Abstract
Implications for practice
The approvals of dabrafenib and trametinib, administered concurrently, provide a new regimen for the treatment of a rare subset of non-small cell lung cancer (NSCLC) and demonstrate how drugs active for treatment of BRAF-mutant tumors in one setting predict efficacy and can provide supportive evidence for approval in another setting. The FDA also approved the first next-generation sequencing oncology panel test for simultaneous assessment of multiple actionable mutations, which will facilitate selection of optimal, personalized therapy. The test was shown to accurately and reliably select patients with NSCLC with the BRAF V600E mutation for whom treatment with dabrafenib and trametinib is the optimal treatment.Free full text

FDA Approval Summary: Dabrafenib and Trametinib for the Treatment of Metastatic Non‐Small Cell Lung Cancers Harboring BRAF V600E Mutations
Abstract
On June 22, 2017, the Food and Drug Administration expanded indications for dabrafenib and trametinib to include treatment of patients with metastatic non‐small cell lung cancer (NSCLC) harboring BRAF V600E mutations. Approval was based on results from an international, multicenter, multicohort, noncomparative, open‐label trial, study BRF113928, which sequentially enrolled 93 patients who had received previous systemic treatment for advanced NSCLC (Cohort B, n =
= 57) or were treatment‐naïve (Cohort C, n
57) or were treatment‐naïve (Cohort C, n =
= 36). All patients received dabrafenib 150 mg orally twice daily and trametinib 2 mg orally once daily. In Cohort B, overall response rate (ORR) was 63% (95% confidence interval [CI] 49%–76%) with response durations ≥6 months in 64% of responders. In Cohort C, ORR was 61% (95% CI 44%–77%) with response durations ≥6 months in 59% of responders. Results were evaluated in the context of the Intergroupe Francophone de Cancérologie Thoracique registry and a chart review of U.S. electronic health records at two academic sites, characterizing treatment outcomes data for patients with metastatic NSCLC with or without BRAF V600E mutations. The treatment effect of dabrafenib 150 mg twice daily was evaluated in 78 patients with previously treated BRAF mutant NSCLC, yielding an ORR of 27% (95% CI 18%–38%), establishing that dabrafenib alone is active, but that the addition of trametinib is necessary to achieve an ORR of >40%. The most common adverse reactions (≥20%) were pyrexia, fatigue, nausea, vomiting, diarrhea, dry skin, decreased appetite, edema, rash, chills, hemorrhage, cough, and dyspnea.
36). All patients received dabrafenib 150 mg orally twice daily and trametinib 2 mg orally once daily. In Cohort B, overall response rate (ORR) was 63% (95% confidence interval [CI] 49%–76%) with response durations ≥6 months in 64% of responders. In Cohort C, ORR was 61% (95% CI 44%–77%) with response durations ≥6 months in 59% of responders. Results were evaluated in the context of the Intergroupe Francophone de Cancérologie Thoracique registry and a chart review of U.S. electronic health records at two academic sites, characterizing treatment outcomes data for patients with metastatic NSCLC with or without BRAF V600E mutations. The treatment effect of dabrafenib 150 mg twice daily was evaluated in 78 patients with previously treated BRAF mutant NSCLC, yielding an ORR of 27% (95% CI 18%–38%), establishing that dabrafenib alone is active, but that the addition of trametinib is necessary to achieve an ORR of >40%. The most common adverse reactions (≥20%) were pyrexia, fatigue, nausea, vomiting, diarrhea, dry skin, decreased appetite, edema, rash, chills, hemorrhage, cough, and dyspnea.
Implications for Practice.
The approvals of dabrafenib and trametinib, administered concurrently, provide a new regimen for the treatment of a rare subset of non‐small cell lung cancer (NSCLC) and demonstrate how drugs active for treatment of BRAF‐mutant tumors in one setting predict efficacy and can provide supportive evidence for approval in another setting. The FDA also approved the first next‐generation sequencing oncology panel test for simultaneous assessment of multiple actionable mutations, which will facilitate selection of optimal, personalized therapy. The test was shown to accurately and reliably select patients with NSCLC with the BRAF V600E mutation for whom treatment with dabrafenib and trametinib is the optimal treatment.
Introduction
An improved understanding of molecular pathways in cancer has led to the development of targeted agents [1]. Based on literature reports, BRAF V600 mutations occur in 2% of all non‐small cell lung cancer (NSCLC), of which half are BRAF V600E (1%–1.5% of NSCLC) [2]. In NSCLC, BRAF V600E is predominantly found in tumors with adenocarcinoma histology [3]. Prior to these approvals, the U.S. Food and Drug Administration (FDA) had not approved any drugs specifically for the treatment of this rare subset of NSCLC. However, demonstration of a large treatment effect on overall response rate (ORR) that is very durable has led to approvals for targeted therapies specifically for the treatment of EGFR T790M‐mutant [4], ALK rearrangement‐positive [5], and ROS‐1‐mutant NSCLC [6].
Dabrafenib and trametinib target BRAF and MEK1/2, respectively, two kinases within the serine/threonine kinase family in the RAS/RAF/MEK/ERK pathway. The clinical benefit and safety of dabrafenib administered with trametinib was verified in two randomized, multicenter trials (the COMBI‐d study [NCT01584648] and the COMBI‐v study [NCT01597908]), demonstrating that concurrent administration of dabrafenib and trametinib improves progression‐free survival (PFS) and overall survival (OS) compared with a BRAF inhibitor alone (dabrafenib or vemurafenib, respectively) for treatment of patients with BRAF V600E or V600K mutation‐positive melanoma [7], [8].
On November 21, 2013, the FDA granted Breakthrough Therapy designation for dabrafenib for the treatment of patients with metastatic BRAF V600E mutation‐positive NSCLC who had received at least one prior line of platinum‐containing chemotherapy, based on a reported ORR of 45% (95% confidence interval [CI] 23%–68%) in 20 patients, of whom 6 of the 9 responding patients had response durations of more than 6 months. On July 15, 2015, the FDA granted Breakthrough Therapy designation for dabrafenib and trametinib, administered concurrently for the treatment of patients with advanced or metastatic BRAF V600E mutation‐positive NSCLC who have received at least one prior line of platinum‐containing chemotherapy, based on a reported ORR of 68% (95% CI 45%–86%) in 22 patients. On October 29, 2015, the FDA designated dabrafenib and trametinib as Orphan Drugs for the treatment of patients with BRAF V600E mutation‐positive NSCLC.
Herein, we summarize the FDA review of the efficacy supplement supporting approval of dabrafenib and trametinib administered concurrently for BRAF V600E mutant NSCLC.
Clinical Trial Design
The FDA primarily relied on data from the study BRF113928 (NCT01336634), an international, multicohort, nonrandomized, open‐label, activity‐estimating, parallel cohort trial [9]. Key eligibility criteria were a histologically or cytologically confirmed diagnosis of NSCLC, American Joint Committee on Cancer stage IV disease, the presence of BRAF V600E mutation confirmed in a Clinical Laboratory Improvement Amendments (CLIA) certified local laboratory, Eastern Cooperative Oncology Group performance status 0–2, and measurable disease according to Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1. Following enrollment, patients’ tumors were centrally confirmed for BRAF V600E mutation status using the next‐generation sequencing (NGS) assay Oncomine Dx Target Test (Thermo Fisher Scientific, Waltham, MA).
The three study arms were:
Cohort A, which enrolled patients with previously treated BRAF V600E‐mutant metastatic NSCLC. All patients received dabrafenib 150 mg orally twice daily.
Cohort B, which enrolled patients with previously treated BRAF V600E‐mutant metastatic NSCLC. All patients received dabrafenib 150 mg orally twice daily and trametinib 2 mg orally once daily.
Cohort C, which enrolled patients with previously untreated BRAF V600E‐mutant metastatic NSCLC. All patients received dabrafenib 150 mg orally twice daily and trametinib 2 mg orally once daily.
The primary efficacy outcome measure was estimation of the ORR in patients enrolled in Cohort B (n =
= 57) or C (n
57) or C (n =
= 36). Results of Cohort A were evaluated to assess efficacy with dabrafenib alone and for indirect comparison to assess the contribution of trametinib in Cohorts B and C. For regulatory purposes, the primary efficacy endpoint was ORR as assessed by an independent review committee (IRC) according to RECIST version 1.1. Duration of response was a key secondary endpoint. Additional secondary efficacy endpoints were PFS, OS, and safety. All endpoints were characterized using descriptive statistics.
36). Results of Cohort A were evaluated to assess efficacy with dabrafenib alone and for indirect comparison to assess the contribution of trametinib in Cohorts B and C. For regulatory purposes, the primary efficacy endpoint was ORR as assessed by an independent review committee (IRC) according to RECIST version 1.1. Duration of response was a key secondary endpoint. Additional secondary efficacy endpoints were PFS, OS, and safety. All endpoints were characterized using descriptive statistics.
Analysis Plan
Formal comparisons between the cohorts were not planned. The sample size for each cohort was based on the following:
Cohort A: A total of 60 patients were needed to exclude an ORR of 10% based on the lower bound of the 95% confidence interval, assuming an observed ORR of 30%.
Cohort B: A total of 40 patients were needed to exclude an ORR of 30% based on the lower bound of the 95% confidence interval, assuming an observed ORR of 55%.
Cohort C: A total of 25 patients were needed to exclude an ORR of 30% based on the lower bound of the 95% confidence interval, assuming an observed ORR of 60%.
Results
A total of 171 patients were enrolled in 11 countries and in 70 sites in the U.S, representing an overenrollment of 46 patients spread equally across all three cohorts. Seventy‐eight patients (46%) were enrolled in Cohort A, 57 patients (33%) were enrolled in Cohort B, and 36 patients (21%) were enrolled in Cohort C. Key demographics and disease characteristics are summarized in Table Table11.
Table 1.
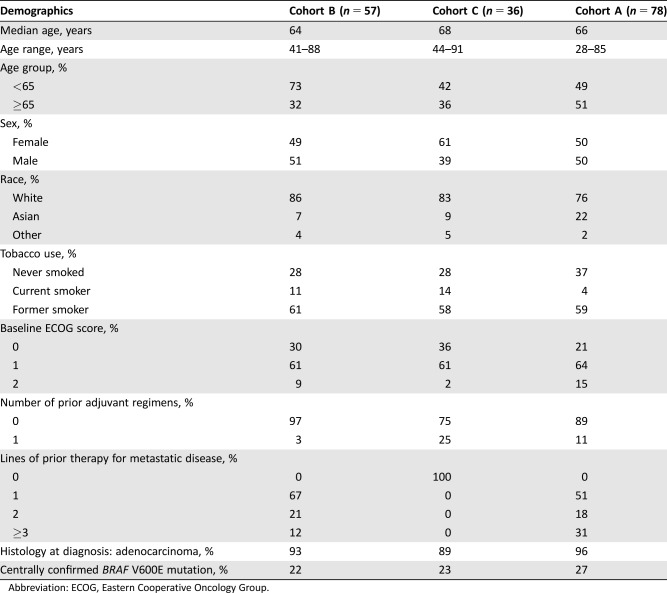
Abbreviation: ECOG, Eastern Cooperative Oncology Group.
Efficacy
Efficacy was assessed in the first 171 patients enrolled, with a minimum follow‐up of 6 months from study entry. Efficacy results are shown in Table Table2.2. In all cohorts, the targeted level of efficacy was achieved, with exclusion of an ORR of ≤10% in Cohort A and an ORR of ≤30% in Cohorts B and C based on the IRC‐assessed ORR. Although evidence of antitumor activity was observed with dabrafenib alone, the addition of trametinib appeared to result in a twofold higher ORR when comparing Cohorts A and B. In contrast, antitumor activity was similar in patients with previously untreated and with previously treated BRAF‐mutant NSCLC. Results based on investigator assessment were similar to those based on IRC assessment.
Table 2.

Abbreviations: CI, confidence interval; CR, complete response; DoR, duration of response; IRC, independent review committee; NE, not estimable; ORR, overall response rate; PR, partial response.
Given their relative rarity, there is little information about whether BRAF V600 mutations are prognostic for better survival or response to chemotherapy. To assess for prognostic effects and put the data observed in the context of the natural history of BRAF‐mutant NSCLC, Novartis provided the results of two registries. The Intergroupe Francophone de Cancérologie Thoracique (IFCT) registry (NCT01700582) was a prospective observational study that provided natural history and ORR outcomes data following treatment with available standard‐of‐care therapies in patients with NSCLC with and without BRAF V600E mutations [10]. Approximately 17,640 patients were screened; of these, 10,322 had results of BRAF V600 status and 189 patients were identified as having BRAF V600E mutation. In patients with BRAF V600E‐mutant NSCLC, the ORR was 30% (95% CI 12.6%–38.6%) following platinum‐based chemotherapy compared with 30% (95% CI 29%–31%) in those with BRAF wild‐type NSCLC. In addition, data obtained in a retrospective review of U.S. electronic health medical records (EHR) from Dana‐Farber and Stanford Medical Centers demonstrated an ORR of 38% (95% CI 18%–62%) following platinum‐based chemotherapy in 21 patients with NSCLC harboring BRAF V600 mutation.
To assess the predictive value of the Oncomine Dx Target Test for the selection of patients with BRAF V600‐mutant NSCLC, a prospective plan for retrospective assessment of BRAF V600E mutation status in tumor samples from patients enrolled in BRF113928 was performed at a central, CLIA‐certified laboratory. Approximately 72 patients had centrally confirmed BRAF V600E mutation. The ORR was similar in this convenience sample to that in the individual cohorts.
Safety
The safety profile of dabrafenib and trametinib concomitantly administered was similar to that previously observed in over 500 patients with metastatic melanoma. Among the 93 patients who received at least one dose of dabrafenib or trametinib, 53 (57%) were exposed to dabrafenib and trametinib for >6 months and 27 (29%) were exposed for ≥1 year. Common adverse reactions occurring in patients receiving dabrafenib and trametinib are listed in Table Table3.3. Adverse reactions resulting in permanent discontinuation occurred in 18% of patients; the most frequent adverse reactions leading to discontinuation were pyrexia, decreased ejection fraction, and respiratory distress (2.2% each). Adverse reactions leading to dose reductions occurred in 35% of patients treated with dabrafenib in combination with trametinib. The most frequent adverse reactions leading to dose reductions were pyrexia (12%) and diarrhea, nausea, and vomiting (4.3% each). Adverse reactions leading to dose interruptions of dabrafenib in combination with trametinib occurred in 62% of patients, and the most common were pyrexia (27%), vomiting (11%), neutropenia (8%), and chills (6%).
Table 3.

Treatment‐emergent laboratory abnormalities occurring in patients receiving dabrafenib and trametinib are listed in Table Table4.4. With a few exceptions, the incidence of treatment‐emergent laboratory abnormalities observed was similar to that observed in patients with melanoma, with the exception of a higher overall incidence of hyponatremia in study BRF113928 (57% vs. 25%) and of grade 3–4 hyponatremia (17% vs. 8%).
Table 4.
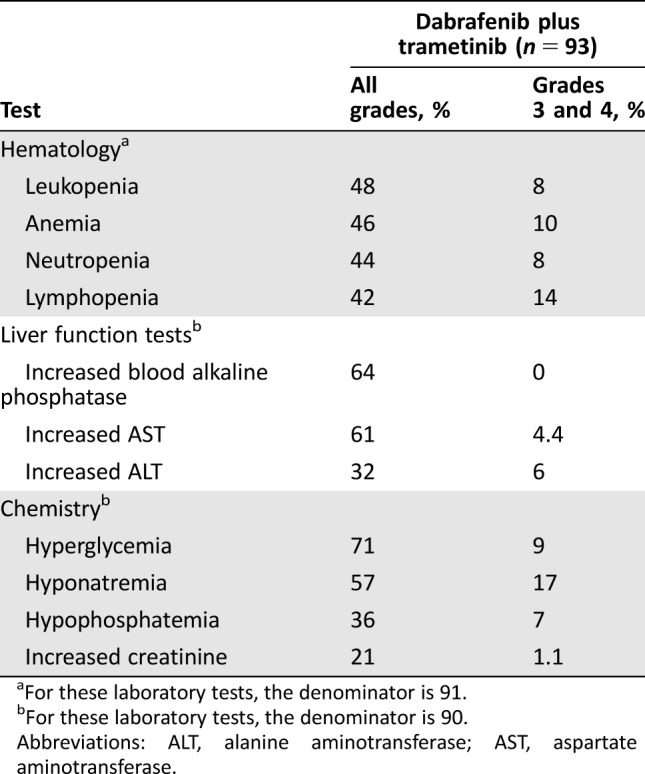
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase.
Discussion
Study BRF113928 demonstrated that dabrafenib is an active single agent for the treatment of BRAF V600E‐positive NSCLC in the second‐line setting; however, the observed ORR of 27% (95% CI 18%–38%) does not represent a meaningful advantage over available therapy based on the lower bound of the confidence interval of 18%, which overlaps with the reported ORR with docetaxel (alone or with ramucirumab), pemetrexed, or pembrolizumab, which are approved drugs for the second‐line treatment of NSCLC.
In contrast, concomitant administration of trametinib and dabrafenib produced a large treatment effect on ORR (63%; 95% CI 49%–76%), in which 64% of patients had responses durable for 6 months or longer. This represents a meaningful advantage over FDA‐approved second line treatments. Similarly, the ORR 61% (95% CI 44%–77%) and durability of responses (59% durable for ≥6 months) with dabrafenib and trametinib in chemotherapy‐naïve patients also represent a meaningful advantage over those observed with platinum‐doublet chemotherapy.
These results were considered in the context of the natural history of BRAF V600‐mutant NSCLC, using data obtained in the IFCT and the U.S. EHR registries. There were limitations in the registry data in that clinicians’ documentation of response was not standardized, and there was a substantial proportion of missing data with regard to BRAF V600 status. However, given the very large number of subjects included in the IFCT registry, the analyses included sufficient information to characterize the responses to first‐line chemotherapy in the subset of patients with NSCLC harboring BRAF V600E mutation and allow the FDA to conclude that BRAF V600 mutation is not predictive for more favorable responses to standard treatment.
The adverse reactions of dabrafenib and trametinib, administered concurrently, have been well characterized in studies of melanoma patients, and no new adverse reactions were identified during this review. Serious risks of dabrafenib and trametinib, administered concurrently, include development of new cutaneous and noncutaneous malignancies, hemorrhage, interstitial lung disease, cardiomyopathy, and serious febrile reactions. Common adverse reactions (occurring in ≥20%) were pyrexia, fatigue, nausea, vomiting, diarrhea, dry skin, decreased appetite, edema, rash, chills, hemorrhage, cough, and dyspnea. The most common grade 3–4 adverse reactions (≥20%) were pyrexia, fatigue, dyspnea, vomiting, rash, hemorrhage, and diarrhea. The higher incidence of hyponatremia observed in patients with NSCLC, compared with those with melanoma, may reflect the underlying disease or use of prior platinum‐based chemotherapy. The FDA concluded that the serious risks of dabrafenib and trametinib, administered concomitantly, were acceptable to patients in light of the large magnitude and durability of overall response rates that were achieved and the incurable nature of the disease, as reflected by 5‐year survival rates of less than 20%.
Based upon the magnitude of the ORR in both first‐ and second‐line treatment, together with the prolonged durability of those responses, the FDA determined that the observed treatment effects provided substantial evidence of effectiveness. In making this determination, the FDA noted that a durable overall response rate may be evidence of direct clinical benefit for patients with uncommon, serious, and life‐threatening cancers, such as unresectable or metastatic basal cell cancers or ROS‐1 positive NSCLC, for which there are unsatisfactory alternatives. This approach also is supported by an FDA meta‐analysis demonstrating that in metastatic NSCLC, a drug with a large magnitude of effect on ORR is likely to result in a large improvement in PFS [11]. Given the magnitude of the ORR observed and the rarity of this subset of NSCLC, the FDA determined that it would not be feasible to conduct randomized trials against a chemotherapy control to determine the treatment effect on a time‐to‐event endpoint such as PFS or OS.
Based on this favorable benefit‐risk assessment, the FDA granted regular approval to dabrafenib and trametinib, administered concurrently, on June 22, 2017, for the treatment of patients with metastatic NSCLC harboring BRAF V600E mutation. Table Table55 summarizes the FDA benefit‐risk analysis. Novartis agreed to a postmarketing commitment to obtain longer follow‐up on all patients enrolled to better characterize the durability of response, because the Kaplan‐Meier estimated medians for duration of response were either unstable or not estimable, and to obtain a preliminary estimate of OS in this population. These OS data may be used to inform the design of future trials in this patient population.
Table 5.
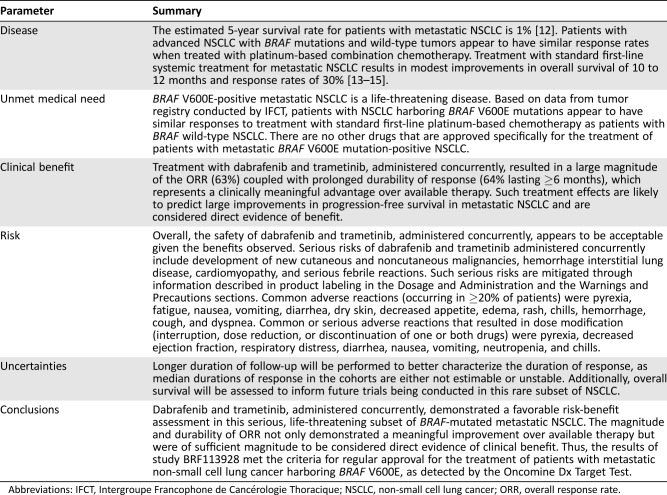
Abbreviations: IFCT, Intergroupe Francophone de Cancérologie Thoracique; NSCLC, non‐small cell lung cancer; ORR, overall response rate.
The FDA concurrently approved the first NGS oncology panel test, Oncomine Dx Target Test, for the detection of BRAF V600E, EGFR mutations, and ROS1 fusions for the selection of patients with NSCLC eligible for treatment with targeted therapies. This panel will facilitate patient management by allowing identification of these molecular abnormalities in a single NSCLC tissue specimen.
Conclusion
The concomitant administration of dabrafenib and trametinib resulted in a durable ORR of a large magnitude, which provided substantial evidence of the effectiveness in a rare subgroup of genetically defined patients with metastatic NSCLC, that is, those harboring BRAF V600E mutation. These results, along with the observed safety profile, provided a favorable overall benefit‐risk assessment for dabrafenib and trametinib for the treatment of patients with BRAF V600E‐mutant NSCLC.
Author Contributions
Conception/design: Lauretta Odogwu, Luckson Mathieu, Gideon Blumenthal, Erin Larkins, Karen Bijwaard, Eunice Y. Lee, Reena Philip, Xiaoping Jiang, Lisa Rodriguez, Patricia Keegan, Richard Pazdur
Collection and/or assembly of data: Lauretta Odogwu, Luckson Mathieu, Gideon Blumenthal, Erin Larkins, Karen Bijwaard, Eunice Y. Lee, Reena Philip, Xiaoping Jiang, Lisa Rodriguez, Patricia Keegan, Richard Pazdur
Data analysis and interpretation: Lauretta Odogwu, Luckson Mathieu, Gideon Blumenthal, Erin Larkins, Karen Bijwaard, Eunice Y. Lee, Reena Philip, Xiaoping Jiang, Lisa Rodriguez, Patricia Keegan, Richard Pazdur
Manuscript writing: Lauretta Odogwu, Luckson Mathieu, Gideon Blumenthal, Kirsten B. Goldberg, Karen Bijwaard, Eunice Y. Lee, Reena Philip, Patricia Keegan, Richard Pazdur
Final approval of manuscript: Lauretta Odogwu, Gideon Blumenthal, Erin Larkins, Kirsten B. Goldberg, Karen Bijwaard, Eunice Y. Lee, Reena Philip, Amy E. McKee, Patricia Keegan, Richard Pazdur
References
Articles from The Oncologist are provided here courtesy of Oxford University Press
Full text links
Read article at publisher's site: https://doi.org/10.1634/theoncologist.2017-0642
Read article for free, from open access legal sources, via Unpaywall:
https://theoncologist.onlinelibrary.wiley.com/doi/pdfdirect/10.1634/theoncologist.2017-0642
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/33117978
Article citations
Trametinib Sensitivity is Defined by a Myeloid Differentiation Profile in Acute Myeloid Leukemia.
Drugs R D, 24(3):489-499, 24 Sep 2024
Cited by: 0 articles | PMID: 39316279 | PMCID: PMC11456044
Targeted therapy of non-small cell lung cancer: mechanisms and clinical trials.
Front Oncol, 14:1451230, 26 Sep 2024
Cited by: 0 articles | PMID: 39391239 | PMCID: PMC11464343
Review Free full text in Europe PMC
Criteria for assessing evidence for biomarker-targeted therapies in rare cancers-an extrapolation framework.
Ther Adv Med Oncol, 16:17588359241273062, 02 Sep 2024
Cited by: 0 articles | PMID: 39229469 | PMCID: PMC11369883
Personalizing Therapy Outcomes through Mitogen-Activated Protein Kinase Pathway Inhibition in Non-Small Cell Lung Cancer.
Biomedicines, 12(7):1489, 05 Jul 2024
Cited by: 0 articles | PMID: 39062063 | PMCID: PMC11275062
Review Free full text in Europe PMC
Trametinib, a MEK1/2 Inhibitor, Protects Mice from Cisplatin- and Noise-Induced Hearing Loss.
Pharmaceuticals (Basel), 17(6):735, 05 Jun 2024
Cited by: 0 articles | PMID: 38931403
Go to all (106) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Clinical Trials (2)
- (1 citation) ClinicalTrials.gov - NCT01584648
- (1 citation) ClinicalTrials.gov - NCT01597908
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Dabrafenib plus trametinib in patients with previously untreated BRAFV600E-mutant metastatic non-small-cell lung cancer: an open-label, phase 2 trial.
Lancet Oncol, 18(10):1307-1316, 11 Sep 2017
Cited by: 439 articles | PMID: 28919011
Adverse Event Management in Patients with BRAF V600E-Mutant Non-Small Cell Lung Cancer Treated with Dabrafenib plus Trametinib.
Oncologist, 24(7):963-972, 31 Dec 2018
Cited by: 9 articles | PMID: 30598499 | PMCID: PMC6656467
Dabrafenib plus trametinib in patients with previously treated BRAF(V600E)-mutant metastatic non-small cell lung cancer: an open-label, multicentre phase 2 trial.
Lancet Oncol, 17(7):984-993, 06 Jun 2016
Cited by: 438 articles | PMID: 27283860 | PMCID: PMC4993103
Dabrafenib in combination with trametinib in the treatment of patients with BRAF V600-positive advanced or metastatic non-small cell lung cancer: clinical evidence and experience.
Ther Adv Respir Dis, 12:1753466618767611, 01 Jan 2018
Cited by: 41 articles | PMID: 29595366 | PMCID: PMC5941661
Review Free full text in Europe PMC

