Abstract
Background
Metformin may have anticancer effects that are independent of its hypoglycemic effects. Retrospective studies have shown that metformin use is associated with decreased incidence of prostate cancer and prostate cancer-specific mortality. Preclinical studies suggesting additive anticancer effects of combining metformin and bicalutamide prompted this clinical trial (NCT02614859).Methods
This open-label, randomized, phase 2 trial enrolled non-diabetic patients with biochemically recurrent prostate cancer, a PSADT of 3-9 months, BMI > 25 and normal testosterone. Patients were randomized 1:2 to observation for an initial 8 weeks (Arm A) or metformin 1000 mg twice daily (Arm B). Bicalutamide 50 mg/day was added after 8 weeks to both arms. The primary objective was to evaluate the number of patients with undetectable PSA ( < 0.2 ng/mL) at the end of 32 weeks. Immune correlatives were assessed as exploratory endpoints.Results
A total of 29 patients were enrolled from March 2015 to January 2020. No difference was seen between the 2 arms in the proportion of patients with undetectable PSA. Modest PSA decrease ranging from 4% to 24% were seen in 40.0% (95% CI: 19.1-64.0%) of patients with metformin monotherapy, compared to 11.1% (95% CI: 0.3-48.3%) in the observation arm. Metformin monotherapy reduced PD-1+ NK cells, and increased NKG2D+ NK cells. The combination of metformin and bicalutamide led to greater reductions in PD-1 expressing NK, CD4+ T, and CD8+ T-cell subsets compared to bicalutamide alone. The trial was stopped early due to predicted inability to achieve its primary endpoint.Conclusions
Although metformin plus bicalutamide was well tolerated, there was no improvement in rates of achieving undetectable PSA at 32 weeks. Metformin monotherapy induced modest PSA declines in 40% of patients after 8 weeks. Metformin, given alone and in combination with bicalutamide, displayed immune modifying effects, primarily within NK and T cells subsets.Trial registration
Trial Registration Number: NCT02614859.Free full text

A randomized phase 2 study of bicalutamide with or without metformin for biochemical recurrence in overweight or obese prostate cancer patients (BIMET-1)
Abstract
Background:
Metformin may have anticancer effects that are independent of its hypoglycemic effects. Retrospective studies have shown that metformin use is associated with decreased incidence of prostate cancer and prostate cancer-specific mortality. Preclinical studies suggesting additive anticancer effects of combining metformin and bicalutamide prompted this clinical trial (NCT02614859).
Methods:
This open-label, randomized, phase 2 trial enrolled non-diabetic patients with biochemically recurrent prostate cancer, a PSADT of 3–9 months, BMI > 25 and normal testosterone. Patients were randomized 1:2 to observation for an initial 8 weeks (Arm A) or metformin 1000 mg twice daily (Arm B). Bicalutamide 50 mg/day was added after 8 weeks to both arms. The primary objective was to evaluate the number of patients with undetectable PSA (< 0.2 ng/mL) at the end of 32 weeks. Immune correlatives were assessed as exploratory endpoints.
Results:
29 patients were enrolled from March 2015 to January 2020. No difference was seen between the 2 arms in the proportion of patients with undetectable PSA. Modest PSA decrease ranging from 4 to 24% were seen in 40.0% (95% CI: 19.1% - 64.0%) of patients with metformin monotherapy, compared to 11.1% (95% CI: 0.3% - 48.3%) in the observation arm. Metformin monotherapy reduced PD-1+ NK cells, and increased NKG2D+ NK cells. The combination of metformin and bicalutamide led to greater reductions in PD-1 expressing NK, CD4+ T, and CD8+ T-cell subsets compared to bicalutamide alone. The trial was stopped early due to predicted inability to achieve its primary endpoint.
Conclusions:
Although metformin plus bicalutamide was well tolerated, there was no improvement in rates of achieving undetectable PSA at 32 weeks. Metformin monotherapy induced modest PSA declines in 40% of patients after 8 weeks. Metformin, given alone and in combination with bicalutamide, displayed immune modifying effects, primarily within NK and T cells subsets.
Introduction
Metformin is a well-tolerated drug that is commonly used to treat diabetes mellitus and insulin resistance. Several retrospective studies have reported that diabetic patients taking metformin had a lower cancer incidence, prompting evaluations of metformin as an anticancer agent. In 2005, Evans et al. first reported an association between decreased cancer incidence and metformin use [1], while subsequent studies showed that diabetic patients treated with metformin were less likely to be diagnosed with cancer and/or to die from it. Post-hoc analysis of the ZODIAC trial showed that patients with type 2 diabetes are overall at increased risk for cancer mortality, however metformin use was associated with lower cancer mortality (HR: 0.43; 95% CI: 0.23–0.80) [2].
Two-thirds of adult males in the United States are overweight or obese, and several studies have demonstrated an association between obesity and incidence of prostate cancer (PCa) [3]. Obese patients have reduced adiponectin levels and increased leptin levels, which may promote PCa growth [4]. In addition, an increased insulin level in a patient with metabolic syndrome can cause an increase in free androgens by decreasing sex hormone-binding globulins, which may also lead to a worse PCa prognosis [5]. In patients who underwent radical prostatectomy, obesity was associated with more aggressive cancer, a higher rate of PSA failure and a trend toward increased risk of positive surgical margins [6]. A meta-analysis of 16 studies analyzed 26,479 PCa patients after their curative treatment and found that an increase in BMI of 5 kg/m2 was associated with a 21% increase in risk of primary treatment failure (HR: 1.21, 95% CI: 1.11–1.31, P < 0.01) [7].
Despite promising preclinical evidence of metformin’s antineoplastic activity, clinical studies of metformin monotherapy in PCa have been somewhat confusing. A phase 2 study enrolled 42 chemotherapy-naïve patients with metastatic castration-resistant prostate cancer (mCRPC) and treated them with metformin 1000 mg twice daily until progression. Metformin showed modest activity: 36% of patients were progression-free at 12 weeks; 9.1% were progression-free at 24 weeks, 2 of whom had PSA declines of ≥ 50% [8]. Another clinical study treated 24 men with newly diagnosed PCa with metformin 500 mg 3 times daily prior to radical prostatectomy for a median of 41 days (range 18–81 days). Compared to baseline biopsy, treatment with metformin reduced the Ki67 proliferation index by 29%, while PSA reduction was not statistically significant (P = 0.08) [9].
Colquhoun and colleagues investigated whether the anticancer activity of metformin could be further improved by combining it with bicalutamide. They randomized LNCaP prostate cancer xenografts on a high-carbohydrate/high-fat diet into 4 treatment groups: control, metformin, bicalutamide, or metformin plus bicalutamide. The study revealed no significant difference in tumor volume in mice treated with metformin monotherapy, while mice treated with the combination therapy had significantly smaller tumors and lower PSA than controls and mice receiving either monotherapy (P < 0.0004). [10].
Based on available preclinical and clinical data, we hypothesized that metformin could enhance the antitumor effects of bicalutamide, effectively delaying the onset of metastases or need for testosterone suppression. Here we present results from the multicenter, open-label, randomized phase 2 study of bicalutamide with or without metformin in overweight or obese patients with BCR.
Materials and Methods
Patient eligibility
The study population was men with biochemical failure after primary PCa therapy with subsequent progression. Eligible patients were required to have had a primary treatment with curative intent (surgery or radiation therapy). Biochemical failure was declared when PSA reached ≥ 0.2 ng/mL after radical prostatectomy, or a PSA rise of 2 ng/mL above the nadir achieved post-radiation (2006 RTOG-ASTRO Consensus Definition). Patients were required to have hormone-sensitive PCa as evidenced by a total serum testosterone level of > 150 ng/dL, a PSA doubling time (PSADT) of 3–9 months calculated per the MSKCC nomogram [11], PSA < 30 ng/mL at study entry, BMI > 25, no evidence of diabetes mellitus (HgbA1c < 6.5% or fasting blood glucose < 126 mg/dL), and no evidence of metastatic disease on conventional imaging. Eligible patients may have had prior neoadjuvant and/or adjuvant therapy (chemotherapy, vaccines, or experimental agents) up to 4 weeks prior to randomization if the PSA rise and PSADT were documented after the testosterone level was > 150 ng/dL. The study was approved by the Institutional Review Boards of the Fox Chase Cancer Center, Temple University, and the National Cancer Institute (NCI) and registered on ClinicalTrials.gov (NCT02614859).
Study design
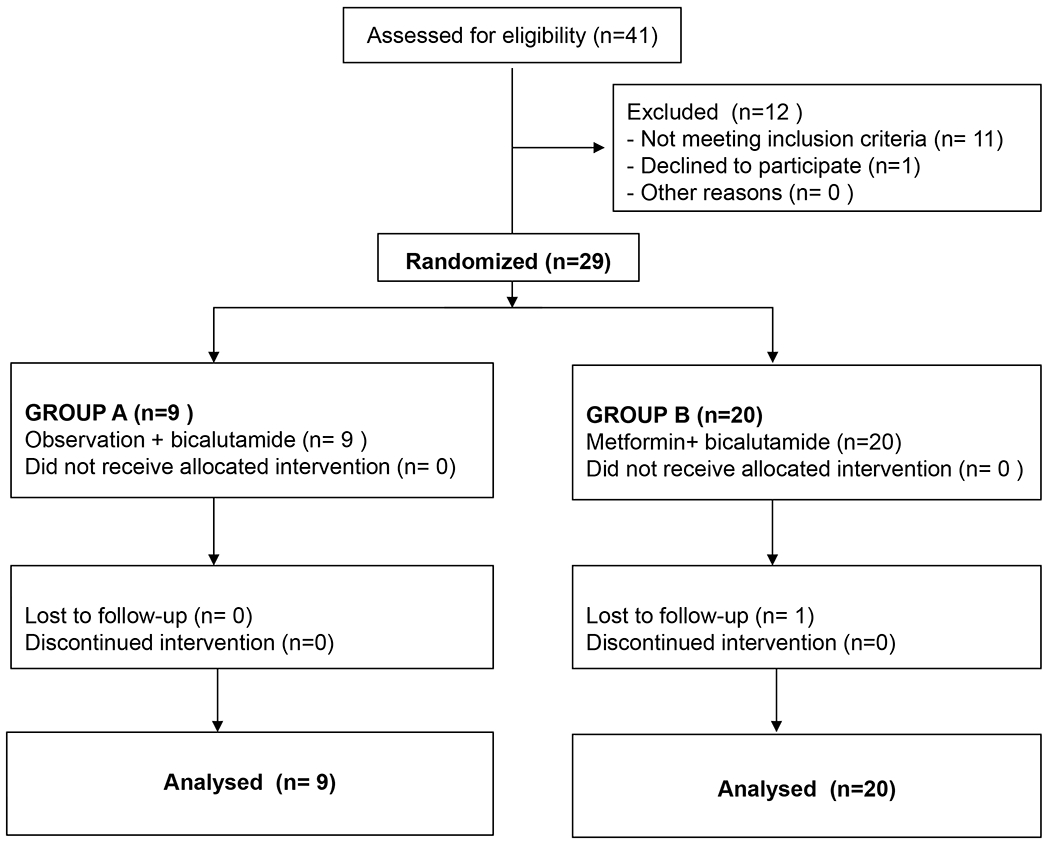
Patients were randomized 1:2 to Arm A (observation x 2 cycles, followed by bicalutamide 50 mg PO daily x 6 cycles) or Arm B (metformin 1000 mg twice daily x 2 cycles, followed by metformin 1000 mg twice daily plus bicalutamide 50 mg PO daily x 6 cycles) (Figure 1) and stratified according to prior definite therapy (radiation vs. surgery). Blood samples were taken at prespecified time points to assess immune activation at baseline, after 8 weeks, and at the end of the trial (32 weeks). The primary endpoint was the proportion of patients with undetectable PSA (< 0.2 ng/mL) at 32 weeks. Adverse events were assessed by the investigators and graded per the NCI’s Common Terminology Criteria for Adverse Events v.4.0. An early stopping rule for futility was set at 39 patients, but due to slow accrual an unplanned interim analysis was undertaken after 29 patients were enrolled.
Immune assays
Research blood was collected at baseline, at week 8, and at week 32 from patients treated at the NCI (5 patients randomized to Arm A and 8 patients randomized to Arm B, including 3 patients with undetectable PSA at the end of study) for exploratory analysis. For serum assays, blood was collected in serum separator tubes, centrifuged, and stored at −80°C prior to analysis. Serum levels of cytokine/soluble factors were evaluated before and after treatment using commercially available kits per the manufacturers’ instruction. Interleukin (IL)-8 was measured by AlphaLISA (PerkinElmer, Waltham, MA, USA), soluble (s) CD27 (sCD27) and sCD40L were measured using Instant ELISA kits (Life Technologies, Carlsbad, CA, USA), and transforming growth factor (TGF)-β1 and Granzyme B were measured using ELISA kits from R&D systems (Minneapolis, MN, USA). For the analysis of peripheral blood mononuclear cell (PBMC) subsets, blood was collected in sodium heparin tubes and PBMCs were isolated after Ficoll-Hypaque density gradient separation. Cells were cryopreserved in 90% heat-inactivated human AB serum and 10% dimethyl sulfoxide at a concentration of 1 x 107 cells/mL prior to analysis. Cryopreserved PBMCs collected from 13 patients with available PBMCs were examined by multicolor flow cytometry using 30 markers in 4 panels to identify 158 peripheral immune cell subsets [12], following methods previously described [13, 14]. Flow cytometry files were acquired on an LSR Fortessa equipped with 5 lasers and analyzed using FlowJo v.9.9.6 for Macintosh, with nonviable cells excluded and negative gates based on fluorescence-minus-one controls. The frequency of all subsets was calculated as a percentage of PBMCs to eliminate any bias that might occur in the smaller populations with fluctuations in leukocyte subpopulations. These studies were performed by the NCI’s Laboratory of Tumor Immunology and Biology.
Statistical analysis
Prespecified statistical assumptions estimated that 20% of patients in Arm A would have undetectable PSA at 32 weeks versus 40% of patients in Arm B. To detect a difference between 20% and 40% with 80% power and type I error < 5%, a cohort of 39 patients was initially planned and, if the study continued, a second cohort of 27 patients was planned to be randomized 1:2 to arms A and B respectively, with a planned final sample size of 66 patients [15]. Analyses were performed on an intention-to-treat basis. Data cut-off was in April 2021. Standard descriptive statistics were used to characterize the study population. Binomial proportions with exact, two-sided 95% confidence intervals or medians with ranges were used to summarize binary and continuous efficacy endpoints, separately by arm. A Wilcoxon rank sum test was used to compare baseline to 32-week changes in Body Mass Index (BMI) between arms. For immune analyses, statistical analyses were performed using GraphPad Prism (GraphPad Software, La Jolla, CA) and RStudio (Boston, MA). Changes in immune parameters between two time points were assessed for statistical significance using a Wilcoxon signed-rank test. Immune parameters compared between treatment arms, as well as between patients with PSA declines vs those without PSA declines, were assessed for the significance of the difference using Wilcoxon rank sum tests. All P values are 2-tailed and reported without adjustment for multiple comparisons; P values < 0.05 were considered statistically significant.
Results
Patient population
Between December 2015 and January 2020, 29 patients were treated at the Fox Chase Cancer Center and Temple University Hospital (both in Philadelphia, PA) and the National Institutes of Health Clinical Center in Bethesda, MD. Nine patients were randomized to Arm A. The mean age was 63 years (range: 54–68). Twenty patients were randomized to Arm B. The mean age was 64 years (range: 53–75). Median PSA at baseline was 5.1 ng/mL in Arm A vs 4.1 ng/mL in Arm B; median Gleason score was 7 in both arms. Patients in Arm A had higher BMI at baseline than patients in Arm B (32.9 vs 29.5), and most patients had radical prostatectomy as their primary curative treatment (77.8% in Arm A and 90% in Arm B). Descriptive characteristics for the overall study population are shown in Table 1.
Table 1
Baseline characteristics and demographic data.
| Randomized N = 29 | ARM A N = 9 | ARM B N = 20 |
|---|---|---|
|
| ||
| Age (years) | ||
| Mean (range) | 63 (54–68) | 64 (53–75) |
|
| ||
| Race, n (%) | ||
| White | 6 (66.7) | 19 (95) |
| African American | 3 (33.3) | 1 (5) |
|
| ||
| Prior Therapies, n (%) | ||
| Radical prostatectomy | 7 (77.8) | 18 (90) |
| Radiation (primary or salvage) | 9 (100) | 19 (95) |
|
| ||
| Gleason Score, | ||
| Median (range) | 7 (7–9) | 7 (6–9) |
|
| ||
| PSA at Baseline, ng/mL | ||
| Median (range) | 5.1 (0.9–22.1) | 4.1 (0.9–16.3) |
|
| ||
| BMI | ||
| Mean (range) | 32.9 (27.0–37.2) | 29.5 (25.5–36.4) |
Safety
The study treatment was safe and well tolerated, and there were no dose reductions or treatment discontinuations due to toxicity. Almost all AEs were grade 1 except for gynecomastia (grade 2 AE in both arms). The most common AEs in Arm A were breast/nipple tenderness (56%), hot flushes (33%), gynecomastia (33%), diarrhea (22%), fatigue (22%), and elevated ALT (22%). The most common AEs in Arm B were breast/nipple tenderness (50%), diarrhea (31%), nausea (21%), and fatigue (21%).
Response to therapy
Of 29 randomized patients, 28 completed all 32 weeks as planned. One patient was lost to follow-up; he was not censored and was considered a PSA non-responder at time of study withdrawal. The primary endpoint of the study was not met. At the end of the study there was no significant difference between the 2 arms in the percent of patients who reached an undetectable PSA of < 0.2 ng/mL (33.3%; 95% CI: 7.5% - 70.1%) in Arm A vs 25%; 95% CI: 8.7% - 49.1%) in Arm B or in the fraction of patients with a PSA decline of ≥ 85% (66.7% (95% CI: 29.9% - 92.5% ) in Arm A vs 50% (95% CI: 27.2% - 72.8%) in Arm B). In addition, 8 patients (40%; 95% CI: 19.1% - 64.0%)) in Arm B had a PSA decline after 8 weeks of metformin monotherapy vs 1 patient ( 11.1%; 95% CI: 0.3% - 48.3%) in the observation arm. Median PSA decline was 9% (range: 4%–24%) after 8 weeks of metformin monotherapy, suggesting modest clinical activity. Efficacy results are summarized in Table 2.
Table 2
Results of treatment
| ENDPOINTS | ARM A (Observation) N = 9 | ARM B (Metformin) N = 20 |
|---|---|---|
| Completed trial (32 weeks) | 9 (100.0%) | 19 (95.0%) |
| Patients with undetectable PSA after 32 weeks (primary endpoint) | 3 (33.3%; 95% CI: 7.5% - 70.1%) | 5 (25.0%; 95% CI: 8.7% - 49.1%) |
| Patients with PSA decline ≥ 85% after 32 weeks | 6 (66.7%; 95% CI: 29.9% - 92.5%) | 10 (50.0%; 95% CI: 27.2% - 72.8%) |
| Patients with PSA decline after 8 weeks (observation vs metformin) | 1 (11.1%; 95% CI: 0.3% - 48.3%) | 8 (40.0%; 95% CI: 19.1% - 64.0%) |
| Median PSA decline after 8 weeks % (range) | N/A* | 9% (4–24) |
| Patients with BMI decline after 32 weeks | 4 (44.4%; 95% CI: 13.7% - 78.8%) | 12 (60.0%; 95% CI: 36.1% - 80.9%) |
| Median BMI change after 32 weeks (range)** | 0.6 (−1, 1.9) | −0.41 (−1.26, 0.9) |
Exploratory Immune analyses
Significant differences were observed when the percent change of specific refined immune subsets was evaluated between the treatment arms. Compared to patients undergoing observation (Arm A), 8 weeks of treatment with metformin alone (Arm B), led to a greater reduction in the frequency of PD-1 expressing NK cells (Figure 2A), and a greater increase in the frequency of Immature NK cells (CD56brCD16−) that express the activating receptor NKG2D (Figure 2B). Compared to Arm A, receiving sequential treatment of metformin followed by metformin plus bicalutamide (Arm B) had greater increases at 32 weeks vs pre in the frequency of double positive (DP, CD56brCD16+) NK cells (Figure 2C), and greater reductions in in the frequency of effector memory (EM, CD45RA−CCR7−) CD4+ T cells, including those that express the immune checkpoint CD73 (Figure 2D, ,E),E), as well as greater decreases in PD-1 expressing EM CD4+ T cells, EM CD8+ T cells, and Mature (CD56dimCD16−) NK cells (Figure 2F–H).
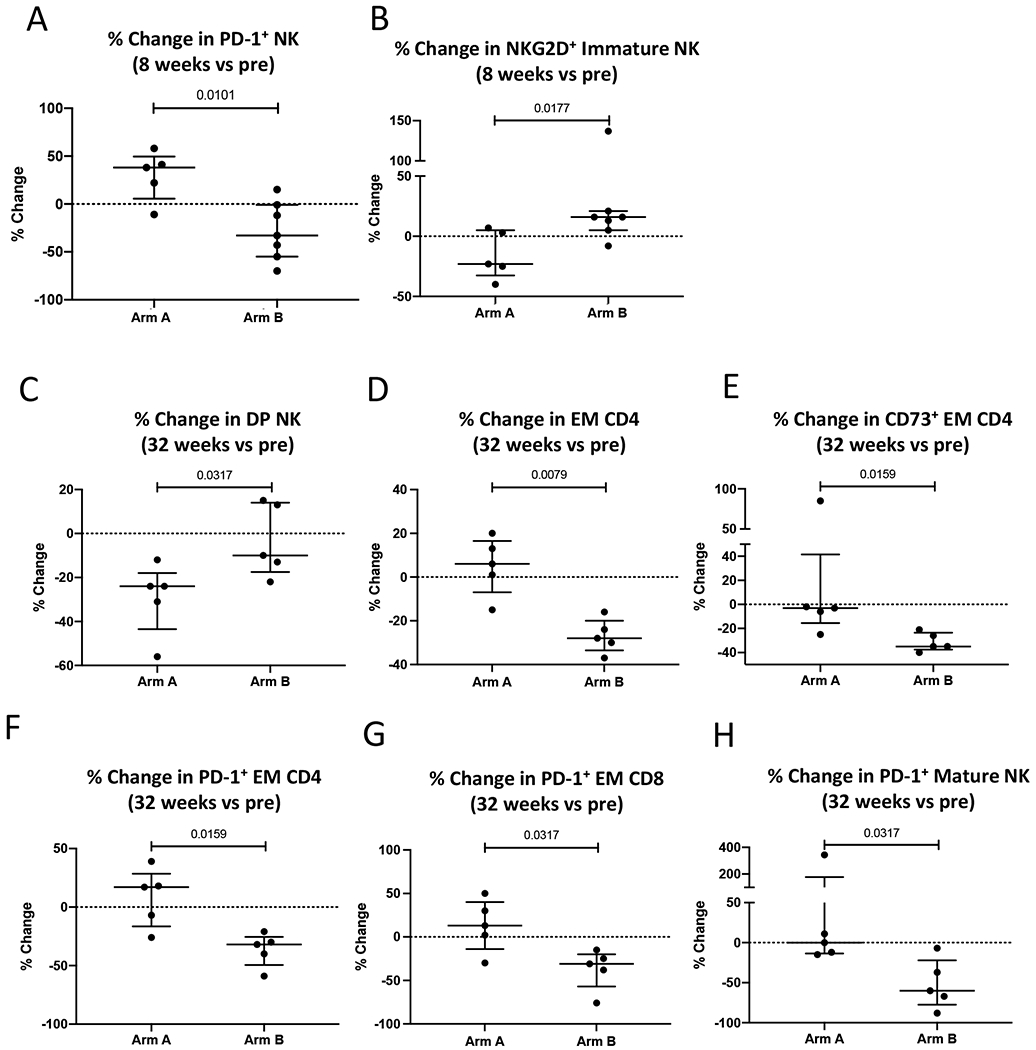
(A-B) Difference in changes in immune parameters after 8 weeks of therapy between Arm A (Observation) vs Arm B (Metformin). (C-H) Differences in changes in immune parameters after 32 weeks of therapy between Arm A (Observation followed by Bicalutamide) vs Arm B (Metformin followed by Metformin plus Bicalutamide). Graphs display values for individual patients plus median and IQR. DP NK (double positive, CD56brCD16+); EM (Effector Memory, CD45RA- CCR7-); Immature NK (CD56brCD16−); Mature NK (CD56dimCD16+).
Discussion
Several recent studies have reported the positive benefit of metformin in the incidence and prognosis of PCa, making it a promising anticancer agent which has been tested in prospective trials. However, extensive research has failed to identify the exact mechanism of metformin’s anticancer effects. Possible mechanisms include activation of the AMPK signaling pathway [2, 16], inhibition of protein synthesis [17], activation of the tumor suppressor p53 [18], induction of apoptosis and/or cell cycle arrest [18], activation of the immune system (protecting tumor-infiltrating lymphocytes from apoptosis) [19], or killing of cancer stem cells [20]. To the best of our knowledge, this is the first randomized, phase 2 trial to evaluate metformin treatment in men with biochemical recurrence of PCa.
From December 2015 to January 2020, 29 patients were randomized and treated on this 3-center, investigator-initiated phase 2 clinical trial. Although as anticipated, treatment was well tolerated with no dose reductions or discontinuations due to safety, the study was not going to reach its primary endpoint showing a difference in the proportion of patients with undetectable PSA values at the end of study and thus was stopped for poor accrual and futility. Although PSADT is a better surrogate for survival than PSA response alone, limited evidence in specific settings (such as hormone-sensitive disease) suggests that PSA response may be used as a surrogate for an ultimate effect on survival and therefore may be used for early stopping in case of futility [21]. Our study showed that fewer participants had undetectable PSA in the combination arm compared to the bicalutamide-only arm, likely due to chance since study did not have enough power due to small sample size.
However, a key secondary endpoint (number of patients with a PSA decline with metformin monotherapy after 8 weeks) was reached. Eight patients (40%) had a PSA decline ranging from −4% to −24%, suggesting modest anticancer activity. It is important to note that all patients had rapidly rising PSA with a PSADT of 3–9 months at baseline, prior to enrolment.
Three other prospective studies of combination treatments with metformin in mCRPC have been published since 2015, when our study was initiated. The combination of metformin plus abiraterone in mCRPC after PSA progression (abiraterone failure) reported no clinical benefit [22], while the combination of metformin and enzalutamide in mCRPC was reported as active. A PSA response of ≥ 50% was observed in 19/24 patients (79%), although this was not a randomized study and patients had not been exposed to enzalutamide [23]. A third study, a randomized phase 2 trial of docetaxel vs docetaxel plus metformin in mCRCP (NCT01796028), was also reported as negative in 2019 [24].
Our immune correlative analyses suggest that metformin, given alone and/or in combination with bicalutamide, has some modest immune potentiating activity, as evidenced by reductions in the frequency of subsets (NK, CD4+ and CD8+ T cells) that express the exhaustion marker PD-1, reductions in CD4+ T cells that express CD73, which is involved in adenosine metabolism and implicated in suppressing an anti-tumor immune response [25], and increases in NK cells that express the activating receptor NKG2D. Others have similarly reported in lung cancer patients with diabetes, that metformin can reduce T cell exhaustion [26].
While this study was negative, observed PSA responses to metformin monotherapy are interesting and warrant further research. This study also demonstrated that metformin can be safely prescribed to non-diabetic patients at higher doses without significant toxicities and with good compliance. Unanswered questions still remain: 1) Is 1000 mg twice daily sufficient therapeutic dosing of metformin as an anticancer agent? 2) What is the duration of PSA response and what is the resistance mechanism to metformin? 3) What is the efficacy in patients with a BMI < 25? and 4) Would diabetic patients with PCa receive the same or greater benefit from metformin given its ability to reduce insulin resistance, insulin levels, and circulating levels of glucose, which can affect cancer progression?
It is hoped that ongoing trials of metformin treatment for PCa, including the Metformin Active Surveillance Trial (MAST), which is recruiting men with low-risk cancer (NCT01864096), and a large phase 3 trial adding metformin to standard therapy for newly diagnosed metastatic castration-sensitive cancer (STAMPEDE – Arm K; NCT00268476) will shed more light on the role of metformin in the treatment of PCa.
Acknowledgments
We thank the patients and associated study staff for their participation in this trial. The authors also thank Bonnie L. Casey for editorial assistance in the preparation of this manuscript. The authors additionally thank Ariana Sabzevari, Angie Schwab, and Keanan Wright for technical assistance with immune assays, and Dr. Samuel Litwin for his assistance with the statistical design for the trial
Funding
This work was supported in part by a grant to the Fox Chase Cancer Center (#P30CA006927) from the National Cancer Institute, National Institutes of Health, and the Intramural Research Program of the Center for Cancer Research, National Cancer Institute, National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.”
Footnotes
Trial Registration Number: NCT02614859
Institutional Review Board Statement
The study protocol was approved by the Institutional Review Boards of the Fox Chase Cancer Center, Temple University, and the Center for Cancer Research, National Cancer Institute. The study was conducted according to the principles of the Declaration of Helsinki and was performed in compliance with Good Clinical Practice guidelines. Written informed consent was obtained from each patient.
Conflicts of Interest
The authors declare that they have no conflict of interest.
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Full text links
Read article at publisher's site: https://doi.org/10.1038/s41391-022-00492-y
Read article for free, from open access legal sources, via Unpaywall:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9309187
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/121552036
Article citations
Targeting Asparagine Metabolism in Well-Differentiated/Dedifferentiated Liposarcoma.
Cancers (Basel), 16(17):3031, 30 Aug 2024
Cited by: 0 articles | PMID: 39272889 | PMCID: PMC11394161
Cancer biology in diabetes update: Focusing on antidiabetic drugs.
J Diabetes Investig, 15(5):525-540, 08 Mar 2024
Cited by: 1 article | PMID: 38456597 | PMCID: PMC11060166
Review Free full text in Europe PMC
Metformin and the Liver: Unlocking the Full Therapeutic Potential.
Metabolites, 14(4):186, 25 Mar 2024
Cited by: 2 articles | PMID: 38668314 | PMCID: PMC11052067
Review Free full text in Europe PMC
Metformin and cancer hallmarks: shedding new lights on therapeutic repurposing.
J Transl Med, 21(1):403, 21 Jun 2023
Cited by: 37 articles | PMID: 37344841 | PMCID: PMC10286395
Review Free full text in Europe PMC
Current status and frontier tracking of clinical trials on Metformin for cancer treatment.
J Cancer Res Clin Oncol, 149(18):16931-16946, 12 Sep 2023
Cited by: 4 articles | PMID: 37698682
Review
Go to all (11) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
Clinical Trials (4)
- (4 citations) ClinicalTrials.gov - NCT02614859
- (1 citation) ClinicalTrials.gov - NCT01864096
- (1 citation) ClinicalTrials.gov - NCT00268476
- (1 citation) ClinicalTrials.gov - NCT01796028
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
An open-label, phase 2 trial of bicalutamide dose escalation from 50 mg to 150 mg in men with CAB and castration resistance. A Canadian Urology Research Consortium Study.
Prostate Cancer Prostatic Dis, 17(4):320-324, 02 Sep 2014
Cited by: 11 articles | PMID: 25179591
Phase I-II trial of weekly bicalutamide in men with elevated prostate-specific antigen and negative prostate biopsies.
Cancer Prev Res (Phila), 2(4):377-384, 31 Mar 2009
Cited by: 4 articles | PMID: 19336728
Tolerability, efficacy and pharmacokinetics of bicalutamide 300 mg, 450 mg or 600 mg as monotherapy for patients with locally advanced or metastatic prostate cancer, compared with castration.
BJU Int, 98(3):563-572, 08 Jun 2006
Cited by: 17 articles | PMID: 16771791
Bicalutamide (Casodex) in the treatment of prostate cancer: history of clinical development.
Prostate, 34(1):61-72, 01 Jan 1998
Cited by: 47 articles | PMID: 9428389
Review
Funding
Funders who supported this work.
Intramural NIH HHS (1)
Grant ID: ZIE BC010843
NCI NIH HHS (1)
Grant ID: P30 CA006927