Abstract
Background
Septic shock is a major public health problem that is associated with up to 50% mortality. Unfavorable outcomes are mainly attributed to multiple organ failure (MOF) resulting from an uncontrolled inflammatory response and ischemia-reperfusion processes. REmote ischemic COnditioning (RECO) is a promising intervention to prevent ischemia-reperfusion injury. We hypothesize that RECO would reduce the severity of septic shock-induced MOF.Methods/design
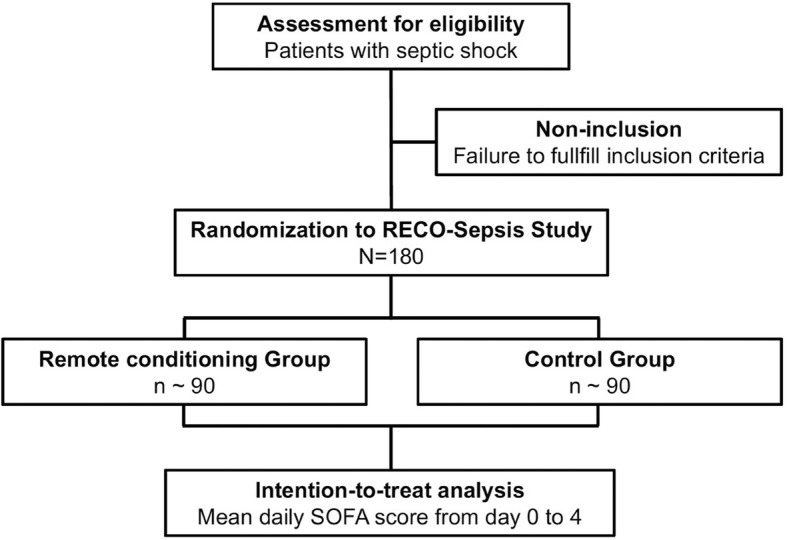
RECO in septic shock patients (RECO-Sepsis study) is an ongoing, prospective, multicenter, randomized, open-label trial, testing whether RECO, as an adjuvant therapy to conventional treatment in septic shock, decreases the severity of MOF as assessed by the Sequential Organ Failure Assessment (SOFA) score. Adult patients admitted to an intensive care unit with documented or suspected infection, lactatemia > 2 mmol/l, and treated with norepinephrine for less than 12 h are potentially eligible for the study. Non-inclusion criteria are: having expressed the wish not to be resuscitated, contraindication for the use of a brachial cuff on both arms, intercurrent disease with an expected life expectancy of less than 24 h, cardiac arrest, and pregnant or breastfeeding women. After enrollment, patients are randomized (n = 180) 1:1 to receive RECO or no adjunctive intervention. RECO consists of four cycles of cuff inflation to 200 mmHg for 5 min and then deflation to 0 mmHg for another 5 min. RECO is performed at inclusion and repeated 12 and 24 h later. The primary endpoint is the mean daily SOFA score up to day 4 after inclusion. Secondary outcomes include the need for organ support, hospital length of stay, and 90-day mortality.Discussion
Results of this proof-of-concept trial should provide information on the efficacy of RECO in patients with septic shock.Trial registration
ClinicalTrials.gov, ID: identifier: NCT03201575 . Registered on 28 June 2017.Free full text

Remote ischemic conditioning in septic shock (RECO-Sepsis): study protocol for a randomized controlled trial
Abstract
Background
Septic shock is a major public health problem that is associated with up to 50% mortality. Unfavorable outcomes are mainly attributed to multiple organ failure (MOF) resulting from an uncontrolled inflammatory response and ischemia-reperfusion processes. REmote ischemic COnditioning (RECO) is a promising intervention to prevent ischemia-reperfusion injury. We hypothesize that RECO would reduce the severity of septic shock-induced MOF.
Methods/design
RECO in septic shock patients (RECO-Sepsis study) is an ongoing, prospective, multicenter, randomized, open-label trial, testing whether RECO, as an adjuvant therapy to conventional treatment in septic shock, decreases the severity of MOF as assessed by the Sequential Organ Failure Assessment (SOFA) score. Adult patients admitted to an intensive care unit with documented or suspected infection, lactatemia > 2
2 mmol/l, and treated with norepinephrine for less than 12
mmol/l, and treated with norepinephrine for less than 12 h are potentially eligible for the study. Non-inclusion criteria are: having expressed the wish not to be resuscitated, contraindication for the use of a brachial cuff on both arms, intercurrent disease with an expected life expectancy of less than 24
h are potentially eligible for the study. Non-inclusion criteria are: having expressed the wish not to be resuscitated, contraindication for the use of a brachial cuff on both arms, intercurrent disease with an expected life expectancy of less than 24 h, cardiac arrest, and pregnant or breastfeeding women. After enrollment, patients are randomized (n
h, cardiac arrest, and pregnant or breastfeeding women. After enrollment, patients are randomized (n =
= 180) 1:1 to receive RECO or no adjunctive intervention. RECO consists of four cycles of cuff inflation to 200
180) 1:1 to receive RECO or no adjunctive intervention. RECO consists of four cycles of cuff inflation to 200 mmHg for 5 min and then deflation to 0
mmHg for 5 min and then deflation to 0 mmHg for another 5 min. RECO is performed at inclusion and repeated 12 and 24
mmHg for another 5 min. RECO is performed at inclusion and repeated 12 and 24 h later. The primary endpoint is the mean daily SOFA score up to day 4 after inclusion. Secondary outcomes include the need for organ support, hospital length of stay, and 90-day mortality.
h later. The primary endpoint is the mean daily SOFA score up to day 4 after inclusion. Secondary outcomes include the need for organ support, hospital length of stay, and 90-day mortality.
Discussion
Results of this proof-of-concept trial should provide information on the efficacy of RECO in patients with septic shock.
Trial registration
ClinicalTrials.gov, ID: identifier: NCT03201575. Registered on 28 June 2017.
Electronic supplementary material
The online version of this article (10.1186/s13063-019-3406-4) contains supplementary material, which is available to authorized users.
Background
Septic shock is a major and growing public health problem affecting millions of patients worldwide annually and 10 to 30% of patients admitted to intensive care units (ICUs) [1]. Despite regularly updated international guidelines and significant improvement in the treatment of septic shock, short-term mortality, which is mostly attributed to multiple organ failure (MOF), still remains as high as 50% [1, 2]. In view of these alarming figures, there is a compelling need for new therapeutic approaches to prevent MOF and thereby improve survival in this disease.
The pathophysiology of septic shock-induced MOF is complex and remains incompletely understood. It is recognized that it results, at least in part, from an uncontrolled systemic inflammatory response and ischemia/reperfusion (I/R) processes related to transient arterial hypotension, low cardiac output, and compromised microcirculation with cellular hypoxia/reoxygenation and mitochondrial dysfunction [2–4]. However, among the first-line treatments recommended for patients with septic shock (i.e., antibiotics, source control of infection, fluids, and vasoactive drugs), none specifically targets I/R injury [2].
REmote ischemic COnditioning (RECO) represents an innovative strategy to protect organs (e.g., brain, heart, kidneys) against the deleterious effect of I/R through activation of cell survival pathways and modulation of the inflammatory response [5–8]. RECO usually consists of applying brief and repeated cycles of non-lethal ischemia alternating with reperfusion by inflating and deflating a blood pressure cuff placed around a limb [5–8]. Over the past few years, this simple, inexpensive, non-invasive, and innocuous intervention has been investigated in numerous clinical trials with promising results [9–13]. For instance, it has been shown that RECO may limit myocardial damage after both acute myocardial infarction [9] and on-pump cardiac surgery [10]. Beyond protection of the heart, RECO has also been reported to be nephroprotective and neuroprotective, suggesting a ubiquitous effect of this intervention in humans [11–13]. Specifically for septic shock, only pre-clinical data are available and these indicate clear benefits of RECO, including survival, in both small and large animals [14–16].
To the best of our knowledge, RECO has not been tested in patients with septic shock. We have, therefore, implemented a multicenter, randomized controlled trial (RECO-Sepsis study) to investigate whether ischemic conditioning applied by inflating/deflating a cuff around an upper limb would limit the severity of MOF in septic shock patients.
Methods/design
Design and setting
The trial protocol was developed in accordance with the SPIRIT (Standard Protocol Items: Recommendations for Interventional Trials) guidelines [17] (Additional file 1). RECO-Sepsis is a prospective, multicenter, randomized, open-label, parallel-group trial, testing whether RECO as an adjuvant therapy to conventional treatment in septic shock would decrease the severity of MOF as measured by the Sequential Organ Failure Assessment (SOFA) score [18–21]. The trial is conducted in French intensive care units (ICUs). An overview of enrollment, interventions, and follow-up of participants in the RECO-Sepsis trial is shown in Figs. 1 and and2.2. The trial is supported by a grant from the French Ministry of Health (Programme Hospitalier de Recherche Clinique Inter-régional 2016) and the study sponsor is the Hospices Civils de Lyon. The study was registered at ClinicalTrials.gov (NCT03201575) on 28 June 2017.
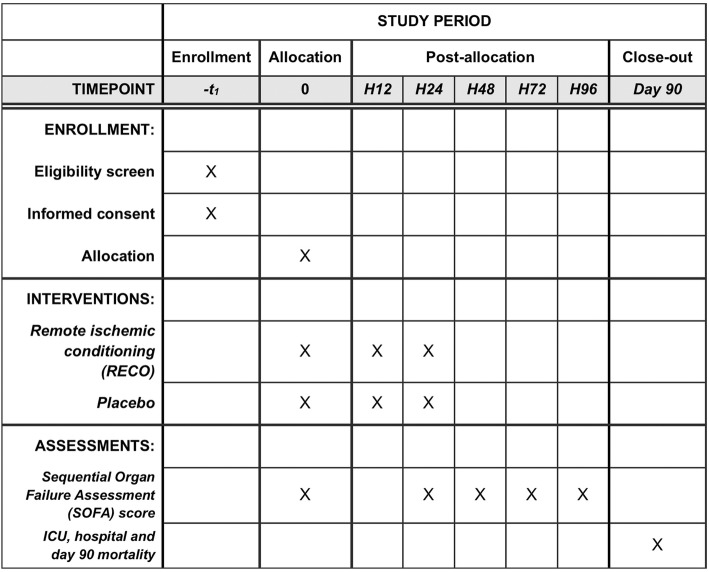
Schedule of enrollment, interventions, and assessments according to Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT). ICU intensive care unit
Ethics
The trial is conducted in accordance with the principles of the Declaration of Helsinki 1964 as revised in 2013 and the International Conference of Harmonization Guidelines for Good Clinical Practice (ICH GCP E6 guidelines). In accordance with French laws, the trial was authorized by the French national drug agency (Agence Nationale de la Sécurité du Médicament, ANSM) on 23 August 2017. The study was also approved by the Institutional Review Board (Comité de Protection des Personnes CCP Ile-de-France X) on 5 September 2017 (n°2017-A01684–49).
Participants
All patients admitted to the participating centers are screened against eligibility criteria. Inclusion criteria are as follows: adult patients (aged 18 years or older); hospitalized in ICU for less than 24
years or older); hospitalized in ICU for less than 24 h, and septic shock evolving for less than 12
h, and septic shock evolving for less than 12 h. Septic shock is defined according to the 2016 revised standard consensus criteria: documented or suspected infection, lactatemia >
h. Septic shock is defined according to the 2016 revised standard consensus criteria: documented or suspected infection, lactatemia > 2
2 mmol/l, and norepinephrine administration to maintain a mean arterial pressure (MAP) above 65
mmol/l, and norepinephrine administration to maintain a mean arterial pressure (MAP) above 65 mmHg after adequate fluid resuscitation, [22]. Non-inclusion criteria are as follows: having expressed the wish not to be resuscitated, contraindication for the use of a brachial cuff on both arms, intercurrent disease with an expected life expectancy of less than 24
mmHg after adequate fluid resuscitation, [22]. Non-inclusion criteria are as follows: having expressed the wish not to be resuscitated, contraindication for the use of a brachial cuff on both arms, intercurrent disease with an expected life expectancy of less than 24 h, cardiac arrest, pregnant or breastfeeding women, previous inclusion in the study or participation in another interventional study, lack of French national health insurance coverage, and being under judicial protection.
h, cardiac arrest, pregnant or breastfeeding women, previous inclusion in the study or participation in another interventional study, lack of French national health insurance coverage, and being under judicial protection.
Informed consent
Informed consent that details the study design and outcomes must be obtained prior to inclusion. If the patient is able to consent but cannot write, an oral consent can be obtained in the presence of an impartial witness, independent of the study investigator. It is expected that, in most cases, it will not be possible to obtain prospective consent from the patient due to incapacity resulting from critical illness and its treatment. Under such conditions, written consent is sought from next of kin. All surviving patients are informed about the trial once they regain capacity and are asked to give consent to their ongoing involvement in the trial. Patient or their relatives can withdraw their consent and discontinue the participation in the study at any time upon request.
Randomization
After consent, eligible patients are randomly assigned to received RECO or not. Randomization is stratified by center on a 1:1 basis in permuted blocks. Randomization is performed online using the ClinSight™ software (Ennov Clinical Software, Paris, France).
Intervention
As soon as possible after randomization to either the RECO group or the Control group, a standard CE-approved blood pressure cuff (Tensiomètre Manopoire Duo Colson®, Drive DeVilbiss Healthcare, Frouard, France) is placed, deflated, around one of the patient’s upper arms. In line with the recommendations for non-invasive measurement of blood pressure, the size of the cuff is chosen to accommodate the patient’s arm [23]. In the RECO group, ischemic conditioning consists of four cycles of cuff inflation to 200 mmHg for 5 min and then deflation to 0
mmHg for 5 min and then deflation to 0 mmHg for another 5 min (40 min total duration of the intervention). Both the safety and the efficacy of this standard protocol has been demonstrated in previous clinical trials [5–7, 9–13]. In the Control group, the cuff is left in place, deflated, for 40
mmHg for another 5 min (40 min total duration of the intervention). Both the safety and the efficacy of this standard protocol has been demonstrated in previous clinical trials [5–7, 9–13]. In the Control group, the cuff is left in place, deflated, for 40 min (sham procedure). The RECO or the sham procedure is repeated at 12 and 24
min (sham procedure). The RECO or the sham procedure is repeated at 12 and 24 h after inclusion. Standard operation procedures (SOP) describing the interventions are available for all investigators. A written checklist is completed by the investigator performing the intervention to ensure protocol adherence. With the exception of the tested intervention, patients receive standard care in accordance with international guidelines [2].
h after inclusion. Standard operation procedures (SOP) describing the interventions are available for all investigators. A written checklist is completed by the investigator performing the intervention to ensure protocol adherence. With the exception of the tested intervention, patients receive standard care in accordance with international guidelines [2].
Primary outcome
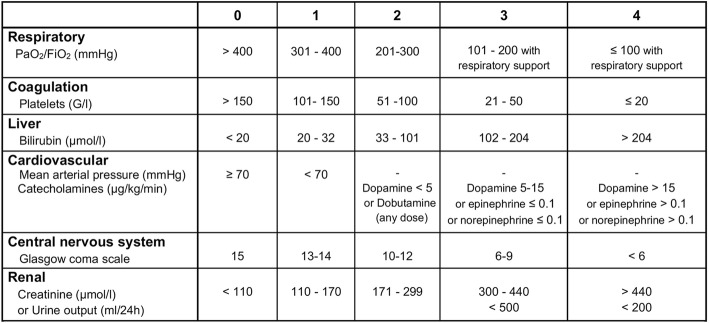
The primary outcome of this trial is the severity of sepsis-induced MOF as assessed by the mean daily SOFA score from inclusion (day 0) to the fourth day after inclusion (day 4) or to the day of death if it occurs before day 4. The SOFA score is an objective tool to quantitatively describe the degree of organ dysfunction over time [18–21]. The mean daily SOFA score, which closely correlates with mortality in critically ill patients [19], is regularly used as a surrogate outcome for death in sepsis trials [24, 25]. The choice of a mean SOFA score helps to solve the “truncated by death” issue as all patients contribute scores and no imputation of data is needed if a patient dies. As shown in Table 1, the SOFA score ranges from 0 to 24 (higher scores indicate more severe organ failure), with 0 to 4 points assigned for each of six organ dysfunctions (i.e., respiratory, coagulation, liver, cardiovascular, central nervous system, and renal). As previously described, a daily SOFA score is calculated using the most abnormal clinical and/or biological values in the previous 24 h (or from the start of the considered 24-h period to death) [18–21]. Predefined rules for dealing with potential missing data have been established.
h (or from the start of the considered 24-h period to death) [18–21]. Predefined rules for dealing with potential missing data have been established.
Table 1
Sequential Organ Failure Assessment (SOFA) score. PaO2 partial pressure of oxygen, FiO2 Fraction of inspired oxygen
Secondary outcomes
The main secondary endpoints include: mean SOFA score from day 0 to day 4 after exclusion of the neurological subscore; daily SOFA scores from day 0 to day 4; daily SOFA subscores for each organ/system from day 0 to day 4; variation of SOFA scores from day 0 to day 4; number of days alive without organ support (i.e., invasive mechanical ventilation, renal replacement therapy, or vasoactive drugs) on day 28; length of ICU and hospital stay; all-cause mortality in ICU, hospital, on day 28 and on day 90.
Data collection
The following data are recorded: demographics, concurrent medical conditions and comorbidities, sepsis diagnosis and treatment, severity of illness and organ dysfunction scores, vital signs and laboratory results, characteristics of the RECO procedure, life-sustaining therapies, and outcomes (follow-up: 90 days). Data are entered into a web-based electronic case report form (eCRF, ClinSight™ software) from patient notes (source) under the supervision of the trial site investigators. A unique numeric identifier is assigned to each patient. All the data entered in the study database are anonymized in order to ensure confidentiality. Assessments are made at inclusion, and on days 1, 2, 3, 4, 28, and 90. From the eCRFs, the trial database will be established. According to French laws, all original records (including informed consent forms, eCRF, and relevant correspondences) will be archived at trial sites for 25
days). Data are entered into a web-based electronic case report form (eCRF, ClinSight™ software) from patient notes (source) under the supervision of the trial site investigators. A unique numeric identifier is assigned to each patient. All the data entered in the study database are anonymized in order to ensure confidentiality. Assessments are made at inclusion, and on days 1, 2, 3, 4, 28, and 90. From the eCRFs, the trial database will be established. According to French laws, all original records (including informed consent forms, eCRF, and relevant correspondences) will be archived at trial sites for 25 years.
years.
Safety
Given that the trial is being conducted in critically ill patients, a significant number of patients are likely to experience adverse events (AEs) not related to the intervention. Therefore, only grade-3 or more AEs are reported. The following clinical outcomes from sepsis will not be recorded as AEs unless the local investigator deems the event to be related to the study: cardiovascular failure, respiratory failure, hepatic failure, renal failure, hematological failure, or neurological failure. While no significant RECO-related AE is expected based on previously published studies, all mechanical complications related to the use of the blood pressure cuff and leading to a medical/surgical intervention are systematically reported as serious AEs to the sponsor center within 24 h and to the relevant authorities in accordance with current French regulations. Any death occurring within 90
h and to the relevant authorities in accordance with current French regulations. Any death occurring within 90 days after inclusion is also reported as a serious AE. All AEs are assessed for relationship with the tested intervention. No data safety monitoring board will be ask to monitor the study.
days after inclusion is also reported as a serious AE. All AEs are assessed for relationship with the tested intervention. No data safety monitoring board will be ask to monitor the study.
Monitoring
Monitoring on site of all centers is performed by the study sponsor. The monitor will review the entries into the eCRF on the basis of source documents. Monitors will review the consent forms and all data contributing to the primary outcome. The investigators must give access to all essential source data and must provide support at all times to the monitor. The monitor also ensures regularly that the trial is conducted according to the protocol and regulatory requirements. In each center, a monitoring is planned after the enrollment of the first two patients and afterwards after every six patients.
Study organization
The RECO-Sepsis study is coordinated by the Clinical Investigation Center (CIC) located in Lyon, France. Each participating center has a senior investigator as principal investigator with strong clinical trial experience. All trial-related processes follow the SOP of the RECO-Sepsis study protocol. The RECO-Sepsis Steering Committee is co-chaired by Martin Cour, MD, PhD and Laurent Argaud, MD, PhD (Claude Bernard University of Lyon 1 – Edouard Herriot Hospital, Lyon, France). The Steering Committee is responsible for the scientific content of the protocol and oversees the trial operations. The Steering Committee will have full access to all the data in the study and will have final responsibility for the decision to submit the results for publication.
Role of the funding source
The funding source has no role in the study design, data collection, data analysis, data interpretation and writing of the final report.
Sample size
No a priori evidence suggests the magnitude of the effect of RECO on the severity of MOF in septic shock patients. Under the alternative hypothesis of an expected difference of half the standard deviation (effect size: 0.5) of the mean daily SOFA score of the first 4 days after inclusion, at least 168 patients have to be enrolled to reject the null hypothesis of a similar mean SOFA score in both arms with a power of 90% (β
days after inclusion, at least 168 patients have to be enrolled to reject the null hypothesis of a similar mean SOFA score in both arms with a power of 90% (β =
= 10%) and type 1 error at α
10%) and type 1 error at α =
= 5% (two-tailed). Even though there is no published data regarding the mean daily SOFA score in patients meeting our eligibility criteria, one can assume a mean value ranging from 8 to 12 with a standard deviation ranging from 4 to 6. Thus, for example, if the standard deviation is found to be 4, then a decrease of 2 or more in the mean daily SOFA score will be needed to reach significance. We planned to recruit an additional 7% patients (n
5% (two-tailed). Even though there is no published data regarding the mean daily SOFA score in patients meeting our eligibility criteria, one can assume a mean value ranging from 8 to 12 with a standard deviation ranging from 4 to 6. Thus, for example, if the standard deviation is found to be 4, then a decrease of 2 or more in the mean daily SOFA score will be needed to reach significance. We planned to recruit an additional 7% patients (n =
= 12) to account for potential loss to follow-up and withdrawal of consent. Thus, the planned sample size is 180 patients (90 patients in each arm). Based on the enrollment capacity of the participating centers, the recruitment is expected to be completed within 18
12) to account for potential loss to follow-up and withdrawal of consent. Thus, the planned sample size is 180 patients (90 patients in each arm). Based on the enrollment capacity of the participating centers, the recruitment is expected to be completed within 18 months. The sample size was calculated with the use of nQuery Advisor, version 5.0 (Statistical solution, Cork, Ireland).
months. The sample size was calculated with the use of nQuery Advisor, version 5.0 (Statistical solution, Cork, Ireland).
Statistical analysis
The trial will end once 180 patients have been enrolled and have completed the 90 days’ follow-up. No interim analysis will be planned for this study. A full statistical analysis plan will be drawn up by the CIC (Lyon) before the trial database is locked. Prior to performing any statistical tests or fitting statistical models, an exploratory analysis of the baseline variables will be completed. The number and pattern of missing data for baseline variables and outcomes will be established by forming appropriate tables and likely causes of any missing values will be investigated. Variables will be expressed as median and interquartile range (IQR) or number and proportion, as appropriate. Statistical analyses of the primary and secondary efficacy endpoints will primarily be based on the intent-to-treat population. The main analysis of the primary endpoint, the mean daily SOFA score from day 0 to day 4, will be performed by fitting a mixed-effects linear model with a fixed effect for the assigned treatment and a random centre effect. Secondary outcomes will be analyzed with appropriate statistical methods including the Wilcoxon rank sum, chi2 and log-rank tests. The same analysis will be performed on the per-protocol population, which will include all randomized patients who have received the intervention in accordance with the allocated group without any major protocol deviation. A probability value of <
days’ follow-up. No interim analysis will be planned for this study. A full statistical analysis plan will be drawn up by the CIC (Lyon) before the trial database is locked. Prior to performing any statistical tests or fitting statistical models, an exploratory analysis of the baseline variables will be completed. The number and pattern of missing data for baseline variables and outcomes will be established by forming appropriate tables and likely causes of any missing values will be investigated. Variables will be expressed as median and interquartile range (IQR) or number and proportion, as appropriate. Statistical analyses of the primary and secondary efficacy endpoints will primarily be based on the intent-to-treat population. The main analysis of the primary endpoint, the mean daily SOFA score from day 0 to day 4, will be performed by fitting a mixed-effects linear model with a fixed effect for the assigned treatment and a random centre effect. Secondary outcomes will be analyzed with appropriate statistical methods including the Wilcoxon rank sum, chi2 and log-rank tests. The same analysis will be performed on the per-protocol population, which will include all randomized patients who have received the intervention in accordance with the allocated group without any major protocol deviation. A probability value of < 0.05 will be considered to indicate statistical significance and 95% confidence intervals will be calculated.
0.05 will be considered to indicate statistical significance and 95% confidence intervals will be calculated.
Dissemination of the results
The final trial report will be submitted to high-quality, peer-reviewed journals regardless of the results. The results of the trial will be reported in accordance with the Consolidated Standards of Reporting Trials (CONSORT) Statement [26, 27]. The Steering Committee will grant authorship depending on personal input according to the Vancouver definitions. All trial sites with at least one randomized patient will be granted at least one authorship. All trial sites and trial site investigators will be acknowledged. Side studies will be allowed if supported by the Steering Committee.
Discussion
The protocol describes the first randomized controlled trial designed to evaluate the efficacy of RECO in septic shock patients. Conduct of the RECO-Sepsis trial is in broad agreement with the international guidelines. The RECO-Sepsis trial has sufficient power to detect a significant decrease in the severity of septic shock-induced MOF, which is known to be strongly correlated with outcomes. We empirically chose to repeat three times the RECO procedure within the first 24 h after randomization to enhance the potential effects of the intervention. We acknowledge that, in absence of comparison data on different modalities of RECO in septic shock, we cannot be certain that our procedure is the most protective in this field. The results of this proof-of-concept trial will not provide a definitive answer on whether RECO is useful to improve long-term survival in septic shock, but are expected to provide essential data for the design of trials with a hard endpoint.
h after randomization to enhance the potential effects of the intervention. We acknowledge that, in absence of comparison data on different modalities of RECO in septic shock, we cannot be certain that our procedure is the most protective in this field. The results of this proof-of-concept trial will not provide a definitive answer on whether RECO is useful to improve long-term survival in septic shock, but are expected to provide essential data for the design of trials with a hard endpoint.
Trial status
The RECO-Sepis trial is currently recruiting patients. Inclusion began on 15 November 2017. The estimated inclusion period is 18 months.
months.
Additional file
Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) 2013 Checklist: recommended items to address in a clinical trial protocol and related documents. (DOC 124 kb)
Acknowledgements
We would like to thank Claire Jossan (CIC) and Sylvie de-la-Salle (CIC) for their help in the development of this protocol. We also thank Dr. Philip Robinson (DRCI, Hospices Civils de Lyon) for help in manuscript preparation.
Funding
The study has received a grant from the French Ministry of Health (Programme Hospitalier de Recherche Clinique Inter-régional 2016).
Availability of data and materials
Not applicable.
Abbreviations
| AE | Adverse event |
| CONSORT | Consolidated Standards of Reporting Trials |
| eCRF | Electronic case report form |
| I/R | Ischemia/reperfusion |
| ICU | Intensive care unit |
| MAP | Mean arterial pressure |
| MOF | Multiple organ failure |
| RECO | REmote ischemic COnditioning |
| SOFA | Sequential Organ Failure Assessment |
| SOP | Standard operating procedure |
| SPIRIT | Standard Protocol Items: Recommendations for Interventional Trials |
Authors’ contributions
MC drafted the first version of the manuscript together with LA. MC, and LA conceived and designed the trial and requested funding from the Ministry of Health. MB and MO provided significant input to the study design. KK, BS, CS, JPQ, FZ, PB contribute to enroll patients and to data collection. MB, KK, BS, CS, JPQ, FZ, PB, and MO critically revised the manuscript. All authors approved the final manuscript.
Ethics approval and consent to participate
The protocol was approved (n°2017-A01684–49) by the Institutional Review Board Comité de Protection des Personnes CCP Ile-de-France X, France. Patients are included after faithful information is given and a signed informed consent is obtained.
Consent for publication
All authors consent to see this protocol article published.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Martin Cour, Phone: (+33)-4-72-11-00-15, Email: [email protected].
Marielle Buisson, Email: [email protected].
Kada Klouche, Email: rf.reilleptnom-uhc@ehcuolk-k.
Radhia Bouzgarrou, Email: rf.dnarreftnomrelc-uhc@uorragzuobr.
Carole Schwebel, Email: rf.elbonerg-uhc@lebewhcsc.
Jean-Pierre Quenot, Email: [email protected].
Fabrice Zeni, Email: rf.enneite-ts-uhc@inezf.
Pascal Beuret, Email: [email protected].
Michel Ovize, Email: [email protected].
Laurent Argaud, Email: [email protected].
References
Articles from Trials are provided here courtesy of BMC
Full text links
Read article at publisher's site: https://doi.org/10.1186/s13063-019-3406-4
Read article for free, from open access legal sources, via Unpaywall:
https://trialsjournal.biomedcentral.com/track/pdf/10.1186/s13063-019-3406-4
Citations & impact
Impact metrics
Citations of article over time
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1186/s13063-019-3406-4
Article citations
Immune and inflammatory mechanism of remote ischemic conditioning: A narrative review.
Brain Circ, 9(2):77-87, 01 Apr 2023
Cited by: 14 articles | PMID: 37576576 | PMCID: PMC10419737
Review Free full text in Europe PMC
Remote ischemic conditioning in septic shock: the RECO-Sepsis randomized clinical trial.
Intensive Care Med, 48(11):1563-1572, 14 Sep 2022
Cited by: 2 articles | PMID: 36100725
Remote ischemic conditioning for acute respiratory distress syndrome in COVID-19.
Am J Physiol Lung Cell Mol Physiol, 320(3):L331-L338, 06 Jan 2021
Cited by: 3 articles | PMID: 33404365 | PMCID: PMC7938644
Review Free full text in Europe PMC
The Role of Heme Oxygenase-1 in Remote Ischemic and Anesthetic Organ Conditioning.
Antioxidants (Basel), 8(9):E403, 16 Sep 2019
Cited by: 10 articles | PMID: 31527528 | PMCID: PMC6770180
Review Free full text in Europe PMC
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Clinical Trials
- (2 citations) ClinicalTrials.gov - NCT03201575
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Remote ischemic conditioning in septic shock: the RECO-Sepsis randomized clinical trial.
Intensive Care Med, 48(11):1563-1572, 14 Sep 2022
Cited by: 2 articles | PMID: 36100725
EXCHANGE-2: investigating the efficacy of add-on plasma exchange as an adjunctive strategy against septic shock-a study protocol for a randomized, prospective, multicenter, open-label, controlled, parallel-group trial.
Trials, 24(1):277, 15 Apr 2023
Cited by: 4 articles | PMID: 37061693 | PMCID: PMC10105400
Remote ischemic conditioning in ST-elevation myocardial infarction as adjuvant to primary angioplasty (RIC-STEMI): study protocol for a randomized controlled trial.
Trials, 16:398, 08 Sep 2015
Cited by: 3 articles | PMID: 26350480 | PMCID: PMC4563839
Remote Ischemic Conditioning in Acute Myocardial Infarction and Shock States.
J Cardiovasc Pharmacol Ther, 25(2):103-109, 11 Dec 2019
Cited by: 16 articles | PMID: 31823646
Review
Funding
Funders who supported this work.

 1,2
1,2