Abstract
Free full text

Cardiovascular and systemic determinants of exercise capacity in people with type 2 diabetes mellitus
Abstract
The global burden of heart failure (HF) is on the rise owing to an increasing incidence of lifestyle related diseases, predominantly type 2 diabetes mellitus (T2D). Diabetes is an independent risk factor for cardiovascular disease, and up to 75% of those with T2D develop HF in their lifetime. T2D leads to pathological alterations within the cardiovascular system, which can progress insidiously and asymptomatically in the absence of conventional risk factors. Reduced exercise tolerance is consistently reported, even in otherwise asymptomatic individuals with T2D, and is the first sign of a failing heart. Because aggressive modification of cardiovascular risk factors does not eliminate the risk of HF in T2D, it is likely that other factors play a role in the pathogenesis of HF. Early identification of individuals at risk of HF is advantageous, as it allows for modification of the reversible risk factors and early initiation of treatment with the aim of improving clinical outcomes. In this review, cardiac and extra-cardiac contributors to reduced exercise tolerance in people with T2D are explored.
Introduction
The global prevalence of heart failure (HF) is growing at an alarming rate. In 2011, approximately 23 million people were living with HF worldwide, a figure that is expected to rise by 46% by 2030.1 In developed countries, up to one in five people are expected to develop HF during their lifetime.2 This is largely owing to the mounting pandemic of lifestyle-related diseases such as obesity and type 2 diabetes mellitus (T2D), which are intimately linked with each other, and with HF development.3 For example, United Kingdom estimates suggest that in the general population the prevalence of diabetes is 6%.4 However, in data from contemporary trials the prevalence of diabetes in HF patients ranges from 35% to 44%,5,6 with a particular predisposition towards HF with preserved ejection fraction (HFpEF). Furthermore, clinical outcomes for diabetes-associated HF are considerably worse for patients with T2D than those without,7 and development of HF in T2D is associated with the greatest risk of death and loss of lifespan than any other cardiovascular complication of T2D.6 HF is one of the most common complications of T2D, second only to peripheral vascular disease.8 Recognizing, preventing and treating HF in T2D is clearly a major priority for healthcare professionals and is considered a national priority in the United Kingdom.9
million people were living with HF worldwide, a figure that is expected to rise by 46% by 2030.1 In developed countries, up to one in five people are expected to develop HF during their lifetime.2 This is largely owing to the mounting pandemic of lifestyle-related diseases such as obesity and type 2 diabetes mellitus (T2D), which are intimately linked with each other, and with HF development.3 For example, United Kingdom estimates suggest that in the general population the prevalence of diabetes is 6%.4 However, in data from contemporary trials the prevalence of diabetes in HF patients ranges from 35% to 44%,5,6 with a particular predisposition towards HF with preserved ejection fraction (HFpEF). Furthermore, clinical outcomes for diabetes-associated HF are considerably worse for patients with T2D than those without,7 and development of HF in T2D is associated with the greatest risk of death and loss of lifespan than any other cardiovascular complication of T2D.6 HF is one of the most common complications of T2D, second only to peripheral vascular disease.8 Recognizing, preventing and treating HF in T2D is clearly a major priority for healthcare professionals and is considered a national priority in the United Kingdom.9
Despite not having overt signs or symptoms of HF or prevalent cardiovascular disease, numerous studies have reported a 20–30% reduction in peak oxygen consumption (VO2peak) in adults with T2D compared with controls.10–13 Exercise limitations occur early in the disease process and may be present in individuals with good glycaemic control14 and in those without clinically apparent cardiovascular disease.14 Importantly, these limitations in physical fitness correlate strongly with increased risk of cardiovascular and all-cause mortality, and HF.15 In combination, it is highly likely that early cardiovascular and systemic disturbances associated with T2D cause significant exercise limitation that predisposes to HF development. Enhanced understanding of the factors directly influencing exercise capacity in people with T2D may lead to the development of strategies to prevent or treat HF, as summarized in Figure 1. This review synthesizes the available evidence assessing predictors of exercise capacity in asymptomatic individuals with T2D. Links to mechanisms limiting exercise capacity specifically in HFpEF are explored. We emphasize the contributions of both cardiovascular and systemic factors that may lead to reduced physical fitness, highlighting areas of unmet research need and future strategies for targeted interventions.

Contributors to reduced exercise capacity in T2D.
Reduced exercise capacity in T2D is a net result of complex interactions between the biomechanics of obesity and frailty and the systemic and cardiovascular factors. Molecular mechanisms involved in the interactions between excess nutrients, adiposity, and chronic inflammation result in insulin resistance, which further propels the vicious cycle of metabolic dysregulation. Adapted from Del Buono MG, et al. J Am Coll Cardiol 2019;73(17):2209-2519
BMI, body mass index; LA, left atrium; LV, left ventricular; T2D, type 2 diabetes mellitus.
Physiological cardiovascular responses to exercise
In health, the cardiovascular system adapts and modifies its parameters in response to increased demand such as physical exertion, with the aim to facilitate tissue perfusion and oxygen delivery.16 At the microcirculatory level, perfusion pressure equates to mean arterial pressure which is regulated by interaction between cardiac output (a combination of heart rate and stroke volume) and systemic vascular resistance.17 During exercise, a positive chronotropic response and increased stroke volume (through re-direction of splanchnic and renal blood flow, increasing preload) result in increased cardiac output. The systemic vascular resistance in exercise-critical tissues such as the myocardium falls through local release of nitric oxide mediators and activity of cyclic guanosine monophosphate.17 The net result is a controlled change in mean arterial pressure, with improved perfusion of the exercise-critical muscle groups, such as the myocardium and skeletal muscle. The inability of one or more of these physiological parameters to adjust on exercise will result in failure of the cardiovascular system to meet tissue oxygen demands, leading to diminished exercise capacity.17
Cardiopulmonary exercise testing offers an assessment of individuals’ functional capacity through evaluation of gas exchange during exercise and thus an assessment of systems involved in both oxygen transport and utilization.18 Cardiopulmonary exercise testing allows for measurement of the volume of tissue oxygen uptake, which is a key parameter that offers insights into cardiac and pulmonary function, as expressed by Fick’s principle, according to which VO2 equates to cardiac output multiplied by the artero-venous gradient [C(a-v)O2].18 During ramp-like exercise, VO2 increases exponentially up to a steady state corresponding to peak exercise, and will adopt different patterns in patients with different aetiologies of HF.18 Any number of perturbations in T2D can interfere with the body’s normal physical responses to increased work, and thus affect the VO2peak.
Systemic contributors to impaired exercise capacity
Metabolic dysregulation and chronic inflammation in T2D
A number of theories have been proposed to explain the pathophysiology of the metabolic dysregulation, insulin resistance and development of endothelial dysfunction in adults with T2D and obesity.20 A chronic inflammatory state is induced by mitochondrial dysfunction and driven by chronic excess of nutrients, in particular the free fatty acids.21–23 Mitochondrial nutrient overload results in metabolic shifts towards generation of reactive oxygen species (ROS), which activate endothelial cytokine production, leading to direct endothelial damage and alterations of insulin signalling.24 This theory is based on the observation of higher and persistently elevated baseline levels of proinflammatory cytokines in obese individuals with T2D and insulin resistance as compared with lean controls.25–31 Furthermore, endothelial inflammation exerts pro-atherogenic effects, further compounding the cardiovascular risk in this cohort.22,32 In addition to promoting fatty streak deposition within the arterial wall, mitochondrial ROS results in reduced bioavailability of nitric oxide, which is essential to normal vascular homeostasis.26 The resultant impaired endothelial vasomotor mechanics are the hallmark of endothelial dysfunction and a key mechanism behind microvascular dysfunction, which is responsible for a range of the pathological sequelae of T2D, including left ventricular (LV) diastolic dysfunction.32–36 ROS toxicity leads to diastolic dysfunction by two mechanisms: first, ROS-mediated cardiomyocyte damage results in inflammation, apoptosis and fibrosis, directly contributing to LV diastolic dysfunction through remodeling,22 and second, ROS interact with endoplasmic reticulum, altering its structure and function primarily by altering the activity of the sarcoplasmic reticulum calcium pump, which is responsible for calcium sequestration during cardiomyocyte relaxation, thus leading to diastolic dysfunction.37 However, a recent systematic review of 11 studies did not show an association between exercise and reduced levels of inflammatory markers in adults with T2D.38
The volume and type of adipose tissue seems to have a significant effect on the propagation of the proinflammatory response. Brown adipose tissue and white adipose tissue play specific roles in energy metabolism and insulin homeostasis.39 Brown adipose tissue has an important role in regulating energy and glucose homeostasis, and has been associated with peripheral insulin resistance and glucose levels.40 Visceral white adipose tissue (around the trunk, upper body or abdomen) appears to be the major source of inflammatory markers in T2D, responsible for the production of inflammatory cytokines, thus contributing to the systemic inflammation and insulin resistance.40 Although insulin resistance has been associated with reduced VO2peak, this association has been described mainly from univariate analyses of small sample subjects with a risk of significantly overfitting the regression models.41 Although some older studies have shown association between glycaemic control (expressed as HbA1c) and exercise capacity,42 newer studies have not confirmed this association.22 Strict glycaemic control alone has not been shown to improve cardiovascular outcomes in patients with T2D.42
Changes within the systemic micro- and macro-vasculature play an important role in maintaining exercise capacity. In a study of 134 asymptomatic adults with T2D, reduced capillary blood flow to skeletal muscle was found and was positively correlated with VO2peak independent of mean arterial pressure and cardiac output.15 This association was driven by capillary blood velocity reserve rather than capillary blood volume reserve, suggesting impaired endothelial vasomotive response to exercise.13 Furthermore, the association of capillary blood flow with VO2peak was independent of mean arterial pressure, cardiac output reserve and other cardiac covariates, suggesting that pre-capillary factors, particularly endothelium-mediated vasodilation, may be responsible.13 In another study, which compared 20 uncomplicated T2Ds with 20 T2Ds with microvascular complications, the latter group had abnormal skeletal muscle capillary responses to periodic contractile exercise,43 thus reflecting an underlying abnormality in microvascular recruitment.20 These findings again implicate endothelium-mediated vasodilation, which is responsible for exercise hyperaemia and known to be impaired in diabetes.17 Insulin increases limb blood flow in a dose dependent fashion; however, this mechanism is ineffective in the presence of insulin resistance and is compounded by reduced local availability of nitric oxide, as present in microvascular dysfunction.11
Clinical predictors
A number of studies evaluating determinants of exercise capacity have linked clinical characteristics to exercise capacity (Table 1). The strongest independent predictors of VO2peak have been age and sex. In the largest to date study of over 5000 participants with T2D, peak exercise capacity was higher for males compared with females and there was a consistent 5–10% reduction in metabolic equivalents of tasks (METs) per decade of life.44,45 Body habitus [both increased waist circumference and body mass index (BMI) ![[gt-or-equal, slanted]](/http/europepmc.org/corehtml/pmc/pmcents/ges.gif) 30
30 kg/m2] were also independently associated with reduced exercise tolerance (all p
kg/m2] were also independently associated with reduced exercise tolerance (all p <
< 0.001).46 In addition, duration and severity of T2D (expressed as insulin resistance) have been linked to reduced VO2peak in adults with T2D.45 However, it is important to note that these associations have been produced from univariate analysis of studies often involving small sample subjects, reported in subjects with T2D regardless of disease severity, mode of assessment of exercise capacity or presence of LV dysfunction, which confounds the findings.
0.001).46 In addition, duration and severity of T2D (expressed as insulin resistance) have been linked to reduced VO2peak in adults with T2D.45 However, it is important to note that these associations have been produced from univariate analysis of studies often involving small sample subjects, reported in subjects with T2D regardless of disease severity, mode of assessment of exercise capacity or presence of LV dysfunction, which confounds the findings.
Table 1.
Clinical determinants of exercise capacity in T2D.
| Author | T2D group(s) | Inclusion/exclusion criteria | Method of assessment | Predictors of ↓ VO2peak | Comments |
|---|---|---|---|---|---|
| Gulsin et al.47 | N = = 247 T2D age 51.8 247 T2D age 51.8 ± ± 11.9 11.9 years, 55% males, HbA1c 7.4 years, 55% males, HbA1c 7.4 ± ± 1.1% 1.1% | Incl.: T2D, asymptomatic | CMR for MPR | Myocardial perfusion reserve (β = = 0.822, p 0.822, p = = 0.006) and E/e′ (β 0.006) and E/e′ (β = = -0.388, p -0.388, p = = 0.001) were independently associated with VO2peak in subjects with T2D 0.001) were independently associated with VO2peak in subjects with T2D | In subjects with T2D, significant correlations were observed between VO2peak and age, T2D duration, BP, absolute and indexed LV volumes, LV EF, LV mass, LV GLS, average E/e′ and MPR |
| Excl.: T1D, IHD, HF, CKD | TTE for E/e′ | ||||
| CPET for VO2peak | |||||
| Vukomanovic et al.48 | N = = 70 T2D, uncomplicated, age 52 70 T2D, uncomplicated, age 52 ± ± 7 7 years, 56% M, HbA1c 7.5 years, 56% M, HbA1c 7.5 ± ± 1.2 controls 1.2 controls | Incl.: T2D | CPET for VO2peak | GLS (–21.6 ± ± 2.8 versus −18.4 2.8 versus −18.4 ± ± 2.3%, p 2.3%, p < < 0.001) and circumferential strain (−22.0 0.001) and circumferential strain (−22.0 ± ± 2.9 versus −19.5 2.9 versus −19.5 ± ± 2.6%, p 2.6%, p < < 0.001) were reduced in DM versus controls. VO2peak significantly lower in T2D (27.0 0.001) were reduced in DM versus controls. VO2peak significantly lower in T2D (27.0 ± ± 4.3 versus 20.7 4.3 versus 20.7 ± ± 4.0 4.0 mL/kg per min, p mL/kg per min, p < < 0.001) 0.001) | Age and sex were not forced into the regression model. Furthermore, subjects were combined with controls in the regression model |
| Excl.: HTN, HF, CAD | TTE for GLS and GCS | ||||
| Kosmala et al.49 | N = = 510 (292 T2D) 510 (292 T2D) | Incl.: asymptomatic patients with T2D, HTN and BMI ![[gt-or-equal, slanted]](/http/europepmc.org/corehtml/pmc/pmcents/ges.gif) 30 30 kg/m2 kg/m2 | Diastolic dysfunction (E/e′ >13) or strain >18% >18% | ↓VO2peak associated with LV strain and presence of LVH (p < < 0.001) 0.001) | ↓VO2peak correlated with ↑components of abnormal values; however, not adjusted for age or sex |
Stage AHF: T2D (n = 186, 70%), HbA1c 7 ± ± 1.6%, log HOMA-IR 0.41 1.6%, log HOMA-IR 0.41 ± ± 0.36 0.36 | |||||
| CPET for VO2peak | Multivariate: presence of stage B HF independently associated with ↓VO2 peak. Stage B HF (>1 imaging variable present) associated with lower VO2peak (beta ¼ 0.20; p < < 0.0001) and METs (beta ¼ 0.21; p 0.0001) and METs (beta ¼ 0.21; p < < 0.0001 0.0001 | ||||
Stage BHF: T2D (n = 106, 44%) HbA1c (7 ± ± 1.4%), log HOMA-IR 0.47 1.4%), log HOMA-IR 0.47 ± ± 0.27 0.27 | |||||
| Excl.: complications of T2D, IHD | |||||
| Fang et al.44 | N = = 170, 53% M, age 56 170, 53% M, age 56 ± ± 10 10 years years | Incl.: T2D | Abnormal EC defined as: METs = = 18 18 × × 0.15 0.15 × age × age | Univariate: ↓EC associated with ↓diastolic function.↑ EC: males (r = = 0.26, p 0.26, p < < 0.001), preserved Em (r 0.001), preserved Em (r = = 0.43, p 0.43, p < < 0.001), and preserved HRR (r 0.001), and preserved HRR (r = = 0.42, p 0.42, p < < 0.001) 0.001) | NS correlation in univariate analysis between METs, LV EF, insulin or cardiovascular drugs |
Excl.: LV EF <50%, IHD <50%, IHD | |||||
Abnormal HRR: ![[less-than-or-eq, slant]](/http/europepmc.org/corehtml/pmc/pmcents/les.gif) 18 beats.min−1, echo: strain, Em 18 beats.min−1, echo: strain, Em |
AHF, stage A heart failure; BHF, stage B heart failure; BMI, body mass index; BP, blood pressure; CAD, coronary artery disease; CMR, cardiac magnetic resonance; CPET, cardiopulmonary exercise test; DM, diabetes mellitus; EC, exercise capacity; E/E’, ratio of early mitral inflow velocity and mitral annular early diastolic velocity; EF, ejection fraction; Em, early mitral inflow velocity; GCS, global circumferential strain; GLS, global longitudinal strain; HF, heart failure; HOMA-IR, homeostatic model assessment of insulin resistance; HRR, heart rate recovery; HTN, hypertension; IHD, ischaemic heart disease; LV, left ventricle; LVH, left ventricular hypertrophy; M, male; MET, metabolic equivalent of task; MPR, myocardial perfusion reserve; T2D, type 2 diabetes; TTE, transthoracic echocardiography; VO2peak, peak oxygen consumption.
Skeletal muscles and anaerobic metabolism
In T2D, microangiopathy contributes to skeletal muscle dysfunction though impaired perfusion and oxygen extraction during exercise. In addition to impaired exercise hyperaemia, skeletal muscle oxygen extraction is impaired in adults with T2D compared with non-diabetic individuals of similar anthropometric features and equally sedentary lifestyle.16 During graded exercise at 60%, 70% and 100% VO2max, those with T2D achieved significantly lower workloads than controls.50 This corresponded to a minimal rise in stroke volume, and no change in cardiac output between 60% and 100% VO2max despite adequate rise in heart rate. Furthermore, VO2max was correlated with a-vˉ O2 difference (19% lower in T2D, p
O2 difference (19% lower in T2D, p <
< 0.001) but not with cardiac output, suggesting that impaired maximal total body O2 extraction contributed to lower VO2max in T2D patients.51 This may be in part explained by presence of diastolic dysfunction; however, the study did not include echocardiographic data. Diabetes-mediated endothelial dysfunction results in reduced local availability of nitric oxide, which is the key mediator of exercise induced hyperaemia within the skeletal muscles.15 The lower a-vˉ
0.001) but not with cardiac output, suggesting that impaired maximal total body O2 extraction contributed to lower VO2max in T2D patients.51 This may be in part explained by presence of diastolic dysfunction; however, the study did not include echocardiographic data. Diabetes-mediated endothelial dysfunction results in reduced local availability of nitric oxide, which is the key mediator of exercise induced hyperaemia within the skeletal muscles.15 The lower a-vˉ O2 difference may suggest that T2Ds had impaired peripheral oxygen extraction and were more reliant on anaerobic metabolism. Impaired VO2max in T2D may therefore be related to poor peripheral oxygen extraction and reliance of anaerobic metabolism,15 which would be explained by presence of endothelial dysfunction.
O2 difference may suggest that T2Ds had impaired peripheral oxygen extraction and were more reliant on anaerobic metabolism. Impaired VO2max in T2D may therefore be related to poor peripheral oxygen extraction and reliance of anaerobic metabolism,15 which would be explained by presence of endothelial dysfunction.
Autonomic dysfunction
Systemically, diabetes-mediated cardiac autonomic dysregulation results in impaired chronotropic response to exercise and in turn lowers myocardial ability to modulate cardiac output through heart rate.52 This confers reduced exercise tolerance through the inability of cardiac output to meet the metabolic demands of tissues. Several studies have reported impaired heart rate response and heart rate recovery in T2D, with positive associations with VO2peak on univariate analysis14,44,45,51 but not in any multivariable analysis. Nevertheless, autonomic dysregulation is one of the many factors contributing to impaired exercise tolerance in T2D.
Frailty
Increasingly, T2D is being recognized as a driver of accelerated metabolic ageing and physical deconditioning, which manifest as a state of physical frailty.53 People with T2D are up to five times more likely to suffer from frailty than individuals without diabetes.54 The diabetes-related frailty phenotype is now regarded as a major contributor to low physical functioning in people with T2D.54 It is, however, distinct from the traditional frailty phenotype that is prevalent in elderly, low body weight people. Rather, frailty in T2D occurs in younger as a well as older age groups, is related to obesity in the presence of sarcopenia, but still manifests as low physical fitness and reduced quality of life.54 To our knowledge, however, no studies have directly evaluated the contribution of frailty in T2D to objective measures of aerobic exercise capacity. Given the considerable impact that frailty will have on exercise capacity, identifying and treating frailty in T2D is an area that warrants further study.
Cardiovascular contributors to impaired exercise capacity
The pathophysiological mechanisms leading to development of diabetic cardiomyopathy
The pathological myocardial alterations characteristic of diabetic cardiomyopathy begin early in the course of T2D and are present in otherwise asymptomatic individuals, suggesting a latent phase of cardiovascular dysfunction.3 Cardiac dysfunction in diabetes is thought to lie on a continuum ranging from asymptomatic diastolic dysfunction though subclinical systolic dysfunction and then overt HF.55 Reduction in exercise tolerance is amongst the first marker of stage B HF (defined as structural or functional LV alterations in the absence of symptoms) in diabetic cardiomyopathy and a 10% reduction in an individual’s exercise tolerance in the presence of detectable cardiomyopathic changes would automatically class them as stage-II HF by the New York Heart Association (NYHA).56 Figure 2 summarizes the stages of progression of diabetic cardiomyopathy, based on the presence of cardiomyopathic changes and symptomatology, with reference to the American College of Cardiology/American Heart Association (ACC/AHA) and NYHA HF classification scores, and Table 2 summarizes cardiac predictors of reduced exercise capacity.
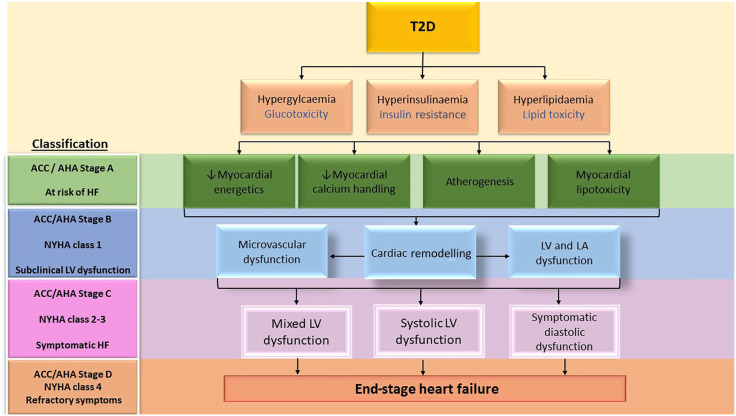
Pathological alterations leading to diabetic cardiomyopathy in relation to stages of progression of heart failure.
ACC/AHA, American College of Cardiology/American Heart Association; HF, heart failure; LA, left atrium; LV, left ventricular; NYHA, New York Heart Association; T2D, type 2 diabetes mellitus.
Table 2.
Cardiac predictors of exercise capacity in T2D.
| Author | T2D group(s) | Inclusion/exclusion criteria | Method of assessment | Predictors of ↓ VO2peak | Comments |
|---|---|---|---|---|---|
| Gulsin et al.47 | N = = 247 T2D age 51.8 247 T2D age 51.8 ± ± 11.9 11.9 years, 55% M, HbA1c 7.4 years, 55% M, HbA1c 7.4 ± ± 1.1% 1.1% | Incl.: T2D, asymptomatic | CMR for MPR | MPR (β = = 0.822, p 0.822, p = = 0.006) and E/e′ (β 0.006) and E/e′ (β = = –0.388, p –0.388, p = = 0.001) were independently associated with VO2peak in subjects with T2D 0.001) were independently associated with VO2peak in subjects with T2D | In subjects with T2D, significant correlations were observed between VO2peak and age, T2D duration, BP, absolute and indexed LV volumes, LV EF, LV mass, LV GLS, average E/e′ and MPR |
| Excl.: T1D, IHD, HF, CKD | TTE for E/e′ | ||||
| CPET for VO2peak | |||||
| Vukomanovic et al.48 | N = = 70 T2D, uncomplicated, age 52 70 T2D, uncomplicated, age 52 ± ± 7 7 years, 56% M, HbA1c 7.5 years, 56% M, HbA1c 7.5 ± ± 1.2 Controls 1.2 Controls | Incl.: T2D | CPET for VO2peak | GLS (−21.6 ± ± 2.8 versus -18.4 2.8 versus -18.4 ± ± 2.3%, p 2.3%, p < < 0.001) and circumferential strain (−22.0 0.001) and circumferential strain (−22.0 ± ± 2.9 versus −19.5 2.9 versus −19.5 ± ± 2.6%, p 2.6%, p < < 0.001) were reduced in T2D versus controls. VO2peak significantly lower in T2D (27.0 0.001) were reduced in T2D versus controls. VO2peak significantly lower in T2D (27.0 ± ± 4.3 versus 20.7 4.3 versus 20.7 ± ± 4.0 4.0 mL/kg per min, p mL/kg per min, p < < 0.001). 0.001). | Age and sex were not forced into the regression model. Furthermore, regression was calculated combined with controls |
| Excl.: HTN, HF, CAD | Echo for GLS and GCS | ||||
| Kosmala et al.49 | N = = 510 (292 T2D) 510 (292 T2D) | Incl.: asymptomatic patients with T2D, HTN and BMI ![[gt-or-equal, slanted]](/http/europepmc.org/corehtml/pmc/pmcents/ges.gif) 30 kg/m2 30 kg/m2 | Diastolic dysfunction (E/e′ >13) or strain >18% >18% | ↓VO2peak associated with LV strain and presence of LVH (p < < 0.001), 0.001), | ↓VO2peak correlated with ↑components of abnormal values; however, not adjusted for age or sex |
Stage A HF: T2D (n = = 186, 70%), HbA1c 7 186, 70%), HbA1c 7 ± ± 1.6%, log HOMA-IR 0.41 1.6%, log HOMA-IR 0.41 ± ± 0.36 0.36 | |||||
Multivariate: presence of stage B HF independently associated with ↓VO2peak. Stage B HF (>1 imaging variable present) associated with lower VO2peak (beta ¼ 0.20; p < < 0.0001) and METs (beta ¼ 0.21; p 0.0001) and METs (beta ¼ 0.21; p < < 0.0001 0.0001 | |||||
| Excl.: complications of T2D, IHD | CPET for VO2peak | ||||
Stage B HF: T2D (N = = 106, 44%) HbA1c (7 106, 44%) HbA1c (7 ± ± 1.4%), log HOMA-IR 0.47 1.4%), log HOMA-IR 0.47 ± ± 0.27 0.27 | |||||
| Fang et al.44 | N = = 170, 53% M, age 56 170, 53% M, age 56 ± ± 10 10 years years | Incl.: T2D | Abnormal EC defined as: METs = = 18 18 × × 0.15 0.15 × × age ageAbnormal HRR: ![[less-than-or-eq, slant]](/http/europepmc.org/corehtml/pmc/pmcents/les.gif) 18 beats.min−1, echo: strain, Em 18 beats.min−1, echo: strain, Em | Univariate: ↓EC associated with ↓diastolic function.↑ EC: males (r = = 0.26, p 0.26, p < < 0.001), preserved Em (r 0.001), preserved Em (r = = 0.43, p 0.43, p < < 0.001) and preserved HRR (r 0.001) and preserved HRR (r = = 0.42, p 0.42, p < < 0.001). 0.001). | NS correlation in univariate analysis between METs, LV EF, insulin or cardiovascular drugs |
| Excl.: LV EF <50%, IHD |
BMI, body mass index; BP, blood pressure; CAD, coronary artery disease; CKD, chronic kidney disease; CMR, cardiac magnetic resonance; CPET, cardiopulmonary exercise test; EC, exercise capacity; E/E′, ratio of early mitral inflow velocity and mitral annular early diastolic velocity; EF, ejection fraction; Em, early mitral inflow velocity; GCS, global circumferential strain; GLS, global longitudinal strain; HF, heart failure; HOMA-IR, homeostatic model assessment of insulin resistance; HRR, heart rate recovery; HTN, hypertension; IHD, ischaemic heart disease; LV, left ventricle; LVH, left ventricular hypertrophy; M, male; MET, metabolic equivalent of task; MPR, myocardial perfusion reserve; T1D, type 1 diabetes; T2D, type 2 diabetes; TTE, transthoracic echocardiography; VO2peak, peak oxygen consumption.
The pathogenesis of diabetic cardiomyopathy is complex and incompletely understood.3 Myocardial steatosis, altered myocardial energetics, and impaired calcium handling have all been implicated. In vivo studies have confirmed elevated myocardial triglyceride content in T2D57,58 and myocardial steatosis has been linked to both diastolic and systolic strain,59 linking steatosis with development of cardiac dysfunction. Myocardial energy metabolism (as assessed by the myocardial Creatinine phosphate/ATP ratio) is reduced in T2D and this is exacerbated by exercise.60 Abnormalities of myocardial calcium handling via impairments in the sarcoplasmic reticulum Ca2+ ATPase (SERCA) have been implicated in HF, but not in T2D. SERCA2a activity declines in late stage HF61 and decreased levels of SERCA2a have been found in cardiac tissues isolated from humans and animals with HF.62 Importantly, low SERCA2 levels have been correlated to poor clinical outcomes.62,63 Therapeutic approaches aiming to boost the myocardial SERCA2a levels have produced disappointing results.61,64 No studies, however, have directly evaluated links between myocardial steatosis, calcium handling, or energetics and impaired aerobic exercise capacity.
The predominant HF phenotype in people with T2D is HFpEF, which accounts for up to 83% of newly diagnosed cases of HF.65 In two contemporary large-scale HF trials of angiotensin-neprilysin inhibitor sacubitril-valsartan – PARAGON-HF52 and PARADIGM-HF5 – prevalence of diabetes was 44% and 35%, respectively, and in our own HFpEF cohort 54% of 140 patients had T2D.66 People with T2D appear to be particularly prone to development of diastolic LV impairment, although systolic LV impairment often co-exists. A significant proportion of patients with diastolic impairment are asymptomatic, which poses a clinical challenge as diastolic dysfunction even in isolation is associated with poor outcomes.3,67 Over the past three decades, the rapid evolution of advanced non-invasive cardiac imaging techniques has enabled detailed evaluation of cardiovascular structure and function in vivo. Application of these techniques has provided key insights to the relationship between cardiovascular function and exercise capacity in T2D, shedding light on early perturbations that may lead to HF (Table 2).
LV diastolic dysfunction
LV diastolic dysfunction is widely regarded as the earliest functional change occurring in diabetic cardiomyopathy.3 The reported prevalence of LV diastolic dysfunction in asymptomatic subjects ranges between 15% and 78%3,66,68 and differs according to imaging technique used. Subclinical diastolic dysfunction is frequently observed in asymptomatic, sedentary T2D even in the absence of microvascular complications, and is associated with impaired exercise tolerance (time and METs achieved).10,50 Several inverse correlations between indices of impaired LV relaxation and VO2peak in asymptomatic individuals have been identified, including smaller cardiac size (LV end-diastolic volume, r =
= 0.67)45 and attenuated increase in stroke volume during exercise,14 suggesting that impaired LV compliance may herald development of diastolic dysfunction. Invasive measurements of pulmonary capillary wedge pressure offer further insight on the impaired LV diastology in diabetes.50 The VO2peak and peak cardiac output were lower in T2D than in controls, and the pulmonary capillary wedge pressure rose significantly more during exercise in T2D than in controls (148% versus 109% increase at peak exercise, p
0.67)45 and attenuated increase in stroke volume during exercise,14 suggesting that impaired LV compliance may herald development of diastolic dysfunction. Invasive measurements of pulmonary capillary wedge pressure offer further insight on the impaired LV diastology in diabetes.50 The VO2peak and peak cardiac output were lower in T2D than in controls, and the pulmonary capillary wedge pressure rose significantly more during exercise in T2D than in controls (148% versus 109% increase at peak exercise, p <
< 0.01).50 However, the numbers included in the study were small and limited to females only, which precludes generalization to the whole population and conclusions on the causative associations of reduced exercise tolerance.
0.01).50 However, the numbers included in the study were small and limited to females only, which precludes generalization to the whole population and conclusions on the causative associations of reduced exercise tolerance.
The pathological myocardial and systemic changes precede development of overt diabetic cardiomyopathy, and can exist even in the absence of symptoms. The clinical importance of these findings is recognized by the ACC/AHA, who classify this as stage B HF (SBHF).
LV systolic dysfunction
Despite the association of T2D with HF, few studies have shown that diabetes causes a reduction in LV ejection fraction (EF), which remains the most utilized form of assessing LV performance. Furthermore, the evidence to suggest a relationship between VO2peak and systolic LV EF is lacking.69,70 Subclinical LV dysfunction, as measured by impaired myocardial strain and strain rates, is increasingly reported in T2D, and affects all layers of myocardium, from apex to base.60,71 Individuals with T2D have reduced global longitudinal strain (GLS) rate compared with controls, and it is detectable with a range of imaging techniques, including speckle tracking echocardiography69 and cardiac magnetic resonance (CMR) feature tracking.72 These impairments with GLS worsen over time,73 inversely correlate with indices of glycaemic control48 and have been found to be an independent predictor of cardiovascular events in longitudinal studies.73 GLS may thus offer an incremental prognostic value in this cohort, especially as GLS has been shown to be superior to LV EF at identifying patients with reduced exercise capacity.74 Several small observational studies have shown that GLS and global circumferential strain (GCS) may be independently associated with VO2peak. In a 100 patient study of adults with T2D, a GLS value of −17.3% had excellent sensitivity of 0.89 [95% confidence interval (CI) 0.79–0.95] and specificity of 0.91 (95% CI 0.71–0.99) to identify patients with a VO2peak of <20 mL/kg per min independent of age and sex.70
mL/kg per min independent of age and sex.70
In another study of 80 asymptomatic T2D, GLS (−21.6 ±
± 2.8 versus −18.4
2.8 versus −18.4 ±
± 2.3%, p
2.3%, p <
< 0.001) and GCS (−22.0
0.001) and GCS (−22.0 ±
± 2.9 versus −19.5
2.9 versus −19.5 ±
± 2.6%, p
2.6%, p <
< 0.001) were significantly reduced in all myocardial layers in T2D patients73 and were associated with lower VO2peak independently of other clinical and echocardiographic parameters of LV structure, and systolic and diastolic function.73
0.001) were significantly reduced in all myocardial layers in T2D patients73 and were associated with lower VO2peak independently of other clinical and echocardiographic parameters of LV structure, and systolic and diastolic function.73
Coronary microvascular dysfunction
Several studies have shown reduced myocardial perfusion reserve (MPR) in T2D, which is now being recognized as part of the pathophysiology of HF in T2D as well as HFpEF.35,60,71,75,76 Our group has assessed the association between aerobic capacity and cardiac structure and function in asymptomatic T2D, using a combination of multiparametric CMR and echocardiography (see Table 2).47 Even after exclusion of subjects with reversible perfusion defects, the overall MPR in the diabetic cohort was lower than in matched controls (2.60 ±
± 1.24 versus 3.54
1.24 versus 3.54 ±
± 1.15, respectively, p
1.15, respectively, p <
< 0.001). On both univariate and multivariable analysis in subjects with T2D, the ratio of early mitral inflow velocity and mitral annular early diastolic velocity (E/e′) (β
0.001). On both univariate and multivariable analysis in subjects with T2D, the ratio of early mitral inflow velocity and mitral annular early diastolic velocity (E/e′) (β =
= −0.388, p
−0.388, p <
< 0.001) and MPR (β
0.001) and MPR (β =
= 0.0822, p
0.0822, p =
= 0.006) were significantly associated with VO2peak independent of age, sex, ethnicity, smoking status and systolic blood pressure.47 This may be explained by the fact that myocardial perfusion must increase incrementally during exercise to meet the metabolic demands of tissues.77 A similar relationship has been documented in patients with severe aortic stenosis.77
0.006) were significantly associated with VO2peak independent of age, sex, ethnicity, smoking status and systolic blood pressure.47 This may be explained by the fact that myocardial perfusion must increase incrementally during exercise to meet the metabolic demands of tissues.77 A similar relationship has been documented in patients with severe aortic stenosis.77
Left atrial dysfunction
Left atrial (LA) enlargement is increasingly recognized for its association with adverse cardiac outcomes, including atrial fibrillation, stroke and heart failure.78
In addition, the LA plays an important role in cardiovascular response to exercise, specifically if LV diastology is also impaired. In diastolic LV dysfunction, prolonged relaxation time leads to a greater dependence on the atrial contribution at end-diastole for optimal filling.79 Diastolic filling time is inversely proportional to heart rate and this association is more pronounced during exercise. Reduced LV relaxation time leads to a greater dependence on atrial contribution at end-diastole for optimal filling.74 Impaired atrial systolic function will compromise cardiac output with effort, which highlights the role of the LA for maintaining exercise capacity.80
Atrial geometry, function and electrophysiological alterations are well-defined in patients with HFpEF and in atrial fibrillation, and have been closely linked with reduced exercise capacity and HF-related outcomes.81–92 However, atrial myopathy in T2D appears distinct. In adults with T2D, abnormalities of LA geometry have been described but the results are contradictory. While some studies reported smaller LA volumes in subjects with T2D89 others have shown the opposite.78 Smaller atrial volumes are observed in T2D in the presence of HFpEF, a disease typically associated with increased LA volumes.86,91,92 We have recently compared patients with HFpEF with and without T2D.66 Despite higher BMI and higher filling pressures (E/e′) than the patients without T2D, the diabetic HFpEFs had smaller LA volumes, suggesting that atrial myopathy in T2D is different from LA dilatation observed in HFpEF.90 T2D has been proposed as an independent risk factor for LA impairment, regardless of co-existent hypertension or presence of LV diastolic dysfunction.89,90 Whilst the link between LA dysfunction and reduced exercise capacity is well established in HFpEF, there is paucity of data in asymptomatic people with T2D. Abnormalities of LA function are utilized as prognostic markers in heterogenous cohorts of HFpEF patients which included T2D: increased indexed LA volume (>32 mL/m2)87,90 and reduced LA peak strain,93–96 reservoir, conduit and pump function66 have all been found to independently correlate with an increased risk of major adverse cardiovascular events50,68,86 and hospitalization for HF.85
mL/m2)87,90 and reduced LA peak strain,93–96 reservoir, conduit and pump function66 have all been found to independently correlate with an increased risk of major adverse cardiovascular events50,68,86 and hospitalization for HF.85
Strategies to improve exercise capacity
Weight loss and exercise
Weight loss confers a number of clinically important benefits in patients with T2D.
Weight loss achieved through bariatric surgery97,98 or low-calorie meal replacement diet58 results in remission to a non-diabetic state, with a strong correlation between the extent of weight loss and reversal of T2D. However, the same effects are not seen in more advanced T2D (defined by insulin therapy) or with longer disease duration.58,97,98 Sustained weight loss also confers direct beneficial cardiovascular effects in obese adults without T2D, with reductions in LV mass, volumes, arterial stiffness and diastolic function as measured by CMR.3 Improved diastolic function, energetics and reduced myocardial triglyceride content, which may confer benefit to exercise tolerance, have been reported in obese individuals following bariatric surgery-mediated weight loss.99 Importantly, bariatric surgery can achieve sustained weight loss (in up to one-fifth of patients), sustained remission of diabetes (in up to one-third of patients) and lower rates of major adverse cardiovascular events (including HF) in people with T2D and obesity.
In addition to benefits on cardiac function, weight loss simply improves physical function in adults with T2D by reducing the biomechanical burden of moving around.47 However, the cardiovascular benefits of weight loss alone do not directly translate to significant improvements in objective measures of aerobic exercise capacity. In fact, a number of studies have reported reduced strength and VO2max in individuals exposed to caloric restriction alone.47 In a study of 52 obese individuals, daily calorific reduction of 20% mediated weight loss of approximately 7% of body mass over a 12 week period and corresponded to an approximately 6% reduction in absolute VO2max, an effect that was attenuated by exercise.99–101 Conversely, exercise alone resulted in 15% improvement in the VO2max, even in the absence of weight loss. The combination of modest exercise (4.4 ±
± 0.5
0.5 h/week) and 20% calorific reduction attenuated the reduction in lean mass and aerobic capacity that occurred with calorific reduction alone. Weight loss achieved through exercise confers the greatest benefits on preservation of lean mass and increase in VO2max, but the required amount of exercise to cause weight loss is substantial (7.4
h/week) and 20% calorific reduction attenuated the reduction in lean mass and aerobic capacity that occurred with calorific reduction alone. Weight loss achieved through exercise confers the greatest benefits on preservation of lean mass and increase in VO2max, but the required amount of exercise to cause weight loss is substantial (7.4 ±
± 0.5
0.5 h/week) and may be challenging to achieve in deconditioned, overweight individuals in the real world.100
h/week) and may be challenging to achieve in deconditioned, overweight individuals in the real world.100
Our group has also assessed the impact of lifestyle interventions on cardiac function and exercise capacity in younger obese adults with T2D.102 We undertook a 12-week randomized trial comparing a supervised exercise programme or low energy meal replacement diet. A significant improvement in the primary outcome of diastolic function was observed in the exercise arm, despite only small reductions in weight, BMI and exercise capacity. By contrast, in the diet arm there was dramatic overall weight loss (median 13.6 kg and fall in BMI of 4.8
kg and fall in BMI of 4.8 kg/m2) accompanied by a mean HbA1c decrease of 0.75%, with 83% of participants achieving T2D remission. However, only a small increase in VO2peak when corrected for body weight (1.9
kg/m2) accompanied by a mean HbA1c decrease of 0.75%, with 83% of participants achieving T2D remission. However, only a small increase in VO2peak when corrected for body weight (1.9 mL/kg per min) was observed, but there was no change in absolute VO2peak.103 There were no significant improvements in myocardial perfusion or remodelling with exercise.103 Although exercise has been found to improve endothelial function, it is possible that the small sample size and short duration (12
mL/kg per min) was observed, but there was no change in absolute VO2peak.103 There were no significant improvements in myocardial perfusion or remodelling with exercise.103 Although exercise has been found to improve endothelial function, it is possible that the small sample size and short duration (12 weeks) of follow-up precluded these effects from fully manifesting in this study. The lack of improvement in VO2peak may be explained by loss of lean tissue mass.103 Even in obese individuals, weight loss resulting from calorific restriction results in loss of lean tissue as fat free mass in a 1:4 ratio with adipose tissue. The predominant site of reduction in lean body mass is the skeletal muscle, which when coupled with possible reductions in the functional capacity of the musculature with weight loss, limit the magnitude of benefits realized.103
weeks) of follow-up precluded these effects from fully manifesting in this study. The lack of improvement in VO2peak may be explained by loss of lean tissue mass.103 Even in obese individuals, weight loss resulting from calorific restriction results in loss of lean tissue as fat free mass in a 1:4 ratio with adipose tissue. The predominant site of reduction in lean body mass is the skeletal muscle, which when coupled with possible reductions in the functional capacity of the musculature with weight loss, limit the magnitude of benefits realized.103
Pharmacological treatments
In addition to improving diabetic control, several antidiabetic treatments have been shown to have cardioprotective effects, but data on their efficacy in improving anaerobic capacity are sparse. Two large randomized controlled trials – LEADER (liraglutide)104 and PIONEER-6 (semaglutide)105,106 – have shown a reduction on atherosclerotic cardiovascular events with glucagon-like receptor 1 agonist treatment, and in the case of semaglutide a nearly 14% weight loss.103 However, neither study had examined improvements in exercise capacity.
The beneficial cardiovascular effects of sodium-dependent glucose linked transporter-2 inhibitor (SGLT2i) therapy are well established. In the largest to date SGLT2i trial, DECLARE-TIMI 58, dapagliflozin reduced the risk of death or hospitalization for HF by 17% even in lower-risk patients with T2D.105 SGLT2is exert cardioprotective effects which may be beneficial to improving exercise tolerance, including favourable changes in LV mass and wall stress, lowering arterial stiffness and improvements in myocardial energetics.106 To our knowledge, only one study to date has examined the effects of SGLT2i on physical function.104 In a randomized, double blinded study of dapagliflozin and exercise versus dapagliflozin and placebo, the dapagliflozin treatment resulted in 15% increase in exercise capacity from baseline compared with exercise and placebo (VO2peak 2.58 ±
± 0.63
0.63 mL/kg per min versus 2.98
mL/kg per min versus 2.98 ±
± 0.63
0.63 mL/kg per min p
mL/kg per min p <
< 0.001).104 The precise mechanisms behind this effect of dapagliflozin are unclear. The proposed mechanisms include improved vascular function,107 decreased arterial stiffness,107 preferential shift to fatty acid oxidation and ketotic metabolism which is favourable to cardiac energetics,108 and weight loss.107 These must be interpreted with caution as none of the studies used HF as an end point, nor assessed the role of SGLT2i in improving exercise capacity on a wider scale. Nevertheless, the finding that SGLT2i may improve exercise capacity is of interest, and deserves further examination in larger studies.
0.001).104 The precise mechanisms behind this effect of dapagliflozin are unclear. The proposed mechanisms include improved vascular function,107 decreased arterial stiffness,107 preferential shift to fatty acid oxidation and ketotic metabolism which is favourable to cardiac energetics,108 and weight loss.107 These must be interpreted with caution as none of the studies used HF as an end point, nor assessed the role of SGLT2i in improving exercise capacity on a wider scale. Nevertheless, the finding that SGLT2i may improve exercise capacity is of interest, and deserves further examination in larger studies.
Conclusion
The scale of T2D prevalence has now reached pandemic proportions. Individuals with T2D are at high risk of cardiovascular mortality and HF. It is widely accepted that people with T2D have a baseline reduction in exercise capacity, which confers increased clinical risk of morbidity and mortality. Exercise intolerance can be present in otherwise asymptomatic individuals, and may be the first sign of HF. A multitude of factors contribute to reduced exercise capacity and HF risk in T2D, including metabolic dysregulation, chronic inflammation, endothelial dysfunction, frailty, cardiac systolic and diastolic dysfunction, impaired myocardial energetics, steatosis, calcium homeostasis, coronary microvascular dysfunction and LA myopathy. Whilst there are a number of clinical scoring systems designed to stratify the risk of development of cardiovascular complications in T2D, none of these have been validated for predicting the reduced exercise tolerance in T2D, and thus helping to identify those at risk of HF. Strategies to improve cardiovascular fitness based on combination of diet and exercise appear to be the most efficacious way towards improving outcomes in those with T2D, although newer glucose-lowering therapies may play a key role in preventing HF development in the future.
Footnotes
Authors Contribution: GPM conceived the idea for the manuscript and decided the overall theme and content. JMB drafted the first draft. JMB and GSG revised the manuscript. All authors critically reviewed and approved the final version.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: GPM is supported by a NIHR Research Professorship (2017-08-ST2-007) and GSG was supported by a BHF Clinical Research Training Fellowship (FS/16/47/32190).
ORCID iDs: Gaurav S. Gulsin  https://orcid.org/0000-0002-1212-505X
https://orcid.org/0000-0002-1212-505X
Gerry P. McCann  https://orcid.org/0000-0002-1740-9270
https://orcid.org/0000-0002-1740-9270
Contributor Information
Joanna M. Bilak, Department of Cardiovascular Sciences, University of Leicester and The National Institute for Health Research (NIHR) Leicester Biomedical Research Centre, Leicester, UK.
Gaurav S. Gulsin, Department of Cardiovascular Sciences, University of Leicester and The National Institute for Health Research (NIHR) Leicester Biomedical Research Centre, Leicester, UK.
Gerry P. McCann, Department of Cardiovascular Sciences, University of Leicester, Glenfield Hospital, Groby Road, Leicester LE39QP, UK.
References
Articles from Therapeutic Advances in Endocrinology and Metabolism are provided here courtesy of SAGE Publications
Full text links
Read article at publisher's site: https://doi.org/10.1177/2042018820980235
Read article for free, from open access legal sources, via Unpaywall:
https://journals.sagepub.com/doi/pdf/10.1177/2042018820980235
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/99198190
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1177/2042018820980235
Article citations
Clinical and Electrocardiographic Predictors of Cardiac Resynchronization Therapy Response That Correlate with the 6 min Walking Test.
J Clin Med, 13(20):6287, 21 Oct 2024
Cited by: 0 articles | PMID: 39458240 | PMCID: PMC11508288
Type 2 diabetes mellitus negatively affects the functional performance of 6-min step test in chronic heart failure: a 3-year follow-up study.
Diabetol Metab Syndr, 16(1):229, 14 Sep 2024
Cited by: 0 articles | PMID: 39272115 | PMCID: PMC11401430
Obesity dominates early effects on cardiac structure and arterial stiffness in people with type 2 diabetes.
J Hypertens, 41(11):1775-1784, 17 Aug 2023
Cited by: 1 article | PMID: 37589719 | PMCID: PMC10592255
Impact of diabetes on remodelling, microvascular function and exercise capacity in aortic stenosis.
Open Heart, 10(2):e002441, 01 Aug 2023
Cited by: 2 articles | PMID: 37586847 | PMCID: PMC10432628
Impact of the Remission of Type 2 Diabetes on Cardiovascular Structure and Function, Exercise Capacity and Risk Profile: A Propensity Matched Analysis.
J Cardiovasc Dev Dis, 10(5):191, 24 Apr 2023
Cited by: 0 articles | PMID: 37233158 | PMCID: PMC10219263
Go to all (8) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Diabetic cardiomyopathy: prevalence, determinants and potential treatments.
Ther Adv Endocrinol Metab, 10:2042018819834869, 27 Mar 2019
Cited by: 49 articles | PMID: 30944723 | PMCID: PMC6437329
Review Free full text in Europe PMC
Asymptomatic Diabetic Cardiomyopathy: an Underrecognized Entity in Type 2 Diabetes.
Curr Diab Rep, 21(10):41, 27 Sep 2021
Cited by: 12 articles | PMID: 34580767
Review
Use of Sodium-Glucose Cotransporter-2 Inhibitors in Clinical Practice for Heart Failure Prevention and Treatment: Beyond Type 2 Diabetes. A Narrative Review.
Adv Ther, 39(2):845-861, 09 Dec 2021
Cited by: 12 articles | PMID: 34881413 | PMCID: PMC8866261
Review Free full text in Europe PMC
Rationale for the Early Use of Sodium-Glucose Cotransporter-2 Inhibitors in Patients with Type 2 Diabetes.
Adv Ther, 36(10):2567-2586, 23 Aug 2019
Cited by: 7 articles | PMID: 31444707 | PMCID: PMC6822830
Review Free full text in Europe PMC
Funding
Funders who supported this work.
British Heart Foundation (1)
Prevalence and determinants of subclinical cardiovascular dysfunction in adults with Type 2 Diabetes Mellitus (Dr Gaurav Gulsin)
Prof Gerald McCann, University of Leicester
Grant ID: FS/16/47/32190
National Institute for Health Research (NIHR) (1)
Heart failure in type 2 diabetes: improving diagnosis and management in a multi-ethnic population.
Prof Gerald McCann, University of Leicester
Grant ID: RP-2017-08-ST2-007

