Abstract
Background
Understanding factors affecting the size and the evolution of the HIV reservoir is essential for the development of curative strategies. This study aimed to assess the impact of antiretroviral therapy (ART) initiated during primary infection (PHI) vs chronic infection (CHI) on the levels and dynamics of integrated HIV-1 DNA, a biomarker of viral persistence.Methods
Integrated and total HIV-1-DNA were measured in the blood of 92 patients treated during PHI (early group) and 41 during CHI (deferred group), at diagnosis, ART initiation, and 12-24 months on treatment.Results
On ART, detectable (>1.78 log10 copies/106 PBMCs) integrated HIV-1 DNA levels were significantly lower in the early vs deferred group (2.99 log10vs 3.29 log10,p = 0.005). The proportion of undetectable integrated HIV-1 DNA tended to be higher in the early group vs deferred group (61 % vs 46 %; p = 0.133).Conclusion
Treatment initiated at PHI limits the levels of integrated HIV-1 DNA in blood. However, initiating treatment at CHI does not allow reaching such low levels in most patients, probably because the stable proviruses at that stage are present in the less prone to elimination long-lived cells. Thus, early ART could provide an opportunity to preparing for functional cure and eradication strategies.Free full text

The build-up of stock of stable integrated proviruses overtime explains the difficulty in reducing HIV-1 DNA levels when treatment is initiated at the chronic stage of the infection
Abstract
Background
Understanding factors affecting the size and the evolution of the HIV reservoir is essential for the development of curative strategies. This study aimed to assess the impact of antiretroviral therapy (ART) initiated during primary infection (PHI) vs chronic infection (CHI) on the levels and dynamics of integrated HIV-1 DNA, a biomarker of viral persistence.
Methods
Integrated and total HIV-1-DNA were measured in the blood of 92 patients treated during PHI (early group) and 41 during CHI (deferred group), at diagnosis, ART initiation, and 12–24 months on treatment.
Results
On ART, detectable (>1.78 log10 copies/106 PBMCs) integrated HIV-1 DNA levels were significantly lower in the early vs deferred group (2.99 log10vs 3.29 log10,p = 0.005). The proportion of undetectable integrated HIV-1 DNA tended to be higher in the early group vs deferred group (61 % vs 46 %; p = 0.133).
Conclusion
Treatment initiated at PHI limits the levels of integrated HIV-1 DNA in blood. However, initiating treatment at CHI does not allow reaching such low levels in most patients, probably because the stable proviruses at that stage are present in the less prone to elimination long-lived cells. Thus, early ART could provide an opportunity to preparing for functional cure and eradication strategies.
1. Introduction
Combined antiretroviral therapy (ART) improved drastically the life expectancy of people living with HIV-1 (PLWHIV). While it suppresses HIV-1 replication to undetectable levels and prevents disease progression, it is unable to eradicate the virus due to the early establishment of reservoirs, in particular in resting CD4+ T cells.1,2 Thus, adherence to treatment in PLWHIV is crucial to prevent virological rebound.
Efforts are deployed worldwide to find curative strategies aimed at reducing viral reservoirs and reinforcing specific immune responses in order to achieve control of viral replication upon ART withdrawal (functional cure). Therefore, understanding the factors affecting the size and the evolution of reservoirs is critical for the development of such strategies.
Total HIV-1 DNA is frequently quantified in cohorts of patients to estimate the size of HIV-1 reservoirs because it is clinically relevant at different stages of the infection.3, 4, 5 It is composed of several forms: 1 and 2-long terminal repeats DNA (1-LTR and 2-LTR), linear unintegrated HIV-1 DNA and integrated HIV-1 DNA. The latter constitutes the stable form of viral persistence and plays a crucial role in the HIV-1 pathogenesis, even if it includes mainly defective genomes.3,6,7
Several studies have described a multiphasic decay of total HIV-1 DNA on ART with a first phase characterized by a rapid decline followed by a slower decrease.8, 9, 10, 11 Treatment initiation in primary infection (PHI) results in lower total HIV-1 DNA levels than when initiated in the chronic stage (CHI).12 However, only scarce studies have analysed the kinetics of integrated HIV-1 DNA on ART and compared the impact of early and deferred treatment on the levels of reservoir biomarkers.12, 13, 14, 15 They include a relatively limited number of patients, which could explain some conflicting results between studies. Moreover Massanella et al. did not compare the levels of integrated HIV-1 DNA between early and deferred treatment groups after several months of treatment.12
We have showed in a previous study in untreated patients that the peak of total HIV-1 DNA during PHI is mainly constituted of unintegrated forms,16 and that HIV-1 DNA is located mostly in short-lived cells with low proliferative capacity at this stage.17,18 During the first year of infection, total HIV-1 DNA levels progressively increase, as does the proportion of integrated forms.16 We have suggested models of HIV-1 DNA dynamics on treatment, depending on the timing of ART initiation, to explain the difference of decay of total HIV-1 DNA in blood when treatment is initiated either in PHI or during CHI. We have hypothesized that the introduction of treatment at PHI, when the integrated HIV-1 DNA level is low and mainly in short-lived cells, not only decreases rapidly total HIV-1 DNA but could also limit the establishment of integrated HIV-1 DNA forms, the stable and most functional form of HIV-1 DNA, in long-lived cells.16 In contrast, we have suggested that the introduction of treatment at CHI, when integrated forms are predominant among total HIV-1 DNA and established in long-lived central memory T-cells, does not permit the decay of the stable proviruses load.16 We have then assumed that early treatment initiation halts the establishment of integrated HIV-1 and stops the evolution of the CD4+ T-cell subset contributions to the HIV-1 reservoirs; these remain mainly composed of short-lived cells when ART is initiated during PHI,17,18 while long-lived cells with high proliferative capacity are the major contributor to HIV-1 DNA when ART is initiated during chronic infection.18,19 This difference might explain the ongoing decrease in total HIV-1 DNA after several years of treatment when ART is initiated at the time of PHI.16
In order to better describe the impact of the ART initiation timing on the dynamics of total HIV-1 DNA and integrated HIV-1 DNA and to evaluate the validity of the previously proposed hypotheses, we have conducted a comparative study between two groups of patients treated either at PHI or during CHI by quantifying the levels of these two biomarkers before and after treatment initiation and by evaluating their kinetics.
2. Material and methods
2.1. Study participants
Patients were selected from the OPTIPRIM 2-ANRS-169 trial and the ANRS-PRIMO CO6 cohort. Both studies were approved by the regional ethics committee (CPP Ile de France) and all participants have given their written consent.
The OPTIPRIM 2-ANRS-169 trial is a multicentric, open-label, phase 3 trial that included patients diagnosed at PHI and treated within a median of two days from diagnosis. Patients were randomized into two groups: group 1 received once-daily dolutegravir plus tenofovir/emtricitabine and group 2 received once-daily darunavir/cobicistat plus tenofovir/emtricitabine.20 Samples taken at baseline and at 12 months after ART initiation were retrieved for analysis. Patients from this trial who consented to be included into the ANRS-PRIMO CO6 cohort and constitute “the early group” of the present study. Both arms were considered within the early group since no difference was observed in the primary outcome of the trial (i.e. total HIV-1 DNA levels).
The ongoing French ANRS-PRIMO cohort enrols patients presenting with PHI, as previously described.9 All are treatment-naïve at inclusion. Patients enrolled in the present study from the PRIMO cohort were those with triple therapy initiated at the chronic phase (24–60 months post-acute infection diagnosis) and who had available blood samples at inclusion into the cohort, at treatment initiation (within a 6 month-interval before) and at 12–24 months post-treatment initiation while still on ART. These patients constitute “the deferred group”. The gender of participants was defined based on self-reporting.
2.2. Analytical methods
Total HIV-1 DNA was quantified in duplicate in thawed whole blood samples using ultrasensitive-real time PCR with Generic HIV-1 DNA Cell kit (Biocentric, France).21 Integrated HIV-1 DNA was measured in quadruplicate with an in-house nested Alu-LTR PCR assay, using the HelaR7Neo cell line as previously described.16 Both results (total HIV-1 DNA and integrated HIV-1 DNA) are reported as log10 copies of HIV-1 DNA (total or integrated) per 106 PBMCs. The quantitation threshold varied depending on the number of available cells tested.16 Integrated HIV-1 DNA loads were reported as undetectable when they were under the highest limit of quantification reported in this study (60 copies/106 PBMCs; 1.78 log10).
2.3. Statistical analysis
The continuous variables were expressed as median with its interquartile range (IQR). For continuous variables, the Mann-Whitney test was used to compare unpaired data set-points and the Wilcoxon matched-pairs signed-rank test for paired values. For categorical variables, Fischer's exact test was used. To assess the viral decay of total HIV-1 DNA, the kinetics was evaluated by calculating the difference between the viral loads at the different set points for each patient. For the early group, the difference was calculated between treatment initiation (at the time of diagnosis in PHI) and at one year after. For the deferred group, the difference was calculated between diagnosis and treatment initiation and between treatment initiation and at one to two years after for each patient. The statistical analysis was repeated after retrieving patients who had a positive viral load (RNA load>50 copies/mL) at the time of HIV-1 DNA quantification while on ART. The statistical analysis was performed using GraphPad Prism 9.3.1 (350), GraphPad Software LLC.
3. Results
3.1. Study participants
Overall, 92 patients who had received treatment during PHI were considered in the early group and 41 who had received treatment at the chronic stage were considered in the deferred group. Out of the 92 patients from the early group, 45 had received a dolutegravir-based regimen and 47 received a darunavir plus cobicistat-based regimen. More than half of patients from the deferred group (n = 23) were on a regimen including two nucleoside/nucleotide reverse-transcriptase inhibitors (NRTIs) and a protease inhibitor (including 11 with darunavir), 16 patients were on a combination of two NRTIs and one non-nucleoside reverse transcriptase inhibitor (NNRTI) and the last two patients were on two NRTIs and an integrase strand transfer inhibitor (INSTI). Patient baseline characteristics are depicted in Table 1. Most were male (93 % for the early group and 86 % for the deferred one). Treatment was initiated at a median of 36.40 months [IQR 30.12; 47.40] from diagnosis for the deferred group and follow-up samples were taken at a median of 24 months18,22 on ART. The median CD4+ T-cell count at diagnosis was 436 cells/μL [315; 609] for the early group and 688 cells/μL [488; 873] for the deferred group. At treatment initiation, the CD4+ T-cell count was 403 [333; 530] cells/μL for the deferred group. At follow-up, 86 patients from the early group (93 %) and 38 (93 %) from the deferred group presented with an undetectable HIV-1 RNA load (<50 copies/mL) (p = 0.72).
Table 1
Baseline characteristics of the participants.
| Variable | Early group (n = 92) | Deferred group (n = 41) | |
|---|---|---|---|
| At diagnosis | At diagnosis | Before ART | |
| Age in years | 36 [28; 49] | 35 [29; 42] | |
| Male gender (%) | 93 | 86 | |
| Time between diagnosis and initiation of treatment | 1 [0; 4] days | 36·40 [30·12; 47·40] months | |
| Plasma HIV-1 RNA load (log10 copies/mL) | 5·81 [5·08; 6·55] | 4·89 [4·10; 5·34] | 4·51 [4·15; 4·86] |
| Total HIV-1 DNA level (log10 copies/106 PBMCs) | 3·90 [3·53; 4·19] | 3·31 [2·87; 3·57] | 3·50 [3·22; 3·82] |
| Undetectable integrated HIV-1 DNA (%) | 51 | 49 | 41 |
| Detectable integrated HIV-1 DNA level (log10 copies/106 PBMCs) | 3·81 [3.13; 4.26] | 2·85 [2·31; 3·22] | 3·88 [3·10; 4·07] |
| CD4+ T-cell count (cells/μL) | 436 [315; 609]b | 688 [488; 873] | 403 [333; 530]b |
| CD8+ T-cell count (cells/μL) | 848 [529; 1294]b | 1115 [749; 1293]a | 1301 [916; 1532] |
| CD4+/CD8+ ratio | 0·51 [0·32; 0·73]b | 0·67 [0·42; 0·88]a | 0·30 [0·25; 0·44]b |
Categorical data are presented as frequencies (N) with their corresponding percentages (%). Continuous variables are presented as median with interquartile range (Me [Q1; Q3]).
3.2. HIV-1 DNA levels at baseline
At the time of primary HIV-1 infection, the median total HIV-1 DNA in the early group was 3.90 [3.53; 4.19] log10 copies/106 PBMCs and significantly higher than in the deferred group, 3.31 [2.87; 3.57] log10 copies/106 (p<0.001). Total HIV-1 DNA levels increased from diagnosis to 3.50 [3.22; 3.82] log10 copies/106 PBMCs at treatment initiation in the deferred group (p = 0.005). This level of HIV-1 DNA remained however significantly lower than that of the early group at the time of treatment initiation (p < 0.001) (Fig. 1a).

Baseline (Fig. 1a and b) and follow up after ART initiation (Fig. 1c and d) of total HIV-1 DNA and integrated HIV-1 DNA for patients treated early in the primary infection (in green) and for patients treated during the chronic phase (in blue). Levels are expressed as log10 copies/106 PBMCs. The bars represent median and interquartile range values. Undetectable levels of integrated HIV-1 DNA were excluded. Unpaired continuous variables were compared using Mann-Whitney test and paired variables were compared using Wilcoxon matched-pairs signed rank test. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Forty-seven patients (51 %) from the early group presented with undetectable levels of integrated HIV-1 DNA at diagnosis vs 20 patients (49 %) in the deferred group (p > 0.999). This percentage tended to decrease between diagnosis and treatment initiation for the deferred group (49 % vs 41 %). When detectable, levels of integrated HIV-1 DNA at diagnosis were higher in the early group vs the deferred group, 3.81 [3.13; 4.26] vs 2.85 [2.31; 3.22] log10 copies/106 PBMCs, p < 0.001. We also noted a significant rise in levels of integrated HIV-1 DNA in the deferred group between diagnosis and treatment initiation, reaching median levels at ART initiation of 3.88 [3.10; 4.07] log10 copies/106 PBMCs (p=0.001). This level of integrated HIV-1 DNA did not significantly differ from that of the early group (p = 0.649) (Fig. 1b).
3.3. Levels of total and integrated HIV-1 DNA during ART
After 12 months of treatment for the early group and a median of 24 months18, 19, 20, 21, 23, 24, 22 for the deferred group, the levels of total HIV-1 DNA differed significantly between both groups, with a median of 2.43 [2.14; 2.80] log10 copies/106 PBMCs in the early group vs 2.96 [2.68; 3.30] log10 copies/106 PBMCs in the deferred group (p < 0.001) (Fig. 1c). The proportion of undetectable integrated HIV-1 DNA tended to be higher in the early group vs the deferred one (61 % vs 46 %; p = 0.133). Similarly to total HIV-1 DNA levels, detectable integrated HIV-1 DNA levels were significantly lower in the early group (2.99 [2.45; 3.23] log10 copies/106 PBMCs vs 3.29 [2.89; 3.71] log10 copies/106 PBMCs) (p = 0.007) (Fig. 1d).
Considering antiretroviral (ARV) regimens, in the early group there was no statistical difference in the levels of detectable integrated HIV-1 DNA between participants receiving dolutegravir (n = 17) and those receiving darunavir- (n = 19) based regimens (3.00 vs 2.99 log10; p = 0.611), similarly to what was described for total HIV-1 DNA in the princeps analysis of the trial (20). In the deferred group, no difference was observed in detectable integrated HIV-1 DNA levels between patients receiving a protease inhibitor (PI)- (n = 11) vs a NNRTIs-based regimen (n = 10) (3.27 vs 3.32 log10 copies/106 PBMCs; p = 0.69). Similarly, levels of total HIV-1 DNA did not differ between both groups (2.96 vs 2.91 log10 copies/106 PBMCs respectively; p = 0.983). When comparing patients receiving PI-based regimens from deferred vs early groups, we noted higher levels of both total and detectable integrated HIV-1 DNA in the deferred group (2.96 vs 2.54 log10 copies/106 PBMCs; p = 0.002 for total HIV-1 DNA and 3.27 (n = 11) vs 2.99 (n = 19) log10 copies/106 PBMCs; p = 0.045 for detectable integrated HIV-1 DNA, respectively). No difference was seen in the loads of both total and detectable integrated HIV-1 DNA according to the ART timing (within 36 months from diagnosis vs later) and baseline CD4+ levels (below vs above 400/mm3) in the deferred group (data not shown).
3.4. Total and integrated HIV-1 DNA evolution
Total HIV-1 DNA decreased between treatment initiation and 12 months of ART in the early group: the median decrease was −1.39 [−1.73; −0.37] log10 copies/106 PBMCs (Fig. 2a and b). Total HIV-1 DNA increased between diagnosis and ART initiation in the deferred group (+0.27 [0.15; 1.1] log10 copies/106 PBMCs). Once ART was initiated, the marker decreased by a median of −0.54 [−0.67; −0.37] log10 copies/106 PBMCs, significantly less than what was observed in the early group (p < 0.001) within a shorter time interval. Overall, the decrease from diagnosis to 24 months on ART in the deferred group was −0.29 [−0.64; 0.55] log10 copies/106 PBMCs (Fig. 2). Same conclusions were reached when excluding patients with HIV-1 RNA load >50 copies/mL on ART.
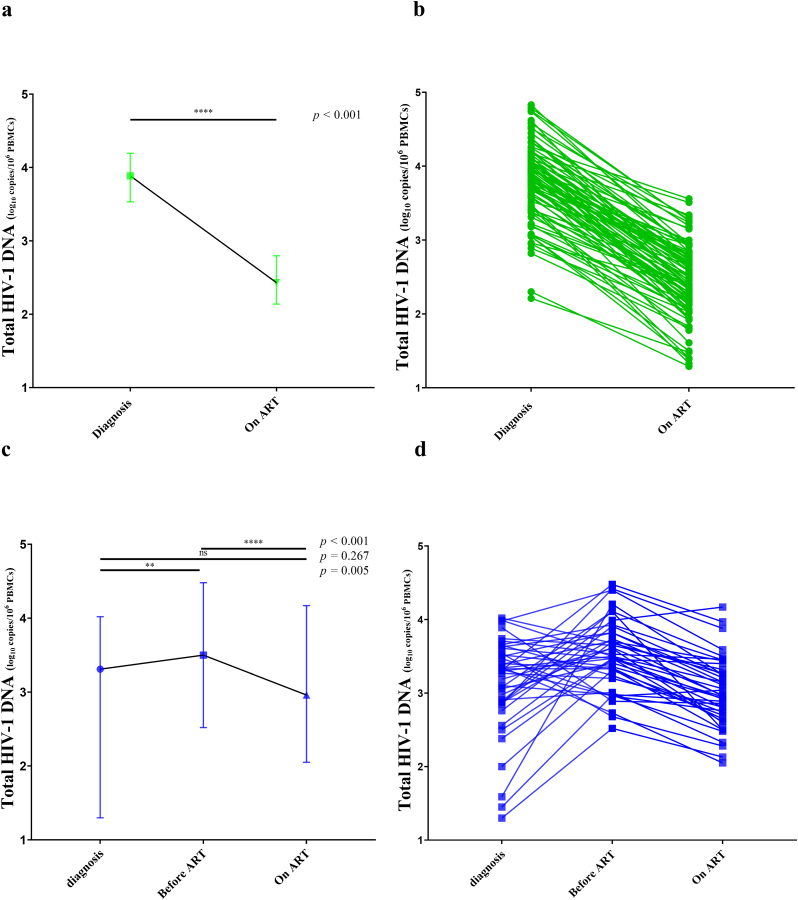
Total HIV-1 DNA evolution. Levels of total HIV-1 DNA are expressed as log10 copies/106 PBMCs. Fig. 2a and c represent the evolution of total HIV-1 DNA loads expressed as medians. The bars in Fig. 2a and c represent median and interquartile range values. Fig. 2b and d depict individual evolution of total HIV-1 DNA loads for early (2b) and deferred groups (2d). Unpaired continuous variables were compared using the Mann-Whitney test and paired variables were compared using Wilcoxon matched-pairs signed-rank test.
Integrated HIV-1 DNA kinetics on treatment also differed between both groups. All patients from the early group with undetectable levels of integrated HIV-1 DNA at diagnosis (n = 46) remained undetectable 12 months on ART. Among 46 patients with detectable levels at diagnosis, 10 patients (22 %) became undetectable on ART. For others (n = 36), levels of detectable integrated HIV-1 DNA significantly decreased from diagnosis to 12 months on ART for the early group with a median of −0.96 [−1.47; −0.43] log10 copies/106 PBMCs.
Among 24 patients from the deferred group who presented with detectable integrated HIV-1 DNA at treatment initiation, two (8 %) had undetectable integrated HIV-1 DNA levels on ART. Levels of detectable integrated HIV-1 DNA were significantly lower at a median of 24 months on ART when compared with levels at treatment initiation. For patients with detectable levels at both time points (n = 22), integrated HIV-1 DNA levels decreased with a median of −0.29 [−0.68; −0.09] log10 copies/106 PBMCs. Among them, only 4 showed a decrease greater than 0.5 log in the levels of this reservoir marker. The others showed small to no decline of integrated HIV-1 DNA on ART. Globally, levels of detectable integrated HIV-1 DNA increased between diagnosis and on ART with a median of +0.44 [0.19; 0.87] log10 copies/106 PBMCs for patients with detectable levels on ART (n = 19) (Fig. 3).
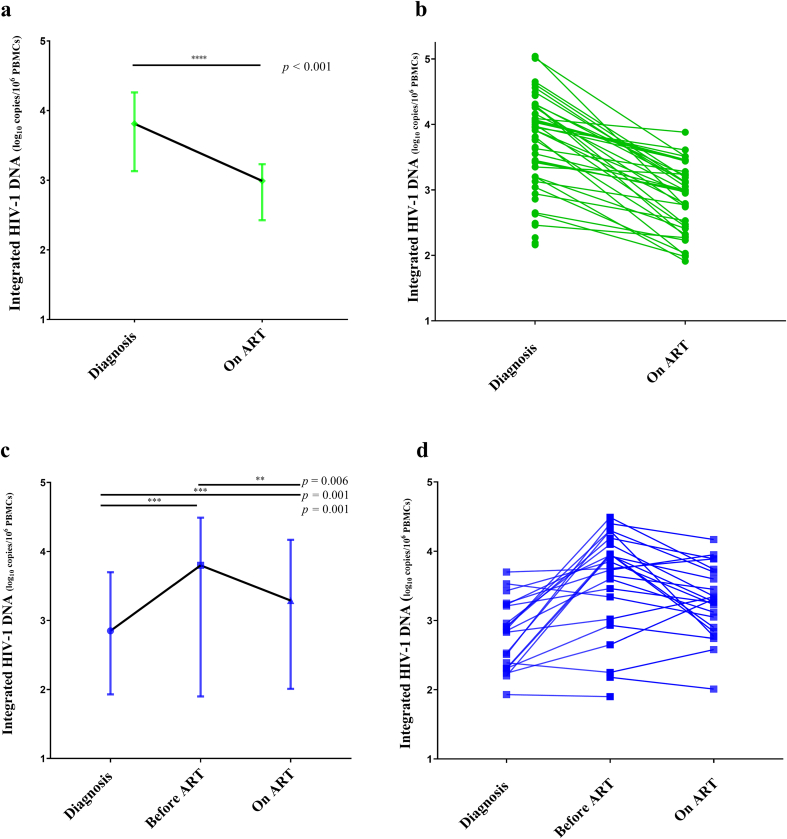
Integrated HIV-1 DNA evolution. Levels of integrated HIV-1 DNA are expressed as log10 copies/106 PBMCs. Undetectable levels of integrated HIV-1 DNA are not represented and were excluded from statistical analysis. Fig. 3a and c represent the evolution of integrated HIV-1 DNA loads expressed as medians and 3b and 3d depict individual evolution of integrated HIV-1 DNA. The bars in Fig. 3a and c represent median and interquartile range values. Unpaired continuous variables were compared using the Mann-Whitney test and paired variables were compared using the Wilcoxon matched-pairs signed-rank test.
4. Discussion
Understanding factors affecting the size and composition of HIV-1 persistence markers is crucial for developing new therapeutic strategies aiming at functional cure. ART halts viral replication but does not eradicate the HIV-1 reservoir. However, several studies have demonstrated that initiating ART during acute infection limits the size of total HIV-1 DNA.9,11, 12, 13 In this study, we aimed to assess the impact of timing of ART on the kinetics and levels of integrated HIV-1 DNA, the most stable persistent form of total HIV-1 DNA.
To our knowledge, this is the largest study to compare the effect of early vs deferred treatment initiation on HIV-1 persistence markers, both total and integrated HIV-1 DNA. Existing studies were more interested in assessing the impact of early and chronic treatment initiation on the kinetics of reservoir markers than describing the size of the integrated HIV-1 DNA reached several months after treatment initiation in both groups.12, 13, 14,23
We have observed higher levels of both total and detectable integrated HIV-1 DNA levels in the early group compared with the deferred one at the time of primary HIV-1 infection diagnosis. This is linked with the very early stage of the infection in the early group, as evidenced by the higher levels of plasma HIV-1 RNA and lower levels of CD4+ T cells than in the deferred group.3,16,24 This suggests the existence of a peak in integrated HIV-1 DNA early in the natural course of the infection that parallels the peak seen with total HIV-1 DNA that rapidly declines. This peak of integrated HIV-1 DNA in blood was not observed in the study by Tremeaux et al. this, likely due to a smaller number of patients with very early diagnosis.16 This peak was previously observed in a group of 19 patients described by Ananworanich et al. during early infection.23 Leyre et al. also described a peak of integrated HIV-1 DNA in lymph nodes but not in blood during primary HIV-1infection.13 At this stage, the integrated form of HIV-1 DNA may be mainly present in short lived cells that are rapidly driven to death due to the clearance of activated and productive infected T cells.9 Previous studies have found that integrated HIV-1 DNA level is low in the early stages of infection, and that the high total HIV-1 DNA levels mainly consist of episomal and linear forms, i.e. less stable forms.11,16 As the untreated infection evolves, the proportion of integrated HIV-1 DNA increases, as seen here for the deferred group, confirming previous observations.13,16,23 Data in the literature indicates that this form of persistence will accumulate in long-lived persistent cells that are less prone to elimination.3,16,24
We have shown that initiating treatment at the acute phase of HIV-1 infection is associated not only with lower levels of total but also of integrated HIV-1 DNA at 12 months of ART than when ART is initiated in the chronic phase. These differences were not linked to the class of ARVs used: no difference was seen between different therapeutic regimens and both total and integrated HIV-1 DNA levels in patients receiving PI-based regimens tended to be higher on ART in the deferred group than in the early group. Patients from the early group receiving INSTI-based regimen did not harbour lower levels of integrated HIV-1 DNA at 12 months on ART than those receiving PI-based regimen, indicating that this decay of integrated HIV-1 DNA is not related to the drug mechanism of action. Our results support on a much larger scale the results of Leyre et al. who showed that ART initiation during acute infection (Fiebig I to III) is associated to a low frequency of cells harbouring integrated HIV-1 DNA in blood, in lymph node and in gut-associated lymphoid tissues13 and those of Koelsch et al. and Pinzone et al.14,15 The decrease of integrated HIV-1 DNA when ART is initiated early could be related to the death of short-lived cells which are the main contributor to the reservoir at that time.9,11,17,18 Our results show that the quantification of total HIV-1 DNA can be misleading when evaluating the dynamics of the reservoir during early treatment because it overestimates its size by considering also labile forms. This is also true with other assays like the intact proviral DNA sssay (IPDA).
On the other hand, even though initiating treatment at the chronic phase is associated with a decay of total HIV-1 DNA, the impact on the integrated HIV-1 DNA is low. This is probably because stable proviruses are the main form of HIV-1 DNA at that stage.13,16 Chomont et al. reported that HIV-1 proviruses are present essentially in long-lived cells that are less prone to elimination during chronic infection, not to mention the fact that current ART is not able to act directly on these integrated forms.19 This stable reservoir is primarily maintained by the persistence of long-lived cells and the proliferation of infected cells as this reservoir is transmitted from mother to daughter cells.13,16,19,22 These factors could explain why the levels of detectable integrated HIV-1 DNA increased between diagnosis and a two year-ART period in the deferred group. Interestingly, most individuals of the chronic group showed small to no decline of integrated HIV-1 DNA on ART and only a few of them a significant decrease in the levels of this reservoir marker driving the small decline seen in the chronic group. This suggests that a subset of individuals maintain a strong ability to clear the virus even during chronic infection. All these findings indicate that initiating treatment during early phase of the infection will have a beneficial impact on disease progression, by limiting the pool of long-lived infected cells with the persistent proviruses, the motor of infection.
Considering all these results, we hereby validate the previously proposed model describing the evolution of HIV-1 DNA forms in blood during early and deferred treatment.16 We confirm that the initial rapid decay of total HIV-1 DNA in early ART could be explained by the elimination of the labile unintegrated forms and the limited establishment of the integrated forms. In contrast, this persistent form accumulates during natural history of the infection in the absence of treatment. Hence the reason why blood reservoirs are little affected by ART initiated in the chronic phase in most patients.
Our study has some limitations. One limitation is the large number of censored samples related to a limited number of sampled cells. Nevertheless, the levels of integrated HIV-1 DNA in these censored samples were low. In addition, follow-up samples were not taken at the same time in both groups. Nevertheless, even though samples for the deferred group were taken at a longer time-frame from treatment initiation compared to the early group, we were able to show that the early group achieved lower levels of total and integrated HIV-1 DNA in a shorter period.
In conclusion, implementing antiretroviral treatment at the early stages of HIV-1 infection prevents the establishment of this stable and less prone to elimination form. This strategy could favour opportunities to achieving functional cure when combined with other interventions.
Funding sources
The ANRS-PRIMO cohort and OPTIPRIM2 trial are sponsored by the ANRS MIE (French National Agency for Research on AIDS and Viral Hepatitis and Emerging Diseases). This work was supported by the ANRS. The funders of the study had no role in study design, data collection, data analysis, data interpretation, writing the report, or decision to submit for publication.
Data sharing statement
Any researcher will be able to obtain the data under reasonable request.
CRediT authorship contribution statement
Gilbert Mchantaf: Methodology, Visualization, Writing – original draft, Writing – review & editing, Formal analysis, Project administration, Investigation. Antoine Cheret: Investigation, Writing – review & editing, Resources, Project administration, Visualization. Adeline Melard: Writing – review & editing, Investigation, Methodology. Asma Essat: Resources, Writing – review & editing, Investigation, Methodology. Elise Gardiennet: Writing – review & editing, Investigation. Rebecca Bauer: Resources, Validation, Writing – review & editing, Formal analysis, Investigation, Methodology, Visualization. Caroline Charre: Writing – review & editing, Investigation. Vincent Meiffredy: Writing – review & editing, Investigation, Resources. Lionel Piroth: Resources, Visualization, Writing – review & editing. Cécile Goujard: Investigation, Project administration, Resources, Writing – review & editing. Laurence Meyer: Formal analysis, Investigation, Methodology, Project administration, Resources, Validation, Visualization, Writing – original draft, Writing – review & editing. Véronique Avettand-Fenoel: Conceptualization, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: V.A.-F. has received grants (to her institution) from the ANRS, ViiV Healthcare and the MSD AVENIR foundation and honoraria and travel grants from ViiV Healthcare and Gilead Sciences, for participation in educational programs and conferences. A.C. reports grants from Gilead Sciences, ViiV Healthcare and Janssen. C.C. reports grants from ViiV Healthcare and Gilead Sciences. Other authors: none to declare.
References
Articles from Journal of Virus Eradication are provided here courtesy of Elsevier
Full text links
Read article at publisher's site: https://doi.org/10.1016/j.jve.2023.100357
Citations & impact
Impact metrics
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/158198628
Article citations
Factors associated with unquantifiable total HIV-1 DNA in peripheral blood in persons living with HIV: An observational study.
J Virus Erad, 10(1):100370, 28 Mar 2024
Cited by: 0 articles | PMID: 38596322 | PMCID: PMC11002866
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Long-term effects of early antiretroviral initiation on HIV reservoir markers: a longitudinal analysis of the MERLIN clinical study.
Lancet Microbe, 2(5):e198-e209, 23 Mar 2021
Cited by: 13 articles | PMID: 34841369 | PMCID: PMC8622834
Early antiretroviral therapy with raltegravir generates sustained reductions in HIV reservoirs but not lower T-cell activation levels.
AIDS, 29(8):911-919, 01 May 2015
Cited by: 27 articles | PMID: 25730509
HIV Proviral Burden, Genetic Diversity, and Dynamics in Viremic Controllers Who Subsequently Initiated Suppressive Antiretroviral Therapy.
mBio, 12(6):e0249021, 16 Nov 2021
Cited by: 11 articles | PMID: 34781741 | PMCID: PMC8693448
Impact of the Timing of Initiation of Antiretroviral Therapy During Primary HIV-1 Infection on the Decay of Cell-Associated HIV-DNA.
Clin Infect Dis, 60(11):1715-1721, 03 Mar 2015
Cited by: 92 articles | PMID: 25737374
Funding
Funders who supported this work.

 , for the for the ANRS PRIMO cohort and the OPTIPRIM2 trial
, for the for the ANRS PRIMO cohort and the OPTIPRIM2 trial