Abstract
Free full text

Collagenous Colitis Possibly Associated with Dipeptidyl Peptidase-4 Inhibitor
Abstract
A 60-year-old man with type 2 diabetes mellitus treated with a dipeptidyl peptidase-4 (DPP-4) inhibitor was referred to our hospital because of his refractory watery diarrhea. Ileocolonoscopy revealed increased capillary growth, fine granular mucosa, and longitudinal mucosal tears mainly in the left side of the colon. A bioptic examination revealed thickened subepithelial collagen bands, thus confirming the diagnosis of collagenous colitis. Systemic steroid therapy was initiated, but his symptoms recurred when tapering the steroid. However, withdrawal of the DPP-4 inhibitor was successful even after the cessation of steroid therapy. We therefore considered his collagenous colitis to have been caused by the DPP-4 inhibitor.
Introduction
Collagenous colitis (CC) is a relatively rare disease characterized by chronic non-bloody watery diarrhea and a thickened subepithelial collagen band (1). CC was first described by Lindström in 1976 (2), and it is classified as microscopic colitis along with lymphocytic colitis. While its etiology remains uncertain, an aberrant immune response to the gut microenvironment may be a possible cause, as patients with CC occasionally suffer from concurrent autoimmune disorders (3). Medication has been suggested as another cause, as a strong likelihood of an association between CC and medications, including aspirin, lansoprazole, ticlopidine, and non-steroidal anti-inflammatory drugs (NSAIDs), has been reported (4). In Japan, NSAIDs have been considered the major cause because of the increasing number of reports of drug-induced CC (5).
We herein report a case of CC possibly caused by a dipeptidyl peptidase-4 (DPP-4) inhibitor. To our knowledge, this is the first case of DPP-4-induced CC.
Case Report
A 60-year-old man with type 2 diabetes mellitus treated by sitagliptin was referred to our hospital because of his refractory watery diarrhea (3-6 times/day) lasting over 2 months. He had been taking sitagliptin 50 mg daily for about 2 years. However, he took no other regular medication, including dietary supplements, herbal medicines, and over-the-counter medicines. During that period, he lost 3 kg, but no clinical symptoms other than diarrhea occurred.
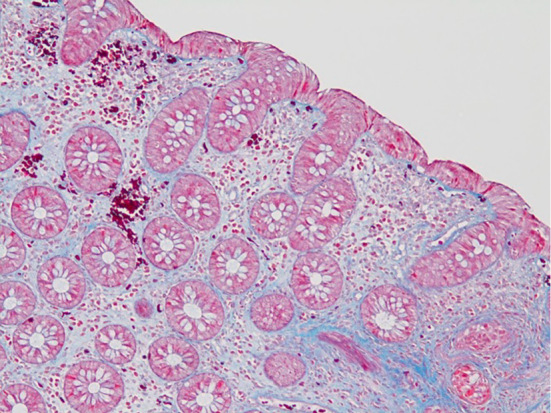
Laboratory data revealed mild anemia (Hb 11.2 g/dL), hypoalbuminemia (3.2 g/dL) and hypokalemia (3.4 mmol/L), while inflammatory biomarkers remained within normal limits (WBC 5,800×103/μL, C-reactive protein 0.06 mg/dL). No pathogenic bacteria were found by stool culture. Abdominal enhanced computer tomography failed to reveal any abnormal findings. However, ileocolonoscopy showed increased capillary growth in the transverse colon and descending colon. In addition, fine granular mucosa with scattered longitudinal mucosal defects was also found in the rectosigmoid colon (Fig. 1). Serial colorectal biopsy specimens revealed chronic inflammatory cell infiltration in the lamina propria and thickened subepithelial collagen bands throughout the colon (Fig. 2). We thus made a diagnosis of CC.

Ileocolonoscopy at the diagnosis demonstrates increased capillary growth in the transverse colon (A), fine granular mucosa with cracking (B), and longitudinal mucosal defects in the rectosigmoid colon (arrows) (C).
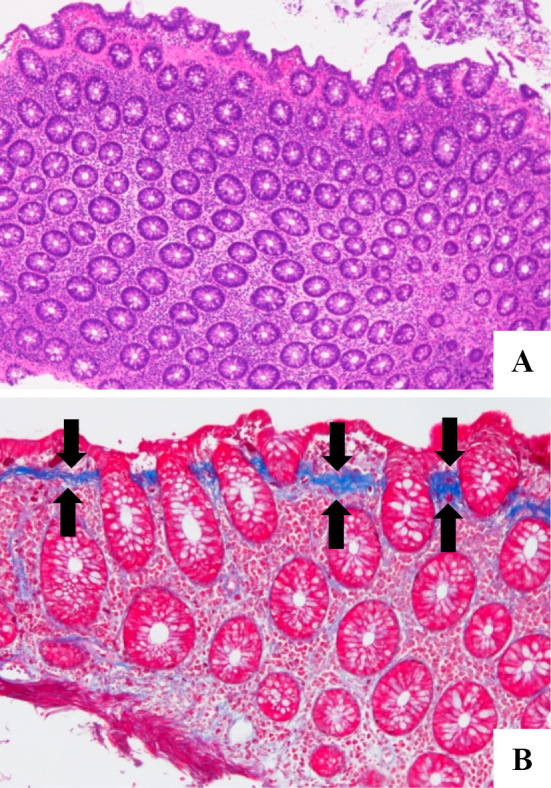
Histological findings of biopsy specimens from the sigmoid colon. Images show chronic inflammatory cell infiltration in the lamina propria (A: Hematoxylin and Eosin staining, ×40) and thickened subepithelial collagen bands (arrows) (B: Azan staining, ×100).
Since a repeat medical interview failed to identify known causative medications or concurrent autoimmune disorders, 20 mg/day of oral prednisolone (PSL) was started. His diarrhea quickly improved, but the symptom recurred after tapering PSL to 10 mg/day. After increasing the PSL dose (15 mg/day) and adding mesalamine (2 g/day), his symptoms improved, but the diarrhea deteriorated again when the PSL dose was reduced to 12.5 mg/day. We then switched sitagliptin to glimepiride, considering the possibility of sitagliptin-induced CC. Consequently, his diarrhea gradually improved within a month after the withdrawal of sitagliptin. PSL and mesalamine were successfully discontinued within the next two months. Under ilecolonoscopy five months after the cessation of sitagliptin, the colorectal mucosa appeared normal, and no longitudinal mucosal defects were detected (Fig. 3).

Ileocolonoscopy five months after the cessation of sitagliptin shows a normal-appearing colonic mucosa.
A histologic examination of the biopsy specimen from the sigmoid colon still showed a thickened subepithelial collagen band, but such histologic findings were absent in the biopsy specimens from other sites of the colorectum (Fig. 4). In the year since the withdrawal of sitagliptin, his clinical course has been uneventful.
Discussion
The etiology of CC is considered multifactorial and is largely unclear (4,6-9). However, considering the strong likelihood of an association of the disease with some medications, drugs are considered a major cause of the development of CC. Medications known to be responsible include proton pump inhibitors (PPIs), ranitidine, aspirin, NSAIDs, ticlopidine, sertraline, and acarbose (4,9-12). In Japan, the majority of cases were reportedly caused by PPIs and NSAIDs (5,10). In our case, sitagliptin was suggested as the causative drug for the development of CC. While acarbose, an α-glucosidase inhibitor, has been already reported as a causative drug among medications for diabetes mellitus (11), a possible association of a DPP-4 inhibitor with CC has never been reported.
It is difficult to determine the causal relationship between drug exposure and microscopic colitis, because the time elapsed between the initiation of causative drug and the onset of diarrhea varies widely. Beaugerie et al. proposed a scoring system to help specify drug-induced microscopic colitis (12). This scoring system comprises “resulting scoring” and “literature scoring”, and the former is determined by the combination of causality score and chronology score. All of the scores were determined based on a thorough review of drug-induced microscopic colitis. When applying this system to our case, the present case was judged to have a “low probability that the drug can cause microscopic colitis”, as the time between the drug onset and the onset of diarrhea was much longer than the values in previous reports (range: 1-112 days) and because no case of sitagliptin-induced CC has ever been reported. However, Yagi et al. recently reported a case series of CC caused by NSAIDs that included cases where several years elapsed between the drug initiation and the onset of diarrhea (13). Given that the present case was negative for pathogenic bacteria in stool culture, concurrent autoimmune disorder, and medications other than sitagliptin, and because his refractory diarrhea improved after the withdrawal of sitagliptin, it seems plausible that the medication was the causative drug for the development of his CC.
CC mainly occurs around middle age predominantly among women (3). Chronic diarrhea is the most common clinical manifestation, but there have been reports of cases with abdominal pain, weight loss, or arthralgias (14). Although colonoscopy usually reveals a normal-appearing colonic mucosa, diminutive mucosal changes, such as an increased capillary growth, erythematous or fine granular mucosa, and cracking or linear mucosal defects, have reportedly been found in CC (15). In particular, linear mucosal defects found in the present case (Fig. 1), known as “mucosal tears”, are considered characteristic of CC (16). However, since CC is confirmed by the histologic finding of a thickened subepithelial collagen band (>10 μm) (17), it is mandatory to take biopsies from several sites of the colorectum, considering the possibility of CC even if colonoscopic findings appear normal (18).
It is important to check all medications in order to discriminate causative drugs when starting treatment, as most cases are reportedly drug-induced CC in Japan. When no causative drug is identified, oral steroids (including budesonide) or aminosalicylic acid is the choice of medication, and immunomodulators such as azathioprine should be considered in refractory cases (1,17,19). In our case, while PSL and the addition of mesalamine were initially applied because no causative drug could be identified, the patient's refractory clinical course raised suspicion of sitagliptin as the causative drug of CC. Eventually, withdrawal of sitagliptin achieved remission of the refractory diarrhea and the improvement of colonoscopic and histologic findings.
In conclusion, it seems possible that sitagliptin can cause CC, although the majority of drug-induced CC cases are caused by PPIs or NSAIDs in Japan. In addition, the elimination of causative drugs should be kept in mind when starting treatment for CC, as drug-induced CC can occur even in patients with long-term use of the causative drug.
The authors state that they have no Conflict of Interest (COI).
References
Articles from Internal Medicine are provided here courtesy of Japanese Society of Internal Medicine
Full text links
Read article at publisher's site: https://doi.org/10.2169/internalmedicine.9040-21
Read article for free, from open access legal sources, via Unpaywall:
https://www.jstage.jst.go.jp/article/internalmedicine/61/18/61_9040-21/_pdf
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Severe spruelike enteropathy and collagenous colitis caused by olmesartan.
BMC Gastroenterol, 21(1):350, 23 Sep 2021
Cited by: 7 articles | PMID: 34556042 | PMCID: PMC8461977
Microscopic colitis related to food supplement containing turmeric: a review of 3 cases.
Acta Gastroenterol Belg, 87(1):34-36, 01 Jan 2024
Cited by: 0 articles | PMID: 38431788
Review
Case Report: Appearance of Various Disease-Specific Antibodies After the Onset of Dipeptidyl Peptidase-4 Inhibitor-Associated Bullous Pemphigoid.
Front Immunol, 13:843480, 03 Mar 2022
Cited by: 0 articles | PMID: 35309321 | PMCID: PMC8927025
Bullous pemphigoid associated with use of dipeptidyl peptidase-4 inhibitor.
CMAJ, 194(20):E705, 01 May 2022
Cited by: 0 articles | PMID: 35609911 | PMCID: PMC9188788