Abstract
Background
Chronic rhinosinusitis with nasal polyps (CRSwNP) is a predominantly type 2-mediated inflammatory disease with high symptom burden and reduced health-related quality of life (HRQoL). This report aimed to comprehensively understand the effects of dupilumab on domains of HRQoL, their individual elements, and health status in patients with severe CRSwNP from phase 3 SINUS-24 (NCT02912468) and SINUS-52 (NCT02898454) trials.Methods
Patients were randomized to dupilumab (n = 438) or placebo (n = 286) for 24 weeks (SINUS-24), or 52 weeks (SINUS-52). Disease-specific HRQoL using 22-item sino-nasal outcome test (SNOT-22), and health status using EuroQoL-visual analog scale (EQ-VAS) was evaluated in the pooled intention-to-treat (ITT) population (Week 24), SINUS-52 ITT (Week 52) and in the subgroups with/without asthma; non-steroidal anti-inflammatory drug-exacerbated respiratory disease (NSAID-ERD); and prior sinus surgery.Results
At baseline, patients had poor disease-specific HRQoL and general health status and identified "Decreased sense of smell/taste" and "Nasal blockage" as the most important symptoms. Dupilumab significantly improved SNOT-22 total, domain (Nasal, Sleep, Function, Emotion, and Ear/facial), and 22-item scores, and EQ-VAS, at Week 24 vs placebo (all p < .0001), with continued improvements to Week 52 in SINUS-52. Improvements occurred irrespective of comorbid asthma, NSAID-ERD, or prior surgery. A significantly greater proportion of dupilumab-treated patients exceeded clinically meaningful thresholds for SNOT-22 total score and EQ-VAS vs placebo (all subgroups p < .05 except patients without surgery at Week 24).Conclusions
Dupilumab treatment led to significant clinically meaningful improvements across all aspects of disease-specific HRQoL, and general health status in patients with severe CRSwNP.Free full text
Dupilumab improves health related quality of life: Results from the phase 3 SINUS studies
Associated Data
Abstract
Background
Chronic rhinosinusitis with nasal polyps (CRSwNP) is a predominantly type 2‐mediated inflammatory disease with high symptom burden and reduced health‐related quality of life (HRQoL). This report aimed to comprehensively understand the effects of dupilumab on domains of HRQoL, their individual elements, and health status in patients with severe CRSwNP from phase 3 SINUS‐24 (NCT02912468) and SINUS‐52 (NCT02898454) trials.
Methods
Patients were randomized to dupilumab (n = 438) or placebo (n = 286) for 24 weeks (SINUS‐24), or 52 weeks (SINUS‐52). Disease‐specific HRQoL using 22‐item sino‐nasal outcome test (SNOT‐22), and health status using EuroQoL‐visual analog scale (EQ‐VAS) was evaluated in the pooled intention‐to‐treat (ITT) population (Week 24), SINUS‐52 ITT (Week 52) and in the subgroups with/without asthma; non‐steroidal anti‐inflammatory drug‐exacerbated respiratory disease (NSAID‐ERD); and prior sinus surgery.
Results
At baseline, patients had poor disease‐specific HRQoL and general health status and identified “Decreased sense of smell/taste” and “Nasal blockage” as the most important symptoms. Dupilumab significantly improved SNOT‐22 total, domain (Nasal, Sleep, Function, Emotion, and Ear/facial), and 22‐item scores, and EQ‐VAS, at Week 24 vs placebo (all p < .0001), with continued improvements to Week 52 in SINUS‐52. Improvements occurred irrespective of comorbid asthma, NSAID‐ERD, or prior surgery. A significantly greater proportion of dupilumab‐treated patients exceeded clinically meaningful thresholds for SNOT‐22 total score and EQ‐VAS vs placebo (all subgroups p < .05 except patients without surgery at Week 24).
Conclusions
Dupilumab treatment led to significant clinically meaningful improvements across all aspects of disease‐specific HRQoL, and general health status in patients with severe CRSwNP.
Abstract
CRSwNP is a predominantly type 2‐mediated inflammatory disease with high symptom burden that impacts HRQoL. In the SINUS‐24 and SINUS‐52 studies, patients with CRSwNP were randomized to dupilumab or placebo. Dupilumab led to significant clinically meaningful improvements across all aspects of disease‐specific HRQoL and general health status in patients with CRSwNP, irrespective of comorbid asthma, NSAID‐ERD, or prior NP surgery.Abbreviations: CRSwNP, chronic rhinosinusitis with nasal polyps; EQ‐VAS, EuroQoL‐visual analog scale; HRQoL, health‐related quality of life; ITT, intent to treat; NSAID‐ERD, non‐steroidal anti‐inflammatory drug‐exacerbated respiratory disease; NP, nasal polyp; SINUS‐24 and SINUS‐52, two placebo‐controlled clinical studies assessing dupilumab in patients with chronic rhinosinusitis with nasal polyps over 24 and 52 weeks, respectively; SNOT‐22, 22‐item Sino‐Nasal Outcome Test
weeks, respectively; SNOT‐22, 22‐item Sino‐Nasal Outcome Test
1. INTRODUCTION
Chronic rhinosinusitis with nasal polyps (CRSwNP) is a predominantly type 2‐mediated inflammatory disease of the nasal cavity and paranasal sinuses associated with significant impact on health‐related quality of life (HRQoL). CRSwNP is diagnosed based on a combination of symptoms, including nasal congestion/blockage, reduction/loss of smell, facial pressure, and anterior/posterior rhinorrhea, with an objective presence of nasal polyps assessed by nasal endoscopy and/or sinus opacification by computed tomography scan. 1
HRQoL is a multidimensional dynamic concept that includes physical, mental, and social domains which are influenced by disease and treatment. 2 , 3 , 4 CRSwNP impacts multiple aspects of HRQoL including mental and physical health, sleep, productivity, cognitive and social functioning, and general health status. 1 , 5 , 6 , 7 , 8 , 9 HRQoL is further worsened in patients with comorbidities, including asthma, non‐steroidal anti‐inflammatory drug‐exacerbated respiratory disease (NSAID‐ERD), or a history of sino‐nasal surgery. 5 , 10 The treatment objectives for CRSwNP are to achieve and maintain disease control, defined as absence of symptoms, improved HRQoL and health status, and improved endoscopic and radiologic outcomes. 1
Dupilumab is a fully human VelocImmune®‐derived monoclonal antibody that blocks interleukin (IL)‐4Rα, the shared receptor component for IL‐4 and IL‐13, which are key and central drivers of type 2 inflammation. 11 , 12 , 13 , 14 In the phase III SINUS‐24 (NCT02912468) and SINUS‐52 (NCT02898454) studies, dupilumab on a background of intranasal corticosteroids, significantly improved endoscopic (nasal polyp score), radiologic (Lund‐McKay computed tomography score), patient‐reported symptoms, and clinical outcomes, in patients with uncontrolled CRSwNP, and was generally well tolerated. 15 Dupilumab is approved for the treatment of CRSwNP in the USA, EU, and Japan.
The objective of these post‐hoc analyses of the SINUS‐24 and SINUS‐52 trials, was to evaluate the effect of dupilumab on multidimensional disease‐specific HRQoL, and general health status measures in patients with severe CRSwNP, including difficult‐to‐treat subgroups.
2. MATERIALS AND METHODS
2.1. Study design and patients
Full details of the phase III, multinational, multicenter, randomized, double‐blind, placebo‐controlled, parallel‐group studies SINUS‐24 and SINUS‐52 have been published previously. 15 Briefly, these studies included adults (≥18 years) with bilateral endoscopic nasal polyp scores (NPS) ≥5 with ≥2 for each nostril and moderate‐to‐severe nasal congestion for ≥8 weeks, and the presence of either rhinorrhea or loss of smell despite receiving systemic corticosteroids in the preceding 2 years or previous sino‐nasal surgery. Patients were stratified by history of prior sinus surgery, asthma/NSAID‐ERD, and country at randomization. Patients received 100 µg mometasone furoate nasal spray in each nostril twice daily throughout the trial period. Rescue treatment with systemic corticosteroids (SCS), sinus surgery, or nasal lavage with saline and/or systemic antibiotics was allowed as per investigator's discretion (see Statistical methods for further details). All patients provided written informed consent before participating in the trials. Local institutional review board or ethics committee at each study center oversaw trial conduct and documentation.
2.2. Treatment
In SINUS‐24, patients were randomized 1:1 to receive subcutaneous (SC) dupilumab 300 mg every 2 weeks (q2w) (n = 143) or placebo q2w (n = 133) for 24 weeks. In SINUS‐52, patients were randomized 1:1:1 to receive dupilumab 300 mg SC q2w for 52 weeks (n = 150); dupilumab 300 mg SC q2w for 24 weeks, then 300 mg SC every 4 weeks for 28 weeks (n = 145); or placebo q2w for 52 weeks (n = 153). The pooled analyses presented here included all patients treated with dupilumab 300 mg q2w or placebo q2w from SINUS‐24 and SINUS‐52 at Week 24. The analyses at Week 52 included patients treated with placebo or dupilumab 300 mg q2w from SINUS‐52 only.
2.3. Outcome measures
Disease‐specific HRQoL was assessed using the 22‐item sino‐nasal outcome test (SNOT‐22; a secondary endpoint from SINUS‐24 and SINUS‐52) score at baseline and Weeks 4 (SINUS‐52 only), 8, 16, 24 (SINUS‐24 and SINUS‐52), 40, and 52 (SINUS‐52 only). SNOT‐22 is a patient‐reported outcome (PRO) that assesses the impact of chronic rhinosinusitis (CRS) on HRQoL with a recall period of 2 weeks. 16 , 17 It includes 22 items, each scored on a Likert‐like scale of 0 (no problem) to 5 (problem as bad as it can be) and allows patients to report up to 5 most important items affecting their health (Table S1). The 22 items are categorized into the following 5 validated domains for CRSwNP: Nasal (8 items), Ear/facial (4 items), Sleep (4 items), Function (3 items), and Emotion (3 items). 18 The total score ranges from 0 to 110, and the domain scores are presented as the average item score per domain (0–5). In each case, higher scores represent worse HRQoL. The SNOT‐22 domain scores allow for both granular understanding on the burden of CRSwNP on a patient's HRQoL, and a comprehensive evaluation of treatment efficacy in addition to objective disease measures. Assessing domain and item scores provides an understanding of individual aspects of CRSwNP which have the greatest impact on patients, helping to identify what “drives” the disease from a patient perspective.
General health status was assessed at baseline and Weeks 16 (SINUS‐52 only), 24 (SINUS‐24 and SINUS‐52), 40, and 52 (SINUS‐52 only) using the EuroQoL‐visual analog scale (EQ‐VAS [0–100] of the EuroQoL 5‐dimension 5‐level; an exploratory endpoint from SINUS‐24 and SINUS‐52), with higher scores indicating better health status, which is a generic, standardized questionnaire developed to provide a simple measure of general health status. 19 EQ‐VAS also provides policy makers with uniform criteria for comparison with other diseases and population normative data. 3 , 20 HRQoL and health status are distinct constructs and it has been shown that patients' ratings of these are influenced by different factors; HRQoL is influenced by emotional well‐being, whereas health status is influenced by physical functioning. 21
2.4. Statistical methods
SNOT‐22 total score was analyzed as absolute and percent change from baseline at Weeks 24 and 52. SNOT‐22 domain scores, SNOT‐22 items, and EQ‐VAS were expressed as absolute change from baseline at Weeks 24 and 52. Individual response thresholds were defined by improvements at Weeks 24 and 52 from baseline meeting or exceeding minimum clinically important differences (MCIDs) for the SNOT‐22 total score (≥8.9) 17 and the EQ‐VAS (≥8). 22 Absolute and/or percent changes from baseline in continuous outcomes were analyzed in the intention‐to‐treat (ITT) population with a hybrid of the worst observation carried forward (WOCF) and multiple imputation methods, followed by an analysis of covariance (ANCOVA) model with the baseline value of the corresponding outcome, treatment, asthma or NSAID‐ERD status, prior surgery history, region (pooled countries), and, for the pooled analyses, the study as covariates. For patients who received SCS or who underwent sinus surgery for any reason, data collected post‐surgery or post‐SCS treatment were set to missing, and the worst post‐baseline value on or before the time of surgery or SCS treatment was used to impute the Week 24 or Week 52 values. For patients who discontinued treatment without rescue by surgery or SCS, a multiple imputation approach was used to impute missing values, using all patients who had not been rescued by surgery or were not receiving SCS. Statistical inference obtained from all imputed data was combined using Rubin's rule. Least squares (LS) mean differences vs placebo along with 95% confidence intervals (CIs) were then calculated.
To assess the effect size of the difference between dupilumab and placebo, Hedges' g of the LS mean difference vs placebo were computed for outcomes without established clinically important differences. 23 These were for (i) percent change from baseline in SNOT‐22 total score, (ii) absolute change from baseline in domain scores, and (iii) top 2 items. An absolute value of 0.5 represents a “medium” effect size and a value of 0.8 a “large” effect size. 23
The proportion of patients achieving response was compared between dupilumab and placebo in the ITT using the Cochran–Mantel–Haenszel test performed on the association between the responder status and treatment group (dupilumab vs placebo), stratified by asthma/NSAID‐ERD status, prior surgery history, region, and for the pooled analyses, the study as covariates. Odds ratios (ORs) with 95% CIs were computed. Patients who were indicated for NP surgery, received SCS for any reason, were considered non‐responders for time points after using SCS or surgery; patients with missing data at the visit of interest were also considered as non‐responders.
Similar analyses on continuous and binary outcomes were performed separately in each of the following subgroups: patients with and without asthma, with and without NSAID‐ERD, and with and without prior NP surgery history at baseline. Comorbid asthma and NSAID‐ERD diagnoses were ascertained by self‐reported medical history.
Correlations between SNOT‐22 and EQ‐VAS outcomes at baseline were computed using the Spearman correlation coefficient for overall treatment groups.
3. RESULTS
3.1. Baseline demographic and disease characteristics
In the pooled ITT population at baseline, patients with CRSwNP had poor disease‐specific HRQoL and general health status was worse than population norms. Mean (SD) SNOT‐22 total score was 50.94 (20.66) (Table 1), the most affected SNOT‐22 domain at baseline was (mean [SD]) Nasal (3.08 [0.82]), followed by Sleep (2.33 [1.40]), Function (2.13 [1.39]), Emotion (1.71 [1.35]), and Ear/facial (1.37 [1.15]), with baseline domain scores being similar across subgroups (data not shown). 59% of patients had comorbid asthma, 28% had NSAID‐ERD, and 63% had prior NP surgery. Mean baseline SNOT‐22 scores in these subgroups were, with asthma: 53.98; without asthma: 46.55; with NSAID‐ERD: 52.86; without NSAID‐ERD: 50.19; with surgery: 51.63; without surgery: 49.72 (Figure S1). In the ITT population and across all subgroups, “Decreased sense of smell/taste” and “Nasal blockage” were identified by patients as the most important SNOT‐22 items affecting their health at baseline (> 80% of patients reported these items as important).
TABLE 1
Baseline demographics and disease characteristics (ITT)
| Pooled SINUS‐24 and SINUS‐52 | |||
|---|---|---|---|
| Placebo (n = 286) |
Dupilumab 300 mg q2w (n = 438) | Overall (n = 724) | |
| Age, mean (SD), years | 51.28 (12.90) | 51.47 (12.79) | 51.39 (12.83) |
| Male sex, n (%) | 165 (57.7) | 272 (62.1) | 437 (60.4) |
| NC score, mean (SD) [range 0–3] | 2.41 (0.54) | 2.39 (0.60) | 2.40 (0.58) |
| TSS, mean (SD) [range 0–9] | 7.18 (1.39) | 7.14 (1.45) | 7.16 (1.43) |
| VAS for overall rhinosinusitis, mean (SD) [range 0–10] | 7.97 (2.14) | 7.82 (2.02) | 7.88 (2.07) |
| SNOT‐22 total score, mean (SD) [range 0–110] a | 52.27 (21.11) | 50.05 (20.33) | 50.94 (20.66) |
| Nasal domain, mean (SD) [range 0–5] a | 3.12 (0.83) | 3.05 (0.81) | 3.08 (0.82) |
| Ear/facial domain, mean (SD) [range 0–5] a | 1.44 (1.23) | 1.33 (1.09) | 1.37 (1.15) |
| Sleep domain, mean (SD) [range 0–5] a | 2.39 (1.41) | 2.29 (1.40) | 2.33 (1.40) |
| Function domain, mean (SD) [range 0–5] a | 2.22 (1.39) | 2.07 (1.39) | 2.13 (1.39) |
| Emotion domain, mean (SD) [range 0–5] a | 1.79 (1.34) | 1.65 (1.36) | 1.71 (1.35) |
| EQ‐VAS (health status), mean (SD) [range 0–100] b | 64.9 (20.6) | 65.8 (20.3) | 65.4 (20.4) |
Abbreviations: EQ‐VAS, EuroQoL‐visual analog scale; HRQoL, health‐related quality of life; ITT, intention to treat; NC, nasal congestion; q2w, every 2 weeks; SD, standard deviation; SNOT‐22, 22‐item sino‐nasal outcome test; TSS, total symptom score; VAS, visual analog scale.
Mean (SD) baseline EQ‐VAS score was 65.4 (20.4), which is below population norms which range from 70.4 to 83.3 by country. 20 There was a weak correlation between SNOT‐22 total score and EQ‐VAS at baseline (Spearman's correlation −0.354), signifying the different concepts these outcomes measure (disease‐specific HRQoL and general health status). The lack of agreement between these outcomes has previously been reported. 24
3.2. Dupilumab efficacy on disease‐specific HRQoL
3.2.1. SNOT‐22 total percent score
At Week 24, the LS mean (standard error) percent change from baseline in SNOT‐22 total score for dupilumab was −56.7% (2.2) and for placebo was −20.1% (2.5) in the ITT population (LS mean difference vs placebo [95% CI] −36.6% [−41.9%, –31.3%]). The effect size for percent change in SNOT‐22 total score was large (absolute Hedges' g > 0.8) at all post‐baseline assessments (data not shown). Dupilumab treatment was associated with significantly greater percentage reductions in SNOT‐22 total score at Week 24 (representing improvement in HRQoL) vs placebo, irrespective of the presence of comorbid asthma or NSAID‐ERD, or history of prior NP surgery with continued improvement through to Week 52 (all p < .0001; Figure 1).
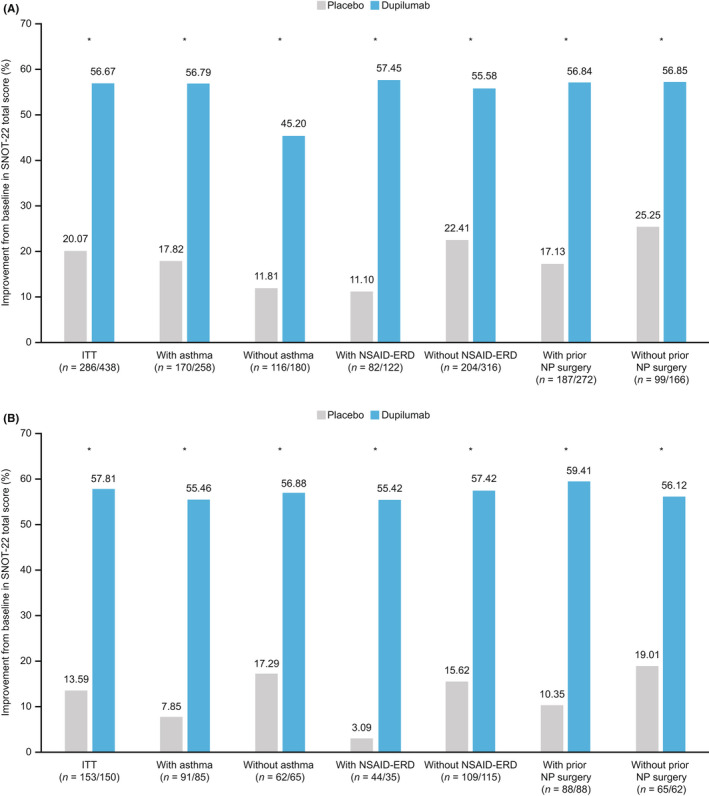
Improvement in percentage change from baseline in SNOT‐22 total score (ITT and subgroups). (A) Pooled SINUS‐24 and SINUS‐52 Week 24. (B) SINUS‐52 Week 52. *p < .0001 least squares mean difference dupilumab vs placebo. ITT, intention to treat; NP, nasal polyp; NSAID‐ERD, non‐steroidal anti‐inflammatory drug‐exacerbated respiratory disease; SNOT‐22, 22‐item sino‐nasal outcome test
3.2.2. SNOT‐22 domain scores
Improvements were observed in all SNOT‐22 domain scores in dupilumab‐treated patients vs placebo at Week 24 in the pooled ITT population, with the greatest improvements (LS mean difference [95% CI]), seen in the Nasal domain −1.16 (−1.31, −1.02), followed by Sleep −0.82 (−0.99, −0.65), Function −0.71 (−0.87, −0.56), Emotion −0.66 (−0.79, −0.52), and Ear/facial −0.60 (−0.72, −0.48) domains (Figure 2; Table S2). Improvements were also seen in subgroups of patients with/without comorbid asthma, prior NP surgery, and NSAID‐ERD, although greater improvements were seen in patients in the difficult‐to‐treat subgroups (Figure 2). At Week 52, dupilumab had the largest LS mean difference vs placebo across all subgroups in the Nasal domain (all p < .0001) followed by either Sleep or Function domains depending on subgroup (Figure 2; Table S2), which were also the most impacted domains at baseline. In the ITT population, a large effect size (>0.8) was observed for the Nasal domain (Hedges' g [95% CI]: −1.2 [−1.4, −1.1] at Week 24 and −1.5 [−1.7, −1.2] at Week 52) and the Ear/facial and Sleep domain at Week 52 (−0.9 [−1.1, −0.6] and −0.8 [−1.1, −0.6], respectively). A medium effect size (>0.5) was observed across all other domains at Week 24 and Week 52 (data not shown).
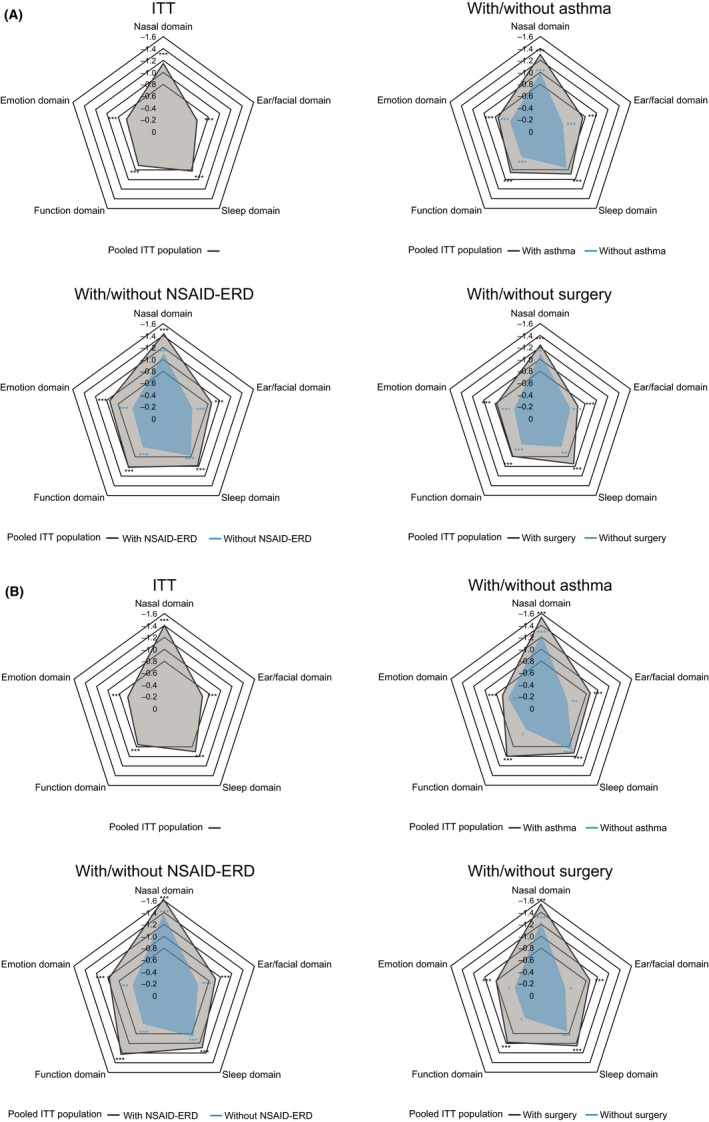
Spider plot of LS mean difference between dupilumab and placebo in change from baseline in SNOT‐22 domain scores at (A) Week 24, and (B) Week 52 (ITT and subgroups). LS mean difference vs placebo in domain scores (range, 0–5) are plotted. Each of the imputed complete data were analyzed by fitting an ANCOVA model with change from baseline at the corresponding visit as the response variable, and the corresponding baseline value, treatment group, asthma/NSAID‐ERD status, prior surgery history, regions, and, for the pooled analyses, study indicator as covariates. Data collected after treatment discontinuation were included. Data post‐SCS or NP surgery were set to missing and imputed by WOCF; other missing data were imputed by multiple imputation methods. *p < .01, **p < .001, ***p < .0001; †p < .05. ANCOVA, analysis of covariance; ITT, intention to treat; LS, least squares; NP, nasal polyp; NSAID‐ERD, non‐steroidal anti‐inflammatory drug‐exacerbated respiratory disease; SCS, systemic corticosteroids; SNOT‐22, 22‐item sino‐nasal outcome test; WOCF, worst observation carried forward
3.2.3. SNOT‐22 items
Dupilumab treatment was associated with significant improvements in all SNOT‐22 items vs placebo, irrespective of the presence of comorbid asthma or NSAID‐ERD, or of prior NP surgery (all p < .05; Figure 3; Figure S2). In the ITT population, LS mean differences (95% CI) in change from baseline between dupilumab and placebo groups were −1.97 (−2.19, −1.75; p < .0001) for “Decreased sense of smell/taste.” The corresponding changes in the subgroups were patients with asthma −2.14 (−2.44, −1.84), without asthma −1.77 (−2.10, −1.44), with NSAID‐ERD −2.12 (−2.54, −1.70), without NSAID‐ERD −1.92 (−2.18, −1.66), prior NP surgery −2.01 (−2.29, −1.73), and no prior NP surgery −1.92 (−2.29, −1.56), respectively (all p < .0001). For “Nasal blockage” LS mean differences (95% CI) in change from baseline between dupilumab and placebo groups were −1.55 (−1.75, −1.36; p < .0001). The corresponding changes in the subgroups were patients with asthma −1.71 (−1.96, −1.46), without asthma −1.32 (−1.64, −1.01), with NSAID‐ERD −1.88 (−2.25, −1.50), without NSAID‐ERD −1.43 (−1.65, −1.20), prior NP surgery −1.58 (−1.83, −1.34), and no prior NP surgery −1.53 (−1.85, −1.20), respectively (all p < .0001). A large effect size was observed from Week 8 (data not shown), continuing to Week 24, for both “Decreased sense of smell/taste” (Hedges' g [95% CI]: Week 24: −1.4 [−1.5, −1.2], Week 52: −1.5 [−1.7, −1.3]) and “Nasal blockage” (Week 24: −1.2 [−1.4, −1.1], Week 52: −1.3 [−1.5, −1.0]).
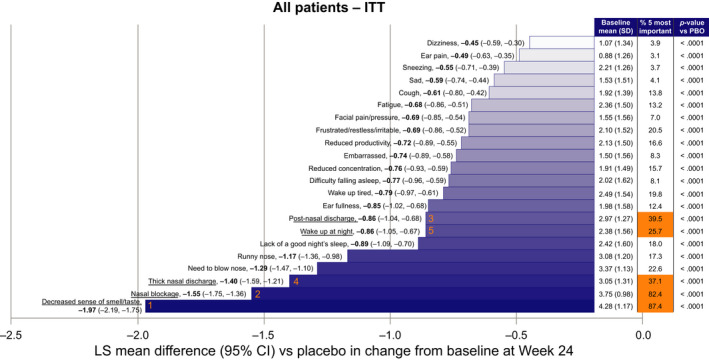
LS mean difference (95% CI) between dupilumab and placebo in change from baseline at Week 24 in SNOT‐22 items (ITT n = 438). Baseline pooled for placebo and dupilumab‐treated patients. “% 5 most important” represents the percentages of patients who considered each item as one of the 5 most important items affecting their health at baseline. The 5 most frequently reported are highlighted in orange and underlined (orange numbers signify order of importance). CI, confidence interval; ITT, intention to treat; LS, least squares; PBO, placebo; SD, standard deviation; SNOT‐22, 22‐item sino‐nasal outcome test
3.2.4. SNOT‐22 responder analysis
A significantly greater proportion of dupilumab‐treated patients exceeded the defined clinically meaningful threshold for SNOT‐22 total score vs placebo at Week 24 and Week 52 (Figure 4). Significant improvements were also observed in subgroups with or without comorbid asthma, prior NP surgery, and without NSAID‐ERD at Week 24 and Week 52 (Figure 4), although greater improvements were seen in patients in the difficult‐to‐treat subgroups.
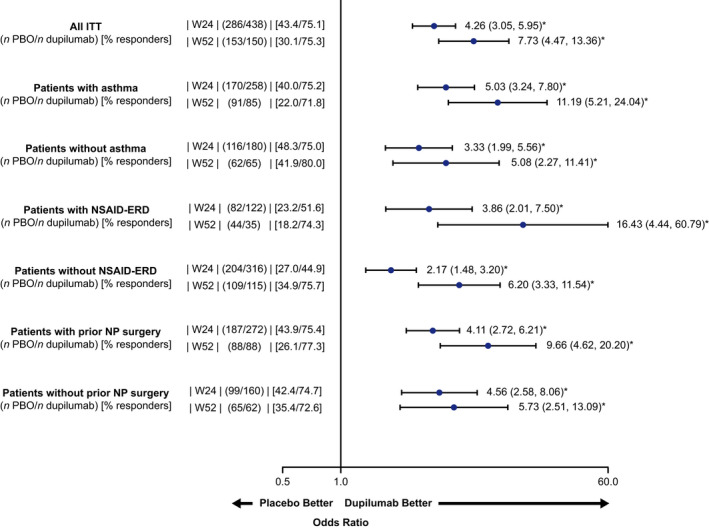
SNOT‐22 total score responder analysis at Weeks 24 and 52 (ITT and subgroups). Treatment responder was defined as an improvement of ≥8.9 from baseline at Weeks 24 and 52 (MCID for SNOT‐22). Odds ratio with 95% confidence interval. *p < .0001, ITT, intention to treat; MCID, minimum clinically important difference; NSAID‐ERD, non‐steroidal anti‐inflammatory drug‐exacerbated respiratory disease; NP, nasal polyp; PBO, placebo; q2w, every 2 weeks; SNOT‐22, 22‐item sino‐nasal outcome test
3.2.5. Dupilumab efficacy on general health status
In the ITT population, dupilumab treatment was associated with significant improvements in EQ‐VAS vs placebo at Weeks 24 and 52, and irrespective of the presence of comorbid asthma, NSAID‐ERD, or prior NP surgery (all p < .05; Table 2). At Weeks 24 and 52, patients treated with dupilumab achieved mean scores within the range of population norms (70.4–83.3 20 ), in the ITT population (Week 24: 76.7; Week 52: 78.2), and across all subgroups (score range 74.3–79.4). In patients treated with placebo, scores remained below population norms at Weeks 24 and 52 (subgroup scores range 64.1–69.5) with the exception of the subgroup without asthma (70.5 at Week 24 and 70.7 at Week 52; Table 2). In the ITT population, a significantly higher proportion of dupilumab‐treated patients achieved a clinically meaningful improvement in EQ‐VAS vs placebo at Week 24 (46.8% vs 25.9%; OR 2.58 [1.85, 3.61]; p < .0001) and Week 52 (50.0% vs 18.3%; OR 4.30 [2.55, 7.27]; p < .0001), with a similar effect vs placebo was seen in all subgroups (except patients without surgery at Week 24; Figure S3); greater improvements were seen in patients in the difficult‐to‐treat subgroups.
TABLE 2
Change from baseline at Weeks 24 and 52 in EQ‐VAS (general health status; ITT and subgroups)
|
Pooled SINUS‐24 and SINUS‐52 Week 24 n placebo/n dupilumab | Baseline mean (SD) | Week 24 mean (SD) | Change from baseline at Week 24 | |||
|---|---|---|---|---|---|---|
| Placebo |
Dupilumab 300 mg q2w | Placebo |
Dupilumab 300 mg q2w | LS mean difference vs placebo (95% CI) | p‐value | |
| ITT (286/438) | 64.9 (20.6) | 65.8 (20.3) | 68.0 (19.9) | 76.7 (16.3) | 8.2 (5.7, 10.7) | <.0001 |
| With asthma (170/258) | 62.6 (20.5) | 64.5 (19.7) | 66.3 (20.0) | 75.3 (16.2) | 8.2 (5.0, 11.5) | <.0001 |
| Without asthma (116/180) | 68.1 (20.5) | 67.8 (21.0) | 70.5 (19.5) | 78.6 (16.1) | 7.9 (3.9, 11.9) | .0001 |
| With NSAID‐ERD (82/122) | 61.4 (19.5) | 63.9 (18.3) | 64.4 (20.3) | 76.7 (16.0) | 11.7 (7.1, 16.3) | <.0001 |
| Without NSAID‐ERD (204/316) | 66.3 (20.9) | 66.5 (21.0) | 69.5 (19.6) | 76.6 (16.4) | 6.8 (3.9, 9.8) | <.0001 |
| With prior NP surgery (187/272) | 65.6 (19.8) | 65.2 (19.1) | 68.0 (20.1) | 76.8 (15.9) | 8.7 (5.6, 11.8) | <.0001 |
| Without prior NP surgery (99/166) | 63.5 (22.2) | 66.8 (22.1) | 68.1 (19.6) | 76.5 (16.9) | 7.6 (3.3, 11.9) | .0006 |
|
SINUS‐52 Week 52 n placebo/n dupilumab | Baseline mean (SD) | Week 52 mean (SD) | Change from baseline at Week 52 | |||
|---|---|---|---|---|---|---|
| Placebo |
Dupilumab 300 mg q2w | Placebo |
Dupilumab 300 mg q2w | LS mean difference vs placebo (95% CI) | p‐value | |
| ITT (153/150) | 63.9 (20.0) | 63.8 (21.8) | 66.8 (20.6) | 78.2 (18.1) | 11.2 (7.2, 15.2) | <.0001 |
| With asthma (91/85) | 60.9 (19.8) | 63.2 (21.0) | 64.1 (20.0) | 77.3 (18.9) | 11.9 (6.7, 17.2) | <.0001 |
| Without asthma (62/65) | 68.2 (19.6) | 64.6 (22.8) | 70.7 (21.0) | 79.3 (17.0) | 10.2 (3.8, 16.5) | .0428 |
| With NSAID‐ERD (44/35) | 62.8 (19.3) | 61.5 (18.3) | 64.6 (20.1) | 74.3 (21.9) | 10.4 (2.4, 18.5) | .0112 |
| Without NSAID‐ERD (109/115) | 64.4 (20.3) | 64.4 (22.7) | 67.7 (20.9) | 79.4 (16.6) | 11.4 (6.8, 16.1) | .0004 |
| With prior NP surgery (88/88) | 64.4 (19.6) | 64.1 (20.0) | 67.1 (21.4) | 78.5 (19.2) | 11.4 (6.2, 16.6) | <.0001 |
| Without prior NP surgery (65/62) | 63.2 (20.6) | 63.4 (24.1) | 66.3 (19.7) | 77.7 (16.4) | 11.0 (4.6, 17.3) | .0063 |
Abbreviations: CI, confidence interval; EQ‐VAS, EuroQoL‐visual analog scale; ITT, intention to treat; LS, least squares; NP, nasal polyp; NSAID‐ERD, non‐steroidal anti‐inflammatory drug‐exacerbated respiratory disease; q2w, every 2 weeks; SD, standard deviation.
4. DISCUSSION
CRSwNP is a type 2 inflammatory disease associated with significant impact on HRQoL. 1 , 25 , 26 Impairment in overall HRQoL in patients with CRSwNP has been previously reported 5 , 7 but not specifically in patients with uncontrolled CRSwNP refractory to available medical and surgical treatment. Here, we expand on previous findings 15 to report on the broad impact of CRSwNP on HRQoL burden, and the effect of dupilumab in improving multiple aspects of HRQoL including the domains and items of SNOT‐22, and general health status in such patients.
SNOT‐22 has been identified as a useful PRO for assessing HRQoL in CRS. 1 , 27 , 28 , 29 , 30 The domains of SNOT‐22, validated in the CRSwNP population, provide valuable information on the dimensions of the patient's life most burdened, inform treatment decision‐making, and are pertinent to personalized medicine. 1 , 18 The EQ‐VAS has been adopted as an attractive tool for rhinosinusitis outcomes research in recent years, as it shows sensitivity to clinical change in rhinosinusitis that supports its use for monitoring patient outcomes. 31 , 32 , 33 , 34
In the present study, patients had high HRQoL burden at baseline as shown by SNOT‐22 total scores, Nasal domain scores (domain including all nasal symptoms) and also in the scores for the Sleep domain (including difficulty falling asleep, waking up at night, lack of a good night's sleep, and waking up tired) and Function domain (e.g., fatigue, reduced productivity, and concentration). These findings illustrate a broad impact of CRSwNP on day‐to‐day living, and a potential impact on societal factors such as productivity loss. More than 80% of the patients reported the SNOT‐22 items “Decreased sense of smell/taste” and “Nasal blockage” as the most important items affecting their health across all subgroups, suggesting that, from a patient's perspective, these are markers of disease severity and thus an important outcome measure of treatment efficacy.
In addition, patients had worse mean EQ‐VAS scores at baseline (approximately 66) than population norms (70.4–83.3) 20 across all subgroups. A previously reported analysis showed that EQ‐VAS scores at baseline in the SINUS‐24 study were lower than those of other chronic conditions: rheumatoid arthritis, type 2 diabetes, and asthma (Global Initiative for Asthma steps 1–3 and 4–5). 35
It has been previously reported that dupilumab treatment resulted in significant and clinically meaningful improvements in SNOT‐22 total score vs placebo in patients with severe CRSwNP. 15 The present analyses expand on these observations, showing significant improvements in percent change from baseline in SNOT‐22 total score associated with dupilumab. Percent change gives a precise description of change over time as it accounts for baseline measurements. This effect was observed in the ITT population and in patient subgroups considered hard to treat, such as those with comorbid asthma or NSAID‐ERD, or who have undergone prior NP surgery. A significantly greater proportion of dupilumab‐treated patients achieved a clinically meaningful SNOT‐22 response, defined as improvement of ≥8.9 on the SNOT‐22 total score 17 in ITT and in all subgroups. Effect sizes were analyzed for outcomes for which clinically meaningful change scores have not yet been established. These are complementary to statistical significance and provide a measure of the magnitude of change between treatment groups without confounding by sample size. In the ITT population, a large effect size was observed for percent change in SNOT‐22 total score by Week 8, and this continued to improve to Week 24. Improvements noted for SNOT‐22 in the placebo group are likely to be the result of optimization of the standard of care component within the context of a clinical trial setting. Such changes were not seen in the placebo group for objective measures assessed in the trials such as nasal polyp score and Lund–Mackay CT score.
Assessing SNOT‐22 domain and item scores provides an understanding of individual aspects of CRSwNP which have the greatest impact on a patient's life and may provide a more complete assessment of a patient's disease than SNOT‐22 total score alone. Dupilumab treatment led to significant improvements in all 5 SNOT‐22 domains at Week 24, with the most marked improvements observed for Nasal, Sleep, and Function domains. A large or medium effect size was reported across all domains at Week 24.
Improvements with dupilumab were significant for all 22 items of SNOT‐22 at Week 24, showing that the decrease in total score with dupilumab treatment was comprehensive, with the greatest improvement in the 2 items considered most important by patients. Continued improvement in all SNOT‐22 items was observed between Weeks 24 and 52, showing that the comprehensive HRQoL improvements achieved with dupilumab were sustained over time, and suggesting that the “maximum” treatment effect was still to be achieved at Week 52. Patients with comorbid asthma, NSAID‐ERD, or prior NP surgery had numerically greater improvements in SNOT‐22 item scores than patients without, probably due to worse baseline scores in these subgroups.
Dupilumab treatment resulted in statistically significant and clinically meaningful improvements in EQ‐VAS, both in change from baseline and in the number of responders in general health status vs placebo in all subgroups. This finding further supports the comprehensive effect of dupilumab, with improvements in multiple elements of HRQoL and general health status, and continued improvement up to Week 52. A limitation of this study is that MCIDs are not established for SNOT‐22 percentage change in total score, domain scores, and individual items scores.
5. CONCLUSIONS
Patients with severe CRSwNP have a wide‐ranging burden on their HRQoL and worse general health status than population norms. In these patients, dupilumab treatment led to significant improvements across all components of disease‐specific HRQoL (including nasal symptoms, sleep, and function), and general health status for which mean scores achieved were within the range of population norms. The results provide stakeholders with insight into the significant impact this disease has on patient quality of life and the effects of dupilumab across multiple measures of HRQoL and overall health status.
CONFLICT OF INTEREST
Stella E. Lee: Allakos, AstraZeneca, GSK, Knopp Biosciences, Sanofi – clinical trial funding; AstraZeneca, Genentech, GSK, Novartis, Regeneron Pharmaceuticals, Inc., Sanofi – advisory board member. Claire Hopkins: GSK, Optinose, Sanofi‐Genzyme, Smith and Nephew – advisory board member. Joaquim Mullol: ALK, AstraZeneca, Genentech, GSK, Menarini, Mitsubishi Tanabe Pharma, MSD, Mylan‐Meda Pharmaceuticals, Novartis, Regeneron Pharmaceuticals, Inc., Sanofi, UCB, Uriach – clinical trial funding, advisory board member, or speaker fees; Mylan‐Meda Pharmaceuticals, Uriach – research grants. Jérôme Msihid, Leda P. Mannent, Yongtao Li, Chien‐Chia Chuang, Asif H. Khan: Sanofi – employees, may hold stock and/or stock options in the company. Isabelle Guillemin: Sanofi – employee at the time of manuscript development. Nikhil Amin, Shahid Siddiqui, Siddhesh Kamat: Regeneron Pharmaceuticals, Inc. – employees and shareholders.
AUTHOR CONTRIBUTIONS
Stella E. Lee and Shahid Siddiqui contributed substantially to the conception and design, and data analysis or interpretation of the data for this manuscript. Claire Hopkins, Joaquim Mullol, Isabelle Guillemin, Leda P. Mannent, Siddhesh Kamat, and Asif H. Khan contributed substantially to the conception and design, data acquisition, and data analysis or interpretation of the data for this manuscript. Jérôme Msihid, Nikhil Amin, Yongtao Li, and Chien‐Chia Chuang contributed substantially to the data analysis or interpretation of the data for this manuscript.
ACKNOWLEDGEMENTS
Research sponsored by Sanofi and Regeneron Pharmaceuticals, Inc. ClinicalTrials.gov Identifiers: NCT02912468 (SINUS‐24) and NCT02898454 (SINUS‐52). Medical writing/editorial assistance provided by Zach Dixon, PhD, of Adelphi Group, funded by Sanofi‐Genzyme and Regeneron Pharmaceuticals, Inc.
Notes
Lee SE, Hopkins C, Mullol J, et al. Dupilumab improves health related quality of life: Results from the phase 3 SINUS studies. Allergy. 2022;77:2211–2221. 10.1111/all.15222 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
Stella E. Lee and Claire Hopkins contributed equally to this article.
REFERENCES
Full text links
Read article at publisher's site: https://doi.org/10.1111/all.15222
Read article for free, from open access legal sources, via Unpaywall:
https://biblio.ugent.be/publication/8747122/file/01HTJ4NCJ2J48BX6MYQ32Y33DD.pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/121057083
Article citations
Reduced Sense of Smell in Patients with Severe Chronic Rhinosinusitis and its Implications for Diagnosis and Management: A Narrative Review.
Adv Ther, 41(12):4384-4395, 09 Oct 2024
Cited by: 0 articles | PMID: 39382822
Review
Canadian Real-World Study Long-Term Clinical Results Using Dupilumab for Chronic Rhinosinusitis With Polyps.
J Otolaryngol Head Neck Surg, 53:19160216241278659, 01 Jan 2024
Cited by: 0 articles | PMID: 39345032 | PMCID: PMC11450752
The "real life" efficacy of dupilumab is independent of initial polyp size and concomitant steroids in CRSwNP.
J Otolaryngol Head Neck Surg, 52(1):56, 06 Sep 2023
Cited by: 1 article | PMID: 37674253 | PMCID: PMC10481502
Biologics for Chronic Rhinosinusitis-A Modern Option for Therapy.
Life (Basel), 13(11):2165, 05 Nov 2023
Cited by: 14 articles | PMID: 38004305
Review
ChatGPT's Skills in Statistical Analysis Using the Example of Allergology: Do We Have Reason for Concern?
Healthcare (Basel), 11(18):2554, 15 Sep 2023
Cited by: 0 articles | PMID: 37761751 | PMCID: PMC10530997
Go to all (12) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Clinical Trials (2)
- (1 citation) ClinicalTrials.gov - NCT02912468
- (1 citation) ClinicalTrials.gov - NCT02898454
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Efficacy and safety of dupilumab in patients with severe chronic rhinosinusitis with nasal polyps (LIBERTY NP SINUS-24 and LIBERTY NP SINUS-52): results from two multicentre, randomised, double-blind, placebo-controlled, parallel-group phase 3 trials.
Lancet, 394(10209):1638-1650, 19 Sep 2019
Cited by: 430 articles | PMID: 31543428
Efficacy and safety of dupilumab in patients with uncontrolled severe chronic rhinosinusitis with nasal polyps and a clinical diagnosis of NSAID-ERD: Results from two randomized placebo-controlled phase 3 trials.
Allergy, 77(4):1231-1244, 01 Oct 2021
Cited by: 23 articles | PMID: 34459002 | PMCID: PMC9292324
Dupilumab improves health-related quality of life in patients with chronic rhinosinusitis with nasal polyposis.
Allergy, 75(1):148-157, 23 Oct 2019
Cited by: 34 articles | PMID: 31306495
Dupilumab: A Review in Chronic Rhinosinusitis with Nasal Polyps.
Drugs, 80(7):711-717, 01 May 2020
Cited by: 20 articles | PMID: 32240527
Review

 1
1

