Abstract
Free full text

Altered Expression of Ion Channels in White Matter Lesions of Progressive Multiple Sclerosis: What Do We Know About Their Function?
Abstract
Despite significant advances in our understanding of the pathophysiology of multiple sclerosis (MS), knowledge about contribution of individual ion channels to axonal impairment and remyelination failure in progressive MS remains incomplete. Ion channel families play a fundamental role in maintaining white matter (WM) integrity and in regulating WM activities in axons, interstitial neurons, glia, and vascular cells. Recently, transcriptomic studies have considerably increased insight into the gene expression changes that occur in diverse WM lesions and the gene expression fingerprint of specific WM cells associated with secondary progressive MS. Here, we review the ion channel genes encoding K+, Ca2+, Na+, and Cl− channels; ryanodine receptors; TRP channels; and others that are significantly and uniquely dysregulated in active, chronic active, inactive, remyelinating WM lesions, and normal-appearing WM of secondary progressive MS brain, based on recently published bulk and single-nuclei RNA-sequencing datasets. We discuss the current state of knowledge about the corresponding ion channels and their implication in the MS brain or in experimental models of MS. This comprehensive review suggests that the intense upregulation of voltage-gated Na+ channel genes in WM lesions with ongoing tissue damage may reflect the imbalance of Na+ homeostasis that is observed in progressive MS brain, while the upregulation of a large number of voltage-gated K+ channel genes may be linked to a protective response to limit neuronal excitability. In addition, the altered chloride homeostasis, revealed by the significant downregulation of voltage-gated Cl− channels in MS lesions, may contribute to an altered inhibitory neurotransmission and increased excitability.
Introduction
Multiple sclerosis (MS) is an inflammatory demyelinating disease of the central nervous system (CNS) affecting more than 2 million people worldwide. MS lesions in CNS white matter (WM) are multiple focal areas of myelin loss accompanied by inflammation, gliosis, phagocytic activity, and axonal damage (Compston and Coles, 2008; Kuhlmann et al., 2017; Filippi et al., 2018; Rommer et al., 2019). Available MS therapies have little benefit for secondary-progressive MS (SPMS) patients, who develop progressive disability after a disease course characterized by inflammatory attacks. Therefore, promoting neuroprotection and remyelination are important therapeutic goals to prevent irreversible neurological deficits and permanent disability.
Ion channels play a fundamental role in maintaining WM integrity and regulating function of axons, interstitial neurons (Sedmak and Judas, 2021), glia, and vascular cells. Dysregulation of ionic homeostasis in the WM during demyelination is decisive for axonal damage and cell death and may interfere with tissue repair processes (Boscia et al., 2020). Furthermore, MS may involve an acquired channelopathy (Waxman, 2001; Schattling et al., 2014). Hence, selectively targeting ion channels in WM represents an attractive strategy to overcome axonal and glial impairment and prevent disease progression.
Recently, transcriptomic studies have considerably increased our insight into gene expression changes occurring in the MS brain (Elkjaer et al., 2019; Jakel et al., 2019; Schirmer et al., 2019). Aiming at identifying the ion channel genes governing WM dysfunction in SPMS brain, we analyzed the recent bulk RNA-sequencing (RNA-seq) datasets by using the MS-Atlas (Elkjaer et al., 2019; Frisch et al., 2020). We put a special emphasis on the distribution of shared and unique genes encoding ion channels in chronic active (CA), active (AL), inactive (IL), and remyelinating (RL) lesions, and normal-appearing white matter (NAWM) compared to control WM (Figures 1A,B, Table 1). We identified uniquely expressed ion channel genes: 34 genes in CA, 9 in IL, 1 in AL, as well as 2 genes in all lesions and NAWM (Figures 1, ,2,2, Table 1). The CA lesions displayed the highest number of upregulated ion channels genes while downregulated ion channels genes were more consistently found in ILs (Figure 1C). Next, we explored recent single-nuclei RNA-seq (snRNA-seq) datasets to identify the expression of dysregulated ion channel genes in cell clusters in the WM of control and SPMS brain (Jakel et al., 2019; Tables 1, ,2,2, Figure 3).
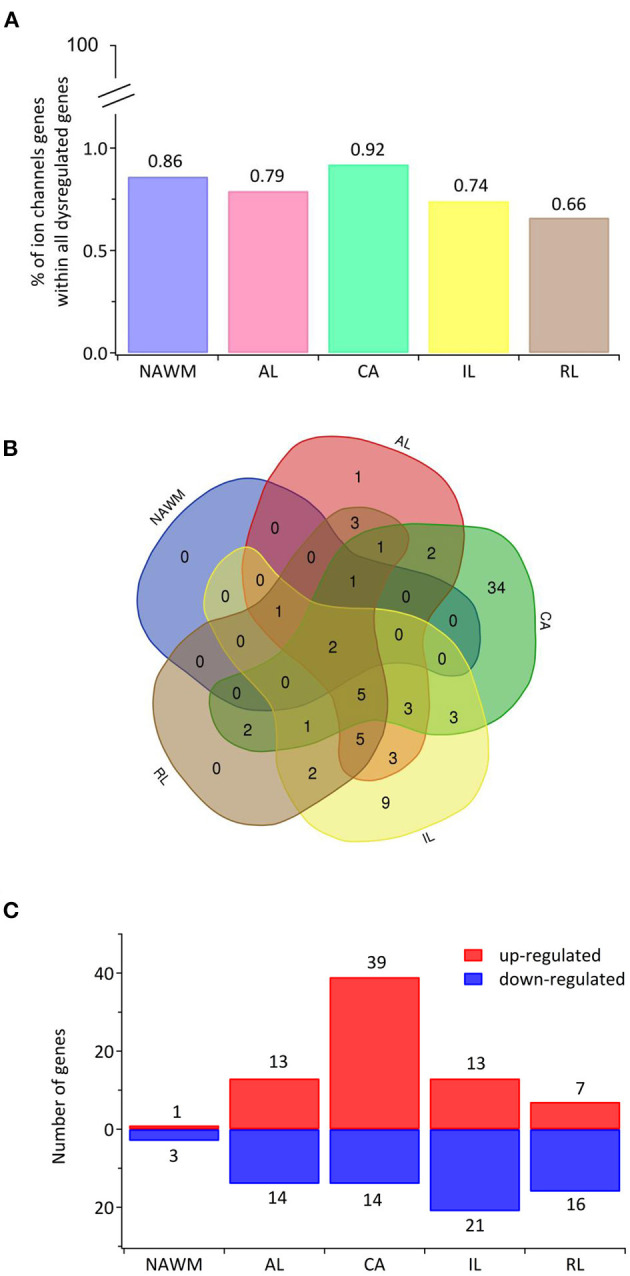
The transcriptional landscape of ion channels in different types of white matter brain lesions from patients with secondary progressive multiple sclerosis. (A) The percentage of significantly differentially expressed genes coding for ion channels among all dysregulated genes and within each lesion type [chronic active (CA), active (AL), inactive (IL), and remyelinating (RL)] and normal-appearing white matter (NAWM) compared to control white matter are indicated. (B) The Venn diagram shows the number of lesion-specific differentially expressed genes coding for ion channels and the number of overlapping genes among the lesion types. (C) The number of significantly differentially upregulated (red) and downregulated (blue) genes in each type of while matter lesion and NAWM compared to control white matter are indicated.
Table 1
Expression and distribution of unique and overlapping genes coding for ion channels within SPMS lesions.
| Protein | Gene | Bulk lesiona | Fold change Up (+)/down (–) regulated (compared to control WM)a | Current type/conductance | Highly expressed in WM clusters of human brainb |
|---|---|---|---|---|---|
| K+ channels | |||||
| Kv 1.1 | KCNA1 | CA | +1.42 | Delayed rectifier | |
| Kv 1.2 | KCNA2 | CA | +1.06 | Delayed rectifier | neuron2 |
| Kv 1.3 | KCNA3 | AL, CA, IL | +1.67 (AL); +1.35 (CA); +1.34 (IL) | Delayed rectifier | |
| Kv 1.4 | KCNA4 | CA | +1.34 | A-type | |
| Kv 1.5 | KCNA5 | AL, RL | +0.86 (AL); +1.36 (RL) | Delayed rectifier | |
| Kv 2.2 | KCNB2 | CA | +1.56 | Delayed rectifier | Neuron1, 2, 3, 4, 5 |
| Kv 2.1 | KCNB1 | CA | +1.26 | Delayed rectifier | Neuron1, 2, 3 |
| Kv 3.3 | KCNC3 | CA | +0.87 | A-type | |
| Kv 3.4 | KCNC4 | AL, IL | +0.81 (AL); +0.72 (IL) | A-type | |
| Kv 4.2 | KCND2 | CA | +0.95 | A-type | OPC, COP, neuron1,3 |
| Kv 4.3 | KCND3 | AL, CA, IL | +0.63 (AL); +0.86 (CA); +0.93 (IL) | A-type | neuron1, 2, 3 |
| Kv 6.1 | KCNG1 | AL, RL | +2.72 (AL); +3.7 (RL) | Modifier of Kv 2 | |
| Kv 7.1 | KCNQ1 | AL, CA | +0.91 (AL); +0.75 (CA) | M-type | |
| Kv 7.2 | KCNQ2 | CA | +0.75 | M-type | neuron1, 2 |
| Kv 7.3 | KCNQ3 | CA | +0.85 | M-type | ImOLGs, neuron1, 2, 3, 5, microglia/macrophages |
| Kv 7.4 | KCNQ4 | AL, CA, IL, RL | +1.19 (AL);+ 0.92 (CA); +1.36 (IL); +2.22 (RL) | M-type | |
| Kv 7.5 | KCNQ5 | CA | +1.69 | M-type | Neuron1, 2, 3, 5 |
| Kv 8.1 | KCNV1 | CA | +1.48 | Modifier of Kv 2 | |
| Kv 9.2 | KCNS2 | CA | +0.90 | Modifier of Kv 2 | |
| Kv 9.3 | KCNS3 | AL, IL, RL, NAWM | −2.72 (AL); −1.5 (IL); −1.98 (RL); −0.71 (NAWM) | Modifier of Kv 2 | |
| Kv 10.1/EAG1 | KCNH1 | CA, IL | +0.81 (CA); +0.93 (IL) | Delayed rectifier | Neuron1, 2, 3 |
| Kv 10. 2/EAG2 | KCNH5 | CA | +1.38 | Delayed rectifier | Neuron2 |
| Kv 11.3/ERG3 | KCNH7 | CA | +1.38 | Delayed rectifier | Neuron1, 2, 3, 5 |
| Kv 12.1/ELK1 | KCNH8 | AL, CA, IL, RL, NAWM | −1.25 (AL); −1.4(CA); −2.05 (IL); −2.38 (RL); −0.62 (NAWM) | Delayed rectifier | Oligo3, Oligo4, Oligo6 |
| TREK1 | KCNK2 | CA | +1.03 | Leak, two pore | |
| TWIK2 | KCNK6 | AL, IL | +1.57 (AL); +0.82 (IL) | Leak, two pore | |
| TREK2 | KCNK10 | AL | −0.65 | Leak, two pore | |
| KCa1.1 | KCNMA1 | AL, CA, IL | +0.69 (AL); +0.87 (CA); +0.7 (IL) | Calcium-Activated | OPC, neuron1, 2, 3, 5, microglia/macrophages |
| KCa2.3 | KCNN3 | IL | −0.7 | Calcium-Activated | Astrocytes1 |
| KNa1.1 | KCNT1 | CA | +1.24 | Sodium-Activated | |
| KNa1.2 | KCNT2 | CA, IL | +0.92 (CA); +1.15 (IL) | Sodium-Activated | Neuron1, 2, 3, pericytes, vascular smooth cells |
| Kir2.1 | KCNJ2 | AL, CA, IL, RL | −0.54 (AL); −0.48 (CA); −0.54 (IL); −0.92 (RL) | Inward rectifier | |
| Kir3.4 | KCNJ5 | AL, CA, RL, NAWM | +2.58 (AL); +1.56 (CA); +1.9 (RL); +1.53 (NAWM) | Inward rectifier | |
| Kir3.2 | KCNJ6 | CA | +1.34 | Inward rectifier | Neuron1, 2, 3 |
| Kir6.1 | KCNJ8 | AL, IL | +0.74 (AL); +0.71 (IL) | Inward rectifier | |
| Kir3.3 | KCNJ9 | CA, RL | −0.52 (CA); −0.9 (RL) | Inward rectifier | |
| Kir4.1 | KCNJ10 | IL, RL | −1.06 (IL); −1.09 (RL) | Inward rectifier | Oligo5 |
| Kir5.1 | KCNJ16 | CA | +1.27 | Inward rectifier | |
| Na+ channels | |||||
| Nav1.1 | SCN1A | CA | +1.12 | TTX-sensitive | OPC, COP, neuron1, 2, 3, 4, 5 |
| Nav1.2 | SCN2A | CA | +1.1 | TTX-sensitive | Neuron1, 2, 3, 4, 5 |
| Nav1.3 | SCN3A | CA | +0.87 | TTX-sensitive | OPC, neuron1, 2, 3, 5 |
| Nav1.6 | SCN8A | CA | +1.15 | TTX-sensitive | Neuron1, 2, 3, 5 |
| Nav1.9 | SCN11A | IL | −1.16 | TTX-resistant | |
| Ca2+ channels | |||||
| Cav1.2 | CACNA1C | CA | +0.56 | L-type | Neuron1, 2, 3, 5, pericytes |
| Cav1.3 | CACNA1D | CA | +0.57 | L-type | Neuron1,3 |
| Cav2.1 | CACNA1A | CA | +0.64 | P/Q-type | OPC, neuron1, 2 |
| Cav2.3 | CACNA1E | CA | +0.97 | P/Q-type | Neuron1, 2, 5 |
| Cav3.1 | CACNA1G | IL | +1.8 | T-type | |
| Cav3.2 | CACNA1H | CA | +1.12 | T-type | |
| Cav3.3 | CACNA1I | CA | +1.03 | T-type | |
| Ryanodine | |||||
| Ryr2 | RYR2 | CA | +0.85 | Ca2+ Release channel | Neuron1, 2, 3 |
| Ryr3 | RYR3 | IL | −0.76 | Ca2+ Release channel | Astrocytes1 |
| TRP channels | |||||
| TRPC1 | TRPC1 | AL, IL, RL | −0.5 (AL); −0.48 (IL); −0.85 (RL) | Ca2+-permeable cation channel | |
| TRPM2 | TRPM2 | IL | +0.92 | Ca2+-permeable cation channel | |
| TRPM3 | TRPM3 | IL, RL | −1.09 (IL); −0.98 (RL) | Ca2+-permeable cation channel | Astrocytes1, neuron1 |
| TRPM6 | TRPM6 | CA, IL, RL | −0.99 (CA); −1.06 (IL); −1.08 (RL) | Ca2+-permeable cation channel | |
| TRPP1 | PKD2 | IL | −0.48 | Ca2+-permeable cation channel | |
| TRPP3 | PKD2L2 | CA | −0.58 | Ca2+-permeable cation channel | |
| TRPV1 | TRPV1 | CA | −1.04 | Ca2+-permeable cation channel | |
| TRPV3 | TRPV3 | AL, CA, IL, RL | −0.51 (AL); −0.72 (CA); −0.5 (IL); −0.74 (RL) | Ca2+-permeable cation channel | |
| TRPV5 | TRPV5 | AL, CA, IL, RL | −1.4 (AL); −1.67 (CA); −1.72 (IL); −2.02 (RL) | Ca2+-permeable cation channel | |
| TRPV6 | TRPV6 | AL, CA, IL, RL, NAWM | −1.77 (AL); −1.97 (IL); −1.32 (CA); −2.23 (RL); 0.86 (NAWM) | Ca2+-permeable cation channel | |
| Cl− channels | |||||
| CLC-2 | CLCN2 | CA | −0.57 | Inward rectification | |
| CLC-4 | CLCN4 | AL, IL, RL | −0.79 (AL); −0.73 (IL); −1.03 (RL) | Cl−/H+ antiporter | |
| CLC-7 | CLCN7 | CA | −0.72 | Cl−/H+ antiporter | |
| Connexins and pannexins | |||||
| Cx43 | GJA1 | AL, CA, RL | +1.53 (AL); +1.12 (CA); +1.19 (RL) | Monovalent and divalent ions | Astrocytes1, astrocytes2 |
| Cx32 | GJB1 | AL, CA, IL, RL | −1.6 (AL); −1.5 (CA); −1.85 (IL); −2.44 (RL) | Monovalent and divalent ions | Oligo5 |
| CX37 | GJA4 | IL | +1.19 | Monovalent and divalent ions | Pericytes |
| Cx47 | GJC2 | AL, CA | −1.62 (AL); −1.74 (CA) | Monovalent and divalent ions | |
| Panx1 Others | PX1 | IL | +0.56 | Monovalent and divalent ions | |
| Piezo2 | PIEZO2 | AL, CA, IL, RL | −0.92 (AL); −1.01 (CA); −0.93 (IL); −1.49 (RL) | Ca2+ -permeable | Oligo1, Oligo6 |
| CFTR | CFTR | AL, CA, IL, RL | −1.22 (AL); −1.37 (CA); −1.77 (IL); −1.86 (RL) | Cl−-permeable | Oligo1 |
| Hv1 | HVCN1 | CA, RL | +0.71 (CA); +0.92 (RL) | H+-selective | |
| Navi2.1 | NALCN | AL, IL, RL | −0.49 (AL); −0.73 (IL); −0.88 (RL) | Sodium leak channel, non-selective | |
| Orai3 | ORAI3 | AL, RL | +0.87 (AL); +1.25 (RL) | Store-Operated Ca2+ entry | |
| Aquaporin 1 | AQP1 | CA, IL | −1.03 (CA); −0.12 (IL) | Water, ammonia, H202 permeability | Astrocytes1, astrocytes2 |
| CATSPERG | CATSPERG | CA | +0.7 | Ca2+ -permeable | |
| CATSPERE | CATSPERE | IL | −0.48 | Ca2+ -permeable | |
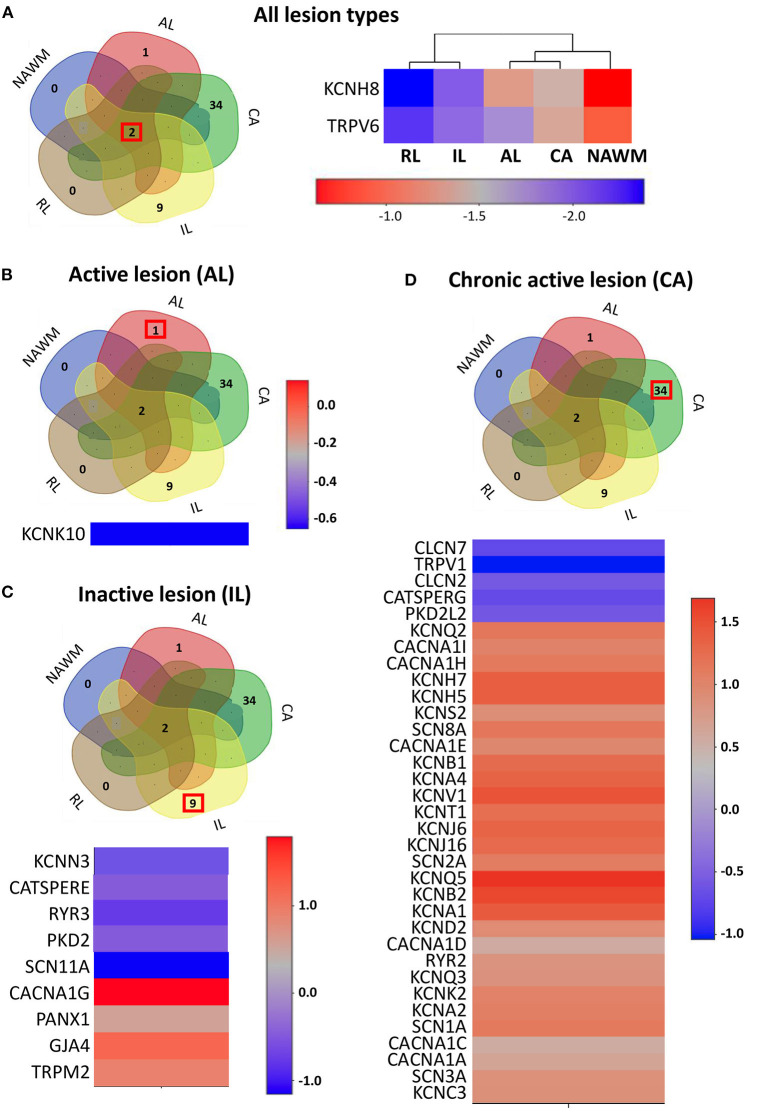
The expression profile of the ion channel genes uniquely expressed in different lesion types. (A) Left panel: The Venn diagram represents the number of overlapping and lesion-specific differentially expressed genes coding for ion channels in chronic active (CA), active (AL), inactive (IL), and remyelinating (RL) lesions and in normal-appearing white matter (NAWM) compared to control white matter. Right panel: The heatmap shows two genes, coding for ion channels KCNH8 and TRPV6 that are significantly altered in all lesion types compared to control white matter. Scale bar indicates fold changes. (B) The Venn diagram, the heatmap, and the scale bar show the single ion channel gene, KCNK10, which is uniquely downregulated in active lesion (AL). (C) The Venn diagram, the heatmap, and the scale bar show the eight genes coding for ion channels that are uniquely significantly differentially dysregulated in inactive lesion (IL). (D) The Venn diagram, the heatmap, and the scale bar show the 33 genes coding for ion channels that are significantly and differentially dysregulated compared to control white matter in chronic active lesion (CA). The red box in Venn diagrams marks the genes that are specifically dysregulated in the corresponding type of lesion.
Table 2
Profiling expression of unique gene in lesions in WM clusters of healthy and SPMS braina.
| Protein | Gene | Neuron | Astrocyte | OPC | COP | ImOLG | Oligo | Microglia | Pericyte |
|---|---|---|---|---|---|---|---|---|---|
| K+ channels | |||||||||
| Kv1.1 | KCNA1 | n.d | n.d | n.d | n.d | n.d | n.d | n.d | n.d |
| Kv1.2 | KCNA2 | + | +/– | +/– | +/– | – | +/– | – | – |
| Kv1.4 | KCNA4 | +/– | – | – | – | – | – | – | – |
| Kv2.1 | KCNB1 | + | +/– | +/– | + | +/– | +/– | – | + |
| Kv2.2 | KCNB2 | ++ | +/– | – | +/– | +/– | +/– | – | – |
| Kv3.3 | KCNC3 | + | +/– | – | +/– | +/– | +/– | +/– | – |
| Kv4.2 | KCND2 | +++ | + | ++++ | +++ | + | +/– | +/– | +/– |
| Kv7.2 | KCNQ2 | + | +/– | + | + | +/– | +/– | – | +/– |
| Kv7.3 | KCNQ3 | +++ | + | + | + | ++ | +/– | +++ | +/– |
| Kv7.5 | KCNQ5 | ++++ | + | +/– | + | + | +/– | +/– | +/– |
| Kv8.1 | KCNV1 | + | – | – | +/– | +/– | – | – | – |
| Kv9.2 | KCNS2 | + | – | – | +/– | – | – | – | – |
| Kv10. 2/EAG2 | KCNH5 | + | +/– | +/– | + | +/– | +/– | – | – |
| Kv11.3/ERG3 | KCNH7 | +++ | +/– | – | + | + | +/– | – | – |
| Kv12.1/ELK1 | KCNH8 | + | +++ | ++ | +++ | ++ | +++ | +/– | +/– |
| TREK1 | KCNK2 | + | +/– | + | +/– | – | – | – | – |
| TREK2 | KCNK10 | + | +/– | +/– | + | +/– | +/– | – | – |
| KCa2.3 | KCNN3 | + | ++ | + | + | + | +/– | +/– | +/– |
| KNa1.1 | KCNT1 | + | – | – | +/– | +/– | – | – | – |
| Kir3.2 | KCNJ6 | + | +/– | + | + | – | + | – | – |
| Kir5.1 | KCNJ16 | – | +/– | + | + | – | – | – | – |
| Na+ channels | |||||||||
| Nav1.1 | SCN1A | ++ | + | +++ | ++ | + | +/– | – | – |
| Nav1.2 | SCN2A | +++ | + | +/– | + | + | +/– | – | +/– |
| Nav1.3 | SCN3A | ++ | +/– | ++ | ++ | + | + | – | +/– |
| Nav1.6 | SCN8A | n.d | n.d | n.d | n.d | n.d | n.d | n.d | n.d |
| Nav1.9 | SCN11A | n.d | n.d | n.d | n.d | n.d | n.d | n.d | n.d |
| Ca2+ channels | |||||||||
| Cav1.2 | CACNA1C | +++ | + | + | + | + | +/– | – | +++ |
| Cav1.3 | CACNA1D | ++ | +/– | + | + | + | +/– | + | – |
| Cav2.1 | CACNA1A | +++ | + | +++ | ++ | + | +/– | + | +/– |
| Cav2.3 | CACNA1E | ++ | +/– | +/– | + | + | +/– | – | – |
| Cav3.1 | CACNA1G | + | – | +/– | +/– | – | – | – | – |
| Cav3.2 | CACNA1H | n.d | n.d | n.d | n.d | n.d | n.d | n.d | n.d |
| Cav3.3 | CACNA1I | + | – | – | +/– | – | – | – | – |
| Ryanodine | |||||||||
| Ryr2 | RYR2 | ++++ | + | +/– | + | + | + | +/– | + |
| Ryr3 | RYR3 | + | +++ | + | + | + | +/– | +/– | +/– |
| TRP channels | |||||||||
| TRPM2 | TRPM2 | + | +/– | – | +/– | + | – | + | +/– |
| TRPP1 | PKD2 | + | ++ | + | ++ | ++ | + | + | + |
| TRPP3 | PKD2L2 | n.d | n.d | n.d | n.d | n.d | n.d | n.d | n.d |
| TRPV1 | TRPV1 | +/– | +/– | – | +/– | – | +/– | – | – |
| TRPV6 | TRPV6 | n.d | n.d | n.d | n.d | n.d | n.d | n.d | n.d |
| Cl− channels | |||||||||
| CLC−2 | CLCN2 | +/– | +/– | +/– | +/– | +/– | + | – | – |
| CLC−7 | CLCN7 | + | + | + | + | + | + | + | +/– |
| Connexins | |||||||||
| Cx37 | GJA4 | – | – | – | – | – | – | – | ++ |
| Pannexin | |||||||||
| Px1 | PANX1 | n.d | n.d | n.d | n.d | n.d | n.d | n.d | n.d |
| Catsper | |||||||||
| CATSPERG | CATSPERG | +/– | +/– | – | +/– | +/– | +/– | – | – |
| CATSPERE | CATSPERE | n.d | n.d | n.d | n.d | n.d | n.d | n.d | n.d |
+/– (log scale > 0.01 ≤ 0.1); + (log scale > 0.1 ≤ 0.5); ++ (log scale > 0.6 ≤ 1); +++ (log scale >1.1 ≤ 1.5); ++++ (log scale > 1.5); – (log scale 0); n.d., not detected; red (+), highly expressed gene in the cluster if compared to the rest of the clusters.
Note, that in the database, some of the clusters encompass both control and MS samples and, therefore, the mean can represent a combination of counts from control and MS brain.
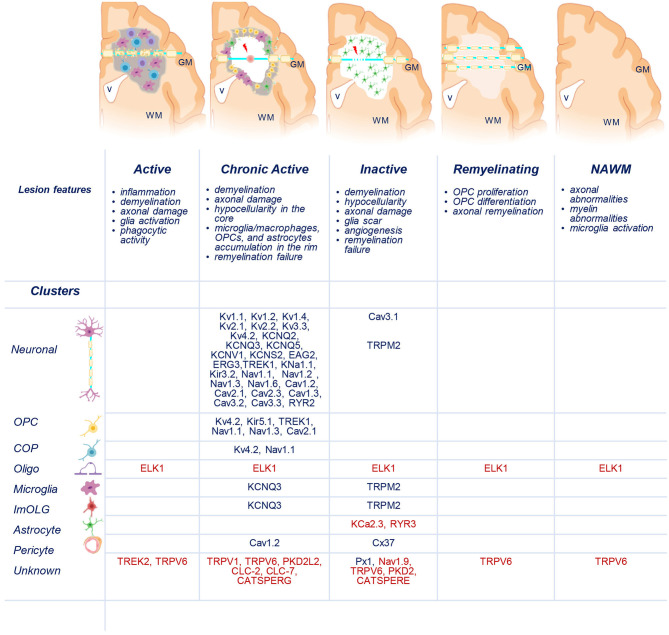
Distribution of uniquely dysregulated genes encoding ion channels in SPMS lesions. Schematic representation of active (AL), chronic active (CA), inactive (IL), and remyelinating (RL) lesions, and normal-appearing white matter (NAWM). Upregulated (blue) and downregulated (red) ion channels encoded by uniquely dysregulated genes are listed according to their expression in the lesions and in neuronal, oligodendrocyte precursor cells (OPCs), committed OPCs (COPs), oligodendrocytes (Oligo), microglia, immune oligo (ImOLG), astrocyte, pericyte, and unknown clusters. GM, gray matter; WM, white matter; v, brain ventricle. Gray areas indicate active inflammatory lesion, white areas indicate demyelinated inactive lesions, red spot indicates tissue damage, red arrow indicates axonal dysfunction. Source icon is from Biorender.com.
The goal of the present review is to discuss the current knowledge on the expression and function of ion channels that turned out to be significantly and uniquely dysregulated in WM lesions of SPMS brain. We summarize the information in the context of human MS and the related experimental models (Tables 1–3, Figure 4).
Table 3
Expression and role of unique dysregulated ion channels in experimental models of MS.
| Gene/protein | Distribution, localization | Cellular functions during physiological conditions | WM in MS models | |
|---|---|---|---|---|
| Alterations | Role | |||
| KCNA1/Kv1.1 | JPN of myelinated axons | Regulate AP propagation and neural excitability | Redistribution to internodes and nodal segments, upregulation | Hyperpolarise axonal Vrest, affect AP threshold, impair AP conduction |
| Microglia, astrocyte (t), OPCs (t) | Proliferation, cell activation | |||
| KCNA2/Kv1.2 | JPN of myelinated axons | Regulate AP propagation and neural excitability | Redistribution to internodes and nodal segments, upregulation | Hyperpolarise axonal Vrest, affect AP threshold, impair AP conduction |
| Reactive astrocyte, microglia, OPC | Proliferation, cell activation | |||
| KCNA4/Kv1.4 | Axons (HP) | Regulate AP propagation and neural excitability | Upregulation in astrocytes and OPCs around EAE lesions | Deficiency ameliorated EAE course in KO mice, but have no effect on demyelination/remyelination in the cuprizone model |
| Reactive astrocyte, OPCs | Proliferation | |||
| KCNB1/Kv2.1 | Soma, proximal dendrites, AIS Microglia, OPCs (t) | Influence AP duration during high frequency firing, regulate neuronal excitability | Unknown in WM Downregulation in motor neurons of GM spinal cord during EAE | Unknown |
| KCNB2/Kv2.2 | Soma, proximal dendrites, AIS Not detected in glia | Influence AP duration during high frequency firing, regulate neuronal excitability | Unknown | Unknown |
| KCNC3/Kv3.3 | Axons, somatodendritic compartment Astrocyte, microglia (t), OPCs (t) | Regulate AP firing at high frequency | Upregulation in some injured WM axons | Unknown |
| KCND2/Kv4.2 | Soma, dendrites Astrocyte (t), OPCs (t), microglia (t) | Regulate threshold for AP initiation and repolarization, frequency-dependent AP broadening, AP back-propagation | Unknown | Unknown |
| KCNQ2/Kv7.2 | AIS, nodes of Ranvier OPCs, microglia | Stabilize Vrest, regulate activity of NaV-channels, accelerate AP upstroke, influence neuronal subthreshold excitability, regulate spike generation, and repetitive firing | Unknown | Unknown |
| KCNQ3/Kv7.3 | AIS, nodes of Ranvier Microglia (pro-inflammatory), OPCs, astrocyte (t) | Stabilize Vrest, regulate activity of NaV-channels, accelerate AP upstroke, influence neuronal subthreshold excitability, regulate spike generation and repetitive firing | Unknown in WM Upregulated in demyelinated neocortical axons of L5 pyramidal neurons in the cuprizone model. | Unknown in WM Ensure AP conduction in demyelinated GM axons, decrease excitability |
| KCNQ5/Kv7.5 | Soma, dendrites Astrocyte, OPCs, microglia | Contributes to AHP currents in the HP | Unknown | Unknown |
| KCNV1/Kv8.1 | Unknown Oligo lineage (t) | Co-assemble with Kv2.1, reduce Kv2.1 current density which may lead to AP broadening and hyper-synchronized high-frequency firing | Unknown | Unknown |
| KCNS2/Kv9.2 | Unknown Oligo lineage (t) | Co-assemble with Kv2.1 | Unknown | Unknown |
| KCNH5/EAG2 | Unknown Astrocyte (t), OPCs (t) | Unknown | Unknown | Unknown |
| KCNH7/ERG3 | Unknown Astrocyte (t), OPCs (t), microglia (t) | Dampen excitability, stabilize Vrest | Unknown | Unknown |
| KCNH8/ELK1 | Unknown OPCs (t) | Unknown | Unknown | Unknown |
| KCNK2/TREK1 | Axons, and node of Ranviers in afferent myelinated nerve Astrocyte, microglia (t) OPCs (t) | Contribute to “leak” K+-current, help establishing and maintaining Vrest, regulate neuronal excitability, ensure AP repolarization at nodes of Ranvier in afferent myelinated fibers Contribute to passive membrane K+ conductance, glutamate release | Unknown | Deficiency aggravates EAE course in KO mice Channel activation reduces CNS immune cell trafficking across BBB and attenuate EAE course |
| KCNK10/TREK2 | Unknown Astrocyte OPCs (t) | Contribute to “leak” K+-current, help establishing and maintaining Vrest
Contribute to K+ buffering, glutamate clearance | Unknown | Unknown |
| KCNT1/KNa1.1 | Soma, axons Astrocytes (t) | Regulate the generation of slow afterhyperpolarization, firing patterns, and setting and stabilizing the Vrest | Unknown | Unknown |
| KCNN3/KCa2.3 | Dendrites, AIS Astrocyte, microglia, oligo lineage (t) | Regulate AP propagation and neuronal excitability, contribute to maintaining Ca2+-homeostasis K+ buffering in astrocytes Microglia proliferation and cytokines production | Unknown | Unknown |
| KCNJ6/Kir3.2 | Somatodendritic compartment Astrocyte, oligo lineage (t) | K+-homeostasis, maintenance of Vrest, hyperpolarization, control of AP firing and neuronal excitability, inhibition of excitatory neurotransmitter release | Unknown | Unknown |
| KCNJ16/Kir5.1 | Somatodendritic compartment, dendritic spines Astrocyte, oligo lineage, microglia (t) | Silent channel when combined with Kir2.1. When combined with Kir4.1, build channels with larger conductance and greater pH-sensitivity. Plays a role in synaptic transmission Chemoreception K+ buffering | Unknown | Unknown |
| SCN1A/Nav1.1 | Somatodendritic compartment, AIS, nodes of Ranvier Microglia, astrocyte, OPCs (t) | Saltatory conduction, maintenance of sustained firing, control of excitability Microglia phagocytosis, cytokine release | Increase or no change; localize along the demyelinated regions | Unknown |
| SCN2A/Nav1.2 | AIS, immature nodes of Ranvier, along the non-myelinated axons Astrocyte, pre-oligodendrocytes | Back-propagation of AP into the somatodendritic compartment, may support slow spike propagation Oligo maturation | Increase of diffuse distribution along demyelinated axons in various mouse models; no change in myelin-deficient rat Upregulated in astrocytes during EAE | Unclear. Suggested: preservation of AP propagation, or axonal damage |
| SCN3A/Nav1.3 | Somatodendritic compartment, along the axons including myelinated fibers Astrocyte oligo lineage (t) | AP initiation and propagation, proliferation and migration of cortical progenitors | No change in the optic nerve | Unknown |
| SCN8A/Nav1.6 | AIS, nodes of Ranvier; low density on cell soma, dendritic shafts, synapses Astrocyte, microglia oligo (t) | AP initiation and propagation, neuronal excitability | Decrease at the nodes of Ranvier, increase of diffuse distribution along the damaged axons, no change at AIS Upregulated in microglia/macrophages during EAE | May trigger Na+ increase in axoplasm, reversal of NCX, and intra-axonal Ca2+ overload. Deletion improves axonal health during EAE |
| SCN11A/Nav1.9 | Soma, proximal processes Negligible in all glial cells (t) | Regulate excitation, control activity-dependent axonal elongation, mediate sustained depolarizing current upon activation of muscarinic receptors | Unknown | Unknown |
| CACNA1C/CaV1.2 | Somatodendritic compartment (synaptically, extrasynaptically), axons, axonal terminals (extrasynaptically), pioneer axons during development Astrocyte, oligo lineage, reactive microglia | Synaptic modulation, propagation of dendritic Ca2+ spikes, regulation of glutamate receptor trafficking, CREB phosphorylation, coupling of excitation to nuclear gene transcription, modulation of long-term potentiation, neurites growth and axonal pathfinding during development Astrogliosis OPCs development and myelination | Unknown | Unknown. Suggested: Neurodegeneration because L-type VGCCs blockers attenuate mitochondrial pathology in nerve fibers and axonal loss Deletion in astrocyte- reduces cell activation and pro-inflammatory mediators release in the cuprizone model Deletion in OPCs reduced remyelination in the cuprizone model |
| CACNA1D/CaV1.3 | Somatodendritic compartment, axonal cylinders Astrocyte, microglia oligo lineage | Pacemaking activity, spontaneous firing, Ca2+-dependent post-burst after-hyperpolarization, Ca2+-dependent intracellular signaling pathways, regulation of morphology of dendritic spines and axonal arbores Oligodendrocyte-axon signaling, release of pro-inflammatory mediators by microglia | Unknown | Unknown. Suggested: neuroprotection because L-type VGCCs blockers attenuate mitochondrial pathology in nerve fibers and axonal loss |
| CACNA1A/CaV2.1 | Axonal synaptic terminals, axonal shafts in WM, somatodendritic compartment Reactive astrocyte OPCs, premyelinating oligo, microglia (t) | Neurotransmitter release at neuronal and neuron-glia synapses, regulation of BK and SK channels, control of neuronal firing, regulation of gene expression, local Ca2+ signaling, and cell survival Calcium influx in oligo upon neuronal activity | Unknown | Unknown |
| CACNA1E/CaV2.3 | Dendritic spines, axonal terminals Astrocyte, oligodendrocyte | Neurotransmitter release, synaptic plasticity, regulation of BK, SK, and KV4.2 channels | Unknown | Unknown |
| CACNA1G/CaV3.1 | Somatodendritic compartment, AIS Astrocyte (t) oligo lineage | Generation and timing of APs, regulation of neuronal excitability, rhythmic AP bursts in thalamus, neuronal oscillations, neurotransmitter release | Unknown | T-cells from KO mice show decreased cytokine release Deficiency in KO mice inhibits the autoimmune response in the EAE model |
| CACNA1H/CaV3.2 | Somatodendritic compartment, AIS Astrocyte oligo lineage | Generation and timing of APs, regulation of neuronal excitability, rhythmic AP bursts in thalamus, neuronal oscillations, neurotransmitter release | Unknown | Unknown |
| CACNA1I/CaV3.3 | Somatodendritic compartment | Generation and timing of APs, regulation of neuronal excitability, rhythmic AP bursts in thalamus, neuronal oscillations, neurotransmitter release | Unknown | Unknown |
| RyR2 | Along ER (also in axons) Astrocyte, oligo lineage | Ca2+ release from the ER into the cytoplasm, vesicle fusion, neurotransmitter release, synaptic plasticity, growth cone dynamics | Unknown | Unknown |
| RyR3 | Along ER (also in axons) Astrocyte, OPCs, oligodendrocytes | Ca2+ release from the ER into the cytoplasm, vesicle fusion, neurotransmitter release, synaptic plasticity, growth cone dynamics Astrocyte motility OPCs development | Unknown | Unknown |
| TRPV1 | Soma, post-synaptic dendritic spines, synaptic vesicles Astrocyte, microglia, oligodendrocytes | Regulation of Ca2+-signaling, synaptic plasticity Astrocyte: migration, chemotaxis, activation during stress, inflammasome activation Microglia: migration, cytokine production, ROS generation, phagocytosis, polarization, cell death | Suggested a main role in regulating microglia inflammatory response | Both detrimental and beneficial effects have been described in EAE disease |
| TRPV6 | Unknown Astrocyte (t) | Unknown | Unknown | Unknown |
| TRPM2 | Soma and neurites in neuronal cultures Microglia, astrocyte (t), oligodendrocyte (t) | Contribute to synaptic plasticity and play an inhibitory role in neurite outgrowth Microglia activation and generation of proinflammatory mediators | Upregulated in monocyte-lineage cells | TRPM2 deficiency reduce monocyte infiltration in EAE |
| PKD2/TRPP1 | ER, primary cilia, and plasma membrane Astrocyte (t), microglia (t), oligo lineage (t) | Maintenance of Ca2+-homeostasis, cell proliferation | Unknown | Unknown |
| PKD2L2/TRPP3 | Unknown Astrocyte (t), microglia (t) | Unknown | Unknown | Unknown |
| CLCN2/CLC-2 | Plasma membranes, intracellular membranes Astrocyte, OPCs, microglia | Maintenance of low intracellular Cl− level, control of cell volume homeostasis, regulation of GABAAR-mediated synaptic inputs, regulation of neuronal excitability Interacts with AQP4 in astrocytes, regulates OPCs differentiation, contribute to volume regulation and phagocytosis in microglia | Unknown | Unknown |
| CLCN7/CLC-7 | Lysosomes Microglia, astrocyte (t), oligo lineage (t) | Suggested function in the neuronal endo-lysosomal pathway Regulate lysosomal acidification in activated microglia | Unknown | Unknown |
| GJA4/CX37 | Largely expressed in vascular cells | Regulate vasomotor activity, endothelial permeability, and maintenance of body fluid balance | Unknown | Unknown |
| PANX1/Px1 | Soma, dendrites, axons Astrocyte, OPCs microglia | Paracrine and autocrine signaling, ATP-sensitive ATP release in complex with P2X7Rs, intercellular propagation of Ca2+-waves, cell differentiation, migration, synaptic plasticity, memory | Unknown | Panx-1 induced ATP release and inflammasome activation contribute to WM damage during EAE Inhibition of Panx1 using pharmacology or gene disruption delays and attenuates disease course in EAE and cuprizone model |
| CATSPERG | Unknown Oligo lineage (t) Microglia (t) | Unknown | Unknown | Unknown |
| CATSPERE | Unknown | Unknown | Unknown | Unknown |
AHP, afterhyperpolarization; AIS, axon initial segment; AP, action potential; BK, big-conductance Ca2+-activated K+-channels; ER, endoplasmatic reticulum; GABAAR, ionotropic gamma aminobutyric acid A receptor; HP, hippocampus; JPN, juxtaparanodal regions; NCX, Na+/Ca2+ exchanger; SCI, spinal cord injury; SK, small-conductance Ca2+-activated K+-channels; SSCx, somatosensory cortex; t, transcipts; Vrest, resting membrane potential.
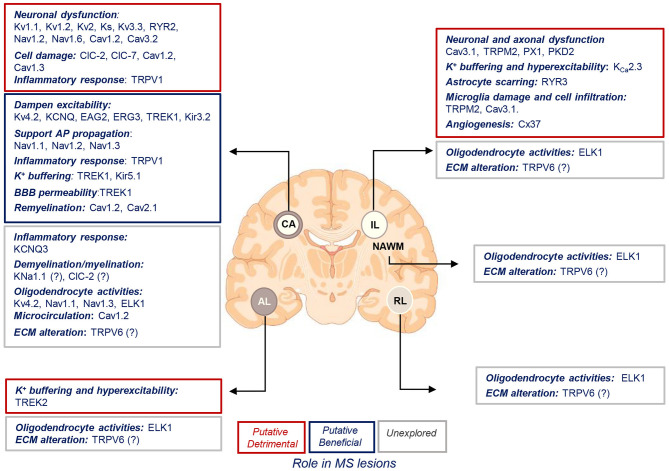
Putative roles of ion channels encoded by uniquely dysregulated genes in SPMS lesions. Schematic illustration of the putative detrimental (red box), putative beneficial (blue box), and unexplored (gray box) functional roles of ion channel encoded by the uniquely dysregulated genes in active (AL), chronic active (CA), inactive (IL), and remyelinating (RL) lesions, and normal-appearing white matter (NAWM) of SPMS brain. Source icon is from Biorender.com.
K+ Channels
Voltage-Gated K+ Channels (Kv)
Kv channels are composed of four α-subunits that assemble as homo- or hetero-tetramers to form a membrane pore. Forty human genes encode for Kv α-subunits representing 12 families. KV1–Kv4 (Shaker, Shab, Shaw, and Shal), KV7 (KCNQ), and KV10–KV12 (eag, erg, and elk) α-subunits produce functional channels, while Kv5, Kv6, Kv8, and Kv9 fail to produce currents when expressed alone in heterologous expression system and are considered modulatory subunits for Kv2-subfamily. The diversity of Kv channels is further increased by the ability of α-subunits to combine with auxiliary subunits, which regulate gating properties.
Kv1.1, Kv1.2, and Kv1.4 (KCNA1, KCNA2, and KCNA4)
KCNA genes encode for low-threshold voltage-activated Kv1 (Kv1.1–1.8) channels, of which Kv1.1–Kv1.6 are expressed in the brain (Chittajallu et al., 2002; Vautier et al., 2004; Vacher et al., 2008; Rasmussen and Trimmer, 2019). Kv1 channels display little/no inactivation, resulting in sustained delayed rectifier K+ currents, with the exception of Kv1.4, which underlies transient A-type K+ current.
Neurons
Kv1.1 expression is highest in the brainstem, while Kv1.4 > Kv1.2 represent the main Kv1 subunits in the hippocampus (Trimmer, 2015). Kv1.1 channels, in association with Kv1.2, cluster in the juxtaparanodal regions of axons under the myelin sheath and regulate action potential (AP) propagation and neural excitability (Wang et al., 1993; Trimmer and Rhodes, 2004; Ovsepian et al., 2016). Mutations of Kv1 channels result in hyper-excitability, episodic ataxia, myokymia, and epilepsy (Allen et al., 2020).
Glia
Mouse astrocytes express low levels of Kv1.1, Kv1.2, and Kv1.4 transcripts (Smart et al., 1997), but Kv1.2 and Kv1.4 expression is high in reactive rat astrocytes (Akhtar et al., 1999). Kv1.1 transcripts and proteins are highly expressed in C6 glioma cells. Rodent oligodendrocyte precursor cells (OPCs) express Kv1.1, Kv1.2, and Kv1.4 transcripts (Attali et al., 1997; Chittajallu et al., 2002; Falcao et al., 2018; Batiuk et al., 2020) but only Kv1.4 and low level of Kv1.2 proteins (Attali et al., 1997; Schmidt et al., 1999). In OPCs and astrocytes, the Kv1 subunits regulate cell growth and cell cycle progression, e.g., Kv1.4 overexpression in vitro increases OPCs proliferation (Schmidt et al., 1999) while deletion decreases it (Gonzalez-Alvarado et al., 2020). Recent RNA-seq did not detect Kv1.1, Kv1.2, and Kv1.4 in mouse microglia (Hammond et al., 2019), but earlier studies found Kv1.1 and Kv1.2 mRNAs and/or proteins in BV2 microglia, rat cultured microglia, and amoeboid microglia within corpus callosum during development, but barely in resting microglia by P21 (Fordyce et al., 2005; Li F. et al., 2008; Wu et al., 2009). In microglia, Kv1.1 and Kv1.2 expression was linked to cell activation (Eder, 1998), and their upregulation induced by lipopolysaccharide (LPS), ATP, or hypoxia is involved in the release of pro-inflammatory cytokines and intracellular production of reactive oxygen species (ROS) and nitric oxide (NO) (Li F. et al., 2008; Wu et al., 2009).
Expression and Function in MS
Bulk RNA-seq found upregulation of Kv1.1, Kv1.2, and Kv1.4 transcripts in CA lesions (Figure 2, Table 1; Elkjaer et al., 2019; Frisch et al., 2020). The snRNA-seq detected significant Kv1.2 expression in neuronal clusters, slight increase of Kv1.4 transcripts in neuronal but not glial clusters, and no Kv1.1 transcript (Tables 1, ,2;2; Jakel et al., 2019).
The CA lesion is characterized by ongoing tissue damage and, functionally, Kv1.2 upregulation in CA lesions may be a hallmark of axonal damage. While recent data found that KCNA1 gene is downregulated during demyelination in the cuprizone model (Martin et al., 2018), in animal models of MS, Kv1.2 (and also Kv1.1) ectopically redistributes to nodes and internodes of WM axons (McDonald and Sears, 1969; Wang et al., 1995; Sinha et al., 2006; Jukkola et al., 2012; Zoupi et al., 2013; Kastriti et al., 2015), while in human MS, the dislocation of Kv1.2 channels is associated with paranodal pathology, particularly in NAWM regions, and contributes to axonal dysfunction (Howell et al., 2010; Gallego-Delgado et al., 2020). The upregulated and redistributed Kv1.2 and Kv1.1 channels may hyperpolarize the axonal resting membrane potential (Vrest), elevate the amount of depolarization necessary for AP initiation, and impair AP conduction (Wang et al., 1995; Sinha et al., 2006; Jukkola et al., 2012). Pharmacological inhibition of Kv1.1 and Kv1.2 channels, e.g., with 4-aminopyridine, enhances axonal conduction and improves MS symptoms (Lugaresi, 2015).
It is difficult to speculate regarding Kv1.4 function in MS because data are not consistent. In animal models of MS and spinal cord injury (SCI), this developmentally restricted subunit re-appears/increases in OPCs, OLs, and astrocytic processes around lesion sites (Herrero-Herranz et al., 2007; Jukkola et al., 2012), but not in WM axons or microglia (Edwards et al., 2002; Jukkola et al., 2012). Mice lacking Kv1.4 exhibit reduced myelin loss in the spinal cord WM during EAE but no change of demyelination/remyelination in the corpus callosum in the cuprizone model (Gonzalez-Alvarado et al., 2020). However, it is unclear whether function of Kv1.4 subunits is relevant for glial cells in human MS because snRNA-seq barely detected Kv1.4 transcripts in glia clusters (Table 2).
Kv2.1 and Kv2.2 (KCNB1 and KCNB2)
Kv2 channels (encoded by KCNB1 and KCNB2 genes) mediate high-voltage-activated slowly inactivating delayed rectifier K+ currents (Guan et al., 2007). Kv2.1 channels can assemble with electrically silent KvS subunits, resulting in greater variability of Kv2 currents (Trimmer, 2015; Johnson et al., 2019).
Neurons
High-density clusters of Kv2.1 and Kv2.2 localize to soma, proximal dendrites, and axonal initial segment (AIS). Kv2 channels influence AP duration during high-frequency firing and regulate neuronal excitability (Guan et al., 2007). Kv2.1 mutations are associated with neonatal encephalopathy epilepsies and neurodevelopmental delays (Torkamani et al., 2014; Thiffault et al., 2015; de Kovel et al., 2017).
Glia
RNA-seq detected KCNB1 gene in mouse OPC and microglia (Falcao et al., 2018; Hammond et al., 2019).
Expression and Function in MS
Bulk RNA-seq revealed upregulation of Kv2.1 and Kv2.2 transcripts in CA lesions of SPMS brain (Figure 2, Table 1; Elkjaer et al., 2019; Frisch et al., 2020), while snRNA-seq found Kv2.1 and Kv2.2 in neuronal clusters (Tables 1, ,2;2; Jakel et al., 2019). During EAE, Kv2.1 protein expression was downregulated in spinal cord motor neurons (Jukkola and Gu, 2015). Remarkably, Kv2.1 channels exist as freely dispersed conducting channels, or form electrically silent somatodendritic clusters (Schulien et al., 2020). Upregulated clustered Kv2.1 channels promote functional coupling of L-type Ca2+ channels in plasma membrane to ryanodine receptors (RyRs) of the endoplasmic reticulum (ER) (Deutsch et al., 2012; Kirmiz et al., 2018; Vierra et al., 2019) and may modulate intracellular Ca2+ level contributing to cell damage, while dispersal of Kv2.1-clusters blocks apoptogenic K+ currents and provides neuroprotection (Sesti et al., 2014; Justice et al., 2017). Hence, to elucidate the functional role of Kv2 upregulation in MS (Table 1), it will be important to determine whether it reflects an increase in clustered or dispersed Kv2 channels.
Kv3.3 (KCNC3)
The KCNC3 gene encodes for the Kv3.3 subunit, which, together with Kv3.1, Kv3.2, and Kv3.4, belongs to the Kv3 channel subfamily (Shaw). The Kv3.3 and Kv3.4 mediate transient A-type K+ currents, while Kv3.1 and Kv3.2 mediate sustained K+ currents.
Neurons
Kv3 channels localize to axonal and somatodendritic domains, and play a critical role in regulating AP firing at high frequency (Rasmussen and Trimmer, 2019). KCNC3 mutations result in spinocerebellar ataxia type-13 and cerebellar neurodegeneration (Rasmussen and Trimmer, 2019).
Glia
Cortical and hippocampal astrocyte cultures express Kv3.3 and Kv3.4 mRNAs and proteins (Bekar et al., 2005; Boscia et al., 2017). KCNC3 mRNA was detected in mouse OPCs and microglia (Larson et al., 2016; Falcao et al., 2018).
Expression and Function in MS
Bulk RNA-seq showed significant Kv3.3 upregulation in CA lesions (Figure 2, Table 1), while snRNA-seq revealed its predominant distribution in neuronal clusters (Table 2; Jakel et al., 2019). Kv3.3 may play a detrimental role in MS because it increases in injured WM axons during EAE progression in mice and in human MS lesions (Jukkola et al., 2017), and the deletion of Kv3.1, which forms hetero-tetramers with Kv3.3, reduced EAE severity in mice (Jukkola et al., 2017).
Kv4.2 (KCND2)
The KCND2 gene encodes for the Kv4.2 subunit that (together with Kv4.1 and Kv4.3) is a member of the Kv4 channel subfamily (Shal) and is highly expressed in the brain (Alfaro-Ruiz et al., 2019). Kv4 channels activate at subthreshold potentials and then inactivate and recover rapidly. They mediate transient A-type K+ current (Bahring et al., 2001; Birnbaum et al., 2004).
Neurons
Kv4.2 subunits are highly expressed in soma and dendrites of hippocampal neurons and interneurons. They regulate the threshold for AP initiation and repolarization, frequency-dependent AP broadening, and AP back-propagation (Nerbonne et al., 2008). Kv4.2 mutations are associated with infant-onset epilepsy and autism.
Glia
Kv4.2-transcripts were found in mouse astrocytes (Bekar et al., 2005) and OPCs, but only at very low levels in microglia (Falcao et al., 2018; Hammond et al., 2019; Batiuk et al., 2020).
Expression and Function in MS
Bulk RNA-seq found significant Kv4.2 upregulation in CA lesions (Figure 2, Table 1; Elkjaer et al., 2019; Frisch et al., 2020). The snRNA-seq reported significant expression of Kv4.2 transcripts in neuronal, OPCs, and committed OPCs (COP) clusters (Table 2; Jakel et al., 2019). Kv4.2 subunit may contribute to oligodendrocyte dysfunction in SPMS brain because dysregulated KCND2 transcripts are associated with oligodendrocyte dysfunction in mental illnesses (Vasistha et al., 2019).
Kv7.2, Kv7.3, and Kv7.5 (KCNQ2, KCNQ3, and KCNQ5)
The KCNQ genes encode for Kv7.1–Kv7.5 (KCNQ1–KCNQ5) family members that underlie a voltage-gated non-inactivating outward K+ current, known as M current (IM).
Neurons
The Kv7.2/3 or Kv7.3/5 hetero-tetramers represent the dominant subunit composition in neurons (Wang et al., 1998; Cooper et al., 2000; Kharkovets et al., 2000), while Kv7.4/Kv7.5 is dominant in vascular smooth muscles (Brueggemann et al., 2014). The Kv7.2- and Kv7.3 subunits co-cluster with Nav channels at AIS and nodes of Ranvier in rodent somatosensory cortex and spinal cord WM and gray matter (GM) (Pan et al., 2006; Cooper, 2011; Battefeld et al., 2014). Kv7.5 localizes to soma and dendrites of cortical and hippocampal neurons and contributes to afterhyperpolarization currents (Tzingounis et al., 2010). The Kv7 channels stabilize Vrest, influence neuronal subthreshold excitability, and regulate spike generation (Jentsch, 2000; Miceli et al., 2008). By reducing the steady-state inactivation of nodal Nav channels, the Kv7 channels increase the availability of transient Nav currents at nodes of Ranvier, thereby accelerating the AP upstroke and elevating short-term axonal excitability (Hamada and Kole, 2015). In the perisomatic region, Kv7 channels counteract the persistent Nav current and restrain repetitive firing (Pan et al., 2006; Cooper, 2011). Variants of KCNQ2/KCNQ3 or KCNQ4 genes cause developmental/epileptic disorders and hearing loss (Soldovieri et al., 2011; Miceli et al., 2013).
Glia
KCNQ3 gene is expressed in spinal cord WM astrocytes (Devaux et al., 2004), while KCNQ5 is expressed in rat retinal astrocytes (Caminos et al., 2015). The KCNQ2-5 mRNAs and proteins were detected in rat cortical OPCs and microglia cultures, while differentiated oligodendrocytes showed weak KCNQ4 expression (Wang et al., 2011; Vay et al., 2020).
Expression and Function in MS
Bulk RNA-seq found upregulation of KCNQ2-3-5 transcripts in CA lesions (Figure 2, Table 1; Elkjaer et al., 2019; Frisch et al., 2020). The snRNA-seq reported KCNQ2-3-5 expression in neuronal clusters and KCNQ3 expression in immune oligodendroglia (ImOLG) and microglia/macrophages clusters (Tables 1, ,2;2; Jakel et al., 2019). Kv7.3 upregulation may reflect increased necessity of the channels along the axons because Kv7.3 subunit extensively redistributes to internodes of acutely and chronically demyelinated GM axons in the cuprizone model (Hamada and Kole, 2015). It is tempting to speculate that Kv7 upregulation may be beneficial during MS. First, Kv7 channels may increase the availability of transient Nav current via membrane hyperpolarization supporting AP conduction in demyelinated axons (Battefeld et al., 2014). Second, Kv7 channels may mitigate inflammation-induced neuronal excitability because, following LPS exposure, the IM inhibition underlies hyperexcitability of hippocampal neurons that is reversed by a nonselective Kv7-opener retigabine (Tzour et al., 2017). Although retigabine also exerts neuroprotective effects in several neurodegenerative conditions (Boscia et al., 2006; Nodera et al., 2011; Wainger et al., 2014; Bierbower et al., 2015; Li et al., 2019; Vigil et al., 2020; Wu et al., 2020), a clinical trial with retigabine analog flupirtine failed to demonstrate neuroprotective effects during MS (Dorr et al., 2018). Furthermore, blockade of Kv7 channels with XE-991 inhibited migration of LPS-treated pro-inflammatory microglia in vitro (Vay et al., 2020), suggesting that these channels may promote the pro-inflammatory role of microglia also during MS. Hence, neuronal and glial Kv7 channels may have diverse functions during MS.
Kv8.1 and Kv9.2 (KCNV1 and KCNS2)
Neurons
KCNV1 and KCNS2 genes encode for electrically silent (KvS) Kv8.1- and Kv9.2 subunits that assemble into hetero-tetrameric channels with Kv2 subunits (Bocksteins, 2016). A number of channelopathies is ascribed to KvS subunits (Salinas et al., 1997a; Liu et al., 2016; Allen et al., 2020), pointing to their important physiological role.
Glia
KCNV1 and KCNS2 transcripts were found in oligodendrocyte lineage cell (Marques et al., 2016).
Expression and Function in MS
Bulk RNA-seq showed upregulation of KCNV1 and KCNS2 genes in CA lesions (Figure 2, Table 1; Elkjaer et al., 2019; Frisch et al., 2020). The snRNA-seq detected KCNV1 and KCNS2 in neuronal clusters (Table 2; Jakel et al., 2019). Co-assembly between Kv8.1 and Kv2.1 reduces Kv2.1 current density (Hugnot et al., 1996; Castellano et al., 1997): the high stoichiometry of the Kv8.1 subunit suppresses surface expression and favors retention of heteromeric channels in the ER (Salinas et al., 1997b). Neurons with reduced Kv2.1-mediated currents demonstrate broadened APs (Du et al., 2000) underlying hyper-synchronized high-frequency firing observed during epilepsy. Hence, upregulated KvS subunits in CA lesions may influence the localization of clustered Kv2 subunits in SPMS brain and affect AP firing and/or propagation.
Eag2, erg3, and elk1 (KCNH5, KCNH7, and KCNH8)
KCNH genes encode for Kv10–Kv12 subfamilies, all orthologs of the Drosophila ether-àgo-go (EAG) channels. They include two eag (Kv10), three eag-related (erg/Kv11), and three eag-like (elk/Kv12) K+ channels that can form heteromeric channels within each subfamily (Rasmussen and Trimmer, 2019).
Neurons
All EAG channels are expressed in the CNS neurons (Ludwig et al., 2000; Papa et al., 2003; Zou et al., 2003), but only erg-mediated currents have been verified using suitable blockers (Bauer and Schwarz, 2018).
Glia
RNA-seq detected KCNH5, KCNH7, and KCNH8 expression in mouse OPCs (Falcao et al., 2018). KCNH5 and KCNH7 genes were found in astrocytes (Batiuk et al., 2020), while only the KCNH7 gene was detected in mouse microglia (Hammond et al., 2019). Erg-type currents were reported in neopallial microglia cultures (Zhou et al., 1998) and hippocampal astrocytes (Emmi et al., 2000; Papa et al., 2003).
Expression and Function in MS
Bulk RNA-seq detected increased KCNH5(eag2) and KCNH7(erg3) transcripts in CA lesions and downregulation of KCNH8(elk1) transcript in all lesions and NAWM (Figure 2, Table 1; Elkjaer et al., 2019; Frisch et al., 2020). The snRNA-seq found significant expression of KCNH5 and KCNH7 transcripts in neuronal clusters and KCNH8 in mature oligodendrocyte clusters (Tables 1, ,2;2; Jakel et al., 2019). The functional role of eag2, erg3, and elk1 during MS may be related to altered neuronal excitability. Indeed, human eag1 and eag2 gain-of-function mutations underlie severe neurological disorders associated with epileptic seizures (Allen et al., 2020). The erg channels that are active at subthreshold potentials stabilize the Vrest and dampen excitability (Fano et al., 2012). Erg3 knockdown in mice increases intrinsic neuronal excitability and enhances seizure susceptibility, while treatment with erg activator reduces epileptogenesis (Xiao et al., 2018). Erg3 expression is decreased in the brain of epilepsy patients. Remarkably, association of KCNH7(erg) intronic polymorphisms with MS pathogenesis was speculated although never substantiated (Martinez et al., 2008; Couturier et al., 2009).
Two-Pore Domain K+ Channels (K2P)
K2P K+ channels are encoded by 15 KCNK genes, stratified into six subfamilies: TWIK, TASK (TWIK-related acid-sensitive), TREK (TWIK-related arachidonic acid activated), THIK (tandem pore domain halothane-inhibited), TALK (TWIK-related alkaline pH-activated), and TRESK (TWIK-related spinal cord) K+ channels (Enyedi and Czirjak, 2010). K2P K+ channels contribute to “leak” K+ current, helping to establish and maintain Vrest (Enyedi and Czirjak, 2010).
TREK1 and TREK2 (KCNK2 and KCNK10)
Neurons and Glia
KCNK2 and KCNK10 genes encode for TREK-1 and TREK-2 channels, which are expressed in neurons, astrocytes, and OPC (Hervieu et al., 2001; Talley et al., 2001; Falcao et al., 2018). Only TREK-1 transcripts were detected in microglia (Hammond et al., 2019). In astrocytes, TREK channels contribute to passive conductance and glutamate release (Zhou et al., 2009; Woo et al., 2012). TREK-1 and TREK-2 may be activated by a wide range of physiological and pathological stimuli reminiscent of inflammatory environment including membrane stretch, heat, intracellular acidosis, and cellular lipids (Ehling et al., 2015).
Expression and Function in MS
Bulk RNA-seq found upregulated TREK-1 transcripts in CA lesions, but a divergent modulation was observed for TREK-2 mRNAs in ALs (Figure 2, Tables 1, ,2;2; Elkjaer et al., 2019; Frisch et al., 2020). KCNK2 and KCNK10 transcripts were detected in neuronal and oligodendrocyte clusters, but scarcely observed in astrocytes (Table 2; Jakel et al., 2019). TREK-1 upregulation in CA lesions most likely reflects a protective response because TREK-1 plays a neuroprotective role during neurological diseases, including MS (Djillani et al., 2019). TREK-1 reduces neuronal excitability by hyperpolarizing the membrane potential (Honore, 2007) and is required for rapid AP repolarization at the node of Ranvier in mammalian afferent myelinated nerves, while TREK-1 loss-of-function retards nerve conduction and impairs sensory responses in animals (Kanda et al., 2019). Treatment of mice with TREK-1 activators, riluzole (Gilgun-Sherki et al., 2003), or alpha-linolenic acid attenuates EAE course (Blondeau et al., 2007), while these effects are reduced in TREK-1−/− mice (Bittner et al., 2014). TREK-1 function is also important for non-neuronal cells because aggravated EAE course in TREK-1−/− mice is associated with increased numbers of infiltrating T cells and higher endothelial expression of ICAM1 and VCAM1 (Bittner et al., 2013), and TREK-1 is reduced in the microvascular endothelium in inflammatory MS brain lesions (Bittner et al., 2013).
TREK-2 downregulation in AL, a lesion type characterized by myelin breakdown and infiltration by inflammatory cells (Elkjaer et al., 2019; Frisch et al., 2020), may contribute to reduced glutamate and K+ buffering and neuronal over-excitation because TREK-2 helps maintain the membrane potential and low extracellular glutamate and K+ level during ischemia (Gnatenco et al., 2002; Rivera-Pagan et al., 2015).
Na+- and Ca2+-Activated K+ Channels
KNa1.1 (KCNT1)
Neurons
The KCNT1 and KCNT2 genes encode for Slack and Slick K+ channels that are activated by Na+ influx (Bhattacharjee and Kaczmarek, 2005). They localize to soma and axons of neurons (Bhattacharjee et al., 2002; Brown et al., 2008; Rizzi et al., 2016) and are involved in the generation of slow after-hyperpolarization, regulation of firing patterns, and setting and stabilizing the Vrest (Franceschetti et al., 2003). Alterations in KCNT1 and KCNT2 genes are linked to early-onset epileptic encephalopathies and Fragile-X-syndrome (Kim and Kaczmarek, 2014).
Glia
RNA-seq detected KCNT1 gene in mouse astrocytes (Batiuk et al., 2020).
Expression and Function in MS
Bulk RNA-seq showed KNa1.1 upregulation in CA lesions (Figure 2, Table 1; Elkjaer et al., 2019; Frisch et al., 2020). SnRNA-seq detected KCNT1 in neuronal clusters (Table 2). KCNT1 function in MS may be related to myelination/demyelination because severely delayed myelination occurs in patients with KCNT1 mutations (Vanderver et al., 2014). Furthermore, KCNT1 is a causative gene in infants with hypomyelinating leukodystrophy showing WM alterations (Arai-Ichinoi et al., 2016), and KCNT1 mutations occur in infant epilepsy associated with delayed myelination, thin corpus callosum, and WM hyper-intensity in MRI (McTague et al., 2013; Shang et al., 2016; Borlot et al., 2020).
KCa2.3, SK3 (KCNN3)
The KCNN3 gene encodes for the SK3 subunit of small-conductance Ca2+-activated K+ channels (SK channels). They mediate Ca2+ gated K+ current and thus couple the increase in intracellular Ca2+ concentration to hyperpolarization of the membrane potential.
Neurons
SK3 channels are found on dendrites and AIS (Abiraman et al., 2018). They play a role in AP propagation and regulation of neuronal excitability (Stocker, 2004). They protect against excitotoxicity by maintaining Ca2+ homeostasis after NMDA receptor activation (Dolga et al., 2011).
Glia
RNA-seq detected intense KCNN3 expression in mouse astrocytes (Batiuk et al., 2020), confirming earlier studies, which showed labeling of GFAP+ processes in the supraoptic nucleus for SK3 channels and suggested the role of SK3 in astrocytic K+ buffering (Armstrong et al., 2005). Oligodendrocyte lineage cells express low levels of KCNN3 mRNA (Falcao et al., 2018), while mouse microglia does not express KCNN3 (Hammond et al., 2019). However, rat microglia in culture expresses the SK3 subunit, which is increased upon microglia activation with LPS (Schlichter et al., 2010). SK3 activation inhibited microglia proliferation, inflammatory IL-6 production, and morphological transformation to macrophages, while blocking SK3 in microglia-reduced neurotoxicity (Dolga et al., 2012).
Expression and Function in MS
Bulk RNA-seq showed significant and unique downregulation of KCNN3 in ILs (Elkjaer et al., 2019; Frisch et al., 2020). SnRNA-seq revealed high KCNN3 expression in astrocyte clusters (Figure 2, Table 1; Jakel et al., 2019). ILs consist of large demyelinated areas devoid of macrophages but filled with scar-forming astrocytes showing reduced ability to buffer glutamate and K+ (Compston and Coles, 2008; Kuhlmann et al., 2017; Filippi et al., 2018; Schirmer et al., 2018). Hence, KCNN3 downregulation in MS may reflect altered function of astrocytes, e.g., K+ buffering (Armstrong et al., 2005), contributing to axonal hyper-excitability and death.
Inward Rectifier K+ Channels (Kir)
KCNJ gene family encodes Kir channels and comprises 16 subunits of Kir1–Kir7 subfamilies categorized into four groups: (1) classical (Kir2.x); (2) G-protein-gated (Kir3.x); (3) ATP-sensitive (Kir6.x); and (4) K+-transport channels (Kir1.x, Kir4.x, Kir5.x, Kir7.x) (Hibino et al., 2010). At a comparable driving force, Kir channels allow greater influx than efflux of K+-ions. Their high open probability at negative transmembrane voltages makes them well-suited to set the Vrest and to control cell excitability.
Kir3.2 (KCNJ6)
KCNJ6 gene encodes for Kir3.2 subunits, also known as G-protein-gated Kir (GIRK2) channels that are effectors for Gi/o-dependent signaling and mediate outward K+ current.
Neurons
Kir3.1/Kir3.2 hetero-tetramers are found in the somatodendritic compartment of neurons. Activation of GIRK channels is mediated by G-protein-coupled receptors including muscarinic, metabotropic glutamate, somatostatin, dopamine, endorphins, endocannabinoids, etc. GIRK channels are important for K+ homeostasis and maintenance of Vrest near the K+ equilibrium potential. GIRK current hyperpolarizes neuronal membrane reducing spontaneous AP firing and inhibiting neurotransmitter release (Luscher and Slesinger, 2010). GIRK signaling contributes to learning/memory, reward, pain, anxiety, schizophrenia, addiction, and other processes (Mayfield et al., 2015). Kir3.2 mutations in mice lead to a loss of K+ selectivity and increased Na+ permeability of the channel, resulting in the weaver phenotype (Liao et al., 1996; Surmeier et al., 1996).
Glia
Astrocytes and Müller cells express Kir3 channels (Raap et al., 2002). Kir3.2 transcripts were detected in the mouse optic nerve (Papanikolaou et al., 2020) and oligodendrocyte lineage (Falcao et al., 2018), but not in microglia (Hammond et al., 2019).
Expression and Function in MS
RNA-seq revealed KCNJ6 upregulation in the CA lesions (Elkjaer et al., 2019; Frisch et al., 2020). The snRNA-seq predominantly found KCNJ6 transcripts in neuronal clusters (Jakel et al., 2019; Table 1). The functional role of Kir3.2 channels in MS may be related to membrane hyperpolarization and compensation of excessive neuronal excitability driving neurodegeneration.
Kir5.1 (KCNJ16)
KCNJ16 gene encodes for Kir5.1 subunit, which forms an electrically silent channel when combined with Kir2.1 (Derst et al., 2001; Pessia et al., 2001), but is functional when combined with Kir4.1 (Konstas et al., 2003). Clustering of heteromeric Kir4.1/Kir5.1 and homomeric Kir5.1 channels on plasmalemma involves the anchoring protein PSD-95 (Tanemoto et al., 2002; Brasko et al., 2017). Heteromeric Kir4.1/Kir5.1 channels exhibit larger channel conductance, greater pH sensitivity, and different expression patterns if compared to Kir4.1 homomers (Tanemoto et al., 2000; Tucker et al., 2000; Pessia et al., 2001; Hibino et al., 2010).
Neurons
In cultures, Kir5.1 immunoreactivity was detected in somatodendritic compartments where PSD-95 immunoreactivity was also localized. The Kir5.1/PSD-95 complex may exist at dendritic spines in vivo and play a role in synaptic transmission (Tanemoto et al., 2002).
Glia
Kir5.1 mRNA is two-fold higher in OPCs (NG2+-glia) vs. astrocytes (Zhang et al., 2014), and mouse brain microglia expresses Kir5.1 transcript too (Hammond et al., 2019). Kir5.1 expression in oligodendrocytes and astrocytes depends on its association with Kir4.1: loss of Kir4.1 reduces Kir5.1, suggesting that altered expression/distribution of Kir5.1 may contribute to the phenotype of Kir4.1 knockout mice (Brasko et al., 2017; Schirmer et al., 2018). The oligodendroglial Kir5.1/Kir4.1 channels are important for K+ clearance (Poopalasundaram et al., 2000; Neusch et al., 2001), long-term maintenance of axonal function, and WM integrity (Kelley et al., 2018; Schirmer et al., 2018). In astrocytes, Kir5.1/Kir4.1 channels contribute to chemoreception, spatial K+ buffering, and breathing control (Mulkey and Wenker, 2011).
Expression and Function in MS
Bulk RNA-seq revealed Kir5.1 upregulation in CA lesions (Table 1; Elkjaer et al., 2019; Frisch et al., 2020). SnRNA-seq detected Kir5.1 in OPCs clusters and scarcely in astrocytes. The KCNJ16 gene is upregulated during demyelination and acute remyelination in mouse cuprizone model (Martin et al., 2018). Upregulation of Kir5.1 may reflect the role of the oligodendroglial Kir4.1/Kir5.1 channels in K+ clearance during MS and may represent a mechanism to compensate Kir4.1 reduction in MS brain (Schirmer et al., 2014). Alternatively, Kir5.1 upregulation may underlie reduced Kir4.1 function in MS because presence of Kir5.1 subunit confers loss of functional activity to Kir4.1/Kir5.1 channels under oxidative stress (Jin et al., 2012).
Voltage-Gated Na+ Channels (Nav)
In the mammalian brain, Nav are composed of α-subunit (260 kDa) and one or several β-subunits (β1–β4, of 33–36 kDa) (Goldin et al., 2000). The α-subunit forms the channel pore and acts as a voltage sensor; β-subunits play a modulatory role and influence voltage dependence, gating kinetics, and surface expression of the channel (Goldin et al., 2000; Yu and Catterall, 2003; Namadurai et al., 2015). The nine NaV1.1–NaV1.9 α-subunits are encoded by the corresponding genes SCN1A–SCN5A and SCN8A–SCN11A. In addition, NaX isoform was described,which is encoded by the SCN6/7A gene.
NaV1.1 (SCN1A)
Neurons
NaV1.1 channels localize to the somatodendritic compartment of principal neurons and AIS of GABAergic interneurons, spinal cord motor neurons, and retinal neurons (Ogiwara et al., 2007; Duflocq et al., 2008; Dumenieu et al., 2017). NaV1.1 channels are also present at the nodes of Ranvier of the cerebellar WM, fimbria, corpus callosum, and spinal cord WM (Ogiwara et al., 2007; Duflocq et al., 2008; O'Malley et al., 2009). They play a role during saltatory conduction along myelinated axons and are essential for maintaining the sustained firing of GABAergic interneurons and Purkinje cells, thus controlling the excitability of neuronal networks (Duflocq et al., 2008; Dumenieu et al., 2017). Mutations in NaV1.1 channels result in various types of epilepsy and reduced volume of brain GM and WM (Lee et al., 2017; Scheffer and Nabbout, 2019).
Glia
Human astrocytes show negligible immunolabelling for NaV1.1 and no upregulation in the WM of MS patients (Black et al., 2010). Transcriptome analysis revealed low level of SCN1A in mouse cortical and hippocampal astrocytes (Batiuk et al., 2020). RNA-seq detected SCN1A in oligodendrocytes and OPCs throughout the CNS (Larson et al., 2016; Marques et al., 2016; Falcao et al., 2018). The functional role of NaV1.1 channels in astrocytes and oligodendroglia remains unknown. Transcriptome studies have not detected SCN1A in microglia prepared from brain homogenates (Hammond et al., 2019), but NaV1.1 protein was found in microglia derived from neonatal rat mixed glial cultures (Black et al., 2009). NaV1.1 channels may be involved in regulation of phagocytosis and/or release of IL-1α, IL-β, and TNF-α from microglia (Black et al., 2009). The NaV1.1 mRNA was detected in astrocytoma, oligodendroglioma, and glioblastoma samples from patients where these channels may contribute to the pathophysiology of brain tumors (Schrey et al., 2002).
Expression and Function in MS
Bulk RNA-seq detected SCN1A upregulation in CA lesions (Figure 2, Table 1; Elkjaer et al., 2019; Frisch et al., 2020). The snRNA-seq revealed significant expression of NaV1.1 transcripts in neuronal, committed OPC, and OPC clusters (Tables 1, ,2;2; Jakel et al., 2019). Experimental models do not provide clues regarding the functional role of NaV1.1 channels in MS: NaV1.1 expression was increased or unaltered in the optic nerve during EAE (Craner et al., 2003; O'Malley et al., 2009), while in the spinal cord, these channels clustered at the nodes of Ranvier and localized along the demyelinated regions (O'Malley et al., 2009). SCN1A upregulation in human MS may reflect the necessity of the channel for redistribution along the demyelinated axons and support of AP propagation.
NaV1.2 (SCN2A)
Neurons
The NaV1.2 channels localize to the AIS, immature nodes of Ranvier, and in non-myelinated axons during early development. As nervous system matures, NaV1.2 channels are replaced by NaV1.6 channels (Boiko et al., 2001; Osorio et al., 2005; Dumenieu et al., 2017), although in some neurons, they remain into adulthood. Nav1.2 channels of the AIS control back-propagation of APs into the somatodendritic compartment, while NaV1.6 channels are being placed at distal parts of the AIS control initiation and propagation of AP into the axon (Boiko et al., 2003; Hu et al., 2009). NaV1.2 channels are also diffusely distributed along non-myelinated axons in the adult CNS where they may support slow spike propagation (Arroyo et al., 2002; Dumenieu et al., 2017).
Glia
NaV1.2 protein was found in rat astrocytes isolated from the spinal cord and optic nerve (Black et al., 1995), but only limited NaV1.2 expression was observed in human astrocytes in control and MS tissue (Black et al., 2010). The RNA-seq detected SCN2A expression in oligodendrocytes and OPCs (Larson et al., 2016; Marques et al., 2016). Knockdown of NaV1.2 in pre-oligodendrocytes of the auditory brainstem resulted in reduced number and length of cellular processes and decreased MBP level, indicating that NaV1.2 channels are important for structural maturation of myelinating cells and myelination (Berret et al., 2017). Microglia expresses no/little functional NaV1.2 channels (Black et al., 2009; Pappalardo et al., 2016; Hammond et al., 2019).
Expression and Function in MS
Bulk RNA-seq detected upregulation of SCN2A gene in CA lesions (Figure 2, Table 1; Elkjaer et al., 2019; Frisch et al., 2020), while snRNA-seq showed abundant SCN2A expression in neuronal clusters (Tables 1, ,2;2; Jakel et al., 2019). The upregulation may reflect re-expression of NaV1.2 protein, in line with previous reports showing diffuse distribution of NaV1.2 channels along the demyelinated axons in human MS lesions within optic nerve and spinal cord (Craner et al., 2004b). Axonal NaV1.2 channels may contribute to preservation of AP propagation and re-establishment of myelin sheathes (Coman et al., 2006), as it occurs during development. On the other hand, NaV1.2 channels may promote axonal damage by increasing the intracellular Na+ concentration that triggers reversal of Na+/Ca2+ exchanger (NCX) and Ca2+ overload in the axoplasm (Friese et al., 2014; Schattling et al., 2016). In line with this, human gain-of-function mutation in the mouse SCN2A gene triggers axonal damage, neurodegeneration, disability, and lethality in the mouse model of MS (Schattling et al., 2016). Expression of “developmental” NaV1.2 channels in axons was also found in animal models of MS, i.e., in adult Shiverer mice that lack myelin (Westenbroek et al., 1992; Boiko et al., 2001), in transgenic mice overexpressing proteolipid protein that initially have normal myelination but then lose myelin (Rasband et al., 2003), and in the demyelinated optic nerve and spinal cord during EAE (Craner et al., 2003, 2004a; Herrero-Herranz et al., 2008). However, other data showed that in chronic spinal cord MS lesions, NaV1.2 channels localize on astrocytic processes surrounding the axons rather than on axons themselves (Black et al., 2007), and NaV1.2 expression/distribution was unchanged in the spinal cord of myelin-deficient rats (Arroyo et al., 2002).
NaV1.3 (SCN3A)
Neurons
NaV1.3 channels are highly expressed in rodent and human CNS throughout the embryonic development (Black and Waxman, 2013). Some studies reported that their expression decreases during the first weeks after birth, while others found NaV1.3 immunoreactivity in GM and/or WM of adult rat and human brain (Whitaker et al., 2001; Lindia and Abbadie, 2003; Thimmapaya et al., 2005; Cheah et al., 2013). NaV1.3 channels mainly localize to the somatodendritic compartment of neurons but were also detected along the axons including myelinated fibers where they may contribute to initiation and propagation of APs (Whitaker et al., 2001; Lindia and Abbadie, 2003; Cheah et al., 2013; Wang et al., 2017). In the developing brain, NaV1.3 channels regulate proliferation and migration of cortical progenitors that do not fire APs (Smith et al., 2018).
Glia
The mRNA and NaV1.3 protein were detected in astrocytes (Black et al., 1995). RNA-seq demonstrated SCN3A expression in oligodendroglial cells and suggested higher expression in OPCs vs. mature oligodendrocytes (Larson et al., 2016; Marques et al., 2016). NaV1.3 expression in microglia was negligible or absent (Black et al., 2009; Hammond et al., 2019). Heterogeneous expression (from weak to strong) of NaV1.3 mRNA occurred in human astrocytoma, oligodendroglial tumors, and glioblastoma (Schrey et al., 2002). Functions of NaV1.3 channels in glia remain unknown.
Expression and Function in MS
Bulk mRNA-seq reported upregulation of SCN3A gene in the CA lesions (Elkjaer et al., 2019; Frisch et al., 2020). The snRNA-seq found significant SCN3A expression in neuronal and OPCs clusters (Jakel et al., 2019; Tables 1, ,2).2). SCN3A upregulation during MS may reflect augmented expression of NaV1.3 protein in axons that is necessary for supporting/re-establishment of AP propagation in injured WM, because increased NaV1.3 levels are known to be associated with higher neuronal firing. For instance, mRNA and NaV1.3 protein were upregulated in spontaneously epileptic rats (Guo et al., 2008), and expression in hippocampal neurons of a novel coding variant SCN3A-K354Q resulted in enhanced Nav1.3 currents, spontaneous firing, and paroxysmal depolarizing shift-like depolarizations of the membrane potential (Estacion et al., 2010).
NaV1.6 (SCN8A)
Neurons
NaV1.6 channels cluster at high-density at the AIS and nodes of Ranvier of GM and WM axons, but can be also located on the soma, dendrites, and synapses although at a lower density (Caldwell et al., 2000; Dumenieu et al., 2017; Johnson et al., 2017; Eshed-Eisenbach and Peles, 2020). The expression level of NaV1.6 channels is low during development, but significantly increases as the nervous system matures (Boiko et al., 2001; Osorio et al., 2005; Dumenieu et al., 2017). In the adult CNS, NaV1.6 channels are the major Na+ channels responsible for initiation and propagation of APs (Boiko et al., 2003; Hu et al., 2009). Loss of Nav1.6 activity results in decreased neuronal excitability, while gain-of-function mutations potentiate excitability (O'Brien and Meisler, 2013). SCN8A mutations in mice result in ataxia, tremor, and dystonia; in humans, SCN8A haploinsufficiency is associated with intellectual disability, while hyperactivity can contribute to pathogenesis of epileptic encephalopathy (O'Brien and Meisler, 2013; Meisler, 2019).
Glia
RNA-seq detected SCN8A transcripts in mouse oligodendrocyte lineage (Marques et al., 2016), but they were negligible in microglia (Hammond et al., 2019). Immunoreactivity for NaV1.6 was observed in cultured spinal cord astrocytes and in brain microglia in vitro and in situ (Reese and Caldwell, 1999; Black et al., 2009; Black and Waxman, 2012; Hossain et al., 2013), but their functional role is unknown.
Expression and Function in MS
Bulk mRNA-seq found upregulation of SCN8A gene in CA lesions (Elkjaer et al., 2019; Frisch et al., 2020), while snRNA-seq did not detect SCN8A transcripts (Jakel et al., 2019) (Tables 1, ,2).2). Upregulation of SCN8A may reflect increased diffuse distribution of the channels along the demyelinated axons; it may be important for remyelination but may also contribute to axonal damage. Re-distribution of NaV1.6 channels, in parallel to their loss from the nodes of Ranvier, was reported previously in chronic, active, and inactive MS plaques within cerebral hemisphere, cerebellum, and spinal cord WM tissue from MS patients (Craner et al., 2004b; Black et al., 2007; Howell et al., 2010; Bouafia et al., 2014), as well as in several CNS regions affected by demyelination in animal models, including optic nerve and spinal cord WM (Craner et al., 2003, 2004a,b; Hassen et al., 2008; Howell et al., 2010). Expression of NaV1.6 channels is disrupted at the nodes of Ranvier of WM axons in Shiverer mice that lack compact myelin (Boiko et al., 2001, 2003), and in transgenic mice overexpressing proteolipid protein that initially have normal myelination but then lose myelin (Rasband et al., 2003). During EAE in animals, NaV1.6 co-localizes with NCX and may contribute to persistent Na+ influx, increased Na+ level in the axoplasm, reversal of NCX, and intra-axonal Ca2+ overload leading to axonal damage (Craner et al., 2004a). Interestingly, robust increase in NaV1.6 expression was detected also in microglia/macrophages and was associated with microglia activation and phagocytosis in human MS brain and in the EAE model (Craner et al., 2005). SCN8A deletion resulted in reduced inflammation and improved axonal health during EAE (Alrashdi et al., 2019). Hence, microglial NaV1.6 may contribute to the pathophysiology of MS as well, yet, snRNA-seq did not detect SCN8A in WM glia clusters (Tables 1, ,22).
NaV1.9 (SCN11A)
Neurons
Although NaV1.9 channels are mainly expressed in sensory ganglia neurons (Wang et al., 2017), NaV1.9 mRNA and/or protein were detected in soma and/or proximal processes of neurons in the olfactory bulb, hippocampus, cerebellar cortex, supraoptic nucleus, and spinal cord of rodents and humans (Jeong et al., 2000; Blum et al., 2002; Subramanian et al., 2012; Wetzel et al., 2013; Black et al., 2014; Kurowski et al., 2015). Information regarding axonal labeling for NaV1.9 is lacking. NaV1.9 channels regulate excitation in hippocampal neurons in concert with BDNF and TrkB, control activity-dependent axonal elongation in spinal cord motoneurons, and mediate sustained depolarizing current upon activation of M1 muscarinic receptors in cortical neurons (Blum et al., 2002; Subramanian et al., 2012; Kurowski et al., 2015). It is uncertain whether, similar to their role in the PNS (Cummins et al., 1999; Wang et al., 2017), NaV1.9 channels contribute to the regulation of Vrest and AP threshold in the CNS neurons.
Voltage-Gated Ca2+ Channels (VGCCs)
The VGCCs are composed of α1-, β-, α2/δ-, and γ-subunits (Catterall, 2011; Zamponi et al., 2015). The pore-forming α1-subunit determines channel activity, whereas other subunits are auxiliary and regulate function of α1-subunit. In mammalian cells, 10 different α1-subunits, encoded by different genes, classify into three subfamilies: CaV1, CaV2, and CaV3 (Catterall, 2011; Zamponi et al., 2015; Alves et al., 2019). Depending on the pharmacological properties and activation voltage of Ca2+ currents, five different types of VGCCs are distinguished: L-type, N-type, P/Q-type, R-type, and T-type.
L-Type VGCCs
The α1-subunit of L-type VGCCs is encoded by CACNA1S (CaV1.1), CACNA1C (CaV1.2), CACNA1D (CaV1.3), or CACNA1F (CaV1.4) genes. High sensitivity to dihydropyridine modulators distinguishes L-type Ca2+ channels from other types of VGCCs. In the CNS, mainly CaV1.2 and CaV1.3 subunits are expressed (Lipscombe et al., 2004; Zamponi et al., 2015), but CaV1.1 subunit was detected in human and rat basal ganglia where it is co-expressed with RyRs in GABAergic neurons (Takahashi et al., 2003).
CaV1.2 (CACNA1C)
Neurons
CaV1.2 channels account for 89% of all Ca2+ currents mediated by L-type VGCCs in the brain (Alves et al., 2019; Enders et al., 2020). In hippocampal neurons, CaV1.2 channels localize to somatodendritic compartment being placed at synapses or extra-synaptically (Joux et al., 2001; Hoogland and Saggau, 2004; Obermair et al., 2004; Tippens et al., 2008; Ortner and Striessnig, 2016), as well as to axons and/or extrasynaptic regions of axonal terminals (Tippens et al., 2008). Within the WM, CaV1.2 channels were identified in the developing rat pioneer axons and the follower axons projecting through the optic nerve, corpus callosum, anterior commissure, lateral olfactory tract, corticofugal fibers, thalamocortical axons, and the spinal cord (Ouardouz et al., 2003; Huang et al., 2012).
CaV1.2 channels open upon membrane depolarization beyond −30 mV, and mediate direct Ca2+ entry from the extracellular space into the cytoplasm. In addition, they may act as voltage sensors, transducing membrane depolarization to the RyRs activation and subsequent Ca2+ release from the ER via the mechanism of Ca2+−induced Ca2+ release (CICR) (Ouardouz et al., 2003; Micu et al., 2016; Vierra et al., 2019). Clustering and functional coupling of plasmalemmal CaV1.2 channels to RyRs of the ER is mediated by the KV2.1 channels (Vierra et al., 2019).
Neuronal CaV1.2 channels are involved in synaptic modulation, propagation of dendritic Ca2+ spikes, regulation of glutamate receptor trafficking, CREB phosphorylation, coupling of excitation to nuclear gene transcription, modulation of long-term potentiation, spatial learning, and fear response (Hofmann et al., 2014; Hopp, 2021). During brain development, spontaneous Ca2+ transients mediated by CaV1.2 channels regulate neurite growth and axonal pathfinding (Huang et al., 2012; Kamijo et al., 2018). Genetic variations in CACNA1C gene are associated with Timothy syndrome, Brugada syndrome, epilepsy, depression, schizophrenia, and autism spectrum disorders (Bhat et al., 2012; Bozarth et al., 2018).
Glia
CaV1.2 channels are expressed in cultured astrocytes and mediate Ca2+ transients upon direct Ca2+ entry and/or subsequent activation of RyRs (D'Ascenzo et al., 2004; Du et al., 2014; Cheli et al., 2016b). Ultrastructural studies found CaV1.2 proteins also in hippocampal astrocytes (Tippens et al., 2008). In vitro, CaV1.2 channels contribute to the mechanism of astrogliosis (Du et al., 2014; Cheli et al., 2016b), and in mouse models of Alzheimer's disease, they were detected in reactive astrocyte associated with Aβ-positive plaques (Willis et al., 2010; Daschil et al., 2013).
CaV1.2 mRNA and/or protein are expressed in oligodendrocytes and their progenitors (Agrawal et al., 2000; Paez et al., 2009, 2012; Fulton et al., 2010; Haberlandt et al., 2011; Cheli et al., 2016a; Larson et al., 2016; Marques et al., 2016; Santiago Gonzalez et al., 2017; Paez and Lyons, 2020; Pitman et al., 2020). CaV1.2 channels may regulate proliferation, migration, survival, or differentiation of OPCs, and myelination (Cheli et al., 2015, 2016a; Paez and Lyons, 2020; Pitman et al., 2020). In human cultured OPCs, static magnetic stimulation augmented CaV1.2 mRNA expression, intracellular Ca2+ levels, and OPC differentiation (Prasad et al., 2017), suggesting a causal relationship between these processes.
Functional expression of CaV1.2 channels in microglia is still debated (Hopp, 2021). Sequencing data showed no/low CACNA1C expression in microglia (Hammond et al., 2019), and no CaV1.2 was found in cultured microglia even upon stimulation with TNF-α/IFN-γ (Schampel et al., 2017). However, increased immunolabelling for α1C-subunit of L-type VGCCs was observed in reactive microglia during excitotoxicity in rat hippocampus (Espinosa-Parrilla et al., 2015).
Expression and Function in MS
Bulk RNA-seq detected increased CACNA1C expression in CA lesions (Figure 2, Table 1; Elkjaer et al., 2019; Frisch et al., 2020). The snRNA-seq showed significant CACNA1C expression in neuronal and pericyte clusters (Jakel et al., 2019; Tables 1, ,2),2), while low expression in OPCs and astrocyte clusters. In mouse models of MS, application of L-type VGCCs blockers reduces brain and spinal cord WM damage, decreases mitochondrial pathology in nerve fibers, attenuates axonal loss, increases oligodendrocyte survival, and promotes remyelination (Brand-Schieber and Werner, 2004; Schampel et al., 2017; Ingwersen et al., 2018; Zamora et al., 2020). These findings suggest that CaV1.2 channels contribute to damage during MS. However, expression and activity of CaV1.2 channel increased in OPCs within the demyelinated lesions in the mouse corpus callosum after cuprizone treatment (Paez et al., 2012), and deletion of CaV1.2 specifically in OPCs resulted in reduced myelination and lower MBP and MOG expression (Santiago Gonzalez et al., 2017). Hence, activity of L-type channels in oligodendroglial lineage is crucial for remyelination in this MS model, but it is unclear whether oligodendroglial CaV1.2 channels also play a role during MS in humans. Upregulation of CaV1.2 channels in pericytes may reflect altered microcirculation in MS lesions, in analogy to the role of L-type VGCCs in pericytes outside the brain (Hashitani and Mitsui, 2019).
CaV1.3 (CACNA1D)
Neurons
CaV1.3 channels localize primarily in neuronal cell bodies and dendrites in GM (Hell et al., 1993; Zhang et al., 2005) but were also found in the developing rat optic nerve, corpus callosum (Huang et al., 2012), and axons in spinal dorsal columns of adult rats where they form clusters with RyR2s (Ouardouz et al., 2003). CaV1.3 channels activate at the membrane potential of −55 mV (Lipscombe et al., 2004) and are important players in generating the pacemaking activity and spontaneous firing (Zuccotti et al., 2011). CaV1.3 channels control Ca2+-dependent post-burst after-hyperpolarization in CA1 pyramidal neurons, and their activity may trigger Ca2+-dependent intracellular signaling pathways (Gamelli et al., 2011; Striessnig et al., 2014). CaV1.3 channels may contribute to the mechanisms of memory because their increased expression correlates with memory loss during aging while their inhibition improves age-related memory deficits (Veng et al., 2003). Deletion of CaV1.3 channels results in increased firing rates of amygdala neurons (probably caused by a reduced slow component of post-burst after-hyperpolarization) and underlies altered fear consolidation in CaV1.3 knockout mice (McKinney et al., 2009). CaV1.3 channels are important for formation of cellular architecture: their various splice variants regulate morphology of dendritic spines while their deletion results in reduced morphology of axonal arbors (Hirtz et al., 2012; Stanika et al., 2016).
Glia
CaV1.3 mRNA and/or protein were detected in cultured or freshly isolated rat brain astrocytes; CaV1.3 channels may mediate intracellular Ca2+ increase directly and via Ca2+-mediated activation of RyRs (Latour et al., 2003; Yan et al., 2013; Du et al., 2014; Enders et al., 2020). CaV1.3 expression increases in reactive astrocytes after status epilepticus in mice, suggesting that role in initiation, maintenance, or spread of seizures (Xu J. H. et al., 2007). Yet, other studies have not found CaV1.3 channels in astrocytes (D'Ascenzo et al., 2004).
CaV1.3 channels are expressed in cortical and hippocampal OPCs where they, in concert with other Ca2+ channels, may mediate Ca2+ entry from the extracellular space and/or trigger CICR from the ER (Haberlandt et al., 2011; Cheli et al., 2015). Knockdown of CaV1.3 reduces Ca2+ influx but does not affect expression level of myelin proteins, proliferation, or morphological differentiation of OPCs (Cheli et al., 2015). In the adult rat spinal cord WM, CaV1.3 channels are expressed by APC-positive oligodendrocytes, may mediate oligodendrocyte-axon signaling, and/or contribute to Ca2+-dependent injury following trauma (Sukiasyan et al., 2009). Static magnetic stimulation may alter CaV1.3 gene expression level in human cultured OPCs (Prasad et al., 2017), suggesting that external manipulations may be a useful approach to modulate L-type VGCCs in oligodendroglial cells during diseases.
RNA-seq detected CACNA1D gene (and its various splice variants) in microglia (Hammond et al., 2019), and its expression increased upon microglia activation (Espinosa-Parrilla et al., 2015). CaV1.3 channels regulate synthesis and release of pro-inflammatory substances from microglia, e.g., NO and TNF-α (Espinosa-Parrilla et al., 2015).
Expression and Function in MS
Bulk RNA-seq showed CACNA1D upregulation in CA lesions (Elkjaer et al., 2019; Frisch et al., 2020), while snRNA-seq detected significant expression of CACNA1D in neuronal clusters (Jakel et al., 2019; Tables 1, ,2).2). Administration of L-type VGCCs blockers resulted in multiple beneficial effects in animal MS models (see above), suggesting that CaV1.3 channels, perhaps in concert with CaV1.2 channels, contribute to tissue damage during MS.
P/Q-Type VGCCs
CaV2.1 (CACNA1A)
The CACNA1A gene encodes the pore-forming α1-subunit of P/Q-type (CaV2.1) VGCCs. Sensitivity to ω-Agatoxin distinguishes Ca2+ currents mediated by these channels.
Neurons
CaV2.1 channels localize on axonal synaptic terminals and play a fundamental role in neurotransmitter release: their direct interaction with the SNARE proteins and synaptotagmin is required for positioning the docked synaptic vesicles near the Ca2+ channels for fast vesicular exocytosis (Rettig et al., 1996; Zamponi et al., 2015; Mochida, 2019). CaV2.1 channels are also present at somatodendritic compartments of neurons (Catterall, 2000; Zamponi et al., 2015; Mochida, 2019) where they co-localize with BK and SK channels and provide Ca2+ for activation of these channels (Berkefeld et al., 2006; Indriati et al., 2013; Irie and Trussell, 2017). Ca2+ enters through the CaV2.1 channels and triggers further Ca2+ release from the intracellular stores upon activation of RyRs on the ER (Berkefeld et al., 2006; Indriati et al., 2013; Irie and Trussell, 2017). These mechanisms control neuronal firing even in the millisecond time scale (Irie and Trussell, 2017). Somatodendritic CaV2.1 channels regulate gene expression, local Ca2+ signaling, and cell survival (Pietrobon, 2010).
CaV2.1 channels are also present in the WM, i.e., corpus callosum and developing optic nerve (Alix et al., 2008; Nagy et al., 2017). In the optic nerve, CaV2.1 channels are transiently clustered in the axolemma at the sites where the underlying vesicular and tubular elements are fusing with the axonal membrane (Alix et al., 2008). Some of these sites later become nodes of Ranvier, and mutations of the α1A-subunit results in malformation of the nodes of Ranvier (Alix et al., 2008). In the corpus callosum, CaV2.1 channels mediate fast release of glutamatergic vesicles at axon-OPC synapses, and blockade of these channels in slices reduces release at axon-glia synapses by 88% (Nagy et al., 2017).
CaV2.1 channels may play a role in nociception because inflammatory and neuropathic pain is altered in mice with deletion of CaV2.1 channels (Pietrobon, 2010). Mutations in the CACNA1A gene underlie familial hemiplegic migraine type 1, spinocerebellar ataxia type 6, and episodic ataxia type 2, and may be associated with increased risk of epilepsy (Pietrobon, 2010; Rajakulendran et al., 2012; Izquierdo-Serra et al., 2020).
Glia
RT-PCR detected α1A-subunit in mouse cortical astrocytes in culture, but CaV2.1 channels did not mediate Ca2+ entry into astrocytes (Cheli et al., 2016b). Exposure of mouse primary astrocytes to β-Amyloid did not affect CaV2.1 transcript level (Daschil et al., 2014). However, increased expression of CaV2.1 channels was observed in reactive astrocytes after status epilepticus in mice, suggesting their role in initiation, maintenance, or spread of seizures (Xu J. H. et al., 2007). CaV2.1 channels are expressed in hippocampal OPCs, and in pre-myelinating oligodendrocytes of the brainstem (Haberlandt et al., 2011; Barron and Kim, 2019). In brainstem oligodendrocytes, opening of CaV2.1 channels is triggered upon depolarization mediated by glutamate (via AMPA receptors) or high K+, as well as upon electrical stimulation of axons (Barron and Kim, 2019), suggesting that CaV2.1 channels mediate Ca2+ influx into the oligodendroglial cells upon neuronal activity in vivo. In this way, neuronal activity may trigger and/or modulate Ca2+-dependent signaling in oligodendroglial cells. RNA-seq detected CACNA1A gene in microglia (Hammond et al., 2019). CaV2.1 channels may contribute to glioblastoma progression because their inhibition reduced proliferation of glioblastoma cells, although to a lesser extent than blockade of N-type channels (Nicoletti et al., 2017).
Expression and Function in MS
Bulk RNA-seq found CACNA1A upregulation in CA lesions (Elkjaer et al., 2019; Frisch et al., 2020). The snRNA-seq revealed significant expression of CACNA1A transcripts in neuronal and OPCs clusters (Jakel et al., 2019; Tables 1, ,2).2). CACNA1A upregulation in MS may reflect the necessity to build new nodes of Ranvier on demyelinated axons within the CA lesions. In oligodendroglial cells, Ca2+ entry through CaV2.1 channels may be required for activation of intracellular signaling pathways necessary for differentiation of OPCs and pre-myelinating oligodendrocytes.
CaV2.3 (CACNA1E), R-Type VGCCs
Neurons
CaV2.3 channels are localized to the dendritic spines and pre-synaptically (Parajuli et al., 2012). CaV2.3-mediated Ca2+ currents activate upon strong membrane depolarization and are distinguished by sensitivity to low NiCl2 concentrations and SNX-482 toxin. Presynaptic R-type channels play a role in neurotransmitter release (Wu et al., 1999; Gasparini et al., 2001) and synaptic plasticity (Dietrich et al., 2003; Yasuda et al., 2003; Takahashi and Magee, 2009), but their efficiency in triggering neurotransmitter release may be lower compared to P/Q- or N-type VGCCs if they are placed distantly from vesicle release sites (Wu et al., 1999). Dendritic R-type channels are coupled to SK channels and provide Ca2+ influx for their activation during excitatory postsynaptic potentials and back-propagating APs (Bloodgood and Sabatini, 2008; Jones and Stuart, 2013). The capacity of dendritic SK channels to promote generation of dendritic Ca2+ spikes also depends on CaV2.3 activation (Bock et al., 2019). Besides, Ca2+ influx via CaV2.3 channels may be necessary for activation of Kv4.2 channels (Wang et al., 2014). The CaV2.3 channels also form complexes with BK channels, and this functional interaction modulates AP properties and short-term plasticity in hippocampal neurons (Gutzmann et al., 2019). Studies in KO mice revealed that Cav2.3 channels are involved in the mechanisms of sleep modulation, fear response, pain, and seizures (Saegusa et al., 2000; Lee et al., 2002; Weiergraber et al., 2007; Siwek et al., 2014; Zamponi et al., 2015; Wormuth et al., 2016). Deletion of CaV2.3 channels in mice resulted in larger infarct size after middle cerebral artery occlusion in vivo and larger Ca2+ entry into the cells upon oxygen-glucose deprivation in slices, suggesting that CaV2.3 channels are protective during ischemic tissue damage (Toriyama et al., 2002).
Glia
In primary astrocyte cultures, mRNA and CaV2.3 proteins were detected using RT-PCR, Western blotting, immunohistochemistry, and electrophysiological recordings (Latour et al., 2003; D'Ascenzo et al., 2004). During myelinogenesis, oligodendrocytes within WM of the brainstem, cerebellum, and telencephalon transiently express CaV2.3 channels, but their expression strongly decreases into adulthood (Chen et al., 2000). Ultrastructural analysis demonstrated CaV2.3 immunoreactivity in soma and processes of oligodendrocytes, paranodal loops, and loose myelin sheaths (Chen et al., 2000). RNA-seq detected only negligible CACNA1 expression in microglia (Hammond et al., 2019).
Expression and Function in MS
Bulk RNA-seq showed CACNA1E upregulation in CA lesions (Elkjaer et al., 2019; Frisch et al., 2020), while snRNA-seq found significant expression of CACNA1E transcripts in neuronal clusters (Jakel et al., 2019; Tables 1, ,2).2). The functional role of CaV2.3 channels in MS is unknown.
T-Type VGCCs
The T-type channels (Cav3) are low-voltage activated Ca2+ channels with α1-subunit being encoded by CACNA1G (Cav3.1), CACNA1H (Cav3.2), or CACNA1I (Cav3.3) gene. They are widely distributed in the brain, spinal cord, and DRGs. Cav3 channels activate around Vrest, show fast inactivation kinetics (Cav3.1 > Cav3.2 > Cav3.3), and mediate tiny Ca2+ currents (Perez-Reyes, 2003; Weiss and Zamponi, 2019). Cav3 channels regulate neuronal excitability and play a role during rhythmic AP bursts of thalamic relay neurons, which underlie generation of neuronal oscillations under physiological (sleep) and pathophysiological (epilepsy) conditions (Suzuki and Rogawski, 1989; Astori et al., 2011). Cav3 channels are involved in regulation of nociceptive pathways, sensory processing, hormone, and neurotransmitter release (Weiss and Zamponi, 2019). Mutations in Cav3 genes are linked to neurodevelopmental, neurological, and psychiatric diseases (Lory et al., 2020). Pharmacological non-selective T-type channel blockers are clinically used as antiepileptic drugs and also show anti-nociceptive effects (Zamponi et al., 2015).
Cav3.1, Ca3.2, and Ca3.3 (CACNA1G, CACNA1H, and CACNA1I)
Neurons
Cav3 isoforms display distinct distribution patterns with prominent somatodendritic expression in thalamic and hippocampal neurons (McKay et al., 2006). Ca2+ imaging and pharmacological experiments showed that Cav3.2 and Cav3.3 subtypes located in the AIS influence the generation and the timing of APs (Bender and Trussell, 2009; Kole and Stuart, 2012). In rodent WM, Cav3 transcripts were detected at low level (Aguado et al., 2016), and information on cellular distribution is lacking.
Glia
Some studies detected Cav3.1 transcripts and proteins in rat cortical astrocytic cultures (Latour et al., 2003), while others found only scarce Cav3.1 expression in cultured astrocytes (Cheli et al., 2016b; Kim et al., 2018). Divergent findings showed that Cav3.2 immunoreactivity was absent (Chen et al., 2015) or present (Li et al., 2017) in rat spinal cord astrocytes. Cav3.1 and Cav3.2 transcripts were detected in clonal oligodendroglial CG4 cell line (Rui et al., 2020) and in OPCs isolated from mouse cortex (Zhang et al., 2014) or hippocampal slices (Haberlandt et al., 2011). In microglia, RNA-seq did not detect the Cav3 isoforms (Hammond et al., 2019).
Expression and Function in MS
Bulk RNA-seq revealed upregulation of Cav3.2 and Cav3.3 genes in CA lesions and upregulation of Cav3.1 in ILs (Elkjaer et al., 2019; Frisch et al., 2020; Table 1). The snRNA-seq detected Cav3.1and Cav3.3 transcripts in neuronal clusters, while it did not detect the Cav3.2 (Jakel et al., 2019; Tables 1, ,2).2). Genome-wide sequencing identified significant association of a Cav3.2 mutation (CACNA1Hp.R1871Q) with patients suffering relapsing-remitting MS (Sadovnick et al., 2017). Cav3 upregulation in MS lesions may be triggered by inflammatory mediators and may contribute to axonal dysfunction. Indeed, prostanoids and hydrogen sulfide modulate Cav3.2 expression and function, and increased Cav3.2 channel activity and axonal accumulation is associated with inflammation and pain (Sadovnick et al., 2017; Chen et al., 2018). T-type currents contribute to Ca2+-mediated injury of spinal cord WM axons triggered by anoxia (Imaizumi et al., 1999) and to peripheral nerve injury (Watanabe et al., 2015). L/T-type VGCC blocker lomerizine prevents retinal ganglion cell death after diffuse axonal injury (Karim et al., 2006).
Animal studies suggest that Cav3.1 upregulation in IL, a lesion type with complete demyelination and substantial axonal loss, may play a detrimental role. Specifically, the Cav3.1-deficient mice are markedly resistant to EAE induction, and this effect may be mediated by lower production of granulocyte–macrophage colony-stimulating factor (a cytokine implicated in EAE susceptibility) by CNS-infiltrating Th1 and Th17 cells (Wang et al., 2016). The Cav3.1 subunit is a functionally predominant T-type channel in CD4+ T cells (Trebak and Kinet, 2019). The Cav3.1-mediated Ca2+ increase is critical for calcineurin-NFAT activation driving transcription of cytokines in T cells, and T cells from Cav3.1-deficient mice show decreased IL-17A, IL-17F, and IL-21 production. The development of isoform-specific modulators should help in establishing the differential role of Cav3 subtypes in MS lesions.
Ryanodine Receptors
RyRs encompass three mammalian isoforms, RyR1–3, which form homo-tetrameric channels on the ER. RyRs are highly conductive Ca2+ channels: they get activated by Ca2+ influx upon plasma membrane depolarization mediating CICR from the ER (Fill and Copello, 2002; Lanner et al., 2010). In the brain, the RyR2s show predominant expression, followed by RyR3s, and then RyR1s (McPherson and Campbell, 1990; Giannini et al., 1995).
RyR2 and RyR3
Neurons
RyRs localize along ER of neurons, including WM axons (Giannini et al., 1995). They play a role in vesicle fusion, neurotransmitter release, synaptic plasticity, and growth cone dynamics (Giannini et al., 1995; Kushnir et al., 2018). RyRs form complexes with L-type Ca2+ channels: RyR1-Cav1.2 and RyR2-Cav1.3 (Ouardouz et al., 2003). WM axons transduce membrane depolarization to Ca2+ release from ER, whereby L-type VGCCs gate RyRs, analogous to “excitation–contraction coupling” in muscles (Ouardouz et al., 2003; Stirling and Stys, 2010). Genetic mutations or oxidative stress can render RyRs leaky to Ca2+ and promote defective signals as observed in neurodegenerative disorders, heart failure, and muscular dystrophy (Kushnir et al., 2018).
Glia
RYR2 and RYR3 transcripts, but only RyR3 protein, were found in cultured astrocytes from mouse brain (Matyash et al., 2002; Kesherwani and Agrawal, 2012). RyR2 transcripts and proteins were upregulated in spinal WM astrocytes after hypoxic injury (Kesherwani and Agrawal, 2012) and SCI (Liao et al., 2016; Pelisch et al., 2017). All RYRs subunits were found in rat optic nerve oligodendrocyte cultures (Ruiz et al., 2010), but RYR3 was selectively expressed in rat cortical OPCs (Haak et al., 2001; Li T. et al., 2018). RyR3s amplify small inward Ca2+ currents in astrocytes and OPC, regulating behavior of these cells (Simpson et al., 1998; Matyash et al., 2002; Haberlandt et al., 2011). RyRs mediate stress response in oligodendrocytes, and RyR inhibition attenuated intracellular Ca2+ overload following AMPA excitotoxicity (Ruiz et al., 2010). RyR1 and RyR2 mRNAs were detected in adult human microglia, whereas only RyR3 was found in fetal microglia (Klegeris et al., 2007). RNA-seq did not detect RYR2 and RYR3 in mouse microglia (Hammond et al., 2019).
Expression and Function in MS
Bulk RNA-seq found upregulation of RyR2 transcripts in CA lesions and downregulation of RyR3 in ILs (Table 1; Elkjaer et al., 2019; Frisch et al., 2020). The snRNA-seq revealed significant expression of RyR2 in neuronal clusters and of RyR3 in the astrocyte1 cluster (Table 1; Jakel et al., 2019). RyR subunits probably play a differential role in perturbed intracellular Ca2+ homeostasis in WM cells of SPMS brain. RyR2 in CA lesions may contribute to axonal dysfunction because intra-axonal Ca2+ overload mediated by RyRs and IP3Rs activates the mitochondrial permeability transition pore and contributes to axonal dieback and degeneration following WM ischemic injury (Ouardouz et al., 2003; Stirling and Stys, 2010; Kesherwani and Agrawal, 2012) and SCI (Stirling et al., 2014; Liao et al., 2016). The RyRs inhibitor ryanodine significantly attenuates mitochondrial dysfunction (Villegas et al., 2014), axonal dieback, and secondary axonal degeneration in injured WM (Thorell et al., 2002; Stirling et al., 2014; Orem et al., 2017). In line, mice with RyR2 gain-of-function mutation exhibit more axonal damage than wild-type controls following SCI (Stirling et al., 2014), while RyR2 knockdown attenuates mitochondrial dysfunction and ER stress and improves functional recovery (Liao et al., 2016).
Functional RyR3s may contribute to astrocyte migration in response to injury, which is important for tissue remodeling and wound healing. In fact, RyR3s control astrocyte motility because astrocytes from RyR3 KO mice display reduced migratory activity (Matyash et al., 2002). Conversely, RyR3 downregulation in ILs may influence the formation of dense astrocytic scar imposing a major barrier to axonal and myelin regeneration. RyR3s also contribute to intracellular Ca2+ transients during OPCs differentiation, while RyR3 inhibition prevents OPCs development (Li T. et al., 2018). Interaction between RyRs and NCX in oligodendrocyte processes may represent an amplification mechanism to generate Ca2+ transients required for oligodendrocyte differentiation in vitro (Casamassa et al., 2016; Hammann et al., 2018; de Rosa et al., 2019; Boscia et al., 2020). However, it remains unclear whether these mechanisms play a role in human MS. The development of selective modulators will help to establish function of RyRs in MS.
TRP Channels
Transient receptor potential (TRP) channels are tetrameric non-selective cation channels which encompass 30 different types (Nilius and Owsianik, 2011). Upon TRP channel activation, the membrane potential depolarizes, leading to activation or inactivation of voltage-gated ion channels and regulation of Ca2+ signaling (Gees et al., 2010). Various intracellular or extracellular stimuli, including chemical and osmotic stress, can trigger activation of TRP channels (Clapham, 2003). TRP channels are involved in pain, regulation of neurotransmitter release, and immune functions. Vanilloid TRP channels (TRPV), melastatin TRP channels (TRPM), and polycystin TRP channels (TRPP) have been detected in WM lesions of patients with progressive MS.
TRPV1
Neurons
In the CNS, TRPV1 channels are mainly localized on cell bodies and dendritic spines, but also in synaptic vesicles (Goswami et al., 2010). TRPV1 channels are activated by exogenous (i.e., capsaicin) or endogenous (i.e., high temperatures, acid pH, anandamide, 2-arachidonoylglycerol, and lipid metabolites) stimuli (Van Der Stelt and Di Marzo, 2004). They play a role in weight, appetite, and energy homeostasis (Derbenev and Zsombok, 2016; Christie et al., 2018); synaptic plasticity (Gibson et al., 2008; Wang et al., 2020); neuropathic pain (Rivat et al., 2018); and regulation of inflammatory response (Kong et al., 2017).
Glia
TRPV1 channels are expressed in astrocytes (Ho et al., 2014), microglia (Sappington and Calkins, 2008), and, to a lesser extent, oligodendrocytes (Gonzalez-Reyes et al., 2013; Marques et al., 2016).
Expression and Function in MS
Bulk RNA-seq showed significant TRPV1 downregulation in CA lesions (Figure 2, Table 1; Elkjaer et al., 2019; Frisch et al., 2020), while snRNA-seq barely detected TRPV1 (Table 2; Jäkel and Williams, 2020). The downregulated TRPV1 in CA lesions may influence neural plasticity and glia response both in the hypocellular inactive demyelinated core and in the hypercellular rim filled with activated glia. However, it is unclear whether dysfunctional TRPV1 has pro- and anti-inflammatory roles, and whether it favors or prevents CA lesion expansion and progression, because experimental findings are inconsistent. In rodents, administration of TRPV1 agonists reduced EAE severity (Tsuji et al., 2010), while the TRPV1 antagonist capsazepine, although ineffective for EAE severity (Paltser et al., 2013), reversed the beneficial effects of the endocannabinoid uptake inhibitor (Cabranes et al., 2005). Beneficial effects of TRPV1 may be mediated by its ability to promote micro-vesicle release from microglia, which enhances glutamatergic transmission in neurons (Marrone et al., 2017). However, on the other hand, TRPV1 stimulation induces the pro-inflammatory phenotype of microglia while downregulation promotes the anti-inflammatory phenotype (Hassan et al., 2014; Marrone et al., 2017). TRPV1 also regulates microglia migration, cytokine production, ROS generation, phagocytosis, and death (Kim et al., 2006; Schilling and Eder, 2009; Miyake et al., 2015). Furthermore, TRPV1 mediates migration and chemotaxis of astrocytes, their activation during stress and injury (Ho et al., 2014), and inflammasome activation. The picture becomes even more complex because TRPV1-KO mice show higher lethality during EAE peak but better recovery in the chronic stage (Musumeci et al., 2011). In addition, genetic deletion of TRPV1 in mice resulted in significant protection in the MOG-EAE model, and less severe breakdown of BBB (Paltser et al., 2013). Interestingly, patients with severe MS progression show over-representation of single-nucleotide polymorphisms (SNPs) in the TRPV1 gene (Paltser et al., 2013) that can affect the expression and activity of the channel and cortical excitability, and modulate pain (Xu H. et al., 2007; Mori et al., 2012; Stampanoni Bassi et al., 2019).
TRPV6
TRPV6 channels are distinguished by high Ca2+ selectivity (van de Graaf et al., 2006) and constitutive activity at low intracellular Ca2+ levels and Vrest (Vennekens et al., 2000). TRPV6 channels can form homo- or hetero-tetramers. TRPV5–6 are mainly expressed in epithelial and bone cells (Hoenderop et al., 2003).
Neurons and Glia
In the mouse brain, TRPV6 channels are expressed in neurons, while transcripts were detected in astrocytes by RNA-seq (Riccio et al., 2002; Nijenhuis et al., 2003; Batiuk et al., 2020).
Expression and Function in MS
Bulk RNA-seq found TRPV6 downregulation in all MS lesion types and in NAWM (Figure 2, Table 1; Elkjaer et al., 2019; Frisch et al., 2020), but snRNA-seq failed to detect TRPV6 transcripts (Table 2; Jakel et al., 2019). Little is known about the functional role of TRPV6 in brain cells. However, TRPV6 deletion in trophoblasts correlates with altered extracellular matrix (ECM) formation in the labyrinth during pregnancy (Winter et al., 2020). Hence, it will be important to investigate whether TRPV6 downregulation contributes to ECM alterations observed in SPMS lesions and believed to be a key remyelination-inhibiting factor.
TRPM2
Neurons
TRPM2 channels are found in cell bodies and neurites (Nagamine et al., 1998; Olah et al., 2009) and often co-localize with a marker of dopaminergic neurons (Bai and Lipski, 2010). They are Ca2+-permeable sensors of various stimuli (Huang et al., 2020), contribute to synaptic plasticity, and inhibit neurite outgrowth (Sita et al., 2018).
Glia
TRPM2 transcripts are intensely expressed in mouse microglia (Malko et al., 2019), but only at lower levels in astrocytes and oligodendrocytes (Choi et al., 2015; Marques et al., 2016; Falcao et al., 2018; Batiuk et al., 2020; Table 3). TRPM2 plays a critical role in microglia activation and generation of pro-inflammatory mediators, thus contributing to neuropathic pain, brain damage due to chronic hypo-perfusion, neonatal hypoxia–ischemia, and amyloid-beta (Malko et al., 2019).
Expression and Function in MS
Bulk RNA-seq showed increased TRPM2 expression in the ILs (Table 1; Elkjaer et al., 2019; Frisch et al., 2020). SnRNA-seq found TRPM2 in neuronal, microglia, and ImOLG clusters. The functional role of TRPM2 channels in ILs, lesions that display reduced microglia density, axonal loss, and upregulation of stress response genes (Elkjaer et al., 2019; Frisch et al., 2020), may be related to neuronal and microglia damage. Indeed, TRPM2 channel is upregulated by diverse pathological stimuli (Malko et al., 2019) and is an important element during oxidative stress, mitochondrial dysfunction (Freestone et al., 2009), and neurodegenerative disorders (Chung et al., 2011). Constitutive TRPM2 activation is triggered by ROS and leads to pathological Ca2+ signaling and cell death (Eisfeld and Luckhoff, 2007; Naziroglu and Luckhoff, 2008). Knockout of TRPM2 gene in mice, or blocking the channels with miconazole, improves pathological outcome in EAE and attenuates painful behavior (Melzer et al., 2012; So et al., 2015; Tsutsui et al., 2018). TRPM2-KO mice show reduction of CXCL2 chemokine production by CNS-infiltrating macrophages and suppressed neutrophil infiltration of the brain tissue (Tsutsui et al., 2018). These findings suggest that TRPM2 may represent a promising target in SPMS.
TRPP1 and TRPP3 (PKD2 and PKD2L2)
The TRPP(PKD2) channels are encoded by TRPP1(PKD2), TRPP2(PKD2L1), and TRPP3(PKD2L2) genes (www.guidetopharmacology.org) and form Ca2+-permeable non-selective cation channels. In the mouse brain, PKD2 and PKD2L2 transcripts are detected in neurons and glia (Table 3). TRPP1 is present on the ER, primary cilia, and plasma membrane, and TRPP3 is widely expressed in fetal tissues (Guo et al., 2000).
Expression and Function in MS
Bulk RNA-seq detected significant downregulation of PKD2 or PKD2L2 in ILs and CA lesions, respectively (Elkjaer et al., 2019; Frisch et al., 2020) (Figure 2, Table 1). SnRNA-seq detected PKD2 transcripts in neuronal and glia clusters but did not detect PKD2L2 (Table 2). It is unclear whether TRPP downregulation in MS lesions is beneficial or detrimental. On one hand, it may be detrimental because TRPP1 and TRPP3 channels are important for maintaining Ca2+ homeostasis and contribute to cell proliferation (Xiao and Quarles, 2010; Xiao et al., 2010), while TRPP1 knockdown results in increased susceptibility to stress-induced cell death in kidney epithelial cells (Brill et al., 2020). On the other hand, overexpression of TRPP contributes to apoptosis (Xiao and Quarles, 2010; Xiao et al., 2010), and TRPP1 is upregulated as a direct consequence of ER and oxidative stress during pathological conditions.
Chloride Channels
ClC channels mediate voltage-dependent transmembrane transport of Cl−. They are expressed in plasmalemma and intracellular membranes forming transmembrane dimers (Weinreich and Jentsch, 2001). ClC proteins can function as Cl− channels or as Cl−/H+ exchangers. ClCs regulate Vrest in skeletal muscle, trans-epithelial Cl− reabsorption in kidneys, and intracellular pH and Cl− concentration through coupled Cl−/H+ exchange in several cell types including brain cells.
CIC-2 (CLCN2)
The CLCN2 gene encodes a voltage- and volume-regulated CIC-2 channel (Chu et al., 1996), essential for efflux of accumulated Cl− and control of cell volume homeostasis. CIC-2 is expressed in neurons and glia (Jentsch et al., 2005) and is upregulated at low osmolarity, cell swelling, and membrane hyperpolarization (Grunder et al., 1992; Clark et al., 1998).
Neurons
CIC-2 localizes on inhibitory interneurons and regulates GABAA receptor-mediated synaptic inputs from basket cells (Foldy et al., 2010). Cl− extrusion by ClC-2 following hyperpolarization ensures the maintenance of low intracellular Cl− concentration following synaptic inhibition (Foldy et al., 2010). The link of ClC-2 mutations with generalized epilepsies in humans suggests an important role of ClC-2 in regulating neuronal excitability (Kleefuss-Lie et al., 2009).
Glia
Astrocytes express ClC-2 that interacts with AQP4 to regulate Cl− influx and efflux (Benfenati et al., 2007). ClC-2 is expressed in microglia and may regulate cell volume and phagocytosis (Ducharme et al., 2007). In oligodendrocyte lineage cells, ClC-2 positively regulates OPCs differentiation (Jentsch and Pusch, 2018) and transcription factors for myelin genes, thus contributing to myelin formation and WM integrity (Hou et al., 2018).
Expression and Function in MS
Bulk RNA-seq showed significant CLCN2 downregulation in CA lesions (Elkjaer et al., 2019; Frisch et al., 2020; Figure 2, Table 1). SnRNA-seq detected CLCN2 transcripts in oligodendrocyte clusters, while they were only faintly observed or absent in other clusters (Table 2). Several findings suggest that CLCN2 downregulation in MS may reflect altered WM integrity and/or contribute to the mechanisms of myelin destruction: first, ClCN2−/− mice exhibit abnormal WM morphology (Blanz et al., 2007); second, loss-of-function CLCN2 mutations lead to leukodystrophy; third, loss of cell adhesion molecule GlialCAM, which binds to ClC-2 in glia, is associated with leukodystrophy (Jeworutzki et al., 2012; Hoegg-Beiler et al., 2014). Of note, though, is a recent report showing that leukodystrophy fully develops only when ClC-2 is disrupted in both astrocytes and oligodendrocytes (Goppner et al., 2020). It remains to be investigated whether CLC-2 loss in glia contributes to the failure of myelin repair in human CA lesions.
CIC-7 (CLCN7)
The CLCN7 gene encodes for the chloride-proton antiporter ClC-7 localized to lysosomes and crucial for function of osteoclasts and brain cells (Kornak et al., 2001; Jentsch and Pusch, 2018).
Neurons and Glia
In mice, neurons and microglia express ClC-7 protein (Kasper et al., 2005; Majumdar et al., 2011; Weinert et al., 2014), while transcripts were found in astrocytes and oligodendrocyte lineage (Falcao et al., 2018; Batiuk et al., 2020). Mutations in the human CLCN7 gene are associated with osteopetrosis and neurodegeneration (Kornak et al., 2001).
Expression and Function in MS
Bulk RNA-seq detected significant CLCN7 downregulation in CA lesions (Elkjaer et al., 2019; Frisch et al., 2020; Figure 2, Table 1). SnRNA-seq found CLCN7 transcripts in neuronal and all glia clusters (Table 2). Functional role of ClC-7 under demyelinating conditions is unknown. In neurons, ClC-7 on lysosomes contributes to the function of the endosomal–lysosomal pathway (Poet et al., 2006; Bose et al., 2021). Lysosomal localization of CLC-7 increases during microglia activation, leading to increased lysosomal acidification and Aβ degradation (Majumdar et al., 2011). ClCN7-deficient mice display widespread WM atrophy, neuronal loss, microglia activation, astrocytosis, and accumulations of storage material in lysosomes (Kornak et al., 2001; Kasper et al., 2005; Pressey et al., 2010). In SPMS lesions, dysfunctional ClC-7 activity may directly affect the luminal pH and Cl− concentrations and lysosomal protein degradation (Wartosch et al., 2009), which, in turn, may lead to neuronal and glial degeneration in the WM.
Connexins
Connexins (Cxs) are transmembrane proteins with channel and non-channel functions. Channel functions include the formation of gap junctions (GJs) and hemichannels (HCs) (Saez et al., 2003; Wang et al., 2013; Gajardo-Gomez et al., 2016), while non-channel functions involve adhesion properties and intracellular signaling (Zhou and Jiang, 2014; Leithe et al., 2018). More than 20 Cxs genes have been described in humans, and 11 of them are expressed in the brain (Willecke et al., 2002; Theis et al., 2005). Cxs are essential players in ionic homeostasis, intercellular Ca2+ signaling and Ca2+ waves propagation, gliotransmission, synaptic transmission and plasticity, brain metabolism, brain–blood barrier development and integrity, and myelination (Takeuchi and Suzumura, 2014). In the WM, GJs are essential for K+ buffering in response to neuronal activity, they facilitate transport of nutrients and ions from oligodendrocyte soma to myelin layers and from astrocytes to oligodendrocytes (Bradl and Lassmann, 2010). In the WM, HCs are involved in metabolic coupling and energy supply to neurons, and provide a major pathway for glucose entry into OPCs and oligodendrocytes (Niu et al., 2016).
Cx37 (GJA4)
Cx37, encoded by GJA4 gene, predominantly builds heterotypic GJs with Cx40 and Cx43 in vascular cells and plays an essential role in vasomotor activity, endothelial permeability, and maintenance of body fluid balance (Falcao et al., 2018; Li et al., 2018).
Expression and Function in MS
Bulk RNA-seq revealed significant GJA4 upregulation in ILs (Elkjaer et al., 2019; Frisch et al., 2020), while snRNA-seq showed high GJA4 expression in pericyte cluster (Jakel et al., 2019; Tables 1, ,2).2). In chronically demyelinated axons, as those within ILs, hypoxia due to imbalance between increased energy demand and reduced ATP production because of mitochondrial dysfunction may drive angiogenesis. However, while providing trophic factors for tissue remodeling, angiogenesis may contribute to hypoperfusion and neurovascular uncoupling (Girolamo et al., 2014). Interestingly, Cx37 knockdown with siRNA in human umbilical vein endothelial cells diminishes capillary branching (Gartner et al., 2012), but Cx37−/− mice develop a more extensive vasculature under ischemic conditions and show enhanced recovery after hind limb ischemia (Fang et al., 2011). In the future, it will be important to investigate whether Cx37 protein contributes to aberrant cerebrovascular and angiogenic responses in human ILs during MS.
Pannexins
The Pannexin (Px) family consists of three members, encoded by Panx1, Panx2, and Panx3 genes. Pannexins do not form GJ in vivo but operate as plasma membrane channels (pannexons) and participate in paracrine and autocrine signaling in brain GM and WM (Sosinsky et al., 2011; Sahu et al., 2014; Dahl, 2015).
Px1 (PANX1)
Px1 is permeable to anions, some negatively charged molecules (glutamate, aspartate, and ATP), and fluorescent dyes (Ma et al., 2012; Yeung et al., 2020). Opening of Px1 may be promoted by voltage, increased intracellular Ca2+, mechanical stress, extracellular K+, oxygen deprivation, caspases cleavage, ATP binding to P2Y or P2X7 receptors, activation of α1-adrenergic, NMDA, and thromboxane receptors (Chiu et al., 2018; Dahl, 2018; Whyte-Fagundes and Zoidl, 2018).
Neurons and Glia
Px1 is distributed in GM and WM regions, including cerebellum, corpus callosum, and fimbria fornix of mice (Bruzzone et al., 2003) and rats (Vogt et al., 2005). Px1 is expressed in neurons, astrocytes, microglia, oligodendrocytes, vascular cells, and peripheral immune cells (Iglesias et al., 2009; Swayne et al., 2010; Orellana et al., 2013; Good et al., 2018; Lapato and Tiwari-Woodruff, 2018). In neurons, Px1 may be co-expressed with Px2 and is found in cell soma, dendrites, and axons (Cone et al., 2013).
Interaction between Px1 and purinergic signaling deserves special attention because Px1 forms complexes with P2X7Rs (Taruno, 2018). Binding of ATP to P2X7R triggers opening of Px1 channels with subsequent ATP release (Locovei et al., 2007; Iglesias et al., 2008; Pelegrin et al., 2008; Chiu et al., 2018). ATP signaling involving Px1 channels regulates neurite outgrowth and synaptic plasticity in neurons, while in glia, it underlies intercellular propagation of Ca2+ waves, cell differentiation, and migration (Giaume et al., 2021).
Expression and Function in MS
Bulk RNA-seq showed significant PANX1 upregulation in ILs (Table 1; Elkjaer et al., 2019; Frisch et al., 2020), but snRNA-seq did not detect PANX1 transcripts (Jakel et al., 2019). ILs are lesions with little/no inflammatory activity but with sharply demarcated hypocellular areas of demyelination and axonal degeneration. Px1 activation is known to enable ATP release, and ATP is a “find me” signal promoting chemotaxis of microglia/macrophages to the injury site for fast clearance of dead cells and a molecule important for myelination (Chekeni et al., 2010; Gajardo-Gomez et al., 2016). Hence, PANX1 upregulation in ILs may be a compensatory mechanism that stimulates glial activity. On the other hand, upregulated Px1 mRNA expression in cerebellum and spinal cord in chronic EAE contributes to WM damage (Lutz et al., 2013). Uncontrolled opening of P2X7R-Px1 complex in response to demyelination triggers excessive glutamate and ATP release, altered Ca2+ dynamics, excitotoxicity, damage of axons, and myelin (Orellana et al., 2011; Crespo Yanguas et al., 2017). Knockout or blockade of Px1 with probenecid in rodents restrains EAE symptoms and results in reduced inflammation and decreased oligodendrocyte damage (Hainz et al., 2017), suggesting that Px1 activity supports damage during MS. More studies are required to establish how Px1 should be modulated in order to halt neurodegeneration during MS.
CatSper Channels
Catsperg and Caspere
Cation channel of spermatozoa (CatSper) is a highly complex multi-subunit voltage-gated Ca2+-permeable ion channel. Four distinct α-subunits (CatSper1–4) and several accessory subunits are encoded by CATSPER genes (Qi et al., 2007). The CatSper channel is essential for the activity of sperm flagellum and sperm fertility (Lishko and Mannowetz, 2018). RNA-seq detected only CATSPERG transcripts in mouse neurons, oligodendrocytes, and microglia (Marques et al., 2016; Hammond et al., 2019; Jakel et al., 2019).
Expression and Function in MS
Bulk RNA-seq found downregulation of the auxiliary subunit gamma (CASPERG) and epsilon (CATSPERE) in CA lesions and ILs, respectively (Table 1; Elkjaer et al., 2019; Frisch et al., 2020). SnRNA-seq did not find CATSPERE transcripts and barely detected CATSPERG in neuronal and glia clusters. It is difficult to speculate on the role of CatSper channels in MS lesions because characterization of these subunits is limited to sperm cells, and no data on CatSper protein expression or function in the brain are available.
Conclusions
Understanding how distinct ion channels regulate CNS ionic homeostasis in WM neurons, axons, glia, and vascular cells under chronic demyelinating conditions is of critical importance for the development of novel therapeutic strategies to prevent neurodegeneration and disability progression and improve functional recovery and repair in MS. Recent Bulk RNA-seq (Elkjaer et al., 2019; Frisch et al., 2020) revealed a considerable number of ion channel genes that are altered in different types of WM lesions of the SPMS brain, particularly in WM CA lesions, a type of lesion that develops in MS patients despite disease-modifying therapy and predicts a more aggressive disease course (Absinta et al., 2019; Elliott et al., 2019). SnRNA-seq found that transcripts for dysregulated ion channels belong to the clusters of neurons, astrocytes, oligodendrocyte lineage, microglia/macrophages, and pericytes (Jakel et al., 2019). The dysregulation of ion channel genes in MS may be detrimental or beneficial for functions of neurons, including interstitial neurons. Intense upregulation of genes encoding voltage-gated Na+ channels in CA lesions may reflect the imbalance of Na+ homeostasis observed in SPMS brain (Inglese et al., 2010). Conversely, the upregulation of a large number of voltage-gated K+ channel genes may be linked to a protective response to limit neuronal excitability. The altered Cl− homeostasis, revealed by the significant downregulation of voltage-gated Cl− channels in MS lesions, may contribute to an altered inhibitory neurotransmission and increased excitability. Depending on the type of alterations, dysregulated ion channels in MS may favor AP propagation and dampen neuronal hyperexcitability or, on the contrary, may contribute to axonal dysfunction and cell death. Altered expression and/or function of ion channels may also influence key properties of glia including proliferation, migration, spatial buffering, cytokine release, cell metabolism, myelin repair, angiogenesis, BBB permeability, and several other important functions.
We described the importance of uniquely dysregulated genes well-known to play a role in WM dysfunction in the MS brain (KCNA1, KCNA2, SCN2A, and SCN8A), or in experimental models of MS (KCNC3, KCNQ3, KCNK2, CACNA1C, CACNA1G, TRPV1, TRPM2, and PANX1). Furthermore, we highlighted the importance of ion channel genes that are uniquely dysregulated in SPMS lesions but have never been previously explored in MS brain. Those genes are expressed in OPC (KCND2, SCN1A, SCN3A, and CACNA1A), ImOLG (KCNQ3), mature oligodendrocyte (KCNH8), microglia (KCNQ3), astrocyte (KCNN3 and RYR3), and pericyte (GJA4 and CACNA1C) clusters of healthy and SPMS brain. It remains to be investigated whether and how the ionic imbalance in different glial cells, particularly oligodendroglia, contributes to impaired recovery and failure of myelin repair.
Several genes, including KCNA1, SCNA8, SCN11A, CACNA1H, PKD2L2 TRPV6, PANX1, and CATSPERE transcripts, were detected in bulk transcriptome (Elkjaer et al., 2019; Frisch et al., 2020), but were not found by snRNA-seq (Jakel et al., 2019). This discrepancy may be explained by several observations: (1) the transcriptional profiling may vary when lesions analyzed by different studies come from different WM regions (Jäkel and Williams, 2020); (2) snRNA-seq analysis lacks information on gene expression in WM axons that may also contain ion channel transcripts; and (3) snRNA-seq only includes RNA transcripts from the nucleus and may therefore lack RNA transcripts from cytoplasm.
Future experiments on dysregulated ion channels predicted by transcriptomic analysis are expected to provide a better understanding of the molecular mechanism of MS progression and may pave the way for the identification of new therapeutic targets to limit lesion expansion, reduce neurological impairment, and stimulate functional recovery.
Author Contributions
FB, MK, ZI, and ME: writing-original draft preparation. FB and MK: writing-review and editing. FB, MK, and ZI: funding acquisition. All authors have read and agreed to the final version of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Eneritz Agirre (Karolinska Institute, Stockholm, Sweden) for helpful discussions.
Glossary
Abbreviations
| AIS | axon initial segment |
| AL | active lesion |
| AP | action potential |
| CA | chronic active lesion |
| Ca2+ | calcium |
| CaV | voltage-gated calcium channels |
| ClC | chloride channels |
| CNS | central nervous system |
| COP | committed OPCs |
| Cx | connexin |
| EAE | experimental autoimmune encephalomyelitis |
| EAG | ether-àgo-go |
| ER | endoplasmic reticulum |
| GM | gray matter |
| HC | hemi-channel |
| IFN-γ | gamma-interferon |
| IL | inactive lesions |
| ImOLG | immune oligodendroglia |
| K2P | two-pore domain K+ channels |
| Kir | inward rectifier potassium channel |
| KO | knockout |
| KV | voltage-gated K+ channels |
| LPS | lipopolysaccharide |
| MS | multiple sclerosis |
| Na+ | sodium |
| NaV | voltage-gated sodium channels |
| NAWM | normal-appearing white matter |
| NCX | sodium calcium exchanger |
| OPCs | oligodendrocyte precursor cells |
| Px | pannexin |
| RL | remyelinating lesion |
| RyR | ryanodine receptor |
| SCI | spinal cord injury |
| SPMS | secondary progressive multiple sclerosis |
| TRP | transient receptor potential |
| WM | white matter. |
Footnotes
Funding. The work of FB was supported by grants from Fondazione Italiana Sclerosi Multipla FISM 2015/R/6 and by Italian Ministry for University and Research PRIN 2017, 20175SA5JJ. The work of MK was supported by Ikerbasque (Basque Foundation for Science), Spanish Ministry of Science and Innovation (Grant No. PID2019-110195RB-I00), and Basque Government. The work of ZI was supported by Lundbeckfonden R118-A11472, Scleroseforeningen R487-A33600-B15690, and R458-A31829-B15690.
References
- Abiraman K., Tzingounis A. V., Lykotrafitis G. (2018). KCa2 channel localization and regulation in the axon initial segment. FASEB J. 32, 1794–1805. 10.1096/fj.201700605R [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Absinta M., Sati P., Masuzzo F., Nair G., Sethi V., Kolb H., et al. . (2019). Association of chronic active multiple sclerosis lesions with disability in vivo. JAMA Neurol. 76, 1474–1483. 10.1001/jamaneurol.2019.2399 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Agrawal S. K., Nashmi R., Fehlings M. G. (2000). Role of L- and N-type calcium channels in the pathophysiology of traumatic spinal cord white matter injury. Neuroscience 99, 179–188. 10.1016/s0306-4522(00)00165-2 [Abstract] [CrossRef] [Google Scholar]
- Aguado C., Garcia-Madrona S., Gil-Minguez M., Lujan R. (2016). Ontogenic changes and differential localization of T-type Ca(2+) channel subunits Cav3.1 and Cav3.2 in mouse hippocampus and cerebellum. Front. Neuroanat. 10:83. 10.3389/fnana.2016.00083 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Akhtar S., McIntosh P., Bryan-Sisneros A., Barratt L., Robertson B., Dolly J. O. (1999). A functional spliced-variant of beta 2 subunit of Kv1 channels in C6 glioma cells and reactive astrocytes from rat lesioned cerebellum. Biochemistry 38, 16984–16992. 10.1021/bi992114x [Abstract] [CrossRef] [Google Scholar]
- Alfaro-Ruiz R., Aguado C., Martin-Belmonte A., Moreno-Martinez A. E., Lujan R. (2019). Expression, cellular and subcellular localisation of Kv4.2 and Kv4.3 channels in the rodent hippocampus. Int. J. Mol. Sci. 20:246. 10.3390/ijms20020246 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Alix J. J., Dolphin A. C., Fern R. (2008). Vesicular apparatus, including functional calcium channels, are present in developing rodent optic nerve axons and are required for normal node of ranvier formation. J. Physiol. 586, 4069–4089. 10.1113/jphysiol.2008.155077 [Abstract] [CrossRef] [Google Scholar]
- Allen N. M., Weckhuysen S., Gorman K., King M. D., Lerche H. (2020). Genetic potassium channel-associated epilepsies: clinical review of the Kv family. Eur. J. Paediatr. Neurol. 24, 105–116. 10.1016/j.ejpn.2019.12.002 [Abstract] [CrossRef] [Google Scholar]
- Alrashdi B., Dawod B., Schampel A., Tacke S., Kuerten S., Marshall J. S., et al. . (2019). Nav1.6 promotes inflammation and neuronal degeneration in a mouse model of multiple sclerosis. J. Neuroinflammation 16:215. 10.1186/s12974-019-1622-1 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Alves V. S., Alves-Silva H. S., Orts D. J. B., Ribeiro-Silva L., Arcisio-Miranda M., Oliveira F. A. (2019). Calcium signaling in neurons and glial cells: role of Cav1 channels. Neuroscience 421, 95–111. 10.1016/j.neuroscience.2019.09.041 [Abstract] [CrossRef] [Google Scholar]
- Arai-Ichinoi N., Uematsu M., Sato R., Suzuki T., Kudo H., Kikuchi A., et al. . (2016). Genetic heterogeneity in 26 infants with a hypomyelinating leukodystrophy. Hum. Genet. 135, 89–98. 10.1007/s00439-015-1617-7 [Abstract] [CrossRef] [Google Scholar]
- Armstrong W. E., Rubrum A., Teruyama R., Bond C. T., Adelman J. P. (2005). Immunocytochemical localization of small-conductance, calcium-dependent potassium channels in astrocytes of the rat supraoptic nucleus. J. Comp. Neurol. 491, 175–185. 10.1002/cne.20679 [Abstract] [CrossRef] [Google Scholar]
- Arroyo E. J., Xu T., Grinspan J., Lambert S., Levinson S. R., Brophy P. J., et al. . (2002). Genetic dysmyelination alters the molecular architecture of the nodal region. J. Neurosci. 22, 1726–1737. 10.1523/JNEUROSCI.22-05-01726.2002 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Astori S., Wimmer R. D., Prosser H. M., Corti C., Corsi M., Liaudet N., et al. . (2011). The Ca(V)3.3 calcium channel is the major sleep spindle pacemaker in thalamus. Proc. Natl. Acad. Sci. U.S.A. 108, 13823–13828. 10.1073/pnas.1105115108 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Attali B., Wang N., Kolot A., Sobko A., Cherepanov V., Soliven B. (1997). Characterization of delayed rectifier Kv channels in oligodendrocytes and progenitor cells. J. Neurosci. 17, 8234–8245. 10.1523/JNEUROSCI.17-21-08234.1997 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Bahring R., Boland L. M., Varghese A., Gebauer M., Pongs O. (2001). Kinetic analysis of open- and closed-state inactivation transitions in human Kv4.2 A-type potassium channels. J. Physiol. 535. (Pt. 1), 65–81. 10.1111/j.1469-7793.2001.00065.x [Abstract] [CrossRef] [Google Scholar]
- Bai J. Z., Lipski J. (2010). Differential expression of TRPM2 and TRPV4 channels and their potential role in oxidative stress-induced cell death in organotypic hippocampal culture. Neurotoxicology 31, 204–214. 10.1016/j.neuro.2010.01.001 [Abstract] [CrossRef] [Google Scholar]
- Barron T., Kim J. H. (2019). Neuronal input triggers Ca(2+) influx through AMPA receptors and voltage-gated Ca(2+) channels in oligodendrocytes. Glia 67, 1922–1932. 10.1002/glia.23670 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Batiuk M. Y., Martirosyan A., Wahis J., de Vin F., Marneffe C., Kusserow C., et al. . (2020). Identification of region-specific astrocyte subtypes at single cell resolution. Nat. Commun. 11:1220. 10.1038/s41467-019-14198-8 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Battefeld A., Tran B. T., Gavrilis J., Cooper E. C., Kole M. H. (2014). Heteromeric Kv7.2/7.3 channels differentially regulate action potential initiation and conduction in neocortical myelinated axons. J. Neurosci. 34, 3719–3732. 10.1523/JNEUROSCI.4206-13.2014 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Bauer C. K., Schwarz J. R. (2018). Ether-a-go-go K(+) channels: effective modulators of neuronal excitability. J. Physiol. 596, 769–783. 10.1113/JP275477 [Abstract] [CrossRef] [Google Scholar]
- Bekar L. K., Loewen M. E., Cao K., Sun X., Leis J., Wang R., et al. . (2005). Complex expression and localization of inactivating Kv channels in cultured hippocampal astrocytes. J. Neurophysiol. 93, 1699–1709. 10.1152/jn.00850.2004 [Abstract] [CrossRef] [Google Scholar]
- Bender K. J., Trussell L. O. (2009). Axon initial segment Ca2+ channels influence action potential generation and timing. Neuron 61, 259–271. 10.1016/j.neuron.2008.12.004 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Benfenati V., Nicchia G. P., Svelto M., Rapisarda C., Frigeri A., Ferroni S. (2007). Functional down-regulation of volume-regulated anion channels in AQP4 knockdown cultured rat cortical astrocytes. J. Neurochem. 100, 87–104. 10.1111/j.1471-4159.2006.04164.x [Abstract] [CrossRef] [Google Scholar]
- Berkefeld H., Sailer C. A., Bildl W., Rohde V., Thumfart J. O., Eble S., et al. . (2006). BKCa-Cav channel complexes mediate rapid and localized Ca2+-activated K+ signaling. Science 314, 615–620. 10.1126/science.1132915 [Abstract] [CrossRef] [Google Scholar]
- Berret E., Barron T., Xu J., Debner E., Kim E. J., Kim J. H. (2017). Oligodendroglial excitability mediated by glutamatergic inputs and Nav1.2 activation. Nat. Commun. 8:557. 10.1038/s41467-017-00688-0 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Bhat S., Dao D. T., Terrillion C. E., Arad M., Smith R. J., Soldatov N. M., et al. . (2012). CACNA1C (Cav1.2) in the pathophysiology of psychiatric disease. Prog. Neurobiol. 99, 1–14. 10.1016/j.pneurobio.2012.06.001 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Bhattacharjee A., Gan L., Kaczmarek L. K. (2002). Localization of the Slack potassium channel in the rat central nervous system. J. Comp. Neurol. 454, 241–254. 10.1002/cne.10439 [Abstract] [CrossRef] [Google Scholar]
- Bhattacharjee A., Kaczmarek L. K. (2005). For K+ channels, Na+ is the new Ca2+. Trends Neurosci. 28, 422–428. 10.1016/j.tins.2005.06.003 [Abstract] [CrossRef] [Google Scholar]
- Bierbower S. M., Choveau F. S., Lechleiter J. D., Shapiro M. S. (2015). Augmentation of M-type (KCNQ) potassium channels as a novel strategy to reduce stroke-induced brain injury. J. Neurosci. 35, 2101–2111. 10.1523/JNEUROSCI.3805-14.2015 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Birnbaum S. G., Varga A. W., Yuan L. L., Anderson A. E., Sweatt J. D., Schrader L. A. (2004). Structure and function of Kv4-family transient potassium channels. Physiol. Rev. 84, 803–833. 10.1152/physrev.00039.2003 [Abstract] [CrossRef] [Google Scholar]
- Bittner S., Ruck T., Fernandez-Orth J., Meuth S. G. (2014). TREK-king the blood-brain-barrier. J. Neuroimmune Pharmacol. 9, 293–301. 10.1007/s11481-014-9530-8 [Abstract] [CrossRef] [Google Scholar]
- Bittner S., Ruck T., Schuhmann M. K., Herrmann A. M., Moha ou Maati H., Bobak N., et al. . (2013). Endothelial TWIK-related potassium channel-1 (TREK1) regulates immune-cell trafficking into the CNS. Nat. Med. 19, 1161–1165. 10.1038/nm.3303 [Abstract] [CrossRef] [Google Scholar]
- Black J. A., Liu S., Waxman S. G. (2009). Sodium channel activity modulates multiple functions in microglia. Glia 57, 1072–1081. 10.1002/glia.20830 [Abstract] [CrossRef] [Google Scholar]
- Black J. A., Newcombe J., Trapp B. D., Waxman S. G. (2007). Sodium channel expression within chronic multiple sclerosis plaques. J. Neuropathol. Exp. Neurol. 66, 828–837. 10.1097/nen.0b013e3181462841 [Abstract] [CrossRef] [Google Scholar]
- Black J. A., Newcombe J., Waxman S. G. (2010). Astrocytes within multiple sclerosis lesions upregulate sodium channel Nav1.5. Brain 133 (Pt. 3), 835–846. 10.1093/brain/awq003 [Abstract] [CrossRef] [Google Scholar]
- Black J. A., Vasylyev D., Dib-Hajj S. D., Waxman S. G. (2014). Nav1.9 expression in magnocellular neurosecretory cells of supraoptic nucleus. Exp. Neurol. 253, 174–179. 10.1016/j.expneurol.2014.01.004 [Abstract] [CrossRef] [Google Scholar]
- Black J. A., Waxman S. G. (2012). Sodium channels and microglial function. Exp. Neurol. 234, 302–315. 10.1016/j.expneurol.2011.09.030 [Abstract] [CrossRef] [Google Scholar]
- Black J. A., Waxman S. G. (2013). Noncanonical roles of voltage-gated sodium channels. Neuron 80, 280–291. 10.1016/j.neuron.2013.09.012 [Abstract] [CrossRef] [Google Scholar]
- Black J. A., Westenbroek R., Minturn J. E., Ransom B. R., Catterall W. A., Waxman S. G. (1995). Isoform-specific expression of sodium channels in astrocytes in vitro: immunocytochemical observations. Glia 14, 133–144. 10.1002/glia.440140208 [Abstract] [CrossRef] [Google Scholar]
- Blanz J., Schweizer M., Auberson M., Maier H., Muenscher A., Hubner C. A., et al. . (2007). Leukoencephalopathy upon disruption of the chloride channel ClC-2. J. Neurosci. 27, 6581–6589. 10.1523/JNEUROSCI.0338-07.2007 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Blondeau N., Petrault O., Manta S., Giordanengo V., Gounon P., Bordet R., et al. . (2007). Polyunsaturated fatty acids are cerebral vasodilators via the TREK-1 potassium channel. Circ. Res. 101, 176–184. 10.1161/CIRCRESAHA.107.154443 [Abstract] [CrossRef] [Google Scholar]
- Bloodgood B. L., Sabatini B. L. (2008). Regulation of synaptic signalling by postsynaptic, non-glutamate receptor ion channels. J. Physiol. 586, 1475–1480. 10.1113/jphysiol.2007.148353 [Abstract] [CrossRef] [Google Scholar]
- Blum R., Kafitz K. W., Konnerth A. (2002). Neurotrophin-evoked depolarization requires the sodium channel Na(V)1.9. Nature 419, 687–693. 10.1038/nature01085 [Abstract] [CrossRef] [Google Scholar]
- Bock T., Honnuraiah S., Stuart G. J. (2019). Paradoxical excitatory impact of SK channels on dendritic excitability. J. Neurosci. 39, 7826–7839. 10.1523/JNEUROSCI.0105-19.2019 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Bocksteins E. (2016). Kv5, Kv6, Kv8, and Kv9 subunits: no simple silent bystanders. J. Gen. Physiol. 147, 105–125. 10.1085/jgp.201511507 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Boiko T., Rasband M. N., Levinson S. R., Caldwell J. H., Mandel G., Trimmer J. S., et al. . (2001). Compact myelin dictates the differential targeting of two sodium channel isoforms in the same axon. Neuron 30, 91–104. 10.1016/s0896-6273(01)00265-3 [Abstract] [CrossRef] [Google Scholar]
- Boiko T., Van Wart A., Caldwell J. H., Levinson S. R., Trimmer J. S., Matthews G. (2003). Functional specialization of the axon initial segment by isoform-specific sodium channel targeting. J. Neurosci. 23, 2306–2313. 10.1523/JNEUROSCI.23-06-02306.2003 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Borlot F., Abushama A., Morrison-Levy N., Jain P., Puthenveettil Vinayan K., Abukhalid M., et al. . (2020). KCNT1-related epilepsy: an international multicenter cohort of 27 pediatric cases. Epilepsia 61, 679–692. 10.1111/epi.16480 [Abstract] [CrossRef] [Google Scholar]
- Boscia F., Annunziato L., Taglialatela M. (2006). Retigabine and flupirtine exert neuroprotective actions in organotypic hippocampal cultures. Neuropharmacology 51, 283–294. 10.1016/j.neuropharm.2006.03.024 [Abstract] [CrossRef] [Google Scholar]
- Boscia F., de Rosa V., Cammarota M., Secondo A., Pannaccione A., Annunziato L. (2020). The Na(+)/Ca(2+) exchangers in demyelinating diseases. Cell Calcium 85:102130. 10.1016/j.ceca.2019.102130 [Abstract] [CrossRef] [Google Scholar]
- Boscia F., Pannaccione A., Ciccone R., Casamassa A., Franco C., Piccialli I., et al. . (2017). The expression and activity of KV3.4 channel subunits are precociously upregulated in astrocytes exposed to abeta oligomers and in astrocytes of Alzheimer's disease Tg2576 mice. Neurobiol. Aging 54, 187–198. 10.1016/j.neurobiolaging.2017.03.008 [Abstract] [CrossRef] [Google Scholar]
- Bose S., He H., Stauber T. (2021). Neurodegeneration upon dysfunction of endosomal/lysosomal CLC chloride transporters. Front. Cell Dev. Biol. 9:639231. 10.3389/fcell.2021.639231 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Bouafia A., Golmard J. L., Thuries V., Sazdovitch V., Hauw J. J., Fontaine B., et al. . (2014). Axonal expression of sodium channels and neuropathology of the plaques in multiple sclerosis. Neuropathol. Appl. Neurobiol. 40, 579–590. 10.1111/nan.12059 [Abstract] [CrossRef] [Google Scholar]
- Bozarth X., Dines J. N., Cong Q., Mirzaa G. M., Foss K., Lawrence Merritt J. 2nd, et al. . (2018). Expanding clinical phenotype in CACNA1C related disorders: From neonatal onset severe epileptic encephalopathy to late-onset epilepsy. Am. J. Med. Genet. A 176, 2733–2739. 10.1002/ajmg.a.40657 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Bradl M., Lassmann H. (2010). Oligodendrocytes: biology and pathology. Acta Neuropathol. 119, 37–53. 10.1007/s00401-009-0601-5 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Brand-Schieber E., Werner P. (2004). Calcium channel blockers ameliorate disease in a mouse model of multiple sclerosis. Exp. Neurol. 189, 5–9. 10.1016/j.expneurol.2004.05.023 [Abstract] [CrossRef] [Google Scholar]
- Brasko C., Hawkins V., De La Rocha I. C., Butt A. M. (2017). Expression of Kir4.1 and Kir5.1 inwardly rectifying potassium channels in oligodendrocytes, the myelinating cells of the CNS. Brain Struct. Funct. 222, 41–59. 10.1007/s00429-016-1199-8 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Brill A. L., Fischer T. T., Walters J. M., Marlier A., Sewanan L. R., Wilson P. C., et al. . (2020). Polycystin 2 is increased in disease to protect against stress-induced cell death. Sci. Rep. 10:386. 10.1038/s41598-019-57286-x [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Brown M. R., Kronengold J., Gazula V. R., Spilianakis C. G., Flavell R. A., von Hehn C. A., et al. . (2008). Amino-termini isoforms of the Slack K+ channel, regulated by alternative promoters, differentially modulate rhythmic firing and adaptation. J. Physiol. 586, 5161–5179. 10.1113/jphysiol.2008.160861 [Abstract] [CrossRef] [Google Scholar]
- Brueggemann L. I., Mackie A. R., Cribbs L. L., Freda J., Tripathi A., Majetschak M., et al. . (2014). Differential protein kinase C-dependent modulation of Kv7.4 and Kv7.5 subunits of vascular Kv7 channels. J. Biol. Chem. 289, 2099–2111. 10.1074/jbc.M113.527820 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Bruzzone R., Hormuzdi S. G., Barbe M. T., Herb A., Monyer H. (2003). Pannexins, a family of gap junction proteins expressed in brain. Proc. Natl. Acad. Sci. U.S.A. 100, 13644–13649. 10.1073/pnas.2233464100 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Cabranes A., Venderova K., de Lago E., Fezza F., Sanchez A., Mestre L., et al. . (2005). Decreased endocannabinoid levels in the brain and beneficial effects of agents activating cannabinoid and/or vanilloid receptors in a rat model of multiple sclerosis. Neurobiol. Dis. 20, 207–217. 10.1016/j.nbd.2005.03.002 [Abstract] [CrossRef] [Google Scholar]
- Caldwell J. H., Schaller K. L., Lasher R. S., Peles E., Levinson S. R. (2000). Sodium channel Na(v)1.6 is localized at nodes of ranvier, dendrites, and synapses. Proc. Natl. Acad. Sci. U.S.A. 97, 5616–5620. 10.1073/pnas.090034797 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Caminos E., Vaquero C. F., Martinez-Galan J. R. (2015). Relationship between rat retinal degeneration and potassium channel KCNQ5 expression. Exp. Eye Res. 131, 1–11. 10.1016/j.exer.2014.12.009 [Abstract] [CrossRef] [Google Scholar]
- Casamassa A., La Rocca C., Sokolow S., Herchuelz A., Matarese G., Annunziato L., et al. . (2016). Ncx3 gene ablation impairs oligodendrocyte precursor response and increases susceptibility to experimental autoimmune encephalomyelitis. Glia 64, 1124–1137. 10.1002/glia.22985 [Abstract] [CrossRef] [Google Scholar]
- Castellano A., Chiara M. D., Mellstrom B., Molina A., Monje F., Naranjo J. R., et al. . (1997). Identification and functional characterization of a K+ channel alpha-subunit with regulatory properties specific to brain. J. Neurosci. 17, 4652–4661. [Europe PMC free article] [Abstract] [Google Scholar]
- Catterall W. A. (2000). Structure and regulation of voltage-gated Ca2+ channels. Annu. Rev. Cell. Dev. Biol. 16, 521–555. 10.1146/annurev.cellbio.16.1.521 [Abstract] [CrossRef] [Google Scholar]
- Catterall W. A. (2011). Voltage-gated calcium channels. Cold Spring Harb. Perspect. Biol. 3:a003947. 10.1101/cshperspect.a003947 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Cheah C. S., Westenbroek R. E., Roden W. H., Kalume F., Oakley J. C., Jansen L. A., et al. . (2013). Correlations in timing of sodium channel expression, epilepsy, and sudden death in dravet syndrome. Channels 7, 468–472. 10.4161/chan.26023 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Chekeni F. B., Elliott M. R., Sandilos J. K., Walk S. F., Kinchen J. M., Lazarowski E. R., et al. . (2010). Pannexin 1 channels mediate 'find-me' signal release and membrane permeability during apoptosis. Nature 467, 863–867. 10.1038/nature09413 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Cheli V. T., Santiago Gonzalez D. A., Namgyal Lama T., Spreuer V., Handley V., Murphy G. G., et al. . (2016a). Conditional deletion of the l-type calcium channel Cav1.2 in oligodendrocyte progenitor cells affects postnatal myelination in mice. J. Neurosci. 36, 10853–10869. 10.1523/JNEUROSCI.1770-16.2016 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Cheli V. T., Santiago Gonzalez D. A., Smith J., Spreuer V., Murphy G. G., Paez P. M. (2016b). L-type voltage-operated calcium channels contribute to astrocyte activation in vitro. Glia 64, 1396–1415. 10.1002/glia.23013 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Cheli V. T., Santiago Gonzalez D. A., Spreuer V., Paez P. M. (2015). Voltage-gated Ca2+ entry promotes oligodendrocyte progenitor cell maturation and myelination in vitro. Exp. Neurol. 265, 69–83. 10.1016/j.expneurol.2014.12.012 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Chen S., Ren Y. Q., Bing R., Hillman D. E. (2000). Alpha 1E subunit of the R-type calcium channel is associated with myelinogenesis. J. Neurocytol. 29, 719–728. 10.1023/a:1010986303924 [Abstract] [CrossRef] [Google Scholar]
- Chen W., Chi Y. N., Kang X. J., Liu Q. Y., Zhang H. L., Li Z. H., et al. . (2018). Accumulation of Cav3.2 T-type calcium channels in the uninjured sural nerve contributes to neuropathic pain in rats with spared nerve injury. Front. Mol. Neurosci. 11:24. 10.3389/fnmol.2018.00024 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Chen Y. L., Tsaur M. L., Wang S. W., Wang T. Y., Hung Y. C., Lin C. S., et al. . (2015). Chronic intrathecal infusion of mibefradil, ethosuximide and nickel attenuates nerve ligation-induced pain in rats. Br. J. Anaesth. 115, 105–111. 10.1093/bja/aev198 [Abstract] [CrossRef] [Google Scholar]
- Chittajallu R., Chen Y., Wang H., Yuan X., Ghiani C. A., Heckman T., et al. . (2002). Regulation of Kv1 subunit expression in oligodendrocyte progenitor cells and their role in G1/S phase progression of the cell cycle. Proc. Natl. Acad. Sci. U.S.A. 99, 2350–2355. 10.1073/pnas.042698399 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Chiu Y. H., Schappe M. S., Desai B. N., Bayliss D. A. (2018). Revisiting multimodal activation and channel properties of pannexin 1. J. Gen. Physiol. 150, 19–39. 10.1085/jgp.201711888 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Choi H. J., Sun D., Jakobs T. C. (2015). Astrocytes in the optic nerve head express putative mechanosensitive channels. Mol. Vis. 21, 749–766. [Europe PMC free article] [Abstract] [Google Scholar]
- Christie S., Wittert G. A., Li H., Page A. J. (2018). Involvement of TRPV1 Channels in energy homeostasis. Front. Endocrinol. 9:420. 10.3389/fendo.2018.00420 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Chu S., Murray C. B., Liu M. M., Zeitlin P. L. (1996). A short CIC-2 mRNA transcript is produced by exon skipping. Nucleic Acids Res. 24, 3453–3457. 10.1093/nar/24.17.3453 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Chung K. K., Freestone P. S., Lipski J. (2011). Expression and functional properties of TRPM2 channels in dopaminergic neurons of the substantia nigra of the rat. J. Neurophysiol 106, 2865–2875. 10.1152/jn.00994.2010 [Abstract] [CrossRef] [Google Scholar]
- Clapham D. E. (2003). TRP channels as cellular sensors. Nature 426, 517–524. 10.1038/nature02196 [Abstract] [CrossRef] [Google Scholar]
- Clark S., Jordt S. E., Jentsch T. J., Mathie A. (1998). Characterization of the hyperpolarization-activated chloride current in dissociated rat sympathetic neurons. J. Physiol. 506 (Pt. 3), 665–678. 10.1111/j.1469-7793.1998.665bv.x [Abstract] [CrossRef] [Google Scholar]
- Coman I., Aigrot M. S., Seilhean D., Reynolds R., Girault J. A., Zalc B., et al. . (2006). Nodal, paranodal and juxtaparanodal axonal proteins during demyelination and remyelination in multiple sclerosis. Brain 129 (Pt. 12), 3186–3195. 10.1093/brain/awl144 [Abstract] [CrossRef] [Google Scholar]
- Compston A., Coles A. (2008). Multiple sclerosis. Lancet 372, 1502–1517. 10.1016/S0140-6736(08)61620-7 [Abstract] [CrossRef] [Google Scholar]
- Cone A. C., Ambrosi C., Scemes E., Martone M. E., Sosinsky G. E. (2013). A comparative antibody analysis of pannexin1 expression in four rat brain regions reveals varying subcellular localizations. Front. Pharmacol. 4:6. 10.3389/fphar.2013.00006 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Cooper E. C. (2011). Made for “anchorin”: Kv7.2/7.3 (KCNQ2/KCNQ3) channels and the modulation of neuronal excitability in vertebrate axons. Semin. Cell Dev. Biol. 22, 185–192. 10.1016/j.semcdb.2010.10.001 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Cooper E. C., Aldape K. D., Abosch A., Barbaro N. M., Berger M. S., Peacock W. S., et al. . (2000). Colocalization and coassembly of two human brain M-type potassium channel subunits that are mutated in epilepsy. Proc. Natl. Acad. Sci. U.S.A. 97, 4914–4919. 10.1073/pnas.090092797 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Couturier N., Gourraud P. A., Cournu-Rebeix I., Gout C., Bucciarelli F., Edan G., et al. . (2009). IFIH1-GCA-KCNH7 locus is not associated with genetic susceptibility to multiple sclerosis in French patients. Eur. J. Hum. Genet. 17, 844–847. 10.1038/ejhg.2008.259 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Craner M. J., Damarjian T. G., Liu S., Hains B. C., Lo A. C., Black J. A., et al. . (2005). Sodium channels contribute to microglia/macrophage activation and function in EAE and MS. Glia 49, 220–229. 10.1002/glia.20112 [Abstract] [CrossRef] [Google Scholar]
- Craner M. J., Hains B. C., Lo A. C., Black J. A., Waxman S. G. (2004a). Co-localization of sodium channel Nav1.6 and the sodium-calcium exchanger at sites of axonal injury in the spinal cord in EAE. Brain 127 (Pt. 2), 294–303. 10.1093/brain/awh032 [Abstract] [CrossRef] [Google Scholar]
- Craner M. J., Lo A. C., Black J. A., Waxman S. G. (2003). Abnormal sodium channel distribution in optic nerve axons in a model of inflammatory demyelination. Brain 126 (Pt. 7), 1552–1561. 10.1093/brain/awg153 [Abstract] [CrossRef] [Google Scholar]
- Craner M. J., Newcombe J., Black J. A., Hartle C., Cuzner M. L., Waxman S. G. (2004b). Molecular changes in neurons in multiple sclerosis: altered axonal expression of Nav1.2 and Nav1.6 sodium channels and Na+/Ca2+ exchanger. Proc. Natl. Acad. Sci. U.S.A. 101, 8168–8173. 10.1073/pnas.0402765101 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Crespo Yanguas S., Willebrords J., Johnstone S. R., Maes M., Decrock E., De Bock M., et al. . (2017). Pannexin1 as mediator of inflammation and cell death. Biochim. Biophys. Acta Mol. Cell Res. 1864, 51–61. 10.1016/j.bbamcr.2016.10.006 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Cummins T. R., Dib-Hajj S. D., Black J. A., Akopian A. N., Wood J. N., Waxman S. G. (1999). A novel persistent tetrodotoxin-resistant sodium current in SNS-null and wild-type small primary sensory neurons. J. Neurosci. 19:RC43. [Europe PMC free article] [Abstract] [Google Scholar]
- Dahl G. (2015). ATP release through pannexon channels. Philos. Trans. R. Soc. Lond. B Biol. Sci. 370:20140191. 10.1098/rstb.2014.0191 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Dahl G. (2018). The Pannexin1 membrane channel: distinct conformations and functions. FEBS Lett. 592, 3201–3209. 10.1002/1873-3468.13115 [Abstract] [CrossRef] [Google Scholar]
- D'Ascenzo M., Vairano M., Andreassi C., Navarra P., Azzena G. B., Grassi C. (2004). Electrophysiological and molecular evidence of L-(Cav1), N- (Cav2.2), and R- (Cav2.3) type Ca2+ channels in rat cortical astrocytes. Glia 45, 354–363. 10.1002/glia.10336 [Abstract] [CrossRef] [Google Scholar]
- Daschil N., Geisler S., Obermair G. J., Humpel C. (2014). Short- and long-term treatment of mouse cortical primary astrocytes with beta-amyloid differentially regulates the mRNA expression of L-type calcium channels. Pharmacology 93, 24–31. 10.1159/000357383 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Daschil N., Obermair G. J., Flucher B. E., Stefanova N., Hutter-Paier B., Windisch M., et al. . (2013). CaV1.2 calcium channel expression in reactive astrocytes is associated with the formation of amyloid-beta plaques in an Alzheimer's disease mouse model. J Alzheimers Dis. 37, 439–451. 10.3233/JAD-130560 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- de Kovel C. G. F., Syrbe S., Brilstra E. H., Verbeek N., Kerr B., Dubbs H., et al. . (2017). Neurodevelopmental disorders caused by de novo variants in KCNB1 genotypes and phenotypes. JAMA Neurol. 74, 1228–1236. 10.1001/jamaneurol.2017.1714 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- de Rosa V., Secondo A., Pannaccione A., Ciccone R., Formisano L., Guida N., et al. . (2019). D-Aspartate treatment attenuates myelin damage and stimulates myelin repair. EMBO Mol. Med. 11:e9278. 10.15252/emmm.201809278 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Derbenev A. V., Zsombok A. (2016). Potential therapeutic value of TRPV1 and TRPA1 in diabetes mellitus and obesity. Semin. Immunopathol. 38, 397–406. 10.1007/s00281-015-0529-x [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Derst C., Karschin C., Wischmeyer E., Hirsch J. R., Preisig-Muller R., Rajan S., et al. . (2001). Genetic and functional linkage of Kir5.1 and Kir2.1 channel subunits. FEBS Lett. 491, 305–311. 10.1016/s0014-5793(01)02202-5 [Abstract] [CrossRef] [Google Scholar]
- Deutsch E., Weigel A. V., Akin E. J., Fox P., Hansen G., Haberkorn C. J., et al. . (2012). Kv2.1 cell surface clusters are insertion platforms for ion channel delivery to the plasma membrane. Mol. Biol. Cell 23, 2917–2929. 10.1091/mbc.E12-01-0047 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Devaux J. J., Kleopa K. A., Cooper E. C., Scherer S. S. (2004). KCNQ2 is a nodal K+ channel. J. Neurosci. 24, 1236–1244. 10.1523/JNEUROSCI.4512-03.2004 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Dietrich D., Kirschstein T., Kukley M., Pereverzev A., von der Brelie C., Schneider T., et al. . (2003). Functional specialization of presynaptic Cav2.3 Ca2+ channels. Neuron 39, 483–496. 10.1016/s0896-6273(03)00430-6 [Abstract] [CrossRef] [Google Scholar]
- Djillani A., Mazella J., Heurteaux C., Borsotto M. (2019). Role of TREK-1 in health and disease, focus on the central nervous system. Front. Pharmacol. 10:379. 10.3389/fphar.2019.00379 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Dolga A. M., Letsche T., Gold M., Doti N., Bacher M., Chiamvimonvat N., et al. . (2012). Activation of KCNN3/SK3/K(Ca)2.3 channels attenuates enhanced calcium influx and inflammatory cytokine production in activated microglia. Glia 60, 2050–2064. 10.1002/glia.22419 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Dolga A. M., Terpolilli N., Kepura F., Nijholt I. M., Knaus H. G., D'Orsi B., et al. . (2011). KCa2 channels activation prevents [Ca2+]i deregulation and reduces neuronal death following glutamate toxicity and cerebral ischemia. Cell Death Dis. 2:e147. 10.1038/cddis.2011.30 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Dorr J., Wernecke K. D., Wurfel J., Bellmann-Strobl J., Siffrin V., Sattler M. B., et al. . (2018). Disease modification in multiple sclerosis by flupirtine-results of a randomized placebo controlled phase II trial. Front. Neurol. 9:842. 10.3389/fneur.2018.00842 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Du J., Haak L. L., Phillips-Tansey E., Russell J. T., McBain C. J. (2000). Frequency-dependent regulation of rat hippocampal somato-dendritic excitability by the K+ channel subunit Kv2.1. J. Physiol. 522 (Pt. 1), 19–31. 10.1111/j.1469-7793.2000.t01-2-00019.xm [Abstract] [CrossRef] [Google Scholar]
- Du T., Liang C., Li B., Hertz L., Peng L. (2014). Chronic fluoxetine administration increases expression of the L-channel gene Cav1.2 in astrocytes from the brain of treated mice and in culture and augments K(+)-induced increase in [Ca(2+)]i. Cell Calcium 55, 166–174. 10.1016/j.ceca.2014.01.002 [Abstract] [CrossRef] [Google Scholar]
- Ducharme G., Newell E. W., Pinto C., Schlichter L. C. (2007). Small-conductance Cl- channels contribute to volume regulation and phagocytosis in microglia. Eur. J. Neurosci. 26, 2119–2130. 10.1111/j.1460-9568.2007.05802.x [Abstract] [CrossRef] [Google Scholar]
- Duflocq A., Le Bras B., Bullier E., Couraud F., Davenne M. (2008). Nav1.1 is predominantly expressed in nodes of ranvier and axon initial segments. Mol. Cell Neurosci. 39, 180–192. 10.1016/j.mcn.2008.06.008 [Abstract] [CrossRef] [Google Scholar]
- Dumenieu M., Oule M., Kreutz M. R., Lopez-Rojas J. (2017). The segregated expression of voltage-gated potassium and sodium channels in neuronal membranes: functional implications and regulatory mechanisms. Front. Cell Neurosci. 11:115. 10.3389/fncel.2017.00115 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Eder C. (1998). Ion channels in microglia (brain macrophages). Am. J. Physiol. 275, C327–342. 10.1152/ajpcell.1998.275.2.C327 [Abstract] [CrossRef] [Google Scholar]
- Edwards L., Nashmi R., Jones O., Backx P., Ackerley C., Becker L., et al. . (2002). Upregulation of Kv 1.4 protein and gene expression after chronic spinal cord injury. J Comp. Neurol. 443, 154–167. 10.1002/cne.10115 [Abstract] [CrossRef] [Google Scholar]
- Ehling P., Cerina M., Budde T., Meuth S. G., Bittner S. (2015). The CNS under pathophysiologic attack–examining the role of K(2)p channels. Pflugers Arch. 467, 959–972. 10.1007/s00424-014-1664-2 [Abstract] [CrossRef] [Google Scholar]
- Eisfeld J., Luckhoff A. (2007). Trpm2. Handb. Exp. Pharmacol. 237–252. 10.1007/978-3-540-34891-7_14 [Abstract] [CrossRef] [Google Scholar]
- Elkjaer M. L., Frisch T., Reynolds R., Kacprowski T., Burton M., Kruse T. A., et al. . (2019). Molecular signature of different lesion types in the brain white matter of patients with progressive multiple sclerosis. Acta Neuropathol. Commun. 7:205. 10.1186/s40478-019-0855-7 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Elliott C., Belachew S., Wolinsky J. S., Hauser S. L., Kappos L., Barkhof F., et al. . (2019). Chronic white matter lesion activity predicts clinical progression in primary progressive multiple sclerosis. Brain 142, 2787–2799. 10.1093/brain/awz212 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Emmi A., Wenzel H. J., Schwartzkroin P. A., Taglialatela M., Castaldo P., Bianchi L., et al. . (2000). Do glia have heart? Expression and functional role for ether-a-go-go currents in hippocampal astrocytes. J. Neurosci. 20, 3915–3925. 10.1523/JNEUROSCI.20-10-03915.2000 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Enders M., Heider T., Ludwig A., Kuerten S. (2020). Strategies for neuroprotection in multiple sclerosis and the role of calcium. Int. J. Mol. Sci. 21:1663. 10.3390/ijms21051663 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Enyedi P., Czirjak G. (2010). Molecular background of leak K+ currents: two-pore domain potassium channels. Physiol. Rev. 90, 559–605. 10.1152/physrev.00029.2009 [Abstract] [CrossRef] [Google Scholar]
- Eshed-Eisenbach Y., Peles E. (2020). The clustering of voltage-gated sodium channels in various excitable membranes. Dev. Neurobiol. 1–11. 10.1002/dneu.2272 [Abstract] [CrossRef] [Google Scholar]
- Espinosa-Parrilla J. F., Martinez-Moreno M., Gasull X., Mahy N., Rodriguez M. J. (2015). The L-type voltage-gated calcium channel modulates microglial pro-inflammatory activity. Mol. Cell Neurosci. 64, 104–115. 10.1016/j.mcn.2014.12.004 [Abstract] [CrossRef] [Google Scholar]
- Estacion M., Gasser A., Dib-Hajj S. D., Waxman S. G. (2010). A sodium channel mutation linked to epilepsy increases ramp and persistent current of Nav1.3 and induces hyperexcitability in hippocampal neurons. Exp. Neurol. 224, 362–368. 10.1016/j.expneurol.2010.04.012 [Abstract] [CrossRef] [Google Scholar]
- Falcao A. M., van Bruggen D., Marques S., Meijer M., Jakel S., Agirre E., et al. . (2018). Disease-specific oligodendrocyte lineage cells arise in multiple sclerosis. Nat. Med. 24, 1837–1844. 10.1038/s41591-018-0236-y [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Fang J. S., Angelov S. N., Simon A. M., Burt J. M. (2011). Cx37 deletion enhances vascular growth and facilitates ischemic limb recovery. Am. J. Physiol. Heart Circ. Physiol. 301, H1872–1881. 10.1152/ajpheart.00683.2011 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Fano S., Caliskan G., Heinemann U. (2012). Differential effects of blockade of ERG channels on gamma oscillations and excitability in rat hippocampal slices. Eur. J. Neurosci. 36, 3628–3635. 10.1111/ejn.12015 [Abstract] [CrossRef] [Google Scholar]
- Filippi M., Bar-Or A., Piehl F., Preziosa P., Solari A., Vukusic S.. (2018). Multiple sclerosis. Nat. Rev. Dis. Primers 4:43. 10.1038/s41572-018-0041-4 [Abstract] [CrossRef] [Google Scholar]
- Fill M., Copello J. A. (2002). Ryanodine receptor calcium release channels. Physiol. Rev. 82, 893–922. 10.1152/physrev.00013.2002 [Abstract] [CrossRef] [Google Scholar]
- Foldy C., Lee S. H., Morgan R. J., Soltesz I. (2010). Regulation of fast-spiking basket cell synapses by the chloride channel ClC-2. Nat. Neurosci. 13, 1047–1049. 10.1038/nn.2609 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Fordyce C. B., Jagasia R., Zhu X., Schlichter L. C. (2005). Microglia Kv1.3 channels contribute to their ability to kill neurons. J. Neurosci. 25, 7139–7149. 10.1523/JNEUROSCI.1251-05.2005 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Franceschetti S., Lavazza T., Curia G., Aracri P., Panzica F., Sancini G., et al. . (2003). Na+-activated K+ current contributes to postexcitatory hyperpolarization in neocortical intrinsically bursting neurons. J. Neurophysiol. 89, 2101–2111. 10.1152/jn.00695.2002 [Abstract] [CrossRef] [Google Scholar]
- Freestone P. S., Chung K. K., Guatteo E., Mercuri N. B., Nicholson L. F., Lipski J. (2009). Acute action of rotenone on nigral dopaminergic neurons–involvement of reactive oxygen species and disruption of Ca2+ homeostasis. Eur. J. Neurosci. 30, 1849–1859. 10.1111/j.1460-9568.2009.06990.x [Abstract] [CrossRef] [Google Scholar]
- Friese M. A., Schattling B., Fugger L. (2014). Mechanisms of neurodegeneration and axonal dysfunction in multiple sclerosis. Nat. Rev. Neurol. 10, 225–238. 10.1038/nrneurol.2014.37 [Abstract] [CrossRef] [Google Scholar]
- Frisch T., Elkjaer M. L., Reynolds R., Michel T. M., Kacprowski T., Burton M., et al. . (2020). Multiple sclerosis atlas: a molecular map of brain lesion stages in progressive multiple sclerosis. Netw. Syst. Med. 3, 122–129. 10.1089/nsm.2020.0006 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Fulton D., Paez P. M., Fisher R., Handley V., Colwell C. S., Campagnoni A. T. (2010). Regulation of L-type Ca++ currents and process morphology in white matter oligodendrocyte precursor cells by golli-myelin proteins. Glia 58, 1292–1303. 10.1002/glia.21008 [Abstract] [CrossRef] [Google Scholar]
- Gajardo-Gomez R., Labra V. C., Orellana J. A. (2016). Connexins and pannexins: new insights into microglial functions and dysfunctions. Front. Mol. Neurosci. 9:86. 10.3389/fnmol.2016.00086 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Gallego-Delgado P., James R., Browne E., Meng J., Umashankar S., Tan L., et al. . (2020). Neuroinflammation in the normal-appearing white matter (NAWM) of the multiple sclerosis brain causes abnormalities at the nodes of Ranvier. PLoS Biol. 18:e3001008. 10.1371/journal.pbio.3001008 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Gamelli A. E., McKinney B. C., White J. A., Murphy G. G. (2011). Deletion of the L-type calcium channel Ca(V) 1.3 but not Ca(V) 1.2 results in a diminished sAHP in mouse CA1 pyramidal neurons. Hippocampus 21, 133–141. 10.1002/hipo.20728 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Gartner C., Ziegelhoffer B., Kostelka M., Stepan H., Mohr F. W., Dhein S. (2012). Knock-down of endothelial connexins impairs angiogenesis. Pharmacol. Res. 65, 347–357. 10.1016/j.phrs.2011.11.012 [Abstract] [CrossRef] [Google Scholar]
- Gasparini S., Kasyanov A. M., Pietrobon D., Voronin L. L., Cherubini E. (2001). Presynaptic R-type calcium channels contribute to fast excitatory synaptic transmission in the rat hippocampus. J. Neurosci. 21, 8715–8721. 10.1523/JNEUROSCI.21-22-08715.2001 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Gees M., Colsoul B., Nilius B. (2010). The role of transient receptor potential cation channels in Ca2+ signaling. Cold Spring Harb. Perspect. Biol. 2:a003962. 10.1101/cshperspect.a003962 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Giannini G., Conti A., Mammarella S., Scrobogna M., Sorrentino V. (1995). The ryanodine receptor/calcium channel genes are widely and differentially expressed in murine brain and peripheral tissues. J. CellBiol. 128, 893–904. 10.1083/jcb.128.5.893 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Giaume C., Naus C. C., Saez J. C., Leybaert L. (2021). Glial connexins and pannexins in the healthy and diseased brain. Physiol. Rev. 101, 93–145. 10.1152/physrev.00043.2018 [Abstract] [CrossRef] [Google Scholar]
- Gibson H. E., Edwards J. G., Page R. S., Van Hook M. J., Kauer J. A. (2008). TRPV1 channels mediate long-term depression at synapses on hippocampal interneurons. Neuron 57, 746–759. 10.1016/j.neuron.2007.12.027 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Gilgun-Sherki Y., Panet H., Melamed E., Offen D. (2003). Riluzole suppresses experimental autoimmune encephalomyelitis: implications for the treatment of multiple sclerosis. Brain Res. 989, 196–204. 10.1016/s0006-8993(03)03343-2 [Abstract] [CrossRef] [Google Scholar]
- Girolamo F., Coppola C., Ribatti D., Trojano M. (2014). Angiogenesis in multiple sclerosis and experimental autoimmune encephalomyelitis. Acta Neuropathol. Commun. 2:84. 10.1186/s40478-014-0084-z [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Gnatenco C., Han J., Snyder A. K., Kim D. (2002). Functional expression of TREK-2 K+ channel in cultured rat brain astrocytes. Brain Res. 931, 56–67. 10.1016/s0006-8993(02)02261-8 [Abstract] [CrossRef] [Google Scholar]
- Goldin A. L., Barchi R. L., Caldwell J. H., Hofmann F., Howe J. R., Hunter J. C., et al. . (2000). Nomenclature of voltage-gated sodium channels. Neuron 28, 365–368. 10.1016/s0896-6273(00)00116-1 [Abstract] [CrossRef] [Google Scholar]
- Gonzalez-Alvarado M. N., Rotger C., Berger L., London B., Haase S., Kuhbandner K., et al. . (2020). Functional role of endogenous Kv1.4 in experimental demyelination. J. Neuroimmunol. 343:577227. 10.1016/j.jneuroim.2020.577227 [Abstract] [CrossRef] [Google Scholar]
- Gonzalez-Reyes L. E., Ladas T. P., Chiang C. C., Durand D. M. (2013). TRPV1 antagonist capsazepine suppresses 4-AP-induced epileptiform activity in vitro and electrographic seizures in vivo. Exp. Neurol. 250, 321–332. 10.1016/j.expneurol.2013.10.010 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Good M. E., Eucker S. A., Li J., Bacon H. M., Lang S. M., Butcher J. T., et al. . (2018). Endothelial cell pannexin1 modulates severity of ischemic stroke by regulating cerebral inflammation and myogenic tone. JCI Insight 3:e96272. 10.1172/jci.insight.96272 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Goppner C., Soria A. H., Hoegg-Beiler M. B., Jentsch T. J. (2020). Cellular basis of ClC-2 Cl- channel-related brain and testis pathologies. J. Biol. Chem. 296:100074. 10.1074/jbc.RA120.016031 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Goswami C., Rademacher N., Smalla K. H., Kalscheuer V., Ropers H. H., Gundelfinger E. D., et al. . (2010). TRPV1 acts as a synaptic protein and regulates vesicle recycling. J. Cell Sci. 123 (Pt. 12), 2045–2057. 10.1242/jcs.065144 [Abstract] [CrossRef] [Google Scholar]
- Grunder S., Thiemann A., Pusch M., Jentsch T. J. (1992). Regions involved in the opening of CIC-2 chloride channel by voltage and cell volume. Nature 360, 759–762. 10.1038/360759a0 [Abstract] [CrossRef] [Google Scholar]
- Guan D., Tkatch T., Surmeier D. J., Armstrong W. E., Foehring R. C. (2007). Kv2 subunits underlie slowly inactivating potassium current in rat neocortical pyramidal neurons. J. Physiol. 581 (Pt. 3), 941–960. 10.1113/jphysiol.2007.128454 [Abstract] [CrossRef] [Google Scholar]
- Guo F., Yu N., Cai J. Q., Quinn T., Zong Z. H., Zeng Y. J., et al. . (2008). Voltage-gated sodium channel Nav1.1, Nav1.3 and beta1 subunit were up-regulated in the hippocampus of spontaneously epileptic rat. Brain Res. Bull. 75, 179–187. 10.1016/j.brainresbull.2007.10.005 [Abstract] [CrossRef] [Google Scholar]
- Guo L., Schreiber T. H., Weremowicz S., Morton C. C., Lee C., Zhou J. (2000). Identification and characterization of a novel polycystin family member, polycystin-L2, in mouse and human: sequence, expression, alternative splicing, and chromosomal localization. Genomics 64, 241–251. 10.1006/geno.2000.6131 [Abstract] [CrossRef] [Google Scholar]
- Gutzmann J. J., Lin L., Hoffman D. A. (2019). Functional coupling of Cav2.3 and BK potassium channels regulates action potential repolarization and short-term plasticity in the mouse hippocampus. Front Cell Neurosci 13, 27. 10.3389/fncel.2019.00027 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Haak L. L., Song L. S., Molinski T. F., Pessah I. N., Cheng H., Russell J. T. (2001). Sparks and puffs in oligodendrocyte progenitors: cross talk between ryanodine receptors and inositol trisphosphate receptors. J. Neurosci. 21, 3860–3870. 10.1523/JNEUROSCI.21-11-03860.2001 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Haberlandt C., Derouiche A., Wyczynski A., Haseleu J., Pohle J., Karram K., et al. . (2011). Gray matter NG2 cells display multiple Ca2+-signaling pathways and highly motile processes. PLoS ONE 6:e17575. 10.1371/journal.pone.0017575 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Hainz N., Wolf S., Beck A., Wagenpfeil S., Tschernig T., Meier C. (2017). Probenecid arrests the progression of pronounced clinical symptoms in a mouse model of multiple sclerosis. Sci. Rep. 7:17214. 10.1038/s41598-017-17517-5 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Hamada M. S., Kole M. H. (2015). Myelin loss and axonal ion channel adaptations associated with gray matter neuronal hyperexcitability. J. Neurosci. 35, 7272–7286. 10.1523/JNEUROSCI.4747-14.2015 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Hammann J., Bassetti D., White R., Luhmann H. J., Kirischuk S. (2018). alpha2 isoform of Na(+),K(+)-ATPase via Na(+),Ca(2+) exchanger modulates myelin basic protein synthesis in oligodendrocyte lineage cells in vitro. Cell Calcium 73, 1–10. 10.1016/j.ceca.2018.03.003 [Abstract] [CrossRef] [Google Scholar]
- Hammond T. R., Dufort C., Dissing-Olesen L., Giera S., Young A., Wysoker A., et al. . (2019). Single-Cell rna sequencing of microglia throughout the mouse lifespan and in the injured brain reveals complex cell-state changes. Immunity 50, 253–271.e256. 10.1016/j.immuni.2018.11.004 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Hashitani H., Mitsui R. (2019). Role of pericytes in the initiation and propagation of spontaneous activity in the microvasculature. Adv. Exp. Med. Biol. 1124, 329–356. 10.1007/978-981-13-5895-1_14 [Abstract] [CrossRef] [Google Scholar]
- Hassan S., Eldeeb K., Millns P. J., Bennett A. J., Alexander S. P., Kendall D. A. (2014). Cannabidiol enhances microglial phagocytosis via transient receptor potential (TRP) channel activation. Br. J. Pharmacol. 171, 2426–2439. 10.1111/bph.12615 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Hassen G. W., Feliberti J., Kesner L., Stracher A., Mokhtarian F. (2008). Prevention of axonal injury using calpain inhibitor in chronic progressive experimental autoimmune encephalomyelitis. Brain Res. 1236, 206–215. 10.1016/j.brainres.2008.07.124 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Hell J. W., Westenbroek R. E., Warner C., Ahlijanian M. K., Prystay W., Gilbert M. M., et al. . (1993). Identification and differential subcellular localization of the neuronal class C and class D L-type calcium channel alpha 1 subunits. J. Cell Biol. 123, 949–962. 10.1083/jcb.123.4.949 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Herrero-Herranz E., Pardo L. A., Bunt G., Gold R., Stuhmer W., Linker R. A. (2007). Re-expression of a developmentally restricted potassium channel in autoimmune demyelination: Kv1.4 is implicated in oligodendroglial proliferation. Am. J. Pathol. 171, 589–598. 10.2353/ajpath.2007.061241 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Herrero-Herranz E., Pardo L. A., Gold R., Linker R. A. (2008). Pattern of axonal injury in murine myelin oligodendrocyte glycoprotein induced experimental autoimmune encephalomyelitis: implications for multiple sclerosis. Neurobiol. Dis. 30, 162–173. 10.1016/j.nbd.2008.01.001 [Abstract] [CrossRef] [Google Scholar]
- Hervieu G. J., Cluderay J. E., Gray C. W., Green P. J., Ranson J. L., Randall A. D., et al. . (2001). Distribution and expression of TREK-1, a two-pore-domain potassium channel, in the adult rat CNS. Neuroscience 103, 899–919. 10.1016/s0306-4522(01)00030-6 [Abstract] [CrossRef] [Google Scholar]
- Hibino H., Inanobe A., Furutani K., Murakami S., Findlay I., Kurachi Y. (2010). Inwardly rectifying potassium channels: their structure, function, and physiological roles. Physiol Rev. 90, 291–366. 10.1152/physrev.00021.2009 [Abstract] [CrossRef] [Google Scholar]
- Hirtz J. J., Braun N., Griesemer D., Hannes C., Janz K., Lohrke S., et al. . (2012). Synaptic refinement of an inhibitory topographic map in the auditory brainstem requires functional Cav1.3 calcium channels. J. Neurosci. 32, 14602–14616. 10.1523/JNEUROSCI.0765-12.2012 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Ho K. W., Lambert W. S., Calkins D. J. (2014). Activation of the TRPV1 cation channel contributes to stress-induced astrocyte migration. Glia 62, 1435–1451. 10.1002/glia.22691 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Hoegg-Beiler M. B., Sirisi S., Orozco I. J., Ferrer I., Hohensee S., Auberson M., et al. . (2014). Disrupting MLC1 and GlialCAM and ClC-2 interactions in leukodystrophy entails glial chloride channel dysfunction. Nat. Commun. 5:3475. 10.1038/ncomms4475 [Abstract] [CrossRef] [Google Scholar]
- Hoenderop J. G., Voets T., Hoefs S., Weidema F., Prenen J., Nilius B., et al. . (2003). Homo- and heterotetrameric architecture of the epithelial Ca2+ channels TRPV5 and TRPV6. EMBO J. 22, 776–785. 10.1093/emboj/cdg080 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Hofmann F., Flockerzi V., Kahl S., Wegener J. W. (2014). L-type CaV1.2 calcium channels: from in vitro findings to in vivo function. Physiol. Rev. 94, 303–326. 10.1152/physrev.00016.2013 [Abstract] [CrossRef] [Google Scholar]
- Honore E. (2007). The neuronal background K2P channels: focus on TREK1. Nat. Rev. Neurosci. 8, 251–261. 10.1038/nrn2117 [Abstract] [CrossRef] [Google Scholar]
- Hoogland T. M., Saggau P. (2004). Facilitation of L-type Ca2+ channels in dendritic spines by activation of beta2 adrenergic receptors. J. Neurosci. 24, 8416–8427. 10.1523/JNEUROSCI.1677-04.2004 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Hopp S. C. (2021). Targeting microglia L-type voltage-dependent calcium channels for the treatment of central nervous system disorders. J. Neurosci. Res. 99, 141–162. 10.1002/jnr.24585 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Hossain M. M., Sonsalla P. K., Richardson J. R. (2013). Coordinated role of voltage-gated sodium channels and the Na+/H+ exchanger in sustaining microglial activation during inflammation. Toxicol. Appl. Pharmacol. 273, 355–364. 10.1016/j.taap.2013.09.011 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Hou X., Zhang R., Wang J., Li Y., Li F., Zhang Y., et al. . (2018). CLC-2 is a positive modulator of oligodendrocyte precursor cell differentiation and myelination. Mol. Med. Rep. 17, 4515–4523. 10.3892/mmr.2018.8439 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Howell O. W., Rundle J. L., Garg A., Komada M., Brophy P. J., Reynolds R. (2010). Activated microglia mediate axoglial disruption that contributes to axonal injury in multiple sclerosis. J. Neuropathol. Exp. Neurol. 69, 1017–1033. 10.1097/NEN.0b013e3181f3a5b1 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Hu W., Tian C., Li T., Yang M., Hou H., Shu Y. (2009). Distinct contributions of Na(v)1.6 and Na(v)1.2 in action potential initiation and backpropagation. Nat. Neurosci. 12, 996–1002. 10.1038/nn.2359 [Abstract] [CrossRef] [Google Scholar]
- Huang C. Y., Chu D., Hwang W. C., Tsaur M. L. (2012). Coexpression of high-voltage-activated ion channels Kv3.4 and Cav1.2 in pioneer axons during pathfinding in the developing rat forebrain. J. Comp. Neurol. 520, 3650–3672. 10.1002/cne.23119 [Abstract] [CrossRef] [Google Scholar]
- Huang Y., Fliegert R., Guse A. H., Lu W., Du J. (2020). A structural overview of the ion channels of the TRPM family. Cell Calcium 85:102111. 10.1016/j.ceca.2019.102111 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Hugnot J. P., Salinas M., Lesage F., Guillemare E., de Weille J., Heurteaux C., et al. . (1996). Kv8.1, a new neuronal potassium channel subunit with specific inhibitory properties towards shab and shaw channels. EMBO J. 15, 3322–3331. [Europe PMC free article] [Abstract] [Google Scholar]
- Iglesias R., Dahl G., Qiu F., Spray D. C., Scemes E. (2009). Pannexin 1: the molecular substrate of astrocyte “hemichannels”. J. Neurosci. 29, 7092–7097. 10.1523/JNEUROSCI.6062-08.2009 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Iglesias R., Locovei S., Roque A., Alberto A. P., Dahl G., Spray D. C., et al. . (2008). P2X7 receptor-Pannexin1 complex: pharmacology and signaling. Am. J. Physiol. Cell Physiol. 295, C752–760. 10.1152/ajpcell.00228.2008 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Imaizumi T., Kocsis J. D., Waxman S. G. (1999). The role of voltage-gated Ca2+ channels in anoxic injury of spinal cord white matter. Brain Res. 817, 84–92. 10.1016/s0006-8993(98)01214-1 [Abstract] [CrossRef] [Google Scholar]
- Indriati D. W., Kamasawa N., Matsui K., Meredith A. L., Watanabe M., Shigemoto R. (2013). Quantitative localization of Cav2.1 (P/Q-type) voltage-dependent calcium channels in Purkinje cells: somatodendritic gradient and distinct somatic coclustering with calcium-activated potassium channels. J. Neurosci. 33, 3668–3678. 10.1523/JNEUROSCI.2921-12.2013 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Inglese M., Madelin G., Oesingmann N., Babb J. S., Wu W., Stoeckel B., et al. . (2010). Brain tissue sodium concentration in multiple sclerosis: a sodium imaging study at 3 tesla. Brain 133 (Pt. 3), 847–857. 10.1093/brain/awp334 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Ingwersen J., De Santi L., Wingerath B., Graf J., Koop B., Schneider R., et al. . (2018). Nimodipine confers clinical improvement in two models of experimental autoimmune encephalomyelitis. J. Neurochem. 146, 86–98. 10.1111/jnc.14324 [Abstract] [CrossRef] [Google Scholar]
- Irie T., Trussell L. O. (2017). Double-Nanodomain coupling of calcium channels, ryanodine receptors, and BK channels controls the generation of burst firing. Neuron 96, 856–870.e854. 10.1016/j.neuron.2017.10.014 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Izquierdo-Serra M., Fernandez-Fernandez J. M., Serrano M. (2020). Rare CACNA1A mutations leading to congenital ataxia. Pflugers Arch. 472, 791–809. 10.1007/s00424-020-02396-z [Abstract] [CrossRef] [Google Scholar]
- Jakel S., Agirre E., Mendanha Falcao A., van Bruggen D., Lee K. W., Knuesel I., et al. . (2019). Altered human oligodendrocyte heterogeneity in multiple sclerosis. Nature 566, 543–547. 10.1038/s41586-019-0903-2 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Jäkel S., Williams A. (2020). What have advances in transcriptomic technologies taught us about human white matter pathologies? Front. Cell Neurosci. 14:238. 10.3389/fncel.2020.00238 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Jentsch T. J. (2000). Neuronal KCNQ potassium channels: physiology and role in disease. Nat. Rev. Neurosci. 1, 21–30. 10.1038/35036198 [Abstract] [CrossRef] [Google Scholar]
- Jentsch T. J., Maritzen T., Zdebik A. A. (2005). Chloride channel diseases resulting from impaired transepithelial transport or vesicular function. J. Clin. Invest. 115, 2039–2046. 10.1172/JCI25470 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Jentsch T. J., Pusch M. (2018). CLC chloride channels and transporters: structure, function, physiology, and disease. Physiol. Rev. 98, 1493–1590. 10.1152/physrev.00047.2017 [Abstract] [CrossRef] [Google Scholar]
- Jeong S. Y., Goto J., Hashida H., Suzuki T., Ogata K., Masuda N., et al. . (2000). Identification of a novel human voltage-gated sodium channel alpha subunit gene, SCN12A. Biochem. Biophys. Res. Commun. 267, 262–270. 10.1006/bbrc.1999.1916 [Abstract] [CrossRef] [Google Scholar]
- Jeworutzki E., Lopez-Hernandez T., Capdevila-Nortes X., Sirisi S., Bengtsson L., Montolio M., et al. . (2012). GlialCAM, a protein defective in a leukodystrophy, serves as a ClC-2 Cl(-) channel auxiliary subunit. Neuron 73, 951–961. 10.1016/j.neuron.2011.12.039 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Jin X., Yu L., Wu Y., Zhang S., Shi Z., Chen X., et al. . (2012). S-Glutathionylation underscores the modulation of the heteromeric Kir4.1-Kir5.1 channel in oxidative stress. J. Physiol. 590, 5335–5348. 10.1113/jphysiol.2012.236885 [Abstract] [CrossRef] [Google Scholar]
- Johnson B., Leek A. N., Tamkun M. M. (2019). Kv2 channels create endoplasmic reticulum/plasma membrane junctions: a brief history of Kv2 channel subcellular localization. Channels 13, 88–101. 10.1080/19336950.2019.1568824 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Johnson K. W., Herold K. F., Milner T. A., Hemmings H. C., Jr., Platholi J. (2017). Sodium channel subtypes are differentially localized to pre- and post-synaptic sites in rat hippocampus. J. Comp. Neurol. 525, 3563–3578. 10.1002/cne.24291 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Jones S. L., Stuart G. J. (2013). Different calcium sources control somatic versus dendritic SK channel activation during action potentials. J. Neurosci. 33, 19396–19405. 10.1523/JNEUROSCI.2073-13.2013 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Joux N., Chevaleyre V., Alonso G., Boissin-Agasse L., Moos F. C., Desarmenien M. G., et al. . (2001). High voltage-activated Ca2+ currents in rat supraoptic neurones: biophysical properties and expression of the various channel alpha1 subunits. J. Neuroendocrinol. 13, 638–649. 10.1046/j.1365-2826.2001.00679.x [Abstract] [CrossRef] [Google Scholar]
- Jukkola P., Gu C. (2015). Regulation of neurovascular coupling in autoimmunity to water and ion channels. Autoimmun. Rev. 14, 258–267. 10.1016/j.autrev.2014.11.010 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Jukkola P., Gu Y., Lovett-Racke A. E., Gu C. (2017). Suppression of inflammatory demyelinaton and axon degeneration through inhibiting Kv3 channels. Front. Mol. Neurosci. 10:344. 10.3389/fnmol.2017.00344 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Jukkola P. I., Lovett-Racke A. E., Zamvil S. S., Gu C. (2012). K+ channel alterations in the progression of experimental autoimmune encephalomyelitis. Neurobiol. Dis. 47, 280–293. 10.1016/j.nbd.2012.04.012 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Justice J. A., Schulien A. J., He K., Hartnett K. A., Aizenman E., Shah N. H. (2017). Disruption of KV2.1 somato-dendritic clusters prevents the apoptogenic increase of potassium currents. Neuroscience 354, 158–167. 10.1016/j.neuroscience.2017.04.034 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Kamijo S., Ishii Y., Horigane S. I., Suzuki K., Ohkura M., Nakai J., et al. . (2018). A critical neurodevelopmental role for L-type voltage-gated calcium channels in neurite extension and radial migration. J. Neurosci. 38, 5551–5566. 10.1523/JNEUROSCI.2357-17.2018 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Kanda H., Ling J., Tonomura S., Noguchi K., Matalon S., Gu J. G. (2019). TREK-1 and TRAAK are principal K(+) channels at the nodes of ranvier for rapid action potential conduction on mammalian myelinated afferent nerves. Neuron 104, 960–971.e967. 10.1016/j.neuron.2019.08.042 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Karim Z., Sawada A., Kawakami H., Yamamoto T., Taniguchi T. (2006). A new calcium channel antagonist, lomerizine, alleviates secondary retinal ganglion cell death after optic nerve injury in the rat. Curr. Eye Res. 31, 273–283. 10.1080/02713680500536647 [Abstract] [CrossRef] [Google Scholar]
- Kasper D., Planells-Cases R., Fuhrmann J. C., Scheel O., Zeitz O., Ruether K., et al. . (2005). Loss of the chloride channel ClC-7 leads to lysosomal storage disease and neurodegeneration. EMBO J. 24, 1079–1091. 10.1038/sj.emboj.7600576 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Kastriti M. E., Sargiannidou I., Kleopa K. A., Karagogeos D. (2015). Differential modulation of the juxtaparanodal complex in multiple sclerosis. Mol. Cell Neurosci. 67, 93–103. 10.1016/j.mcn.2015.06.005 [Abstract] [CrossRef] [Google Scholar]
- Kelley K. W., Ben Haim L., Schirmer L., Tyzack G. E., Tolman M., Miller J. G., et al. . (2018). Kir4.1-dependent astrocyte-fast motor neuron interactions are required for peak strength. Neuron 98, 306–319.e307. 10.1016/j.neuron.2018.03.010 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Kesherwani V., Agrawal S. K. (2012). Upregulation of RyR2 in hypoxic/reperfusion injury. J. Neurotrauma 29, 1255–1265. 10.1089/neu.2011.1780 [Abstract] [CrossRef] [Google Scholar]
- Kharkovets T., Hardelin J. P., Safieddine S., Schweizer M., El-Amraoui A., Petit C., et al. . (2000). KCNQ4, a K+ channel mutated in a form of dominant deafness, is expressed in the inner ear and the central auditory pathway. Proc. Natl. Acad. Sci. U.S.A. 97, 4333–4338. 10.1073/pnas.97.8.4333 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Kim G. E., Kaczmarek L. K. (2014). Emerging role of the KCNT1 Slack channel in intellectual disability. Front. Cell Neurosci. 8:209. 10.3389/fncel.2014.00209 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Kim J. W., Oh H. A., Lee S. H., Kim K. C., Eun P. H., Ko M. J., et al. . (2018). T-Type calcium channels are required to maintain viability of neural progenitor cells. Biomol. Ther. 26, 439–445. 10.4062/biomolther.2017.223 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Kim S. R., Kim S. U., Oh U., Jin B. K. (2006). Transient receptor potential vanilloid subtype 1 mediates microglial cell death in vivo and in vitro via Ca2+-mediated mitochondrial damage and cytochrome c release. J. Immunol. 177, 4322–4329. 10.4049/jimmunol.177.7.4322 [Abstract] [CrossRef] [Google Scholar]
- Kirmiz M., Palacio S., Thapa P., King A. N., Sack J. T., Trimmer J. S. (2018). Remodeling neuronal ER-PM junctions is a conserved nonconducting function of Kv2 plasma membrane ion channels. Mol. Biol. Cell 29, 2410–2432. 10.1091/mbc.E18-05-0337 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Kleefuss-Lie A., Friedl W., Cichon S., Haug K., Warnstedt M., Alekov A., et al. . (2009). CLCN2 variants in idiopathic generalized epilepsy. Nat. Genet. 41, 954–955. 10.1038/ng0909-954 [Abstract] [CrossRef] [Google Scholar]
- Klegeris A., Choi H. B., McLarnon J. G., McGeer P. L. (2007). Functional ryanodine receptors are expressed by human microglia and THP-1 cells: their possible involvement in modulation of neurotoxicity. J. Neurosci. Res. 85, 2207–2215. 10.1002/jnr.21361 [Abstract] [CrossRef] [Google Scholar]
- Kole M. H., Stuart G. J. (2012). Signal processing in the axon initial segment. Neuron 73, 235–247. 10.1016/j.neuron.2012.01.007 [Abstract] [CrossRef] [Google Scholar]
- Kong W. L., Peng Y. Y., Peng B. W. (2017). Modulation of neuroinflammation: role and therapeutic potential of TRPV1 in the neuro-immune axis. Brain. Behav. Immun. 64, 354–366. 10.1016/j.bbi.2017.03.007 [Abstract] [CrossRef] [Google Scholar]
- Konstas A. A., Korbmacher C., Tucker S. J. (2003). Identification of domains that control the heteromeric assembly of Kir5.1/Kir4.0 potassium channels. Am. J. Physiol. Cell Physiol. 284, C910–917. 10.1152/ajpcell.00479.2002 [Abstract] [CrossRef] [Google Scholar]
- Kornak U., Kasper D., Bosl M. R., Kaiser E., Schweizer M., Schulz A., et al. . (2001). Loss of the ClC-7 chloride channel leads to osteopetrosis in mice and man. Cell 104, 205–215. 10.1016/s0092-8674(01)00206-9 [Abstract] [CrossRef] [Google Scholar]
- Kuhlmann T., Ludwin S., Prat A., Antel J., Bruck W., Lassmann H. (2017). An updated histological classification system for multiple sclerosis lesions. Acta Neuropathol. 133, 13–24. 10.1007/s00401-016-1653-y [Abstract] [CrossRef] [Google Scholar]
- Kurowski P., Gawlak M., Szulczyk P. (2015). Muscarinic receptor control of pyramidal neuron membrane potential in the medial prefrontal cortex (mPFC) in rats. Neuroscience 303, 474–488. 10.1016/j.neuroscience.2015.07.023 [Abstract] [CrossRef] [Google Scholar]
- Kushnir A., Wajsberg B., Marks A. R. (2018). Ryanodine receptor dysfunction in human disorders. Biochim. Biophys. Acta. Mol. Cell Res. 1865 (11 Pt. B), 1687–1697. 10.1016/j.bbamcr.2018.07.011 [Abstract] [CrossRef] [Google Scholar]
- Lanner J. T., Georgiou D. K., Joshi A. D., Hamilton S. L. (2010). Ryanodine receptors: structure, expression, molecular details, and function in calcium release. Cold Spring Harb. Perspect. Biol. 2:a003996. 10.1101/cshperspect.a003996 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Lapato A. S., Tiwari-Woodruff S. K. (2018). Connexins and pannexins: At the junction of neuro-glial homeostasis & disease. J. Neurosci. Res. 96, 31–44. 10.1002/jnr.24088 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Larson V. A., Zhang Y., Bergles D. E. (2016). Electrophysiological properties of NG2(+) cells: Matching physiological studies with gene expression profiles. Brain Res. 1638 (Pt. B):138–160. 10.1016/j.brainres.2015.09.010 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Latour I., Hamid J., Beedle A. M., Zamponi G. W., Macvicar B. A. (2003). Expression of voltage-gated Ca2+ channel subtypes in cultured astrocytes. Glia 41, 347–353. 10.1002/glia.10162 [Abstract] [CrossRef] [Google Scholar]
- Lee S. C., Choi S., Lee T., Kim H. L., Chin H., Shin H. S. (2002). Molecular basis of R-type calcium channels in central amygdala neurons of the mouse. Proc. Natl. Accad. Sci. U.S.A. 99, 3276–3281. 10.1073/pnas.052697799 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Lee Y. J., Yum M. S., Kim M. J., Shim W. H., Yoon H. M., Yoo I. H., et al. . (2017). Large-scale structural alteration of brain in epileptic children with SCN1A mutation. Neuroimage Clin. 15, 594–600. 10.1016/j.nicl.2017.06.002 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Leithe E., Mesnil M., Aasen T. (2018). The connexin 43 C-terminus: a tail of many tales. Biochim. Biophys. Acta Biomembr. 1860, 48–64. 10.1016/j.bbamem.2017.05.008 [Abstract] [CrossRef] [Google Scholar]
- Li F., Lu J., Wu C. Y., Kaur C., Sivakumar V., Sun J., et al. . (2008). Expression of Kv1.2 in microglia and its putative roles in modulating production of proinflammatory cytokines and reactive oxygen species. J. Neurochem. 106, 2093–2105. 10.1111/j.1471-4159.2008.05559.x [Abstract] [CrossRef] [Google Scholar]
- Li L., Li J., Zuo Y., Dang D., Frost J. A., Yang Q. (2019). Activation of KCNQ channels prevents paclitaxel-induced peripheral neuropathy and associated neuropathic pain. J. Pain 20, 528–539. 10.1016/j.jpain.2018.11.001 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Li T., Wang L., Ma T., Wang S., Niu J., Li H., et al. . (2018). Dynamic calcium release from endoplasmic reticulum mediated by ryanodine receptor 3 is crucial for oligodendroglial differentiation. Front. Mol. Neurosci. 11:162. 10.3389/fnmol.2018.00162 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Li Y., Tatsui C. E., Rhines L. D., North R. Y., Harrison D. S., Cassidy R. M., et al. . (2017). Dorsal root ganglion neurons become hyperexcitable and increase expression of voltage-gated T-type calcium channels (Cav3.2) in paclitaxel-induced peripheral neuropathy. Pain 158, 417–429. 10.1097/j.pain.0000000000000774 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Li Z., Zhang S., Cao L., Li W., Ye Y. C., Shi Z. X., et al. . (2018). Tanshinone IIA and Astragaloside IV promote the angiogenesis of mesenchymal stem cell-derived endothelial cell-like cells via upregulation of Cx37, Cx40 and Cx43. Exp. Ther. Med. 15, 1847–1854. 10.3892/etm.2017.5636 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Liao B., Zhang Y., Sun H., Ma B., Qian J. (2016). Ryanodine receptor 2 plays a critical role in spinal cord injury via induction of oxidative stress. Cell Physiol. Biochem. 38, 1129–1137. 10.1159/000443063 [Abstract] [CrossRef] [Google Scholar]
- Liao Y. J., Jan Y. N., Jan L. Y. (1996). Heteromultimerization of G-protein-gated inwardly rectifying K+ channel proteins GIRK1 and GIRK2 and their altered expression in weaver brain. J. Neurosci. 16, 7137–7150. [Europe PMC free article] [Abstract] [Google Scholar]
- Lindia J. A., Abbadie C. (2003). Distribution of the voltage gated sodium channel Na(v)1.3-like immunoreactivity in the adult rat central nervous system. Brain Res. 60, 132–141. 10.1016/s0006-8993(02)03802-7 [Abstract] [CrossRef] [Google Scholar]
- Lipscombe D., Helton T. D., Xu W. (2004). L-type calcium channels: the low down. J. Neurophysiol. 92, 2633–2641. 10.1152/jn.00486.2004 [Abstract] [CrossRef] [Google Scholar]
- Lishko P. V., Mannowetz N. (2018). CatSper: a unique calcium channel of the sperm flagellum. Curr. Opin. Physiol. 2, 109–113. 10.1016/j.cophys.2018.02.004 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Liu X., Hernandez N., Kisselev S., Floratos A., Sawle A., Ionita-Laza I., et al. . (2016). Identification of candidate genes for familial early-onset essential tremor. Eur. J. Hum. Genet. 24, 1009–1015. 10.1038/ejhg.2015.228 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Locovei S., Scemes E., Qiu F., Spray D. C., Dahl G. (2007). Pannexin1 is part of the pore forming unit of the P2X(7) receptor death complex. FEBS Lett. 581, 483–488. 10.1016/j.febslet.2006.12.056 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Lory P., Nicole S., Monteil A. (2020). Neuronal Cav3 channelopathies: recent progress and perspectives. Pflugers Arch. 472, 831–844. 10.1007/s00424-020-02429-7 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Ludwig J., Weseloh R., Karschin C., Liu Q., Netzer R., Engeland B., et al. . (2000). Cloning and functional expression of rat eag2, a new member of the ether-a-go-go family of potassium channels and comparison of its distribution with that of eag1. Mol. Cell Neurosci. 16, 59–70. 10.1006/mcne.2000.0851 [Abstract] [CrossRef] [Google Scholar]
- Lugaresi A. (2015). Pharmacology and clinical efficacy of dalfampridine for treating multiple sclerosis. Expert. Opin. Drug Metab. Toxicol. 11, 295–306. 10.1517/17425255.2015.993315 [Abstract] [CrossRef] [Google Scholar]
- Luscher C., Slesinger P. A. (2010). Emerging roles for G protein-gated inwardly rectifying potassium (GIRK) channels in health and disease. Nat. Rev. Neurosci. 11, 301–315. 10.1038/nrn2834 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Lutz S. E., Gonzalez-Fernandez E., Ventura J. C., Perez-Samartin A., Tarassishin L., Negoro H., et al. . (2013). Contribution of pannexin1 to experimental autoimmune encephalomyelitis. PLoS ONE 8:e66657. 10.1371/journal.pone.0066657 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Ma W., Compan V., Zheng W., Martin E., North R. A., Verkhratsky A., et al. . (2012). Pannexin 1 forms an anion-selective channel. Pflugers Arch. 463, 585–592. 10.1007/s00424-012-1077-z [Abstract] [CrossRef] [Google Scholar]
- Majumdar A., Capetillo-Zarate E., Cruz D., Gouras G. K., Maxfield F. R. (2011). Degradation of Alzheimer's amyloid fibrils by microglia requires delivery of ClC-7 to lysosomes. Mol. Biol. Cell 22, 1664–1676. 10.1091/mbc.E10-09-0745 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Malko P., Syed Mortadza S. A., McWilliam J., Jiang L. H. (2019). TRPM2 channel in microglia as a new player in neuroinflammation associated with a spectrum of central nervous system pathologies. Front. Pharmacol. 10:239. 10.3389/fphar.2019.00239 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Marques S., Zeisel A., Codeluppi S., van Bruggen D., Mendanha Falcao A., Xiao L., et al. . (2016). Oligodendrocyte heterogeneity in the mouse juvenile and adult central nervous system. Science 352, 1326–1329. 10.1126/science.aaf6463 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Marrone M. C., Morabito A., Giustizieri M., Chiurchiu V., Leuti A., Mattioli M., et al. . (2017). TRPV1 channels are critical brain inflammation detectors and neuropathic pain biomarkers in mice. Nat. Commun. 8:15292. 10.1038/ncomms15292 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Martin N. A., Nawrocki A., Molnar V., Elkjaer M. L., Thygesen E. K., Palkovits M., et al. . (2018). Orthologous proteins of experimental de- and remyelination are differentially regulated in the CSF proteome of multiple sclerosis subtypes. PLoS ONE 13:e0202530. 10.1371/journal.pone.0202530 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Martinez A., Santiago J. L., Cenit M. C., de Las Heras V., de la Calle H., Fernandez-Arquero M., et al. . (2008). IFIH1-GCA-KCNH7 locus: influence on multiple sclerosis risk. Eur. J. Hum. Genet. 16, 861–864. 10.1038/ejhg.2008.16 [Abstract] [CrossRef] [Google Scholar]
- Matyash M., Matyash V., Nolte C., Sorrentino V., Kettenmann H. (2002). Requirement of functional ryanodine receptor type 3 for astrocyte migration. FASEB J 16, 84–86. 10.1096/fj.01-0380fje [Abstract] [CrossRef] [Google Scholar]
- Mayfield J., Blednov Y. A., Harris R. A. (2015). Behavioral and genetic evidence for girk channels in the cns: role in physiology, pathophysiology, and drug addiction. Int. Rev. Neurobiol. 123, 279–313. 10.1016/bs.irn.2015.05.016 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- McDonald W. I., Sears T. A. (1969). Effect of demyelination on conduction in the central nervous system. Nature. 221, 182–183. 10.1038/221182a0 [Abstract] [CrossRef] [Google Scholar]
- McKay B. E., McRory J. E., Molineux M. L., Hamid J., Snutch T. P., Zamponi G. W., et al. . (2006). Ca(V)3 T-type calcium channel isoforms differentially distribute to somatic and dendritic compartments in rat central neurons. Eur. J. Neurosci. 24, 2581–2594. 10.1111/j.1460-9568.2006.05136.x [Abstract] [CrossRef] [Google Scholar]
- McKinney B. C., Sze W., Lee B., Murphy G. G. (2009). Impaired long-term potentiation and enhanced neuronal excitability in the amygdala of Ca(V)1.3 knockout mice. Neurobiol. Learn. Mem. 92, 519–528. 10.1016/j.nlm.2009.06.012 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- McPherson P. S., Campbell K. P. (1990). Solubilization and biochemical characterization of the high affinity [3H]ryanodine receptor from rabbit brain membranes. J. Biol. Chem. 265, 18454–18460. [Abstract] [Google Scholar]
- McTague A., Appleton R., Avula S., Cross J. H., King M. D., Jacques T. S., et al. . (2013). Migrating partial seizures of infancy: expansion of the electroclinical, radiological and pathological disease spectrum. Brain 136 (Pt. 5), 1578–1591. 10.1093/brain/awt073 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Meisler M. H. (2019). SCN8A encephalopathy: mechanisms and models. Epilepsia 60 (Suppl. 3), S86–S91. 10.1111/epi.14703 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Melzer N., Hicking G., Gobel K., Wiendl H. (2012). TRPM2 cation channels modulate T cell effector functions and contribute to autoimmune CNS inflammation. PLoS ONE 7:e47617. 10.1371/journal.pone.0047617 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Miceli F., Soldovieri M. V., Ambrosino P., Barrese V., Migliore M., Cilio M. R., et al. . (2013). Genotype-phenotype correlations in neonatal epilepsies caused by mutations in the voltage sensor of K(v)7.2 potassium channel subunits. Proc. Natl. Acad. Sci. U.S.A. 110, 4386–4391. 10.1073/pnas.1216867110 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Miceli F., Soldovieri M. V., Martire M., Taglialatela M. (2008). Molecular pharmacology and therapeutic potential of neuronal Kv7-modulating drugs. Curr. Opin. Pharmacol. 8, 65–74. 10.1016/j.coph.2007.10.003 [Abstract] [CrossRef] [Google Scholar]
- Micu I., Plemel J. R., Lachance C., Proft J., Jansen A. J., Cummins K., et al. . (2016). The molecular physiology of the axo-myelinic synapse. Exp. Neurol. 276, 41–50. 10.1016/j.expneurol.2015.10.006 [Abstract] [CrossRef] [Google Scholar]
- Miyake T., Shirakawa H., Nakagawa T., Kaneko S. (2015). Activation of mitochondrial transient receptor potential vanilloid 1 channel contributes to microglial migration. Glia 63, 1870–1882. 10.1002/glia.22854 [Abstract] [CrossRef] [Google Scholar]
- Mochida S. (2019). Presynaptic calcium channels. Int. J. Mol. Sci. 20:2217. 10.3390/ijms20092217 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Mori F., Ribolsi M., Kusayanagi H., Monteleone F., Mantovani V., Buttari F., et al. . (2012). TRPV1 channels regulate cortical excitability in humans. J. Neurosci. 32, 873–879. 10.1523/JNEUROSCI.2531-11.2012 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Mulkey D. K., Wenker I. C. (2011). Astrocyte chemoreceptors: mechanisms of H+ sensing by astrocytes in the retrotrapezoid nucleus and their possible contribution to respiratory drive. Exp. Physiol. 96, 400–406. 10.1113/expphysiol.2010.053140 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Musumeci G., Grasselli G., Rossi S., De Chiara V., Musella A., Motta C., et al. . (2011). Transient receptor potential vanilloid 1 channels modulate the synaptic effects of TNF-alpha and of IL-1beta in experimental autoimmune encephalomyelitis. Neurobiol. Dis. 43, 669–677. 10.1016/j.nbd.2011.05.018 [Abstract] [CrossRef] [Google Scholar]
- Nagamine K., Kudoh J., Minoshima S., Kawasaki K., Asakawa S., Ito F., et al. . (1998). Molecular cloning of a novel putative Ca2+ channel protein (TRPC7) highly expressed in brain. Genomics 54, 124–131. 10.1006/geno.1998.5551 [Abstract] [CrossRef] [Google Scholar]
- Nagy B., Hovhannisyan A., Barzan R., Chen T. J., Kukley M. (2017). Different patterns of neuronal activity trigger distinct responses of oligodendrocyte precursor cells in the corpus callosum. PLoS Biol. 15:e2001993. 10.1371/journal.pbio.2001993 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Namadurai S., Yereddi N. R., Cusdin F. S., Huang C. L., Chirgadze D. Y., Jackson A. P. (2015). A new look at sodium channel beta subunits. Open Biol. 5:140192. 10.1098/rsob.140192 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Naziroglu M., Luckhoff A. (2008). A calcium influx pathway regulated separately by oxidative stress and ADP-Ribose in TRPM2 channels: single channel events. Neurochem. Res. 33, 1256–1262. 10.1007/s11064-007-9577-5 [Abstract] [CrossRef] [Google Scholar]
- Nerbonne J. M., Gerber B. R., Norris A., Burkhalter A. (2008). Electrical remodelling maintains firing properties in cortical pyramidal neurons lacking KCND2-encoded A-type K+ currents. J. Physiol. 586, 1565–1579. 10.1113/jphysiol.2007.146597 [Abstract] [CrossRef] [Google Scholar]
- Neusch C., Rozengurt N., Jacobs R. E., Lester H. A., Kofuji P. (2001). Kir4.1 potassium channel subunit is crucial for oligodendrocyte development and in vivo myelination. J Neurosci 21, 5429–5438 [Europe PMC free article] [Abstract] [Google Scholar]
- Nicoletti N. F., Erig T. C., Zanin R. F., Roxo M. R., Ferreira N. P., Gomez M. V., et al. . (2017). Pre-clinical evaluation of voltage-gated calcium channel blockers derived from the spider P. nigriventer in glioma progression. Toxicon 129, 58–67. 10.1016/j.toxicon.2017.02.001 [Abstract] [CrossRef] [Google Scholar]
- Nijenhuis T., Hoenderop J. G., van der Kemp A. W., Bindels R. J. (2003). Localization and regulation of the epithelial Ca2+ channel TRPV6 in the kidney. J. Am. Soc. Nephrol. 14, 2731–2740. 10.1097/01.asn.0000094081.78893.e8 [Abstract] [CrossRef] [Google Scholar]
- Nilius B., Owsianik G. (2011). The transient receptor potential family of ion channels. Genome Biol. 12:218. 10.1186/gb-2011-12-3-218 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Niu J., Li T., Yi C., Huang N., Koulakoff A., Weng C., et al. . (2016). Connexin-based channels contribute to metabolic pathways in the oligodendroglial lineage. J. Cell Sci. 129, 1902–1914. 10.1242/jcs.178731 [Abstract] [CrossRef] [Google Scholar]
- Nodera H., Spieker A., Sung M., Rutkove S. (2011). Neuroprotective effects of Kv7 channel agonist, retigabine, for cisplatin-induced peripheral neuropathy. Neurosci. Lett. 505, 223–227. 10.1016/j.neulet.2011.09.013 [Abstract] [CrossRef] [Google Scholar]
- Obermair G. J., Szabo Z., Bourinet E., Flucher B. E. (2004). Differential targeting of the L-type Ca2+ channel alpha 1C (CaV1.2) to synaptic and extrasynaptic compartments in hippocampal neurons. Eur. J. Neurosci. 19, 2109–2122. 10.1111/j.0953-816X.2004.03272.x [Abstract] [CrossRef] [Google Scholar]
- O'Brien J. E., Meisler M. H. (2013). Sodium channel SCN8A (Nav1.6): properties and de novo mutations in epileptic encephalopathy and intellectual disability. Front. Genet. 4, 213. 10.3389/fgene.2013.00213 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Ogiwara I., Miyamoto H., Morita N., Atapour N., Mazaki E., Inoue I., et al. . (2007). Nav1.1 localizes to axons of parvalbumin-positive inhibitory interneurons: a circuit basis for epileptic seizures in mice carrying an Scn1a gene mutation. J. Neurosci. 27, 5903–5914. 10.1523/JNEUROSCI.5270-06.2007 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Olah M. E., Jackson M. F., Li H., Perez Y., Sun H. S., Kiyonaka S., et al. . (2009). Ca2+-dependent induction of TRPM2 currents in hippocampal neurons. J. Physiol. 587 (Pt. 5), 965–979. 10.1113/jphysiol.2008.162289 [Abstract] [CrossRef] [Google Scholar]
- O'Malley H. A., Shreiner A. B., Chen G. H., Huffnagle G. B., Isom L. L. (2009). Loss of Na+ channel beta2 subunits is neuroprotective in a mouse model of multiple sclerosis. Mol. Cell Neurosci. 40, 143–155. 10.1016/j.mcn.2008.10.001 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Orellana J. A., Froger N., Ezan P., Jiang J. X., Bennett M. V., Naus C. C., et al. . (2011). ATP and glutamate released via astroglial connexin 43 hemichannels mediate neuronal death through activation of pannexin 1 hemichannels. J. Neurochem. 118, 826–840. 10.1111/j.1471-4159.2011.07210.x [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Orellana J. A., Montero T. D., von Bernhardi R. (2013). Astrocytes inhibit nitric oxide-dependent Ca(2+) dynamics in activated microglia: involvement of ATP released via pannexin 1 channels. Glia 61, 2023–2037. 10.1002/glia.22573 [Abstract] [CrossRef] [Google Scholar]
- Orem B. C., Pelisch N., Williams J., Nally J. M., Stirling D. P. (2017). Intracellular calcium release through IP3R or RyR contributes to secondary axonal degeneration. Neurobiol. Dis. 106, 235–243. 10.1016/j.nbd.2017.07.011 [Abstract] [CrossRef] [Google Scholar]
- Ortner N. J., Striessnig J. (2016). L-type calcium channels as drug targets in CNS disorders. Channels 10, 7–13. 10.1080/19336950.2015.1048936 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Osorio N., Alcaraz G., Padilla F., Couraud F., Delmas P., Crest M. (2005). Differential targeting and functional specialization of sodium channels in cultured cerebellar granule cells. J. Physiol. 569 (Pt. 3), 801–816. 10.1113/jphysiol.2005.097022 [Abstract] [CrossRef] [Google Scholar]
- Ouardouz M., Nikolaeva M. A., Coderre E., Zamponi G. W., McRory J. E., Trapp B. D., et al. . (2003). Depolarization-induced Ca2+ release in ischemic spinal cord white matter involves L-type Ca2+ channel activation of ryanodine receptors. Neuron 40, 53–63. 10.1016/j.neuron.2003.08.016 [Abstract] [CrossRef] [Google Scholar]
- Ovsepian S. V., LeBerre M., Steuber V., O'Leary V. B., Leibold C., Oliver Dolly J. (2016). Distinctive role of KV1.1 subunit in the biology and functions of low threshold K(+) channels with implications for neurological disease. Pharmacol. Ther. 159, 93–101. 10.1016/j.pharmthera.2016.01.005 [Abstract] [CrossRef] [Google Scholar]
- Paez P. M., Cheli V. T., Ghiani C. A., Spreuer V., Handley V. W., Campagnoni A. T. (2012). Golli myelin basic proteins stimulate oligodendrocyte progenitor cell proliferation and differentiation in remyelinating adult mouse brain. Glia 60, 1078–1093. 10.1002/glia.22336 [Abstract] [CrossRef] [Google Scholar]
- Paez P. M., Fulton D., Colwell C. S., Campagnoni A. T. (2009). Voltage-operated Ca(2+) and Na(+) channels in the oligodendrocyte lineage. J. Neurosci. Res. 87, 3259–3266. 10.1002/jnr.21938 [Abstract] [CrossRef] [Google Scholar]
- Paez P. M., Lyons D. A. (2020). Calcium signaling in the oligodendrocyte lineage: regulators and consequences. Annu. Rev. Neurosci. 43, 163–186. 10.1146/annurev-neuro-100719-093305 [Abstract] [CrossRef] [Google Scholar]
- Paltser G., Liu X. J., Yantha J., Winer S., Tsui H., Wu P., et al. . (2013). TRPV1 gates tissue access and sustains pathogenicity in autoimmune encephalitis. Mol. Med. 19, 149–159. 10.2119/molmed.2012.00329 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Pan Z., Kao T., Horvath Z., Lemos J., Sul J. Y., Cranstoun S. D., et al. . (2006). A common ankyrin-G-based mechanism retains KCNQ and NaV channels at electrically active domains of the axon. J. Neurosci. 26, 2599–2613. 10.1523/JNEUROSCI.4314-05.2006 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Papa M., Boscia F., Canitano A., Castaldo P., Sellitti S., Annunziato L., et al. . (2003). Expression pattern of the ether-a-gogo-related (ERG) K+ channel-encoding genes ERG1, ERG2, and ERG3 in the adult rat central nervous system. J Comp Neurol 466, 119–135. 10.1002/cne.10886 [Abstract] [CrossRef] [Google Scholar]
- Papanikolaou M., Butt A. M., Lewis A. (2020). A critical role for the inward rectifying potassium channel Kir7.1 in oligodendrocytes of the mouse optic nerve. Brain Struct. Funct. 225, 925–934. 10.1007/s00429-020-02043-4 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Pappalardo L. W., Black J. A., Waxman S. G. (2016). Sodium channels in astroglia and microglia. Glia 64, 1628–1645. 10.1002/glia.22967 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Parajuli L. K., Nakajima C., Kulik A., Matsui K., Schneider T., Shigemoto R., et al. . (2012). Quantitative regional and ultrastructural localization of the Ca(v)2.3 subunit of R-type calcium channel in mouse brain. J. Neurosci. 32, 13555–13567. 10.1523/JNEUROSCI.1142-12.2012 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Pelegrin P., Barroso-Gutierrez C., Surprenant A. (2008). P2X7 receptor differentially couples to distinct release pathways for IL-1beta in mouse macrophage. J. Immunol. 180, 7147–7157. 10.4049/jimmunol.180.11.7147 [Abstract] [CrossRef] [Google Scholar]
- Pelisch N., Gomes C., Nally J. M., Petruska J. C., Stirling D. P. (2017). Differential expression of ryanodine receptor isoforms after spinal cord injury. Neurosci. Lett. 660, 51–56. 10.1016/j.neulet.2017.09.018 [Abstract] [CrossRef] [Google Scholar]
- Perez-Reyes E. (2003). Molecular physiology of low-voltage-activated t-type calcium channels. Physiol. Rev. 83, 117–161. 10.1152/physrev.00018.2002 [Abstract] [CrossRef] [Google Scholar]
- Pessia M., Imbrici P., D'Adamo M. C., Salvatore L., Tucker S. J. (2001). Differential pH sensitivity of Kir4.1 and Kir4.2 potassium channels and their modulation by heteropolymerisation with Kir5.1. J. Physiol. 532 (Pt. 2), 359–367. 10.1111/j.1469-7793.2001.0359f.x [Abstract] [CrossRef] [Google Scholar]
- Pietrobon D. (2010). CaV2.1 channelopathies. Pflugers Arch. 460, 375–393. 10.1007/s00424-010-0802-8 [Abstract] [CrossRef] [Google Scholar]
- Pitman K. A., Ricci R., Gasperini R., Beasley S., Pavez M., Charlesworth J., et al. . (2020). The voltage-gated calcium channel CaV1.2 promotes adult oligodendrocyte progenitor cell survival in the mouse corpus callosum but not motor cortex. Glia 68, 376–392. 10.1002/glia.23723 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Poet M., Kornak U., Schweizer M., Zdebik A. A., Scheel O., Hoelter S., et al. . (2006). Lysosomal storage disease upon disruption of the neuronal chloride transport protein ClC-6. Proc. Natl. Acad. Sci. U.S.A. 103, 13854–13859. 10.1073/pnas.0606137103 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Poopalasundaram S., Knott C., Shamotienko O. G., Foran P. G., Dolly J. O., Ghiani C. A., et al. . (2000). Glial heterogeneity in expression of the inwardly rectifying K(+) channel, Kir4.1, in adult rat CNS. Glia 30, 362–372. 10.1002/(sici)1098-1136(200006)30:4<362::aid-glia50>3.0.co;2-4 [Abstract] [CrossRef] [Google Scholar]
- Prasad A., Teh D. B. L., Blasiak A., Chai C., Wu Y., Gharibani P. M., et al. . (2017). Static magnetic field stimulation enhances oligodendrocyte differentiation and secretion of neurotrophic factors. Sci. Rep. 7:6743. 10.1038/s41598-017-06331-8 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Pressey S. N., O'Donnell K. J., Stauber T., Fuhrmann J. C., Tyynela J., Jentsch T. J., et al. . (2010). Distinct neuropathologic phenotypes after disrupting the chloride transport proteins ClC-6 or ClC-7/Ostm1. J. Neuropathol. Exp. Neurol. 69, 1228–1246. 10.1097/NEN.0b013e3181ffe742 [Abstract] [CrossRef] [Google Scholar]
- Qi H., Moran M. M., Navarro B., Chong J. A., Krapivinsky G., Krapivinsky L., et al. . (2007). All four CatSper ion channel proteins are required for male fertility and sperm cell hyperactivated motility. Proc. Natl. Acad. Sci. U.S.A. 104, 1219–1223. 10.1073/pnas.0610286104 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Raap M., Biedermann B., Braun P., Milenkovic I., Skatchkov S. N., Bringmann A., et al. . (2002). Diversity of Kir channel subunit mRNA expressed by retinal glial cells of the guinea-pig. Neuroreport 13, 1037–1040. 10.1097/00001756-200206120-00012 [Abstract] [CrossRef] [Google Scholar]
- Rajakulendran S., Kaski D., Hanna M. G. (2012). Neuronal P/Q-type calcium channel dysfunction in inherited disorders of the CNS. Nat. Rev. Neurol. 8, 86–96. 10.1038/nrneurol.2011.228 [Abstract] [CrossRef] [Google Scholar]
- Rasband M. N., Kagawa T., Park E. W., Ikenaka K., Trimmer J. S. (2003). Dysregulation of axonal sodium channel isoforms after adult-onset chronic demyelination. J. Neurosci. Res. 73, 465–470. 10.1002/jnr.10675 [Abstract] [CrossRef] [Google Scholar]
- Rasmussen H. B., Trimmer J. S. (2019). The Voltage-Dependent K+ Channel Family. Oxford: Oxford University Press. [Google Scholar]
- Reese K. A., Caldwell J. H. (1999). Immunocytochemical localization of NaCh6 in cultured spinal cord astrocytes. Glia 26, 92–96. 10.1002/(sici)1098-1136(199903)26:1<92::aid-glia10>3.0.co;2-4 [Abstract] [CrossRef] [Google Scholar]
- Rettig J., Sheng Z. H., Kim D. K., Hodson C. D., Snutch T. P., Catterall W. A. (1996). Isoform-specific interaction of the alpha1A subunits of brain Ca2+ channels with the presynaptic proteins syntaxin and SNAP-25. Proc. Natl. Acad. Sci. U.S.A. 93, 7363–7368. 10.1073/pnas.93.14.7363 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Riccio A., Medhurst A. D., Mattei C., Kelsell R. E., Calver A. R., Randall A. D., et al. . (2002). mRNA distribution analysis of human TRPC family in CNS and peripheral tissues. Brain Res. Mol. Brain Res. 109, 95–104. 10.1016/s0169-328x(02)00527-2 [Abstract] [CrossRef] [Google Scholar]
- Rivat C., Sar C., Mechaly I., Leyris J. P., Diouloufet L., Sonrier C., et al. . (2018). Inhibition of neuronal FLT3 receptor tyrosine kinase alleviates peripheral neuropathic pain in mice. Nat Commun 9, 1042. 10.1038/s41467-018-03496-2 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Rivera-Pagan A. F., Rivera-Aponte D. E., Melnik-Martinez K. V., Zayas-Santiago A., Kucheryavykh L. Y., Martins A. H., et al. . (2015). Up-regulation of TREK-2 potassium channels in cultured astrocytes requires de novo protein synthesis: relevance to localization of TREK-2 channels in astrocytes after transient cerebral ischemia. PLoS ONE. 10:e0125195. 10.1371/journal.pone.0125195 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Rizzi S., Knaus H. G., Schwarzer C. (2016). Differential distribution of the sodium-activated potassium channels slick and slack in mouse brain. J. Comp. Neurol. 524, 2093–2116. 10.1002/cne.23934 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Rommer P. S., Milo R., Han M. H., Satyanarayan S., Sellner J., Hauer L., et al. . (2019). Immunological aspects of approved MS therapeutics. Front. Immunol. 10:1564. 10.3389/fimmu.2019.01564 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Rui Y., Pollitt S. L., Myers K. R., Feng Y., Zheng J. Q. (2020). Spontaneous local calcium transients regulate oligodendrocyte development in culture through store-operated Ca(2+) entry and release. eNeuro 7. 10.1523/ENEURO.0347-19.2020 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Ruiz A., Matute C., Alberdi E. (2010). Intracellular Ca2+ release through ryanodine receptors contributes to AMPA receptor-mediated mitochondrial dysfunction and ER stress in oligodendrocytes. Cell Death Dis. 1:e54. 10.1038/cddis.2010.31 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Sadovnick A. D., Traboulsee A. L., Zhao Y., Bernales C. Q., Encarnacion M., Ross J. P., et al. . (2017). Genetic modifiers of multiple sclerosis progression, severity and onset. Clin. Immunol. 180, 100–105. 10.1016/j.clim.2017.05.009 [Abstract] [CrossRef] [Google Scholar]
- Saegusa H., Kurihara T., Zong S., Minowa O., Kazuno A., Han W., et al. . (2000). Altered pain responses in mice lacking alpha 1E subunit of the voltage-dependent Ca2+ channel. Proc. Natl. Acad. Sci. U.S.A. 97, 6132–6137. 10.1073/pnas.100124197 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Saez J. C., Berthoud V. M., Branes M. C., Martinez A. D., Beyer E. C. (2003). Plasma membrane channels formed by connexins: their regulation and functions. Physiol. Rev. 83, 1359–1400. 10.1152/physrev.00007.2003 [Abstract] [CrossRef] [Google Scholar]
- Sahu G., Sukumaran S., Bera A. K. (2014). Pannexins form gap junctions with electrophysiological and pharmacological properties distinct from connexins. Sci. Rep. 4:4955. 10.1038/srep04955 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Salinas M., de Weille J., Guillemare E., Lazdunski M., Hugnot J. P. (1997a). Modes of regulation of shab K+ channel activity by the Kv8.1 subunit. J. Biol. Chem. 272, 8774–8780. 10.1074/jbc.272.13.8774 [Abstract] [CrossRef] [Google Scholar]
- Salinas M., Duprat F., Heurteaux C., Hugnot J. P., Lazdunski M. (1997b). New modulatory alpha subunits for mammalian Shab K+ channels. J. Biol. Chem. 272, 24371–24379. 10.1074/jbc.272.39.24371 [Abstract] [CrossRef] [Google Scholar]
- Santiago Gonzalez D. A., Cheli V. T., Zamora N. N., Lama T. N., Spreuer V., Murphy G. G., et al. . (2017). Conditional deletion of the L-type calcium channel Cav1.2 in NG2-positive cells impairs remyelination in mice. J. Neurosci. 37, 10038–10051. 10.1523/JNEUROSCI.1787-17.2017 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Sappington R. M., Calkins D. J. (2008). Contribution of TRPV1 to microglia-derived IL-6 and NFkappaB translocation with elevated hydrostatic pressure. Invest Ophthalmol. Vis. Sci. 49, 3004–3017. 10.1167/iovs.07-1355 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Schampel A., Volovitch O., Koeniger T., Scholz C. J., Jorg S., Linker R. A., et al. . (2017). Nimodipine fosters remyelination in a mouse model of multiple sclerosis and induces microglia-specific apoptosis. Proc. Natl. Acad. Sci. U.S.A. 114, E3295–E3304. 10.1073/pnas.1620052114 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Schattling B., Eggert B., Friese M. A. (2014). Acquired channelopathies as contributors to development and progression of multiple sclerosis. Exp. Neurol. 262 (Pt. A), 28–36. 10.1016/j.expneurol.2013.12.006 [Abstract] [CrossRef] [Google Scholar]
- Schattling B., Fazeli W., Engeland B., Liu Y., Lerche H., Isbrandt D., et al. . (2016). Activity of NaV1.2 promotes neurodegeneration in an animal model of multiple sclerosis. JCI Insight 1:e89810. 10.1172/jci.insight.89810 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Scheffer I. E., Nabbout R. (2019). SCN1A-related phenotypes: epilepsy and beyond. Epilepsia 60 (Suppl. 3), S17–S24. 10.1111/epi.16386 [Abstract] [CrossRef] [Google Scholar]
- Schilling T., Eder C. (2009). Importance of the non-selective cation channel TRPV1 for microglial reactive oxygen species generation. J. Neuroimmunol. 216, 118–121. 10.1016/j.jneuroim.2009.07.008 [Abstract] [CrossRef] [Google Scholar]
- Schirmer L., Mobius W., Zhao C., Cruz-Herranz A., Ben Haim L., Cordano C., et al. . (2018). Oligodendrocyte-encoded Kir4.1 function is required for axonal integrity. Elife 7:e36428. 10.7554/eLife.36428 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Schirmer L., Srivastava R., Kalluri S. R., Bottinger S., Herwerth M., Carassiti D., et al. . (2014). Differential loss of KIR4.1 immunoreactivity in multiple sclerosis lesions. Ann. Neurol. 75, 810–828. 10.1002/ana.24168 [Abstract] [CrossRef] [Google Scholar]
- Schirmer L., Velmeshev D., Holmqvist S., Kaufmann M., Werneburg S., Jung D., et al. . (2019). Neuronal vulnerability and multilineage diversity in multiple sclerosis. Nature 573, 75–82. 10.1038/s41586-019-1404-z [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Schlichter L. C., Kaushal V., Moxon-Emre I., Sivagnanam V., Vincent C. (2010). The Ca2+ activated SK3 channel is expressed in microglia in the rat striatum and contributes to microglia-mediated neurotoxicity in vitro. J. Neuroinflammation 7:4. 10.1186/1742-2094-7-4 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Schmidt K., Eulitz D., Veh R. W., Kettenmann H., Kirchhoff F. (1999). Heterogeneous expression of voltage-gated potassium channels of the shaker family (Kv1) in oligodendrocyte progenitors. Brain Res. 843, 145–160. 10.1016/s0006-8993(99)01938-1 [Abstract] [CrossRef] [Google Scholar]
- Schrey M., Codina C., Kraft R., Beetz C., Kalff R., Wolfl S., et al. . (2002). Molecular characterization of voltage-gated sodium channels in human gliomas. Neuroreport 13, 2493–2498. 10.1097/00001756-200212200-00023 [Abstract] [CrossRef] [Google Scholar]
- Schulien A. J., Yeh C. Y., Orange B. N., Pav O. J., Hopkins M. P., Moutal A., et al. . (2020). Targeted disruption of Kv2.1-VAPA association provides neuroprotection against ischemic stroke in mice by declustering Kv2.1 channels. Sci. Adv. 6:eaaz8110. 10.1126/sciadv.aaz8110 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Sedmak G., Judas M. (2021). White matter interstitial neurons in the adult human brain: 3% of cortical neurons in quest for recognition. Cells 10:190. 10.3390/cells10010190 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Sesti F., Wu X., Liu S. (2014). Oxidation of KCNB1 K(+) channels in central nervous system and beyond. World J. Biol. Chem. 5, 85–92. 10.4331/wjbc.v5.i2.85 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Shang K. W., Zhang Y. H., Yang X. L., Liu A. J., Yang Z. X., Liu X. Y., et al. . (2016). [Clinical features and gene mutations in epilepsy of infancy with migrating focal seizures]. Zhonghua Er Ke Za Zhi 54, 735–739. 10.3760/cma.j.issn.0578-1310.2016.10.005 [Abstract] [CrossRef] [Google Scholar]
- Simpson P. B., Holtzclaw L. A., Langley D. B., Russell J. T. (1998). Characterization of ryanodine receptors in oligodendrocytes, type 2 astrocytes, and O-2A progenitors. J Neurosci. Res. 52, 468–482. 10.1002/(SICI)1097-4547(19980515)52:4<468::AID-JNR11>3.0.CO;2-# [Abstract] [CrossRef] [Google Scholar]
- Sinha K., Karimi-Abdolrezaee S., Velumian A. A., Fehlings M. G. (2006). Functional changes in genetically dysmyelinated spinal cord axons of shiverer mice: role of juxtaparanodal Kv1 family K+ channels. J. Neurophysiol. 95, 1683–1695. 10.1152/jn.00899.2005 [Abstract] [CrossRef] [Google Scholar]
- Sita G., Hrelia P., Graziosi A., Ravegnini G., Morroni F. (2018). TRPM2 in the brain: role in health and disease. Cells 7:82. 10.3390/cells7070082 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Siwek M. E., Muller R., Henseler C., Broich K., Papazoglou A., Weiergraber M. (2014). The CaV2.3 R-type voltage-gated Ca2+ channel in mouse sleep architecture. Sleep 37, 881–892. 10.5665/sleep.3652 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Smart S. L., Bosma M. M., Tempel B. L. (1997). Identification of the delayed rectifier potassium channel, Kv1.6, in cultured astrocytes. Glia 20, 127–134. 10.1002/(sici)1098-1136(199706)20:2<127::aid-glia4>3.0.co;2-6 [Abstract] [CrossRef] [Google Scholar]
- Smith R. S., Kenny C. J., Ganesh V., Jang A., Borges-Monroy R., Partlow J. N., et al. . (2018). Sodium channel SCN3A (NaV1.3) regulation of human cerebral cortical folding and oral motor development. Neuron 99, 905–913.e907. 10.1016/j.neuron.2018.07.052 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- So K., Haraguchi K., Asakura K., Isami K., Sakimoto S., Shirakawa H., et al. . (2015). Involvement of TRPM2 in a wide range of inflammatory and neuropathic pain mouse models. J. Pharmacol. Sci. 127, 237–243. 10.1016/j.jphs.2014.10.003 [Abstract] [CrossRef] [Google Scholar]
- Soldovieri M. V., Miceli F., Taglialatela M. (2011). Driving with no brakes: molecular pathophysiology of Kv7 potassium channels. Physiology 26, 365–376. 10.1152/physiol.00009.2011 [Abstract] [CrossRef] [Google Scholar]
- Sosinsky G. E., Boassa D., Dermietzel R., Duffy H. S., Laird D. W., MacVicar B., et al. . (2011). Pannexin channels are not gap junction hemichannels. Channels 5, 193–197. 10.4161/chan.5.3.15765 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Stampanoni Bassi M., Gentile A., Iezzi E., Zagaglia S., Musella A., Simonelli I., et al. . (2019). Transient receptor potential vanilloid 1 modulates central inflammation in multiple sclerosis. Front. Neurol. 10:30. 10.3389/fneur.2019.00030 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Stanika R., Campiglio M., Pinggera A., Lee A., Striessnig J., Flucher B. E., et al. . (2016). Splice variants of the CaV1.3 L-type calcium channel regulate dendritic spine morphology. Sci. Rep. 6:34528. 10.1038/srep34528 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Stirling D. P., Cummins K., Wayne Chen S. R., Stys P. (2014). Axoplasmic reticulum Ca(2+) release causes secondary degeneration of spinal axons. Ann. Neurol. 75, 220–229. 10.1002/ana.24099 [Abstract] [CrossRef] [Google Scholar]
- Stirling D. P., Stys P. K. (2010). Mechanisms of axonal injury: internodal nanocomplexes and calcium deregulation. Trends. Mol. Med. 16, 160–170. 10.1016/j.molmed.2010.02.002 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Stocker M. (2004). Ca(2+)-activated K+ channels: molecular determinants and function of the SK family. Nat. Rev. Neurosci. 5, 758–770. 10.1038/nrn1516 [Abstract] [CrossRef] [Google Scholar]
- Striessnig J., Pinggera A., Kaur G., Bock G., Tuluc P. (2014). L-type Ca(2+) channels in heart and brain. Wiley Interdiscip. Rev. Membr. Transp. Signal 3, 15–38. 10.1002/wmts.102 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Subramanian N., Wetzel A., Dombert B., Yadav P., Havlicek S., Jablonka S., et al. . (2012). Role of Na(v)1.9 in activity-dependent axon growth in motoneurons. Hum. Mol. Genet. 21, 3655–3667. 10.1093/hmg/dds195 [Abstract] [CrossRef] [Google Scholar]
- Sukiasyan N., Hultborn H., Zhang M. (2009). Distribution of calcium channel Ca(V)1.3 immunoreactivity in the rat spinal cord and brain stem. Neuroscience 159, 217–235. 10.1016/j.neuroscience.2008.12.011 [Abstract] [CrossRef] [Google Scholar]
- Surmeier D. J., Mermelstein P. G., Goldowitz D. (1996). The weaver mutation of GIRK2 results in a loss of inwardly rectifying K+ current in cerebellar granule cells. Proc. Natl. Acad. Sci. U.S.A. 93, 11191–11195. 10.1073/pnas.93.20.11191 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Suzuki S., Rogawski M. A. (1989). T-type calcium channels mediate the transition between tonic and phasic firing in thalamic neurons. Proc. Natl. Acad. Sci. U.S.A. 86, 7228–7232. 10.1073/pnas.86.18.7228 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Swayne L. A., Sorbara C. D., Bennett S. A. (2010). Pannexin 2 is expressed by postnatal hippocampal neural progenitors and modulates neuronal commitment. J. Biol. Chem. 285, 24977–24986. 10.1074/jbc.M110.130054 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Takahashi H., Magee J. C. (2009). Pathway interactions and synaptic plasticity in the dendritic tuft regions of CA1 pyramidal neurons. Neuron 62, 102–111. 10.1016/j.neuron.2009.03.007 [Abstract] [CrossRef] [Google Scholar]
- Takahashi Y., Jeong S. Y., Ogata K., Goto J., Hashida H., Isahara K., et al. . (2003). Human skeletal muscle calcium channel alpha1S is expressed in the basal ganglia: distinctive expression pattern among L-type Ca2+ channels. Neurosci. Res. 45, 129–137. 10.1016/s0168-0102(02)00204-3 [Abstract] [CrossRef] [Google Scholar]
- Takeuchi H., Suzumura A. (2014). Gap junctions and hemichannels composed of connexins: potential therapeutic targets for neurodegenerative diseases. Front. Cell Neurosci. 8:189. 10.3389/fncel.2014.00189 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Talley E. M., Solorzano G., Lei Q., Kim D., Bayliss D. A. (2001). Cns distribution of members of the two-pore-domain (KCNK) potassium channel family. J. Neurosci. 21, 7491–7505. 10.1523/JNEUROSCI.21-19-07491.2001 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Tanemoto M., Fujita A., Higashi K., Kurachi Y. (2002). PSD-95 mediates formation of a functional homomeric Kir5.1 channel in the brain. Neuron 34, 387–397. 10.1016/s0896-6273(02)00675-x [Abstract] [CrossRef] [Google Scholar]
- Tanemoto M., Kittaka N., Inanobe A., Kurachi Y. (2000). In vivo formation of a proton-sensitive K+ channel by heteromeric subunit assembly of Kir5.1 with Kir4.1. J. Physiol. 525 (Pt. 3), 587–592. 10.1111/j.1469-7793.2000.00587.x [Abstract] [CrossRef] [Google Scholar]
- Taruno A. (2018). ATP release channels. Int. J. Mol. Sci. 19:808. 10.3390/ijms19030808 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Theis M., Sohl G., Eiberger J., Willecke K. (2005). Emerging complexities in identity and function of glial connexins. Trends Neurosci. 28, 188–195. 10.1016/j.tins.2005.02.006 [Abstract] [CrossRef] [Google Scholar]
- Thiffault I., Speca D. J., Austin D. C., Cobb M. M., Eum K. S., Safina N. P., et al. . (2015). A novel epileptic encephalopathy mutation in KCNB1 disrupts Kv2.1 ion selectivity, expression, and localization. J. Gen. Physiol. 146, 399–410. 10.1085/jgp.201511444 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Thimmapaya R., Neelands T., Niforatos W., Davis-Taber R. A., Choi W., Putman C. B., et al. . (2005). Distribution and functional characterization of human Nav1.3 splice variants. Eur. J. Neurosci. 22, 1–9. 10.1111/j.1460-9568.2005.04155.x [Abstract] [CrossRef] [Google Scholar]
- Thorell W. E., Leibrock L. G., Agrawal S. K. (2002). Role of RyRs and IP3 receptors after traumatic injury to spinal cord white matter. J. Neurotrauma 19, 335–342. 10.1089/089771502753594909 [Abstract] [CrossRef] [Google Scholar]
- Tippens A. L., Pare J. F., Langwieser N., Moosmang S., Milner T. A., Smith Y., et al. . (2008). Ultrastructural evidence for pre- and postsynaptic localization of Cav1.2 L-type Ca2+ channels in the rat hippocampus. J. Comp. Neurol. 506, 569–583. 10.1002/cne.21567 [Abstract] [CrossRef] [Google Scholar]
- Toriyama H., Wang L., Saegusa H., Zong S., Osanai M., Murakoshi T., et al. . (2002). Role of Ca(v) 2.3 (alpha1E) Ca2+ channel in ischemic neuronal injury. Neuroreport 13, 261–265. 10.1097/00001756-200202110-00018 [Abstract] [CrossRef] [Google Scholar]
- Torkamani A., Bersell K., Jorge B. S., Bjork R. L., Jr., Friedman J. R., Bloss C. S., et al. . (2014). De novo KCNB1 mutations in epileptic encephalopathy. Ann. Neurol. 76, 529–540. 10.1002/ana.24263 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Trebak M., Kinet J. P. (2019). Calcium signalling in T cells. Nat. Rev. Immunol. 19, 154–169. 10.1038/s41577-018-0110-7 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Trimmer J. S. (2015). Subcellular localization of K+ channels in mammalian brain neurons: remarkable precision in the midst of extraordinary complexity. Neuron 85, 238–256. 10.1016/j.neuron.2014.12.042 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Trimmer J. S., Rhodes K. J. (2004). Localization of voltage-gated ion channels in mammalian brain. Annu. Rev. Physiol. 66, 477–519. 10.1146/annurev.physiol.66.032102.113328 [Abstract] [CrossRef] [Google Scholar]
- Tsuji F., Murai M., Oki K., Seki I., Ueda K., Inoue H., et al. . (2010). Transient receptor potential vanilloid 1 agonists as candidates for anti-inflammatory and immunomodulatory agents. Eur. J. Pharmacol. 627, 332–339. 10.1016/j.ejphar.2009.10.044 [Abstract] [CrossRef] [Google Scholar]
- Tsutsui M., Hirase R., Miyamura S., Nagayasu K., Nakagawa T., Mori Y., et al. . (2018). TRPM2 exacerbates central nervous system inflammation in experimental autoimmune encephalomyelitis by increasing production of CXCL2 chemokines. J. Neurosci. 38, 8484–8495. 10.1523/JNEUROSCI.2203-17.2018 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Tucker S. J., Imbrici P., Salvatore L., D'Adamo M. C., Pessia M. (2000). pH dependence of the inwardly rectifying potassium channel, Kir5.1, and localization in renal tubular epithelia. J. Biol. Chem. 275, 16404–16407. 10.1074/jbc.C000127200 [Abstract] [CrossRef] [Google Scholar]
- Tzingounis A. V., Heidenreich M., Kharkovets T., Spitzmaul G., Jensen H. S., Nicoll R. A., et al. . (2010). The KCNQ5 potassium channel mediates a component of the afterhyperpolarization current in mouse hippocampus. Proc. Natl. Acad. Sci. U.S.A. 107, 10232–10237. 10.1073/pnas.1004644107 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Tzour A., Leibovich H., Barkai O., Biala Y., Lev S., Yaari Y., et al. . (2017). KV 7/M channels as targets for lipopolysaccharide-induced inflammatory neuronal hyperexcitability. J. Physiol. 595, 713–738. 10.1113/JP272547 [Abstract] [CrossRef] [Google Scholar]
- Vacher H., Mohapatra D. P., Trimmer J. S. (2008). Localization and targeting of voltage-dependent ion channels in mammalian central neurons. Physiol. Rev. 88, 1407–1447. 10.1152/physrev.00002.2008 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- van de Graaf S. F., Hoenderop J. G., Bindels R. J. (2006). Regulation of TRPV5 and TRPV6 by associated proteins. Am. J. Physiol. Renal Physiol. 290, F1295–1302. 10.1152/ajprenal.00443.2005 [Abstract] [CrossRef] [Google Scholar]
- Van Der Stelt M., Di Marzo V. (2004). Endovanilloids. Putative endogenous ligands of transient receptor potential vanilloid 1 channels. Eur. J. Biochem. 271, 1827–1834. 10.1111/j.1432-1033.2004.04081.x [Abstract] [CrossRef] [Google Scholar]
- Vanderver A., Simons C., Schmidt J. L., Pearl P. L., Bloom M., Lavenstein B., et al. . (2014). Identification of a novel de novo p.Phe932Ile KCNT1 mutation in a patient with leukoencephalopathy and severe epilepsy. Pediatr. Neurol. 50, 112–114. 10.1016/j.pediatrneurol.2013.06.024 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Vasistha N. A., Johnstone M., Barton S. K., Mayerl S. E., Thangaraj Selvaraj B., Thomson P. A., et al. . (2019). Familial t(1;11) translocation is associated with disruption of white matter structural integrity and oligodendrocyte-myelin dysfunction. Mol. Psychiatry 24, 1641–1654. 10.1038/s41380-019-0505-2 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Vautier F., Belachew S., Chittajallu R., Gallo V. (2004). Shaker-type potassium channel subunits differentially control oligodendrocyte progenitor proliferation. Glia 48, 337–345. 10.1002/glia.20088 [Abstract] [CrossRef] [Google Scholar]
- Vay S. U., Flitsch L. J., Rabenstein M., Moniere H., Jakovcevski I., Andjus P., et al. . (2020). The impact of hyperpolarization-activated cyclic nucleotide-gated (HCN) and voltage-gated potassium KCNQ/Kv7 channels on primary microglia function. J. Neuroinflammation 17:100. 10.1186/s12974-020-01779-4 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Veng L. M., Mesches M. H., Browning M. D. (2003). Age-related working memory impairment is correlated with increases in the L-type calcium channel protein alpha1D (Cav1.3) in area CA1 of the hippocampus and both are ameliorated by chronic nimodipine treatment. Brain Res. Mol. Brain Res. 110, 193–202. 10.1016/s0169-328x(02)00643-5 [Abstract] [CrossRef] [Google Scholar]
- Vennekens R., Hoenderop J. G., Prenen J., Stuiver M., Willems P. H., Droogmans G., et al. . (2000). Permeation and gating properties of the novel epithelial Ca(2+) channel. J. Biol. Chem. 275, 3963–3969. 10.1074/jbc.275.6.3963 [Abstract] [CrossRef] [Google Scholar]
- Vierra N. C., Kirmiz M., van der List D., Santana L. F., Trimmer J. S. (2019). Kv2.1 mediates spatial and functional coupling of L-type calcium channels and ryanodine receptors in mammalian neurons. Elife 8:e49953. 10.7554/eLife.49953 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Vigil F. A., Bozdemir E., Bugay V., Chun S. H., Hobbs M., Sanchez I., et al. . (2020). Prevention of brain damage after traumatic brain injury by pharmacological enhancement of KCNQ (Kv7, “M-type”) K(+) currents in neurons. J. Cereb. Blood Flow Metab. 40, 1256–1273. 10.1177/0271678X19857818 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Villegas R., Martinez N. W., Lillo J., Pihan P., Hernandez D., Twiss J. L., et al. . (2014). Calcium release from intra-axonal endoplasmic reticulum leads to axon degeneration through mitochondrial dysfunction. J. Neurosci. 34, 7179–7189. 10.1523/JNEUROSCI.4784-13.2014 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Vogt A., Hormuzdi S. G., Monyer H. (2005). Pannexin1 and Pannexin2 expression in the developing and mature rat brain. Brain Res. Mol. Brain Res. 141, 113–120. 10.1016/j.molbrainres.2005.08.002 [Abstract] [CrossRef] [Google Scholar]
- Wainger B. J., Kiskinis E., Mellin C., Wiskow O., Han S. S., Sandoe J., et al. . (2014). Intrinsic membrane hyperexcitability of amyotrophic lateral sclerosis patient-derived motor neurons. Cell Rep. 7, 1–11. 10.1016/j.celrep.2014.03.019 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Wang H., Allen M. L., Grigg J. J., Noebels J. L., Tempel B. L. (1995). Hypomyelination alters K+ channel expression in mouse mutants shiverer and Trembler. Neuron 15, 1337–1347. 10.1016/0896-6273(95)90012-8 [Abstract] [CrossRef] [Google Scholar]
- Wang H., Kunkel D. D., Martin T. M., Schwartzkroin P. A., Tempel B. L. (1993). Heteromultimeric K+ channels in terminal and juxtaparanodal regions of neurons. Nature 365, 75–79. 10.1038/365075a0 [Abstract] [CrossRef] [Google Scholar]
- Wang H., Zhang X., Xue L., Xing J., Jouvin M. H., Putney J. W., et al. . (2016). Low-Voltage-Activated CaV3.1 calcium channels shape T helper cell cytokine profiles. Immunity 44, 782–794. 10.1016/j.immuni.2016.01.015 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Wang H. S., Pan Z., Shi W., Brown B. S., Wymore R. S., Cohen I. S., et al. . (1998). KCNQ2 and KCNQ3 potassium channel subunits: molecular correlates of the M-channel. Science 282, 1890–1893. 10.1126/science.282.5395.1890 [Abstract] [CrossRef] [Google Scholar]
- Wang J., Ou S. W., Wang Y. J. (2017). Distribution and function of voltage-gated sodium channels in the nervous system. Channels 11, 534–554. 10.1080/19336950.2017.1380758 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Wang K., Lin M. T., Adelman J. P., Maylie J. (2014). Distinct Ca2+ sources in dendritic spines of hippocampal CA1 neurons couple to SK and Kv4 channels. Neuron 81, 379–387. 10.1016/j.neuron.2013.11.004 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Wang N., De Bock M., Decrock E., Bol M., Gadicherla A., Vinken M., et al. . (2013). Paracrine signaling through plasma membrane hemichannels. Biochim. Biophys. Acta 1828, 35–50. 10.1016/j.bbamem.2012.07.002 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Wang R., Tu S., Zhang J., Shao A. (2020). Roles of TRP channels in neurological diseases. Oxid. Med. Cell Longev. 2020:7289194. 10.1155/2020/7289194 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Wang W., Gao X. F., Xiao L., Xiang Z. H., He C. (2011). K(V)7/KCNQ channels are functionally expressed in oligodendrocyte progenitor cells. PLoS ONE 6:e21792. 10.1371/journal.pone.0021792 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Wartosch L., Fuhrmann J. C., Schweizer M., Stauber T., Jentsch T. J. (2009). Lysosomal degradation of endocytosed proteins depends on the chloride transport protein ClC-7. FASEB J. 23, 4056–4068. 10.1096/fj.09-130880 [Abstract] [CrossRef] [Google Scholar]
- Watanabe M., Ueda T., Shibata Y., Kumamoto N., Shimada S., Ugawa S. (2015). Expression and regulation of Cav3.2 T-Type calcium channels during inflammatory hyperalgesia in mouse dorsal root ganglion neurons. PLoS ONE 10:e0127572. 10.1371/journal.pone.0127572 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Waxman S. G. (2001). Acquired channelopathies in nerve injury and MS. Neurology 56, 1621–1627. 10.1212/wnl.56.12.1621 [Abstract] [CrossRef] [Google Scholar]
- Weiergraber M., Henry M., Radhakrishnan K., Hescheler J., Schneider T. (2007). Hippocampal seizure resistance and reduced neuronal excitotoxicity in mice lacking the Cav2.3 E/R-type voltage-gated calcium channel. J. Neurophysiol. 97, 3660–3669. 10.1152/jn.01193.2006 [Abstract] [CrossRef] [Google Scholar]
- Weinert S., Jabs S., Hohensee S., Chan W. L., Kornak U., Jentsch T. J. (2014). Transport activity and presence of ClC-7/Ostm1 complex account for different cellular functions. EMBO Rep. 15, 784–791. 10.15252/embr.201438553 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Weinreich F., Jentsch T. J. (2001). Pores formed by single subunits in mixed dimers of different CLC chloride channels. J. Biol. Chem. 276, 2347–2353. 10.1074/jbc.M005733200 [Abstract] [CrossRef] [Google Scholar]
- Weiss N., Zamponi G. W. (2019). T-type calcium channels: from molecule to therapeutic opportunities. Int. J. Biochem. Cell Biol. 108, 34–39. 10.1016/j.biocel.2019.01.008 [Abstract] [CrossRef] [Google Scholar]
- Westenbroek R. E., Noebels J. L., Catterall W. A. (1992). Elevated expression of type II Na+ channels in hypomyelinated axons of shiverer mouse brain. J. Neurosci. 12, 2259–2267 [Europe PMC free article] [Abstract] [Google Scholar]
- Wetzel A., Jablonka S., Blum R. (2013). Cell-autonomous axon growth of young motoneurons is triggered by a voltage-gated sodium channel. Channels 7, 51–56. 10.4161/chan.23153 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Whitaker W. R., Faull R. L., Waldvogel H. J., Plumpton C. J., Emson P. C., Clare J. J. (2001). Comparative distribution of voltage-gated sodium channel proteins in human brain. Brain Res. Mol. Brain Res. 88, 37–53. 10.1016/s0169-328x(00)00289-8 [Abstract] [CrossRef] [Google Scholar]
- Whyte-Fagundes P., Zoidl G. (2018). Mechanisms of pannexin1 channel gating and regulation. Biochim. Biophys. Acta Biomembr. 1860, 65–71. 10.1016/j.bbamem.2017.07.009 [Abstract] [CrossRef] [Google Scholar]
- Willecke K., Eiberger J., Degen J., Eckardt D., Romualdi A., Guldenagel M., et al. . (2002). Structural and functional diversity of connexin genes in the mouse and human genome. Biol. Chem. 383, 725–737. 10.1515/BC.2002.076 [Abstract] [CrossRef] [Google Scholar]
- Willis M., Kaufmann W. A., Wietzorrek G., Hutter-Paier B., Moosmang S., Humpel C., et al. . (2010). L-type calcium channel CaV 1.2 in transgenic mice overexpressing human AbetaPP751 with the London (V717I) and Swedish (K670M/N671L) mutations. J. Alzheimers Dis. 20, 1167–1180. 10.3233/JAD-2010-091117 [Abstract] [CrossRef] [Google Scholar]
- Winter M., Weissgerber P., Klein K., Lux F., Yildiz D., Wissenbach U., et al. . (2020). Transient receptor potential vanilloid 6 (TRPV6) proteins control the extracellular matrix structure of the placental labyrinth. Int. J. Mol. Sci. 21:9674. 10.3390/ijms21249674 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Woo D. H., Han K. S., Shim J. W., Yoon B. E., Kim E., Bae J. Y., et al. . (2012). TREK-1 and Best1 channels mediate fast and slow glutamate release in astrocytes upon GPCR activation. Cell 151, 25–40. 10.1016/j.cell.2012.09.005 [Abstract] [CrossRef] [Google Scholar]
- Wormuth C., Lundt A., Henseler C., Muller R., Broich K., Papazoglou A., et al. . (2016). Review: Cav2.3 R-type voltage-gated Ca(2+) channels - functional implications in convulsive and non-convulsive seizure activity. Open Neurol. J. 10, 99–126. 10.2174/1874205X01610010099 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Wu C. Y., Kaur C., Sivakumar V., Lu J., Ling E. A. (2009). Kv1.1 expression in microglia regulates production and release of proinflammatory cytokines, endothelins and nitric oxide. Neuroscience 158, 1500–1508. 10.1016/j.neuroscience.2008.11.043 [Abstract] [CrossRef] [Google Scholar]
- Wu L. G., Westenbroek R. E., Borst J. G., Catterall W. A., Sakmann B. (1999). Calcium channel types with distinct presynaptic localization couple differentially to transmitter release in single calyx-type synapses. J. Neurosci. 19, 726–736. [Europe PMC free article] [Abstract] [Google Scholar]
- Wu Z., Li L., Xie F., Xu G., Dang D., Yang Q. (2020). Enhancing KCNQ channel activity improves neurobehavioral recovery after spinal cord injury. J. Pharmacol. Exp. Ther. 373, 72–80. 10.1124/jpet.119.264010 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Xiao K., Sun Z., Jin X., Ma W., Song Y., Lai S., et al. . (2018). ERG3 potassium channel-mediated suppression of neuronal intrinsic excitability and prevention of seizure generation in mice. J. Physiol. 596, 4729–4752. 10.1113/JP275970 [Abstract] [CrossRef] [Google Scholar]
- Xiao Y., Lv X., Cao G., Bian G., Duan J., Ai J., et al. . (2010). Overexpression of Trpp5 contributes to cell proliferation and apoptosis probably through involving calcium homeostasis. Mol. Cell Biochem. 339, 155–161. 10.1007/s11010-009-0379-8 [Abstract] [CrossRef] [Google Scholar]
- Xiao Z. S., Quarles L. D. (2010). Role of the polycytin-primary cilia complex in bone development and mechanosensing. Ann. N. Y. Acad. Sic. 1192, 410–421. 10.1111/j.1749-6632.2009.05239.x [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Xu H., Tian W., Fu Y., Oyama T. T., Anderson S., Cohen D. M. (2007). Functional effects of nonsynonymous polymorphisms in the human TRPV1 gene. Am. J. Physiol. Renal Physiol. 293, F1865–1876. 10.1152/ajprenal.00347.2007 [Abstract] [CrossRef] [Google Scholar]
- Xu J. H., Long L., Tang Y. C., Hu H. T., Tang F. R. (2007). Ca(v)1.2, Ca(v)1.3, and Ca(v)2.1 in the mouse hippocampus during and after pilocarpine-induced status epilepticus. Hippocampus 17, 235–251. 10.1002/hipo.20263 [Abstract] [CrossRef] [Google Scholar]
- Yan E., Li B., Gu L., Hertz L., Peng L. (2013). Mechanisms for L-channel-mediated increase in [Ca(2+)]i and its reduction by anti-bipolar drugs in cultured astrocytes combined with its mRNA expression in freshly isolated cells support the importance of astrocytic L-channels. Cell Calcium 54, 335–342. 10.1016/j.ceca.2013.08.002 [Abstract] [CrossRef] [Google Scholar]
- Yasuda R., Sabatini B. L., Svoboda K. (2003). Plasticity of calcium channels in dendritic spines. Nat. Neurosci. 6, 948–955. 10.1038/nn1112 [Abstract] [CrossRef] [Google Scholar]
- Yeung A. K., Patil C. S., Jackson M. F. (2020). Pannexin-1 in the CNS: emerging concepts in health and disease. J. Neurochem. 154, 468–485. 10.1111/jnc.15004 [Abstract] [CrossRef] [Google Scholar]
- Yu F. H., Catterall W. A. (2003). Overview of the voltage-gated sodium channel family. Genome Biol. 4:207. 10.1186/gb-2003-4-3-207 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Zamora N. N., Cheli V. T., Santiago Gonzalez D. A., Wan R., Paez P. M. (2020). Deletion of voltage-gated calcium channels in astrocytes during demyelination reduces brain inflammation and promotes myelin regeneration in mice. J. Neurosci. 40, 3332–3347. 10.1523/JNEUROSCI.1644-19.2020 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Zamponi G. W., Striessnig J., Koschak A., Dolphin A. C. (2015). The physiology, pathology, and pharmacology of voltage-gated calcium channels and their future therapeutic potential. Pharmacol. Rev. 67, 821–870. 10.1124/pr.114.009654 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Zhang H., Maximov A., Fu Y., Xu F., Tang T. S., Tkatch T., et al. . (2005). Association of CaV1.3 L-type calcium channels with shank. J. Neurosci. 25, 1037–1049. 10.1523/JNEUROSCI.4554-04.2005 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Zhang Y., Chen K., Sloan S. A., Bennett M. L., Scholze A. R., O'Keeffe S., et al. . (2014). An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J. Neurosci. 34, 11929–11947. 10.1523/JNEUROSCI.1860-14.2014 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Zhou J. Z., Jiang J. X. (2014). Gap junction and hemichannel-independent actions of connexins on cell and tissue functions–an update. FEBS Lett. 588, 1186–1192. 10.1016/j.febslet.2014.01.001 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Zhou M., Xu G., Xie M., Zhang X., Schools G. P., Ma L., et al. . (2009). TWIK-1 and TREK-1 are potassium channels contributing significantly to astrocyte passive conductance in rat hippocampal slices. J. Neurosci. 29, 8551–8564. 10.1523/JNEUROSCI.5784-08.2009 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Zhou W., Cayabyab F. S., Pennefather P. S., Schlichter L. C., DeCoursey T. E. (1998). HERG-like K+ channels in microglia. J. Gen. Physiol. 111, 781–794. 10.1085/jgp.111.6.781 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Zou A., Lin Z., Humble M., Creech C. D., Wagoner P. K., Krafte D., et al. . (2003). Distribution and functional properties of human KCNH8 (Elk1) potassium channels. Am. J. Physiol. Cell Physiol. 285, C1356–1366. 10.1152/ajpcell.00179.2003 [Abstract] [CrossRef] [Google Scholar]
- Zoupi L., Markoullis K., Kleopa K. A., Karagogeos D. (2013). Alterations of juxtaparanodal domains in two rodent models of CNS demyelination. Glia 61, 1236–1249. 10.1002/glia.22511 [Abstract] [CrossRef] [Google Scholar]
- Zuccotti A., Clementi S., Reinbothe T., Torrente A., Vandael D. H., Pirone A. (2011). Structural and functional differences between L-type calcium channels: crucial issues for future selective targeting. Trends Pharmacol. Sci. 32, 366–375. 10.1016/j.tips.2011.02.012 [Abstract] [CrossRef] [Google Scholar]
Articles from Frontiers in Cellular Neuroscience are provided here courtesy of Frontiers Media SA
Full text links
Read article at publisher's site: https://doi.org/10.3389/fncel.2021.685703
Read article for free, from open access legal sources, via Unpaywall:
https://www.frontiersin.org/articles/10.3389/fncel.2021.685703/pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/108189280
Article citations
circRNAs as Epigenetic Regulators of Integrity in Blood-Brain Barrier Architecture: Mechanisms and Therapeutic Strategies in Multiple Sclerosis.
Cells, 13(16):1316, 06 Aug 2024
Cited by: 0 articles | PMID: 39195206 | PMCID: PMC11352526
Review Free full text in Europe PMC
Adolescent binge ethanol impacts H3K9me3-occupancy at synaptic genes and the regulation of oligodendrocyte development.
Front Mol Neurosci, 17:1389100, 22 May 2024
Cited by: 0 articles | PMID: 38840776
Therapeutic potential of thymoquinone and its nanoformulations in neuropsychological disorders: a comprehensive review on molecular mechanisms in preclinical studies.
Naunyn Schmiedebergs Arch Pharmacol, 397(6):3541-3564, 27 Nov 2023
Cited by: 0 articles | PMID: 38010395
Review
Modelling TDP-43 proteinopathy in Drosophila uncovers shared and neuron-specific targets across ALS and FTD relevant circuits.
Acta Neuropathol Commun, 11(1):168, 20 Oct 2023
Cited by: 0 articles | PMID: 37864255 | PMCID: PMC10588218
A Case of Coexistent Spinocerebellar Ataxia Type 2 and Primary Progressive Multiple Sclerosis-Coincidental or Associated?
Cerebellum, 23(3):1235-1238, 16 Sep 2023
Cited by: 0 articles | PMID: 37715888
Go to all (15) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Molecular signature of different lesion types in the brain white matter of patients with progressive multiple sclerosis.
Acta Neuropathol Commun, 7(1):205, 11 Dec 2019
Cited by: 45 articles | PMID: 31829262 | PMCID: PMC6907342
Unique RNA signature of different lesion types in the brain white matter in progressive multiple sclerosis.
Acta Neuropathol Commun, 7(1):58, 25 Apr 2019
Cited by: 6 articles | PMID: 31023379 | PMCID: PMC6482546
Connecting Immune Cell Infiltration to the Multitasking Microglia Response and TNF Receptor 2 Induction in the Multiple Sclerosis Brain.
Front Cell Neurosci, 14:190, 07 Jul 2020
Cited by: 8 articles | PMID: 32733206 | PMCID: PMC7359043
Neuronal injury in chronic CNS inflammation.
Best Pract Res Clin Anaesthesiol, 24(4):551-562, 29 Nov 2010
Cited by: 81 articles | PMID: 21619866
Review
Funding
Funders who supported this work.
Lundbeck Foundation (1)
Grant ID: R347-2020-2454

