with 400 ppm as the foliar spray may positively affect C. deodara seedlings against ozone. The lowest percent change in diameter was shown by the seedling inoculated by Consortium 2 (C2) under ozone stress, i.e., 0.14%.
3.4. Biochemical assay
3.4.1. Effect of treatments on chlorophyll content
From March to August, nitrogen stress, ozone stress, combined ozone and nitrogen stresses, and EDU were applied. After three months of treatment, chlorophyll a and b were measured. The highest total chlorophyll content was measured in C2 which was 13.0 μg/ml while C1+O3 was lowest i.e. 2.43 μg/ml as compared to control. This means that seedlings inoculated with fungal consortium 2 (C2), i.e., Picea Smithiana have a positive impact on the growth of C. deodara. An increase in chlorophyll indicates a high photosynthetic rate [60].
Chlorophyll a and b increased in C2 treated seedlings as compared to control. A significant difference showed treatment effects as compared to the control (Fig. 4A and B).

(A–B) Represents the measurement of chlorophyll a and b with combined N and O3 stresses using C1, C2, and C3 fungal consortium, which differ significantly with p < 0.05.
According to different studies, EDU has been found to be effective as an O3 protectant. Fig. 5 shows that when EDU400-O3 was used, there was an increase in chlorophyll a and a decrease in chlorophyll b content. The total chlorophyll content in plants treated with O3 and 400 ppm EDU increased 14%, indicating that this concentration might be ideal for achieving a high rate of photosynthesis in plants under high O3 stress [61].

(A–B) Represents the measurement of Chlorophyll a, b, and (C) represents total chlorophyll in deodara needles treated with two EDU concentrations and O3.
A significant difference was observed in EDU400-O3 and EDU-400 compared to the control. However, no statistical significance was found among EDU-400 and seedlings treated with EDU and high O3. A decrease in chlorophyll b in EDU-600 treated plant indicates a negative effect of EDU as it is known to be toxic in higher concentrations (Fig. 5-B). Total chlorophyll increase was observed in plants treated with EDU-400 and O3 (Fig. 5-C). There is a significant difference among total chlorophyll concentrations in all treated plants, as indicated by p < 0.05.
3.4.2. Effect of treatments on SOD, CAT, and APX activities
SOD activity is measured in terms of units per milligram of protein (U/mg) in ozone and nitrogen-stressed plants C. deodara. The activity was found to be lower in control compared to the other treatments. There was a significant increase in C2 treated plants than Control. Among N and O3 treatments, EDU600-O3 showed the lowest activity and is significant (Fig. 6-A). EDU-treated C. deodara plants showed less SOD activity than ozone and nitrogen-stressed plants. The order of SOD activity was EDU400-O3> EDU-600 > EDU-400 > EDU600-O3> Control (Fig. 6-B).

(A) Represents SOD activity in ozone, and nitrogen stressed C. deodara plants, (B) shows SOD activity in ozone stressed C. deodara plants applied with EDU.
Catalase activity was highest in control i.e. 3.1 U/g FW, whereas the lowest was in C2+N as 1.1 U/g FW (Fig. 7-A). When treated with EDU against O3, a least significant increase was seen in control plants and EDU400-O3 treated seedlings showed an increase in CAT activity (Fig. 7-B).

(A) Represents catalase activity in ozone, and nitrogen stressed C. deodara plants, (B) shows Catalase activity in ozone stressed C. deodara plants applied with EDU.
All C. deodara seedlings showed increased APX activity compared to the control (Fig. 8). The highest activity was shown by plants inoculated with C2, which coincides with SOD. This shows a significant difference in APX activity in C2 and C1 compared to the control. While high nitrogen and ozone had no significant difference when applied in combination with fungal consortiums. It was evident from Fig. 8-A that C3, along with 100 ppb O3, was also significantly different from the control. A significant increase in APX activity was observed in EDU400 and EDU600 compared to the control. In contrast, no significant differences were observed between EDU400-O3 and Control (Fig. 8-B).
3.5. Total protein quantification
Protein content was measured in μg/mL by plotting a bovine serum albumin standard curve (Fig. 9-A) using the Bradford assay. After six months of the experiment, it was found that the highest percent increase in total protein content was measured in the consortium (C1+C2+C3), followed by nitrogen treatment with seedlings inoculated with the consortium. The lowest protein content was seen in seedlings treated with EDU-600 compared to the control. Among EDU-treated plants, high total protein content was observed in EDU fumigated with O3. The quality of total protein content was measured through SDS-PAGE electrophoresis, and the highest protein content was shown in a consortium in Fig. 9-B. In contrast, the protein content was lower in the control C. Different protein bands of variable intensities can be seen in Fig. 9-C.
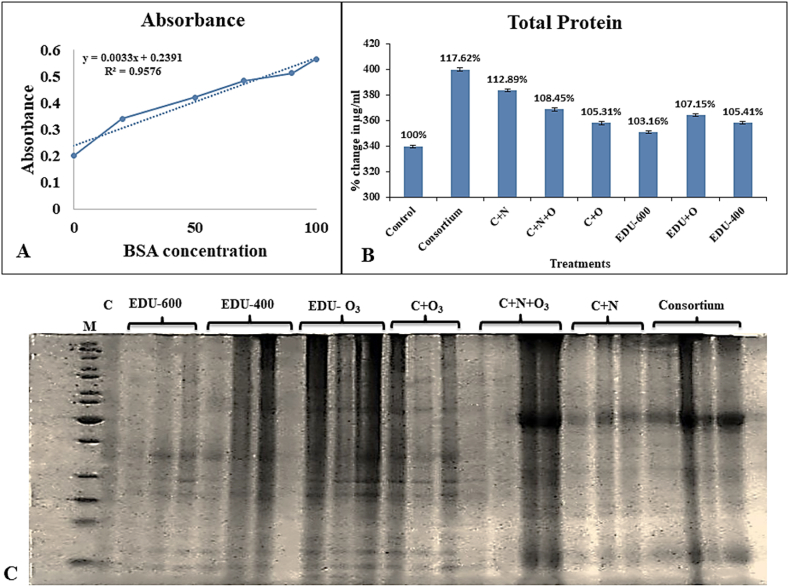
(A) Represents the Bovine Serum Albumin Standard Curve, (B) represents data labels on average total protein content after six months of stress using different treatments. Numerical show percent change among treatments, (C) represents protein visualization using SDS PAGE with different sizes of proteins.
4. Discussion
This study aims to identify the effect of ozone and nitrogen stress in combination to see the effect on growth of deodar seedlings. Also, fungal species from the rhizosphere of different conifer trees were isolated and applied to the conifer plant seedlings as a consortium. Compared to the control, all treated plants have shown an increase and decrease in plant height and diameter respectively. Among all the treatments, EDU-400, C3, and C3+ O3 had shown an increase in height (Fig. 3-A). A little or no difference was seen in the diameter because the growth of conifer trees is slow. Among other treatments, there was a slight difference in height and diameter. As suggested by Feng [62] biomass, shoot height, shoot diameter, and leaf size all experienced significant declines due to high O3, which ranged from 83 to 150 ppb. It was reported that plant biomass increased when inoculated with fungal stains compared to those grown without inoculation, which clearly showed that fungal strain significantly affects pines’ growth [63]. It was also reported that different isolated fungal stains had different effects on the plant biomass [64]. According to models that use tree physiology and forest stand mechanics, the indirect impacts of O3 on development may increase over time. Additional stresses such as prolonged drought and excessive nitrogen deposition may amplify these effects, and antagonistic interactions between species may change [4].
Ozone enters into the plant system and disrupts the cellular machinery resulting in altered chlorophyll content, stomatal conductance, and photosynthetic rate. Decreased chlorophyll in plants is one of the most common effects of O3 stress [65]. The chlorophyll and carotenoid contents in the leaves of Toona sinensis seedlings reduced under drought stress [66]. Degradation of chlorophyll is associated with lipid peroxidation of chloroplast membranes and degradation with ribulose 1,5-bisphosphate carboxylase [67]. Membrane lipid peroxidation can cause membrane permeability change, and reactive oxygen molecules can enter the chloroplast through membrane channels or infiltration [68]. In various review publications, the process of O3 impacts on plant activity has been outlined [51,69]. Stomatal closure is a significant reaction to O3 consumption, even though stomata may respond more slowly than in fresh air (i.e., stomatal unresponsiveness) [70]. In this study, a variation in overall chlorophyll content was observed. Among chlorophyll a and b, an increase was observed among seedlings treated with C2. This means that C2 positively affected the growth of deodara seedlings. The lowest content was shown in C3+O3, i.e., O3 stress (Fig. 4). The combined effect of O3+ N reduced the seedlings’ chlorophyll a and b contents. In poplar clones, elevated O3 reduced leaf N concentration in the photosynthetic apparatus, which seems consistent with our results [13].
Among total chlorophyll content, the highest increase was shown by C2. This means that C2 positively affects the growth of deodara inoculated with fungal strain. C2+N showed the least because of nitrogen stress. These results are consistent with the conclusions of Watanabe which studied the risk of O3 and N stress on the sensitivity of F. crenata, Cryptomeria japonica, Pinus densiflora, and Larix kaempferi [24]. Utriainen and Holopainen [71] studied the O3 stress effect of Pinus sylvestris seedlings under N application conditions, and the results showed that N application decreased shoot and root dry mass under elevated O3 stress. However, there was also no significant interaction between the N-applied effect on photosynthetic pigments and the O3 inhibitory effect. The effects of O3 and N treatment on plant photosynthetic pigments may be independently regulated by different physiological mechanisms in plants [72,73].
The harmful effect of ozone injury can be overcome through EDU by its scavenging activity on physiological parameters and antioxidant-mediated defense reaction [74]. In the present study, elevated ozone concentration was 100 ppb daily during the 5-h treatment period (10:00 a.m.-3:00 p.m.). Most Asian countries like China, India, and Bangladesh are experiencing high levels of ozone concentration during crop-growing seasons [75]. Currently, China is the largest emitter of NOx, which is ozone precursor gas and daily ozone concentration reaches 50 ppb in some regions during the cropping season. Among other significant countries in Asia, India has reported an eight-days ozone concentration of 100 ppb during the spring season [76]. An increase was observed in chlorophyll a and b among the seedlings treated with EDU alone and with ozone stress. These results are in according with the study of [40] in which they observed an increase in the chlorophyll content in rice. Knudson [77] studied the effect of ozone on P. Vulgaris and suggested a correlation between decreased chlorophyll content and visible ozone injury.
Antioxidant enzymes are pivotal in scavenging the free radicals produced due to biotic and abiotic stresses [78]. The activity of antioxidant enzymes is related to stress and the subsequent production of free radicals [79]. SODs are the metalloproteins that catalyze the dismutation of superoxide free radicals (O−2) to H2O2 and O2 and are considered the first line of defense against oxidative damage caused by superoxide radicals [80].
Under ozone and nitrogen stress, C. deodara plants showed antioxidant enzyme activity. SOD activity was observed to be higher in treatments than in control. The change in SOD activities were in this order: C2, C2+N + O3, C3+N + O3, and C3+O3> Control and were highly significant. While among treatments, no significance was observed. This trend was also previously observed by Santos et al., who concluded that cowpea plants showed higher SOD activity in salt-stressed plants than in normal ones [81]. During oxidative stress, SOD activity increases mostly due to an increase in superoxide radicals. During normal conditions, the conversion of superoxide into hydrogen peroxide occurs due to the action of SOD [82]. This study showed an increase in SOD activity in C2 with a significant effect compared to the control and follows Xiong [83] who also studied an increase in SOD activity when inoculated with ECM fungi. Present results also showed increased SOD activity in C. deodara seedlings inoculated with C2+N + O3. The results of SOD activity in this study was also in accordance with previous studies by [84,85], in which SOD activity was increased when inoculated with ECM and significantly impacted root activity. Yet, an increase in antioxidant activity is vital, no matter what the season should be.
Ascorbate peroxidase (APX) also showed higher activity in C2 treated plants. A significant increase in APX inoculated with C2 was observed while in Control plant this activity was minor. Thus, highest APX activity was seen in the following order of seedlings: C2>C1>C3+O3. EDU is an antiozonant that showed its activity against ozone; hence, EDU-treated plants required less APX activity than ozone and nitrogen-stressed plants. A significant difference was observed in EDU and O3-treated plants (EDU400-O3 and EDU600-O3) compared to control, and no significance was observed in APX among plants treated with EDU (Fig. 8-B). These results were in accordance with the results obtained by Gupta and Tiwari in which they recently concluded that under ozone and nitrogen stress in tropical legumes, APX activity was higher than in typical plants [86]. According to [87], APX activity increases in an ozone-exposed plant without the application of EDU. But, when EDU was applied to bean plants exposed to high O3, a decrease in antioxidant enzymes was seen, especially in APX activity. Their result suggests that EDU positively impacts bean plants’ antioxidant enzymes.
The activity of CAT was observed to be the highest in C2 treatments. These findings implied that since the control plant was not exposed to any stress, antioxidant enzyme activity was the least in it. Ozone and nitrogen-stressed plants showed higher activities than EDU-treated ones because, in these treatments, an antiozonant (EDU) is already present to cope with the stress. These results followed those obtained by Pandey et al., 2018 who observed that catalase activity in Triticum aestivum under ozone and nitrogen stress was much higher than in non-stressed plants [88].
Total protein content showed high values in consortium-inoculated seedlings compared to control group, indicating that the consortium had a positive impact on C. deodara under fungal application (Fig. 9B and C). Minor stress variations can cause significant proteome changes, indicating damage or adaptation [89]. In parallel, the lowest protein content was observed in seedlings with EDU-600. The optimum EDU concentration for protecting plants from O3 damage is 500 ppm. Abiotic stress exposure to plant cells results in an array of differences in protein expression levels. Several factors affect protein expression under stress conditions, such as targeting or translocation of protein and post-translational modifications [90]. Exposure to stress regulates the group of functionally related proteins, which includes metabolism-related proteins, storage, and protein synthesis are not directly involved in defense mechanisms [91]. When a plant undergoes stress, it starts producing misfolded, and cysteine proteases degrade aggregated proteins. Still, over-expression of these genes may lead to the degradation of valuable proteins, so their hemostasis needs to be maintained [92].
5. Conclusions
This study demonstrated the impact of high ozone and nitrogen pollutants on Cedrus deodara seedling in open top chamber and how the ECM association and EDU help C. deodara to overcome these stresses. The increase in chlorophyll content, SOD and CAT activities implies positive effect of fungal consortium on C. deodara plants. A significant difference was also observed in EDU and O3-treated plants (EDU400-O3 and EDU600-O3) as compared to control, and no significant difference was observed in APX in EDU-treated plants. In conclusion, this study highlights for the first time the importance of selected ECM fungi species and EDU to reduce the high ozone and nitrogen stresses’ impact on C. deodara and both could be used for the propagation of C. deodara in forest ecosystem. However, it is suggested to perform long term experiments using EDU and ECM and study the EDU mode of action to reduce O3 stress at molecular level. Clarifying the precise mechanisms underlying the reported effects, investigating new stressors, and evaluating long-term consequences on forest ecosystems should be the main goals of future research.
Funding
This study was financially supported by the Higher Education Commission (HEC) of Pakistan in National Research Program for Universities (NRPU) projects with project No: 531 1/Federal/NRP U/R&D/HEC/2016.
Data availability statement
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
CRediT authorship contribution statement
Hafiz Muhammad Ansab Jamil: Writing – original draft, Formal analysis, Data curation. Mansour K. Gatasheh: Writing – review & editing, Visualization, Software, Funding acquisition. Rafiq Ahmad: Writing – review & editing, Validation, Supervision, Resources, Project administration, Methodology, Formal analysis, Conceptualization. Khalid Elfaki Ibrahim: Writing – review & editing, Funding acquisition. Sabaz Ali Khan: Writing – review & editing, Resources, Methodology, Investigation. Usman Irshad: Writing – review & editing, Resources, Methodology. Muhammad Shahzad: Writing – review & editing, Software, Methodology, Formal analysis. Arshad Mehmood Abbasi: Writing – review & editing, Software, Resources, Funding acquisition, Formal analysis, Conceptualization.
Acknowledgments
The authors extend their appreciation to the Researchers Supporting Project number (RSP2024R393), King Saud University, Riyadh, Saudi Arabia for providing article publication charges.
References
Articles from Heliyon are provided here courtesy of Elsevier
Full text links
Read article at publisher's site: https://doi.org/10.1016/j.heliyon.2024.e28635
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Identification and inoculation of fungal strains from Cedrus deodara rhizosphere involve in growth and alleviation of high nitrogen stress.
Saudi J Biol Sci, 27(1):524-534, 26 Nov 2019
Cited by: 2 articles | PMID: 31889878 | PMCID: PMC6933180
Ethylenediurea protects against ozone phytotoxicity not by adding nitrogen or controlling stomata in a stomata-unresponsive hybrid poplar.
Sci Total Environ, 875:162672, 08 Mar 2023
Cited by: 2 articles | PMID: 36894106
Ethylenediurea offers moderate protection against ozone-induced rice yield loss under high ozone pollution.
Sci Total Environ, 806(pt 3):151341, 30 Oct 2021
Cited by: 8 articles | PMID: 34728207
Use of the antiozonant ethylenediurea (EDU) in Italy: verification of the effects of ambient ozone on crop plants and trees and investigation of EDU's mode of action.
Environ Pollut, 157(5):1453-1460, 01 Nov 2008
Cited by: 27 articles | PMID: 18977576
Review
Funding
Funders who supported this work.

![[low asterisk]](/http/europepmc.org/corehtml/pmc/pmcents/x204E.gif)