Abstract
Free full text

In Vivo Expansion and Antitumor Activity of Coinfused CD28- and 4-1BB-Engineered CAR-T Cells in Patients with B Cell Leukemia
Abstract
Several recent clinical trials have successfully incorporated a costimulatory domain derived from either CD28 or 4-1BB with the original CD3ζ T cell activating domain to form second-generation chimeric antigen receptors (CARs) that can increase the responsiveness and survival of CAR-engineered T (CAR-T) cells. However, a rigorous assessment of the individual benefits of these costimulatory components relative to the in vivo performance of infused T cells in patients is still lacking. Therefore, we have designed a study that allows us to investigate and compare the impact of different costimulatory signal domains on CAR-T cells in vivo. Patients with B cell leukemia were infused with a mixture of two types of CD19-specific CAR-T cells, individually bearing CD28 (28ζ) and 4-1BB (BBζ) costimulatory signaling domains. We found that such a clinical procedure was feasible and safe. Complete remission (CR) was observed in five of seven enrolled patients, with two patients exhibiting durable CR lasting more than 15 months. The in vivo expansion pattern of 28ζ and BBζ CAR-T cells varied significantly among individual patients. These results confirm a feasible method of comparing different CAR designs within individual patients, potentially offering objective insights that may facilitate the development of optimal CAR-T cell-based immunotherapies.
Introduction
Adoptive immunotherapy through the infusion of autologous T cells genetically modified with chimeric antigen receptors (CARs) has proven to be a promising mode of treatment for tumors, in particular, hematological malignancies.1, 2, 3, 4, 5 Particularly, CD19-targeted CAR-T cells have shown encouraging clinical responses against various B cell malignancies, including B cell lymphoma,6, 7, 8, 9 chronic lymphocytic leukemia (B-CLL)10, 11 and acute lymphoblastic leukemia (B-ALL).12, 13, 14, 15, 16, 17, 18 Although a variety of forms of CAR-T cell therapy have displayed potent effects on disease clearance, the behavior of essential components of CARs and their impact on CAR-T cell expansion and persistence remains unclear, leaving the mechanisms of long-term therapeutic efficacy undefined.4
CARs consist of an extracellular antigen-recognition domain, such as an antibody single-chain variable fragment (scFv), and intracellular signaling domains, such as the CD3ζ chain of the T cell receptor.19 These first-generation CARs often become anergic after infusion and elicit limited T cell antitumor effects.20, 21 To overcome this, second- and third-generation CARs were developed. These CARS incorporate additional costimulatory cytoplasmic domains, such as CD28, 4-1BB (CD137), OX40, and ICOS, either individually or in combination.1, 22, 23 Such costimulatory elements have been shown to enhance proliferation, persistence, and functionality of CAR-T cells.24 Various studies have reported differing clinical results for CARs that have the same target but incorporate different costimulatory elements. For instance, CD19-specific CAR-T cells with CD28 or 4-1BB costimulatory domains showed similar response rates in patients with B-ALL,12, 14, 17 while CAR-Ts with 4-1BB costimulatory domains appeared to have superior efficacy to those with CD28 domains in patients with B-CLL.11, 25 However, it is difficult to directly compare these two costimulatory domains because of several variations in clinical trial design, as well as differences in the scFv, gene transfer vectors, and T cell culture protocols. Thus, a direct comparison between CD28 and 4-1BB using the same viral vector may bring some insight to their impact on CAR-T expansion, persistence, and clinical efficacy, leading to new pathways for improving CAR design.
In this single-institution study, we directly compared the roles of CD28 and 4-1BB in CD19 CARs for patients with relapsed/refractory B cell leukemia by infusing a mixture of second-generation anti-CD19 CAR-T cells engineered with CD28 (28ζ) and 4-1BB (BBζ) co-stimulatory domains at a 1:1 ratio. We report the safety and feasibility of these mixed CAR-T infusions in conjunction with a low-dose cyclophosphamide and fludarabine preconditioning regimen in seven patients with relapsed/refractory B-ALL. No statistical difference was observed in CAR expression or T cell subtypes and function in our in vitro studies. Interestingly, there was also no significant difference in the in vivo expansion of T cells bearing BBζ or 28ζ CARs in the seven patients studied here; however, some patients displayed preferential expansion of either BBζ or 28ζ CARs, indicating that the optimal CAR structure may be patient specific.
Results
Preclinical Evaluations Show that the Mixture of 28ζ and BBζ CAR-T Cells Perform Equally as well as Individual 28ζ or BBζ CAR-T Cells
In this study, we first constructed γ-retroviral vectors encoding the second-generation 28ζ and BBζ CD19-targeting CAR molecules (Figure 1A). To evaluate the functionality of these CAR constructs, human primary T cells from five donors were individually transduced with one of these two CAR vectors. All of the CAR primary blood mononuclear cell (PBMC) populations were separately expanded under the same conditions, as described in Materials and Methods, to generate 28ζ and BBζ CAR-T cells. We then combined the 28ζ and BBζ cells from the same donor at a 1:1 ratio, based on the CAR expression, to form a CAR-T cell mixture (Mix) and compared the in vitro and in vivo functionality with that of individual 28ζ and BBζ CAR-T cells. As shown in Figure 1B, on average, > 50% of CD3+ T cells expressed CAR constructs equipped with signaling domains consisting of either CD28 or 4-1BB, and the two constructs were expressed at comparable levels, with no significant difference.
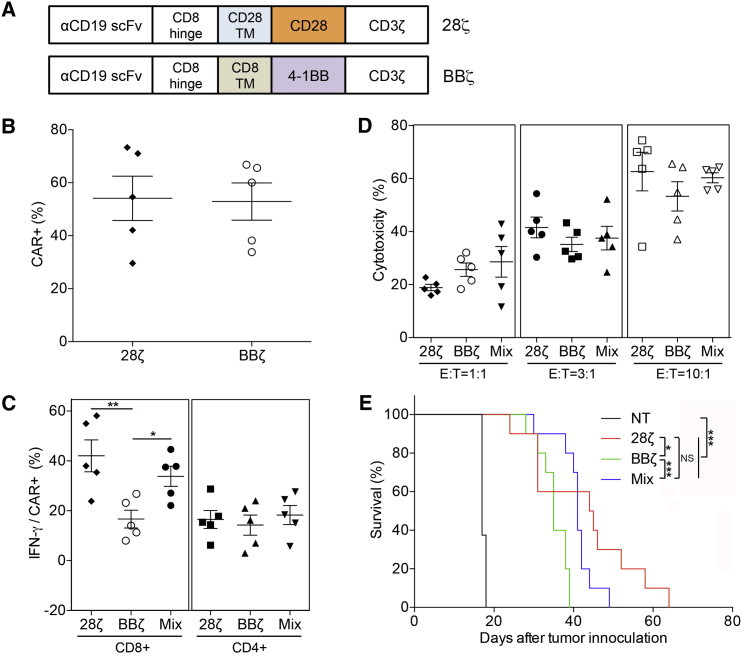
In Vitro and In Vivo Characterization of Mixed 28ζ/BBζ CAR-T Cells
(A) Schematics of the anti-CD19 CAR constructs with either CD28 (28ζ) or 4-1BB (BBζ) costimulatory signaling domain. The single-chain variable fragment (scFv) component is derived from anti-CD19 antibody FMC63. (B) Cell-surface CAR expression on T cells from five donors transduced with either 28ζ or BBζ CAR was determined by flow cytometry. (C) Production of IFN-γ by CD8 and CD4 T cells from 28ζ, BBζ, or Mixed (28ζ:BBζ = 1:1) group after incubation with Raji cells (n = 5). (D) In vitro cytotoxicity of 28ζ, BBζ, or Mixed CAR-T cells against Raji cells at various E:T ratios (1:1, 3:1 and 10:1) (n = 5). (E) Survival of NOG mice inoculated with 0.3 × 106 Raji tumor cells and subsequently treated with 28ζ, BBζ, or Mixed CAR-T cells per mouse (3 × 106 per mouse, n = 10 for each group); non-transduced (NT) T cell group was included as a control (n = 8). Data in (B)–(D) represent means ± SEM. p values were calculated using Student’s paired t test with a two-tailed distribution (*p < 0.05; **p < 0.01; ***p < 0.001).
We subsequently measured the effects of CD28 and 4-1BB signaling domains on the production of interferon (IFN)-γ in response to Raji cells, a CD19-expressing target cell line. As shown in Figure 1C, CD4+ T cells transduced with 28ζ and BBζ CARs produced a similar amount of IFN-γ when cultured with Raji cells for 6 hr. Not surprisingly, the mixture of CD4+ 28ζ and BBζ CAR-T cells expressed the same amount of IFN-γ as the individual CD4+ 28ζ and BBζ CAR-T cells. On the other hand, CD8+ 28ζ CAR-T cells produced significantly more IFN-γ than CD8+ BBζ CAR-T cells (p = 0.0085), indicating that there is a functional difference in the two signaling domains in this CD8+ effector T cell population. The mixture of the two CD8+ CAR-T cells produced similar amounts of IFN-γ as the CD8+ 28ζ CAR-T cells, and significantly more than the CD8+ BBζ CAR-T cells (p = 0.0131). We next tested how the whole CD3+ CAR-T cell populations would respond to different effector-to-target (E:T) ratios. When the mixed group was incubated with Raji cells at various E:T ratios, the level of cytotoxicity was similar to that of individual 28ζ and BBζ CAR-T cells (Figure 1D). However, in a preclinical mouse model, the in vivo antitumor activity of the 28ζ and mixed groups performed significantly better than the BBζ group (p = 0.0419, p = 0.0005, respectively), but the 28ζ and mixed group were not statistically different (p = 0.1388) (Figure 1E).
Characterization of 28ζ and BBζ CAR-T Cells Prepared for Seven Enrolled Patients
Between January 2016 and June 2016, six patients with refractory/relapse B-ALL and one patient with B-CLL were enrolled, and all patients received an infusion of mixed CAR-T cells. Patients ranged from 7–45 years old, and all had primary refractory disease, but without ever having achieved a minimal residual disease (MRD)-negative remission, even after many intensive chemotherapy treatments. As shown in Table 1, one patient had previously undergone allogeneic hematopoietic stem cell transplantation (HSCT). Three patients had measurable CNS leukemia, and one patient had testicular leukemia. Six patients received preconditioning chemotherapy followed by an infusion of 1:1 mixed 28ζ/BBζ CAR-T cells at a dose of 1 × 106 cells/kg. A detailed description of the patient specific prior therapies, preconditioning, and CAR T cell culture times is shown in Table S1. Due to the limits of the clinical situation, patient five did not receive preconditioning, but still received a 1:1 mixed infusion of CAR-T cells. After 14 days with no response to therapy, this patient was removed from the trial. This patient was subsequently given preconditioning followed by another round of CD19 CAR-T cell therapy, which also proved unsuccessful. This follow up treatment is outside of the scope of the trial results discussed here and is therefore not presented in this paper.
Table 1
Patient Demographic Characteristics and Clinical Responses
| Patients | Sex | Age (years) | Leukemia | Previous Treatment | Number of Treatment Failures | Marrow Blast (% of Mononuclear) Post-CAR | CNS Status | Response |
|---|---|---|---|---|---|---|---|---|
| Pt 1 | M | 7 | B-ALL | C | 4 | < 0.01 | 2 | CR (MRD−) |
| Pt 2 | F | 44 | B-CLL/SLL | C | 1 | 0.50 | CR | |
| Pt 3 | F | 26 | B-ALL | C, HSCT | 1 | < 0.01 | CR (MRD−) | |
| Pt 4 | F | 36 | B-ALL | C | 3 | < 0.01 | 2 | CR (MRD−) |
| Pt 5 | M | 45 | B-ALL | C | 1 | 85.50 | PD | |
| Pt 6 | M | 26 | B-ALL | C | 1 | < 0.01 | 1 | CR (MRD−) |
| Pt 7 | M | 14 | B-ALL | C | 3 | 82.60 | PD |
B-ALL, B cell acute lymphoblastic leukemia; B-CLL/SLL, B cell chronic lymphocytic leukemia/small lymphocytic lymphoma; CR, complete response; F, female; HSCT, hematopoietic stem cell transplant; M, male; MRD–, minimum residue disease negative; PD, progressive disease; Pt, patient.
To manufacture CD19 CAR-T cells, half of each patient’s T cells were transduced with 28ζ CAR, and the other half was transduced with BBζ CAR. At the end of T cell expansion, 28ζ and BBζ CAR-T cells were characterized for CAR expression and T cell phenotypes by flow cytometry. This CAR expression measurement was used to calculate the 1:1 ratio for the mixed group. Representative fluorescence-active cell sorting (FACS) plots of the surface expression of CD4, CD8, CD62L, CD45RO, and CAR in transduced cells from one patient are shown in Figure 2A. The mean 28ζ CAR transduction efficiency from all seven patients was 65.84% in CD8+ T cells and 67.64% in CD4+ T cells, which did not statistically differ from BBζ CAR expression (Figure 2B). The expression of CD45RO and CD62L, both associated with central memory phenotype (TCM), was assessed in 28ζ and BBζ CAR-T cells from all patients enrolled. As shown in Figure 2C, this TCM population was more enriched in the BBζ CAR group compared with the 28ζ group in both CD8+ and CD4+ T cells, although it should be noted that there were large variations among patients, and this trend was not statistically significant. In contrast, the 28ζ CAR-T cell population tended to incorporate a higher proportion of the effector memory phenotype (TEM) in both CD8+ and CD4+ T cells (Figure 2D). This observation is consistent with a previous report, which stated that the difference in differentiation status between 28ζ and BBζ CAR-T cells could be more significant after culturing transduced T cells for 21 days.26

CAR Expression and Phenotypic Profiles of Manufactured 28ζ or BBζ CAR-T Cells for Seven Enrolled Patients
(A) Representative FACS analysis of CAR and surface marker (CD4, CD8, CD62L, and CD45RO) expression of CAR-T cells produced for patient four. (B–D) Collective analysis of CAR expression (B), T central memory (TCM) composition (C), and T effector memory (TEM) composition (D) of 28ζ or BBζ CAR-T cells for seven patients.
Safety Evaluation
All toxicities occurring within 30 days of the CAR-T therapy were graded and reported for all seven patients, with leukocytopenia being the most frequent and severe (Table 2). Six out of seven patients experienced CAR-T-related adverse effects (AEs) of any grade, with grade 3 and 4 events reported in two (28%) and two (28%) patients, respectively. Only one patient experienced a grade 1 cytokine release syndrome (CRS).
Table 2
Grades of Treatment-Emergent Adverse Events
| Patient | Fever | Fatigue | Anemia | Leukocytopenia | Thrombocytopenia | Heart Failure | Elevated AST/ALT | Cytokine Release Syndrome | Nausea | Headache | Encephalopathy | Nervous System Disorder |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pt 1 | 1 | 3 | ||||||||||
| Pt 2 | 2 | |||||||||||
| Pt 3 | 1 | 2 | 1 | 1 | ||||||||
| Pt 4 | 1 | 2 | 3 | |||||||||
| Pt 5 | 2 | 2 | 2 | 4 | 4 | 1 | ||||||
| Pt 6 | 1 | 2 | 4 | |||||||||
| Pt 7 |
Adverse event is graded per CTCAE, version 4.03. Cytokine release syndrome was graded per a modified grading system described by Lee et al.39 Pt, patient.
Efficacy Assessment
Five of seven (71%) patients achieved a complete remission (CR) response within 1 month after infusion of the mixture of 28ζ and BBζ CAR-T cells at a 1:1 ratio (Figures 3A and 3B). Of the responders, one patient remained in CR for 18 months post-CAR-T infusion, and another was in ongoing CR at 15 months post-CAR-T infusion (Figure 3A). Median overall survival was 12 months (CI = 95%, 0.5–23.5) and progression-free survival was 5 months (CI = 95%, 0.0–15.5) for all patients enrolled (Figures 3B and 3C).
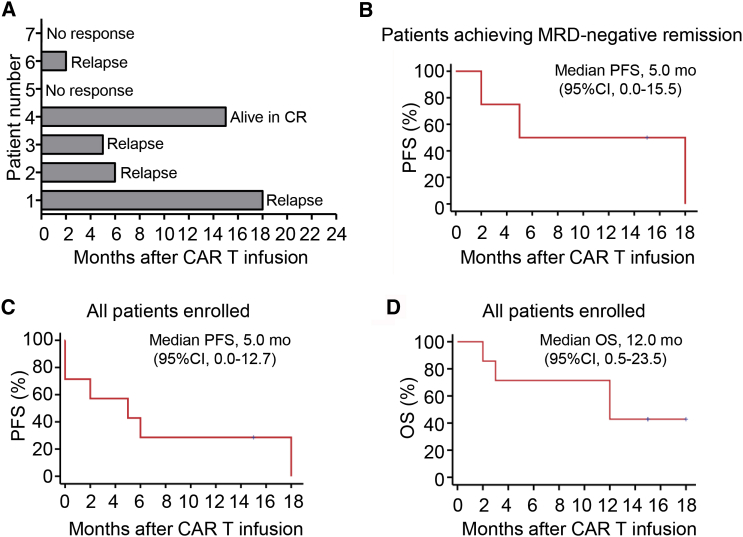
Clinical Efficacy After Infusion of a 1:1 Mixture of Autologous CD19-Specific 28ζ and BBζ CAR-T Cells
(A) Swimmer plot showing the duration of response and survival post-infusion with mixed CAR-T cells. (B) Progression-free survival after CAR-T infusion for patients with B-ALL who had MRD-negative remission. (C) Progression-free survival after CAR-T infusion for all enrolled patients (n = 7). (D) Overall survival after CAR-T infusion for all patients enrolled (n = 7). Data in (B) and (D) were estimated by Kaplan Meier approach for the seven-patient cohort.
The in vivo expansion kinetics of 28ζ and BBζ CAR-T cells was monitored by qPCR in all seven patients. As shown in Figure 4A, six of seven patients had detectable circulating CAR-T cells. The 28ζ CAT-T cell expansion peaked around day 9, while the average peak expansion of circulating BBζ CAR-T cells was observed at day 13, although this difference was not statistically significant (Figure 4B). Patient seven, who did not respond to CAR-T treatment, failed to expand the CAR-T cells. Patient five, who did not receive preconditioning treatment, did show detectable CAR-T expansion but this expansion did not result in a clinical response to therapy. For the other five patients, detectable CAR-T expansion coincided with complete response, indicating that the expansion and persistence of CAR-T cells were correlated with clinical response when the full treatment regime is followed (Figure 4C). Both the magnitude of CAR-T cell expansion and the difference between 28ζ and BBζ CAR cells are highly variable among the patients, but there is no significant difference between the area under the curve (AUC) of 28ζ and BBζ (Figure 4D). Interestingly, the majority of the patients showed similar expansion of the two CARs, while patient three had 10-fold higher expansion of BBζ compared with 28ζ.
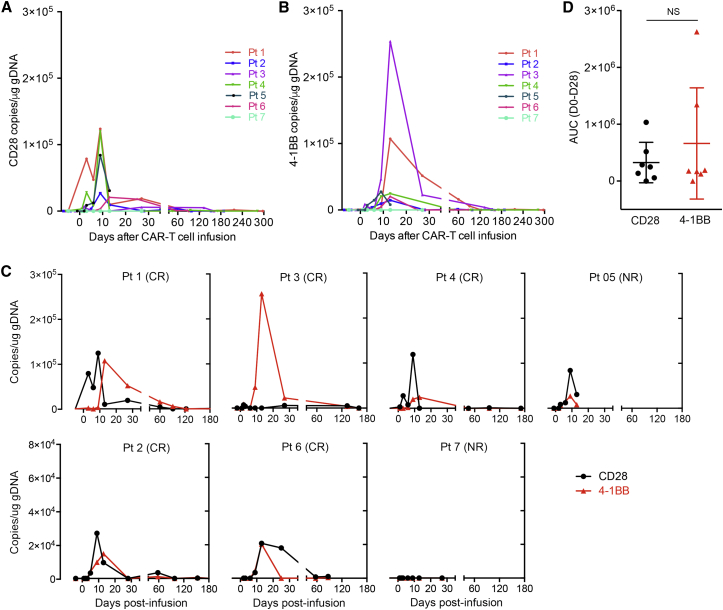
In Vivo Expansion Kinetics of 28ζ and BBζ CAR-T Cells in Seven Patients after Autologous T Cell Infusion
(A) The expansion of 28ζ CAR-T cells in peripheral blood is shown as integrated CD28 copies per microgram of DNA for seven patients. (B) The expansion of BBζ CAR-T cells in peripheral blood is shown as integrated 4-1BB copies per microgram of DNA for seven patients. (C) The expansion of 28ζ and BBζ CAR-T cells in the blood for each patient. The integrated transgene copies were determined by qPCR. (D) The area under curve (AUC) representing the area under the integrated CAR copies per microgram of DNA for CD28 and 4-1BB CARs from day 0 to day 28 post CAR-T infusion. p values were calculated using Student’s paired t test with a two-tailed distribution (NS, no significance; p > 0.05).
In order to investigate the correlation between disease relapse and B cell recovery in responding patients, circulating CD19+ B cells were measured by flow cytometry. Of the five responding patients, four patients had undetectable circulating CD19+ B cells after treatment with lympho-depleting chemotherapy and CAR-T therapy (Figure 5A). Patients one and four are still alive with CR corresponding to the undetectable circulating CD19+ B cells at 180 days post-infusion, while patients two and six relapsed after achieving CR. Their relapse coincided with a gradual restoration of circulating CD19+ B cells.
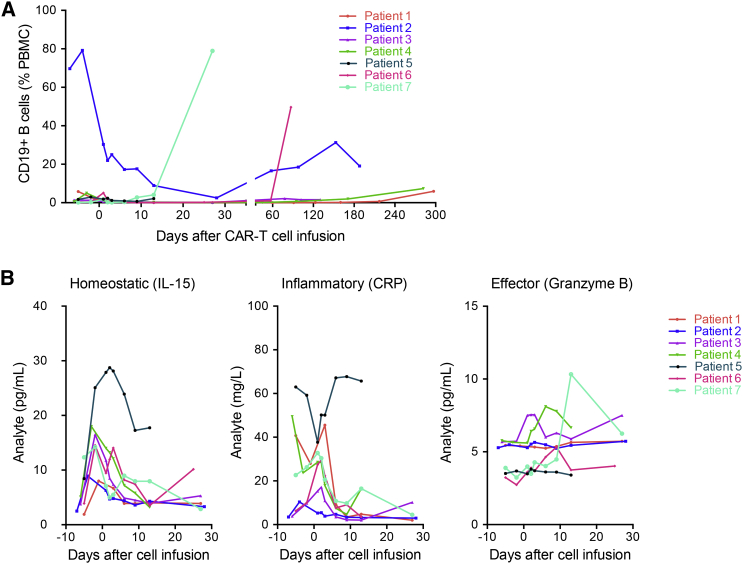
Analysis of Circulating B Cells and Serum Biomarkers for Patients Infused with a Mixture of 28ζ and BBζ CAR-T Cells
(A) Time course of circulating CD19+ B cells estimated by flow cytometry. (B) Time course of serum levels of IL-15, CRP by ELISA, and granzyme B by a cytometric bead assay.
Expansion of CD19 CAR-T cells in responding patients was mirrored by sequential induction, expansion, and general clearance of the homeostatic cytokine interleukin-15 (IL-15), a marker of inflammation (C-reactive protein, CRP), and the cytotoxic T cell effector serine protease granzyme B, as shown in Figure 5B. Notably, IL-15 was elevated after preconditioning with cyclophosphamide and fludarabine. Significantly, these three markers showed very different dynamics for patient five, who did not receive pretreatment, compared with all other patients in the trial, confirming that the pretreatment indeed plays an important role in how the rest of the immune system reacts to CAR-T therapy.
Discussion
First-generation CAR design consists of an extracellular antigen-recognition domain, typically an scFv, and an intracellular signaling domain, predominantly derived from CD3ζ signaling chain, to provide an activation signal for T cells. However, first-generation CAR-T cells have shown limited efficacy in clinical trials, owing to the lack of long-term T cell expansion.27, 28, 29, 30 To overcome this limitation, second-generation CARs were designed by incorporating an additional costimulatory signaling domain into the backbone of the first-generation CARs, providing a secondary signal to tune the response of the T cells. It has been demonstrated that adding the CD28 signaling moiety to CD3ζ could enhance proliferation and persistence of first-generation CD19 CAR-T cells when both second-generation and first-generation CAR-T cells were infused simultaneously into patients with non-Hodgkin lymphoma (NHL).30 More studies reported that second-generation CD19 CAR-T cells with either CD28 or 4-1BB costimulatory elements were able to demonstrate clinical efficacy in the treatment of various B cell malignancies, including B-ALL, CLL, and B cell lymphoma.6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18 However, the optimal signaling domains to be used in second-generation CAR-T cells remain largely undetermined.
Therefore, in the present study, we directly compared the CD28 and 4-BB costimulatory signaling domains by infusing relapsed/refractory B cell leukemia patients with a mixture of CD19 CAR-T cells that express either the 4-1BB or CD28 domains. Our in vitro studies indicated that cytotoxicity was similar for 28ζ and BBζ CARs, but 28ζ CARs showed an enhanced ability to secrete IFN-γ (Figure 1). Previous studies have reported mechanistic differences and differences in metabolic profiles of CAR-T cells with these two signaling domains.26 For instance, CD28 signaling initiates a signaling cascade that increases the cell’s reliance on metabolic reprogramming toward aerobic glycolysis, favoring the differentiation and survival of T effector cells,26 whereas 4-1BB signaling tends to be associated with more T memory cells, as assisted by the oxidative breakdown of fatty acids.31, 32 One study revealed that CD28 costimulation augments exhaustion induced by persistent CAR signaling, whereas 4-1BB costimulation reduces this exhaustion, suggesting that CD19 CAR-T cells incorporating the 4-1BB domain may be more persistent than those with CD28.33 Furthermore, preclinical in vivo studies indicated that CARs with the 4-1BB costimulatory domain could produce superior proliferation and persistence over that induced by CD28.34, 35
Although both CD28 and 4-1BB second-generation CARs can induce substantial T cell proliferation, analysis of clinical trials using either CD28 or 4-1BB CARs revealed that CARs containing the 4-1BB appeared to persist longer.11, 25 However, it should be noted that different viral vectors were employed in these studies. Specifically, CD28 CARs were delivered by γ-retroviral vectors, and 4-1BB CARs were delivered by lentiviral vectors. As opposed to γ-retroviral vectors, lentiviral vectors can transduce less-proliferative and non-dividing cells.36 Thus, it is conceivable that lentiviral vectors would have a higher probability of modifying less-differentiated and more naive T cells and that the resulting CAR-T cells would have a greater chance of surviving, irrespective of the choice of costimulatory domains. On the contrary, our study design employed the same type of viral vectors and therefore reduces the impact of T cell manufacturing conditions on the analysis of the clinical benefits of CARs engineered with different costimulatory components.
Our study demonstrates that it is feasible and safe to infuse this mixture of CD19 CAR-T cells in patients with B cell leukemia and that objective clinical responses could be achieved by such co-infusion. Moreover, we found that there is no significant difference between the expansion kinetics or AUC of 28ζ and BBζ CAR T cells in patients (Figure 4). Several clinical studies reported differences between these two costimulatory domains, but drawing any firm conclusion could be complicated by the many variations between clinical trials. Interestingly, we did see that one patient preferentially expanded BBζ over 28ζ CAR T cells. This could indicate that, for a subset of patients, there may be an optimal CAR structure; however, more patients would need to be treated by this method to see any significant trends. To the best of our knowledge, this is the first clinical study to directly compare these two costimulatory domains under the same viral vector setting. Importantly, our method of comparing the in vivo expansion and persistence of CAR-modified T cells with CD28 or 4-1BB in individual patients could bypass many clinical variables, such as patient-to-patient variation of disease status and condition of immune system, both of which would have confounded an objective assessment of in vivo characteristics of these infused genetically modified T cells. Consequently, our approach could facilitate the development of optimal CAR designs for T cell immunotherapy against cancer.
Materials and Methods
Study Design and Participants
We performed a phase I open-label clinical trial to assess the feasibility and safety of infusing a mixture of autologous T cells modified to express the CD19-specific 28ζ and BBζ CARs at a 1:1 ratio, based on CAR expression levels, in patients with relapsed or refractory CD19+ B-ALL and B-CLL (ClinicalTrials.gov NCT02685670). Patients were screened and treated in the Second Affiliated Hospital of Henan University of Traditional Chinese Medicine between January 2016 and April 2017.
Eligible patients were aged 4–70 years with no detectable leukemia in the cerebrospinal fluid (CSF) (CNS1) and those with CNS2 leukemia (< 5 white blood cells per μL and cytology positive for blasts) without clinically evident neurological change. Patients with CNS2 and neurological changes or CNS3 leukemia or isolated extramedullary leukemia were excluded. Enrolled patients received fludarabine/cyclophosphamide (FC) preconditioning chemotherapy (cyclophosphamide, 200~500 mg/m2, at day −4; fludarabine, 20~30 mg/m2, at days −4 to −2), followed by a single-dose intravenous infusion of 1 × 106 cells/kg CAR-T cells on day 0, except for patient one who was administered with a 3-day split-dose regimen (10%, 30%, and 60% of the total dose). The primary endpoint of this study was the safety of CAR-T cell infusion. The secondary endpoints included studies to compare the in vivo expansion and persistence of infused 28ζ and BBζ CAR-T cells, as well as the morphological and molecular antitumor responses.
Generation of Retroviral Vectors
The retroviral vectors encoding anti-CD19 CARs were constructed based on a modified Moloney Murine Leukemia Virus (Mo-MLV)-based vector described previously.37 The 28ζ CAR consisted of anti-CD19 scFv FMC63, a CD8 hinge region, CD28 transmembrane and cytoplasmic regions, and a CD3ζ cytoplasmic region.38 The BBζ CAR consisted of anti-CD19 scFv FMC63, a CD8 hinge and transmembrane region, and 4-1BB and CD3ζ cytoplasmic regions.39 The cDNA sequences encoding these CARs were codon-optimized, synthesized by Integrated DNA Technologies (Coralville, IA), and cloned into retroviral vectors to yield plasmids RV.28ζ and RV.BBζ. The clinical-grade retroviral producer cell lines for making RV.28ζ and RV.BBζ vectors were generated with the use of the PG13 gibbon ape leukemia virus packaging cell line (CRL-10686, ATCC) as described previously.40 One clone with the highest titer for each CAR was chosen and expanded to generate a seed bank and then a master cell bank. The clones were released for Good Market Practice (GMP)-grade vector production after the completion of safety testing, including the measurement of replication-competent retrovirus.
CAR-T Cell Production
Thawed PBMCs from healthy donors were cultured in T cell medium (TCM) containing X-vivo15 serum-free medium (Lonza, Allendale, NJ), 5% (vol/vol) GemCell human serum antibody AB (Gemini Bio Products, West Sacramento, CA), 1% (vol/vol) Glutamax-100 × (GIBCO Life Technologies), 10mM HEPES buffer (Corning), 1% (vol/vol) penicillin/streptomycin (Corning), and 12.25 mM N-Acetyl-L-cysteine (Sigma). The culture was supplemented with 50–100 IU/mL human IL-2. The PBMCs were activated and expanded using Dynabeads human T-expander CD3/CD28 (Invitrogen) at a bead:PBMC ratio of 1:1. The retroviral supernatants were spin-loaded onto non-tissue culture-treated 12-well plates coated with 15-μg retronectin (Clontech Laboratories, Mountain View, CA) per well by centrifuging 2 hr at 2,000 rpm at 32°C. Activated PBMCs were resuspended at the concentration of 106 cells/mL in TCM, containing 50 IU/mL recombinant human IL-2, and then added to the vector-loaded 12-well plate. The plates were spun at 600 g at 32°C for 30 min and incubated at 37°C overnight. The media was replenished to keep cell densities between 0.5 and 1.5 × 106 cells/mL. During ex vivo expansion, culture medium was replenished, and T cell density was maintained between 0.5 and 1 × 106 cells/mL.
In Vitro Functional Analysis of CAR-T Cells
A previously reported, a flow cytometry-based T cell cytotoxicity assay,38 was employed to measure the cytotoxic response of CAR-T cells in the presence of target Raji cells. Mixed 28ζ/BBζ CAR-T cells, 28ζ CAR-T cells, and BBζ CAR-T cells were incubated with Raji cells at various E:T ratios (1:1, 3:1, 10:1), or K562 cells as a negative control. After 4 hr, cells were harvested, stained for 7-ADD, and subjected to flow cytometry analysis (MACSQuant Analyzer 10; Miltenyi Biotec, Germany), using the established protocol. A standard intracellular cytokine staining protocol was used to assess the ability of CAR-T cells to produce IFN-γ upon Raji cell stimulation (E:T ratio of 1:1).
In Vivo Antitumor Activity of CAR-T Cells in a Mouse Model
Six- to eight-week-old female NOG (NOD/Shi-scid/IL-2Rγnull) mice (The Vital River Laboratory Animal Technology Beijing, China) were housed in the specific pathogen-free animal facility of Shanghai Research Center for Model Organisms. All experimental animal procedures were performed in compliance with the institutional ethical requirements and approved by the Committee of Shanghai Research Center for Model Organisms for the Use and Care of Animals. To assess the antitumor effects, NOG mice were inoculated with 0.3 × 106 Raji tumor cells intravenously. One day later, mice were injected with 3 × 106 CAR-T cells. Mice developing hind limb paralysis, which indicates tumor progression, were euthanized. Survival curves were generated by GraphPad Prism version 5.0 (GraphPad Software, San Diego, CA).
Trial Procedures
Patients were enrolled in this study after screening and confirmation of eligibility; they also underwent leukapheresis to obtain PBMCs. Frozen T cells containing PBMC fractions were shipped to the central cell processing facility. More than 1 × 107 CD3+ T cells obtained from anti-CD3/28 magnetic bead separation were stimulated with the same beads (1:1 bead-to-cell ratio), supplemented with recombinant human IL-2 (50–100 units/mL), for 48 hr. One half of the cells were transduced with retroviral vector encoding 28ζ CAR, and the other half was transduced with retroviral vector encoding BBζ CAR, using the standard retronectin transduction protocol.38 Following the expansion protocol, the T cell product was washed, measured for CAR expression level, T cell phenotypes, potency, and sterility, and then cryopreserved. After lot release testing, the final T cell products were thawed and tested for viability and CAR expression. The 28ζ and BBζ CAR-T cells were then mixed at a 1:1 ratio based on the amount of viable CAR expressing cells and shipped back to the clinical sites within 6 hr for infusion. Patient specific CAR-T cell culture times are listed in Table S1.
Before receiving CAR-T infusion, patients (excluding patient 5) were given cyclophosphamide and fludarabine preconditioning. On day 0, hospitalized patients received a single intravenous infusion of 28ζ and BBζ CAR-T cells mixed in a 1:1 ratio at a target dose of 1 × 106 CAR+ T cells/kg. They remained hospitalized for recovery through day 14 or until all CAR-T-related non-hematological toxicities returned to grade ≤ 1 or baseline.
Patients were instructed to return to the hospital at day 28, and every month between month 2 and month 6. All patients completing the visit at 6 months were followed for survival and disease status every 3 months thereafter through month 24. Beginning with month 24, if possible, patients were instructed to return to the clinic once annually for up to 15 years.
Biomarker Analysis
Multi-parametric flow cytometry was used for analysis of various PBMC and CAR-T samples. The CAR+ T cells were stained with fluorescent-labeled antibodies against CD3, CD4, CD8, CD45RA, CD45RO, and CD62L (BioLegend, San Diego, CA). CAR detection was performed with biotin-labeled polyclonal goat anti-mouse F(ab)2 antibodies (Jackson Immunoresearch, West Grove, PA) and BV421-labeled streptavidin (BioLegend).
The presence, expansion and persistence of 28ζ and BBζ CAR-T cells in the blood were monitored by qPCR. Genomic DNA was isolated from PBMC samples using MiniBEST Universal Genomic DNA Extraction Kit (Takara), quantified by spectrophotometer, and stored at −80°C. The qPCR analysis on genomic DNA samples was performed to detect the integrated CD28/41BB transgene sequence using the following primer pair and specific probe.
CD28 sense primer: 5′- TGGGCATTCCGACTACATGA-3′
CD28 antisense primer: 5′- TGGCTGGTAGTGCTTTCTGGTT-3′
CD28 probe: 5′-VIC- TGACCCCTAGAAGGC-3′
41BB sense primer: 5′- GGTCCTTCTCCTGTCACTGGTT-3′
41BB antisense primer: 5′- CGGCCTCTCTTCACGACACT-3′
41BB probe: 5′-FAM- TCACCCTTTACTGCAGGTT-3′
A parallel qPCR detection on the CDKN1A gene (Genebank: Z85996) was performed as a control to quantify the amount of genomic DNA using the following primer pair and specific probe.
CDKN1A sense primer: 5′- GAAAGCTGACTGCCCCTATTTG-3′
CDKN1A antisense primer: 5′-GAGAGGAAGTGCTGGGAACAAT-3′,
CDKN1A probe: 5′-VIC- CTCCCCAGTCTCTTT-3′
Levels of serum cytokines, chemokines, immune effector molecules, and markers of macrophage activating syndrome (MAS) were assessed in the clinic, and samples were also cryopreserved and shipped back to the central facility for additional measurement. Cytokine levels of IL-6, IL-15, CRP, Granzyme B, tumor necrosis factor-alpha, and IFN-γ were measured from serum samples collected by standard methods. Measurements of IL-6, Granzyme B, tumor necrosis factor-alpha, and IFN-γ cytokines were performed by flow cytometry using the BD Cytometric Bead Array (BD Biosciences), according to the manufacturer’s instructions. Measurements of IL-15 and CRP were performed using an ELISA kit from R&D Systems.
Statistical Analysis
Statistical analysis was performed using Student’s paired t test with a two-tailed distribution. P value < 0.05 is considered to be significant. The results were generated by GraphPad Prism version 5.0 software.
Author Contributions
C.Z., Q.L., B.Z., and P.W. designed the trial and experiments, analyzed data, and wrote the paper. Y.L. analyzed data and wrote the paper. J.A.R performed experiments, analyzed data, and wrote the paper. R.W., Q.M., L.S., F.H., Z.S., T.J., R.X., B.W., J.C., and H.F. performed experiments and analyzed the data.
Conflicts of Interest
Q.L., B.Z., and P.W. have served as members of Scientific Advisory Board for HRAIN Biotechnology. P.W. is also a member of Board of Directors of HRAIN Biotechnology. F.H., Z.S., T.J., R.X., B.W., J.C., H.F., and Y.L. are employees of HRAIN Biotechnology.
Acknowledgments
We thank the patients who participated in this study and their families, as well as the study staff and health care providers at The Second Affiliated Hospital of Henan University of Traditional Chinese Medicine. This study was supported by HRAIN Biotechnology, which manufactured CAR-T cells and provided other study materials. This work was also supported by the National Cancer Institute of the NIH under award numbers R01CA170820, R01EB017206, P01CA132681, and F31CA200242 and the National Nature Science Foundation of China under award number 81620108023.
Footnotes
Supplemental Information includes one table and can be found with this article online at https://doi.org/10.1016/j.ymthe.2018.01.022.
Supplemental Information
References
Articles from Molecular Therapy are provided here courtesy of The American Society of Gene & Cell Therapy
Full text links
Read article at publisher's site: https://doi.org/10.1016/j.ymthe.2018.01.022
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc6079368?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/33091530
Article citations
A CAR enhancer increases the activity and persistence of CAR T cells.
Nat Biotechnol, 30 Jul 2024
Cited by: 2 articles | PMID: 39079964
Generation of allogeneic CAR-NKT cells from hematopoietic stem and progenitor cells using a clinically guided culture method.
Nat Biotechnol, 14 May 2024
Cited by: 4 articles | PMID: 38744947
BCKDK modification enhances the anticancer efficacy of CAR-T cells by reprogramming branched chain amino acid metabolism.
Mol Ther, 32(9):3128-3144, 11 May 2024
Cited by: 1 article | PMID: 38734897
Comparative performance of scFv-based anti-BCMA CAR formats for improved T cell therapy in multiple myeloma.
Cancer Immunol Immunother, 73(6):100, 17 Apr 2024
Cited by: 0 articles | PMID: 38630291 | PMCID: PMC11024081
An alternative fully human anti-BCMA CAR-T shows response for relapsed or refractory multiple myeloma with anti-BCMA CAR-T exposures previously.
Cancer Gene Ther, 31(3):420-426, 15 Dec 2023
Cited by: 1 article | PMID: 38102463 | PMCID: PMC10940153
Go to all (47) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Clinical Trials
- (1 citation) ClinicalTrials.gov - NCT02685670
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Combined CD28 and 4-1BB Costimulation Potentiates Affinity-tuned Chimeric Antigen Receptor-engineered T Cells.
Clin Cancer Res, 25(13):4014-4025, 12 Apr 2019
Cited by: 87 articles | PMID: 30979735 | PMCID: PMC7477921
In Vivo Fate and Activity of Second- versus Third-Generation CD19-Specific CAR-T Cells in B Cell Non-Hodgkin's Lymphomas.
Mol Ther, 26(12):2727-2737, 13 Sep 2018
Cited by: 143 articles | PMID: 30309819 | PMCID: PMC6277484
Reversible Transgene Expression Reduces Fratricide and Permits 4-1BB Costimulation of CAR T Cells Directed to T-cell Malignancies.
Cancer Immunol Res, 6(1):47-58, 27 Oct 2017
Cited by: 59 articles | PMID: 29079655 | PMCID: PMC5963729
A comparison of chimeric antigen receptors containing CD28 versus 4-1BB costimulatory domains.
Nat Rev Clin Oncol, 18(11):715-727, 06 Jul 2021
Cited by: 134 articles | PMID: 34230645
Review
Funding
Funders who supported this work.
HRAIN Biotechnology
NCI NIH HHS (3)
Grant ID: R01 CA170820
Grant ID: F31 CA200242
Grant ID: P01 CA132681
NIBIB NIH HHS (1)
Grant ID: R01 EB017206
NIH (4)
Grant ID: P01CA132681
Grant ID: R01EB017206
Grant ID: R01CA170820
Grant ID: F31CA200242
National Cancer Institute
National Nature Science Foundation of China (1)
Grant ID: 81620108023
 and
and 
