Abstract
Objective
To determine the steroid-sparing effect of methotrexate (MTX) in patients with symptomatic generalized myasthenia gravis (MG).Methods
We performed a 12-month multicenter, randomized, double-blind, placebo-controlled trial of MTX 20 mg orally every week vs placebo in 50 acetylcholine receptor antibody-positive patients with MG between April 2009 and August 2014. The primary outcome measure was the prednisone area under the dose-time curve (AUDTC) from months 4 to 12. Secondary outcome measures included 12-month changes of the Quantitative Myasthenia Gravis Score, the Myasthenia Gravis Composite Score, Manual Muscle Testing, the Myasthenia Gravis Quality of Life, and the Myasthenia Gravis Activities of Daily Living.Results
Fifty-eight patients were screened and 50 enrolled. MTX did not reduce the month 4-12 prednisone AUDTC when compared to placebo (difference MTX - placebo: -488.0 mg, 95% confidence interval -2,443.4 to 1,467.3, p = 0.26); however, the average daily prednisone dose decreased in both groups. MTX did not improve secondary measures of MG compared to placebo over 12 months. Eight participants withdrew during the course of the study (1 MTX, 7 placebo). There were no serious MTX-related adverse events. The most common adverse event was nonspecific pain (19%).Conclusions
We found no steroid-sparing benefit of MTX in MG over 12 months of treatment, despite being well-tolerated. This study demonstrates the challenges of conducting clinical trials in MG, including difficulties with recruitment, participants improving on prednisone alone, and the need for a better understanding of outcome measure variability for future clinical trials.Classification of evidence
This study provides Class I evidence that for patients with generalized MG MTX does not significantly reduce the prednisone AUDTC over 12 months of therapy.Free full text

A randomized controlled trial of methotrexate for patients with generalized myasthenia gravis
Abstract
Objective:
To determine the steroid-sparing effect of methotrexate (MTX) in patients with symptomatic generalized myasthenia gravis (MG).
Methods:
We performed a 12-month multicenter, randomized, double-blind, placebo-controlled trial of MTX 20 mg orally every week vs placebo in 50 acetylcholine receptor antibody–positive patients with MG between April 2009 and August 2014. The primary outcome measure was the prednisone area under the dose-time curve (AUDTC) from months 4 to 12. Secondary outcome measures included 12-month changes of the Quantitative Myasthenia Gravis Score, the Myasthenia Gravis Composite Score, Manual Muscle Testing, the Myasthenia Gravis Quality of Life, and the Myasthenia Gravis Activities of Daily Living.
Results:
Fifty-eight patients were screened and 50 enrolled. MTX did not reduce the month 4–12 prednisone AUDTC when compared to placebo (difference MTX − placebo: −488.0 mg, 95% confidence interval −2,443.4 to 1,467.3, p = 0.26); however, the average daily prednisone dose decreased in both groups. MTX did not improve secondary measures of MG compared to placebo over 12 months. Eight participants withdrew during the course of the study (1 MTX, 7 placebo). There were no serious MTX-related adverse events. The most common adverse event was nonspecific pain (19%).
Conclusions:
We found no steroid-sparing benefit of MTX in MG over 12 months of treatment, despite being well-tolerated. This study demonstrates the challenges of conducting clinical trials in MG, including difficulties with recruitment, participants improving on prednisone alone, and the need for a better understanding of outcome measure variability for future clinical trials.
Classification of evidence:
This study provides Class I evidence that for patients with generalized MG MTX does not significantly reduce the prednisone AUDTC over 12 months of therapy.
Myasthenia gravis (MG) is the most prevalent (7.8/100,000) acquired disorder of the neuromuscular junction, causing significant morbidity.1 Symptoms include difficulties with vision, speech, swallowing, breathing, and strength. The mainstays of treatment are disease-modifying agents: corticosteroids alone or in combination with immunosuppressive drugs. The only immunosuppressive drugs shown to be effective for MG in randomized placebo-controlled studies are azathioprine and cyclosporine.2,3 Azathioprine's steroid-sparing benefit was discernable after 15 months of treatment. Use of cyclosporine is limited by hypertension and renal toxicity. Two mycophenolate mofetil studies did not show benefit; however, the results were hampered by concerns about study design (short duration between 3 and 6 months and choice of primary outcome).4,5 There is still a need for alternative immunosuppressive drugs in MG.
Methotrexate (MTX), a selective inhibitor of dihydrofolate reductase, is an immunosuppressant used in many autoimmune diseases.6,–9 The potential advantages of MTX include oral weekly dosing, a moderate side effect profile, and inexpensive generic preparations. Prior uncontrolled studies of MTX in MG suggested that MTX reduced symptoms or decreased corticosteroid dose in 38%–87% of patients.10,–12 One single-blind uncontrolled study showed similar efficacy to azathioprine at 10 months.13
We performed a randomized, placebo-controlled trial of MTX in MG using standardized outcomes agreed upon in a consensus MG statement.14 We chose the prednisone area under the dose-time curve (AUDTC) as our primary outcome as we believed this most closely mimics the clinician's experience of treating MG, where prednisone dosing varies by visit based on symptoms.
METHODS
Trial design and classification of evidence.
We performed a 12-month randomized, double-blind, placebo-controlled study at 19 sites in the United States and Canada. This interventional study provides Class I evidence that MTX does not reduce the prednisone AUDTC compared to placebo over 12 months of treatment in participants with antibody-positive generalized MG.
Standard protocol approvals, registrations, and patient consents.
The trial was approved by institutional review boards at each site. Written informed consent was obtained by all participants, in accordance with the Declaration of Helsinki and the principles of Good Clinical Practice. This study was registered at clinicaltrials.gov (NCT00814138).
Participants.
Eligible participants were aged at least 18 years; had a diagnosis of generalized MG (Myasthenia Gravis Foundation of America [MGFA] Class II, II, or IV)15 with positive acetylcholine receptor antibodies; and were treated with a stable dose of ≥10 mg/d of prednisone (or equivalent alternate day dosing) for at least 30 days prior to enrolling. Participants were ineligible if they had taken MTX for MG within the last 2 years; if they had a thymoma, tumor, active infection, or interstitial lung disease; if they had thymectomy in the previous 3 months; if they had taken steroid-sparing immunosuppressive drugs within the last 60 days prior to screening; or if they had contraindication to taking MTX (e.g., use of daily nonsteroidal anti-inflammatory drugs, renal or hepatic disease).
The trial was conducted by The Methotrexate in MG Investigators of the Muscle Study Group between April 2009 and September 2014 at 19 sites in the United States and Canada. The study started with 6 sites, but 13 additional sites (including 2 Canadian sites) were added in 2010 because of difficulties with recruitment.
Interventions.
MTX was purchased at the University of Iowa Research Pharmacy. Drug and placebo were overencapsulated. Participants were randomized to either oral MTX (2.5 mg tablets) or matching placebo tablets for 12 months. Participants titrated dosing starting with 4 tablets per week, then increasing by 2 tablets every 2 weeks until they reached 8 tablets weekly (20 mg of MTX or equivalent placebo).
Investigators used a standardized protocol to taper prednisone if participants improved or increase prednisone if participants worsened. Starting at the month 3 visit, if the participant demonstrated clinical improvement according to MGFA postintervention status guidelines prednisone dose was tapered monthly (Supplemental tables e-1 and e-2 on the Neurology® Web site at Neurology.org).15
The prednisone was increased at any time during the study if the patient worsened and the principal investigator believed it was in the best interest of the patient. For patients on ≥15 mg prednisone daily, prednisone daily dose was increased by 20 mg, and for patients on <15 mg daily, prednisone was increased by 10 or 20 mg at the physician's discretion (or equivalent for every other day dosing).
Outcomes and measures.
Baseline characteristics included sex, age, and self-reported race/ethnicity. Quantitative Myasthenia Gravis Score (QMG), Myasthenia Gravis Composite Score (MGC), manual muscle testing (MMT), and the Myasthenia Gravis Activities of Daily Living scale (MG-ADL) were performed at baseline and monthly for 12 months.
The primary outcome measure was the 9-month prednisone AUDTC (months 4–12).14 We chose the prednisone AUDTC because we believed this more closely mimicked prednisone dosing in the clinic where dosage often varies by visit based on MG symptoms. A reduction of prednisone AUDTC demonstrates that patients improved on clinical grounds, and a difference between MTX and placebo could prove a steroid-sparing effect of MTX. Month 4–12 was chosen because it was likely that a steroid-sparing effect of MTX would not occur immediately.
The QMG is a 39-point ordinal scale (0 = normal, 39 = severely affected) that measures ocular, bulbar, and extremity fatigue and strength, along with respiratory function.16 A change of 4 is associated with a sustained clinical improvement.3,17 MG-ADL is an 8-item patient-reported scale developed to assess MG symptoms and their effects on daily activities.18 A 2-point change is considered clinically meaningful.19 MG MMT measures the strength of muscle groups in the face, neck, and extremities and determines the extent of weakness using an ordinal scale.20 The Myasthenia Gravis Quality of Life scale is a 15-item patient-reported scale indicating how MG affects quality of life.21 The MGC is made up of components of QMG, MG-ADL, and MMT, which were found to be the most responsive in prior MG trials.22 MGC ranges from 0 (not affected) to 50 (severely affected), and a change of 3 is believed to be clinically meaningful.
Safety was assessed by standard adverse event reporting.
Randomization and blinding.
The randomization plan was developed by the Department of Biostatistics at the University of Kansas Medical Center. Randomization (1:1 ratio treatment or placebo) was stratified by baseline prednisone doses: ≥30 mg day or <30 mg day, or the equivalent for every other day dosing. Investigators, evaluators, and participants were blinded to treatment allocation.
Sample size.
Lacking prior information about prednisone AUDTC with MTX, we used the prednisone AUDTC from a prior study of azathioprine in MG for sample size calculations.2 Data (obtained from a protocol) showed a mean approximately 3 times its SD in the prednisone plus placebo group, and the mean prednisone area under the curve (AUC)/SD value based on the pooled data from both groups is about 2. We assume a mean AUC/SD ratio of 2.5 for the placebo group and equal variances between treatment groups. Twenty patients in each study arm provides 0.8 power of detecting an effect size of 0.784 (Cohen d), which is equivalent to a 31.4% reduction in the mean AUC/SD in the methotrexate group over 9 months of treatment, using a one-sided 2-sample t test. To allow for 20% dropouts, we recruited a total of 50 participants. For reporting purposes, all analyses were done using 2-sided tests.
Statistical analysis.
Descriptive statistics (mean and SD, or median, minimum, and maximum) were used to describe the study population. Balanced randomization was tested using a 2-sample t test if normality assumption was satisfied by the Shapiro-Wilk normality test; otherwise, a Wilcoxon rank sum test was used.
Outcome variables were analyzed in an intent-to-treat fashion. In the original protocol, no specific missing data imputation method was specified, as required for the intent-to-treat analysis. Prior to data analysis, multiple imputation method assuming missing at random was chosen as the primary imputation approach.23 For 2 participants with one missing monthly prednisone dose, single imputation was used. The other missing monthly doses were imputed using multiple imputations based on a multiple linear regression model with independent variables such as prednisone dose at month 3, treatment, stratum (baseline prednisone ≥30 mg day or <30 mg day), and time trend. Five replicates of imputations were used. For each imputed dataset, prednisone AUDTC was calculated first and Wilcoxon rank sum test was used for between-group comparisons.
For secondary outcomes, a similar multiple imputation approach was used. The 12-month change scores were calculated as the difference between month 12 (or 11, if month 12 was missing) and baseline. When the final measure was missing, the 12-month change scores were imputed based on a multivariate linear model with control for baseline score, stratum, and treatment, and between-group tests of secondary outcomes were done using 2-sample t test or Wilcoxon rank sum test if normality assumption was violated. Due to the skewed distributions for some outcomes, median values were used. The standard errors of each group median and the difference between group medians were estimated by using 1,000 random bootstrapping samples.
As a primary method for imputing missing data was not prespecified, a post hoc sensitivity analysis using different imputation approaches was conducted. This included the last observation carried forward (LOCF) and highest dose (or worst measurement) carried forward for confirmed worsening cases combined with LOCF (for other dropouts).23 The first approach captures each patient's last known status in the study, and the second approach makes the assumption that worsening patients would get worse after dropping out of the study.
For the primary outcome, a significance level of 0.05 was considered significant. For the 5 secondary outcomes, a significance level of 0.01 was used based on a Bonferroni correction for multiplicity. All statistical tests were 2-sided. Analysis was performed using STATA 13.1 (StataCorp LP, College Station, TX).
RESULTS
Eligible participants were screened between April 2009 and December 2013. Of the 58 participants who were screened, 8 were ineligible (2 due to difficulty breathing, 2 due to elevated liver function tests, 2 due to lack of weakness, and 2 chose not to participate after screening; figure). Fifty participants were randomized to treatment, 25 to MTX and 25 to placebo. Eight participants withdrew (7 on placebo) due to the following reasons: 3 due to worsening of symptoms, 1 due to myalgias, 1 due to comorbid illness, 1 due to elevated liver transaminases, 1 due to death (stroke), and 1 due to travel problems (MTX group). Forty-two participants completed 12 months of follow-up. All participants were included in analysis per the intent-to-treat analysis plan. Missing data represented no more than 12.5% of 600 possible observations of any outcome (12 months × 50 participants).
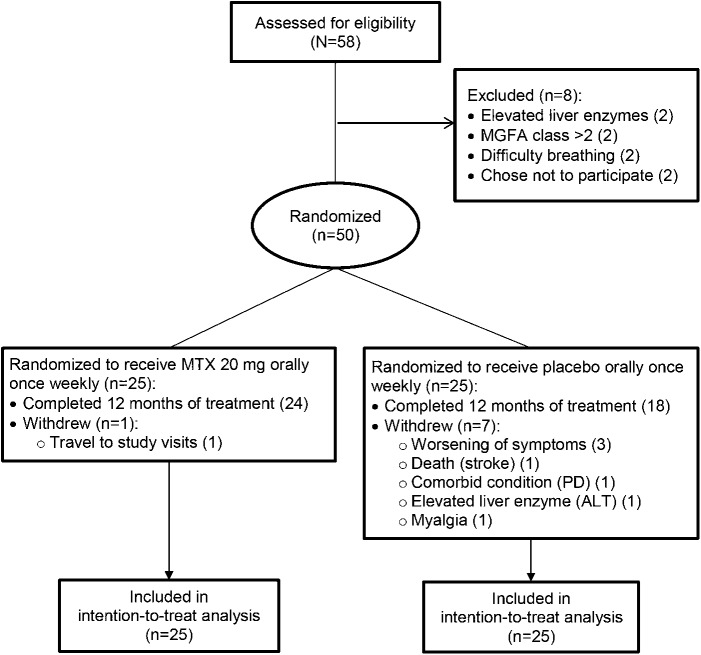
Fifty participants were randomized, 25 to each treatment group. Eight participants withdrew during the course of the study (1 methotrexate, 7 placebo). Fifty participants were included in analysis per the intention-to-treat analysis plan. ALT = alanine aminotransferase; MGFA = Myasthenia Gravis Foundation of America; MTX = methotrexate; PD = Parkinson disease.
Baseline characteristics.
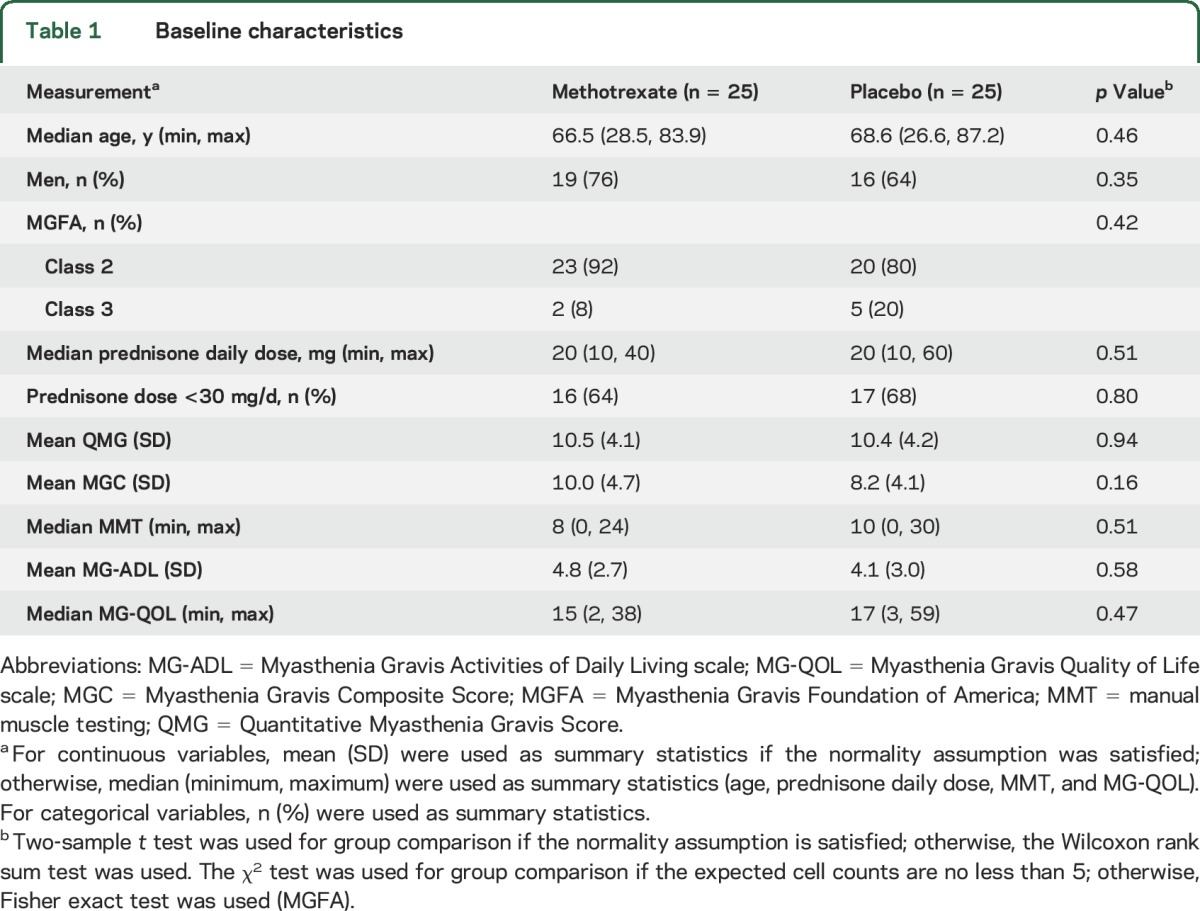
The groups were comparable in regards to age, sex, and clinical MG parameters (table 1). Both median prednisone dose and prednisone <30 mg stratification was balanced between groups.
Table 1
Baseline characteristics
Outcomes and measures.
We saw no difference in 4–12 months prednisone AUDTC between MTX and placebo participants (MTX 2,996.6 mg, 95% confidence interval [CI] 1,495.9–4,497.3 vs placebo 3,484.7, 95% CI 2,151.8–4,817.5; p = 0.26; table 2, figure e-1A). The estimated between-group difference (methotrexate/placebo) for the prednisone AUDTC is −488.0 (95% CI −2,443.4 to 1,467.3), which corresponds to a difference in the mean daily prednisone dose of −1.9 mg/d (95% CI −9.7 to 5.8).
Table 2
Outcome measures
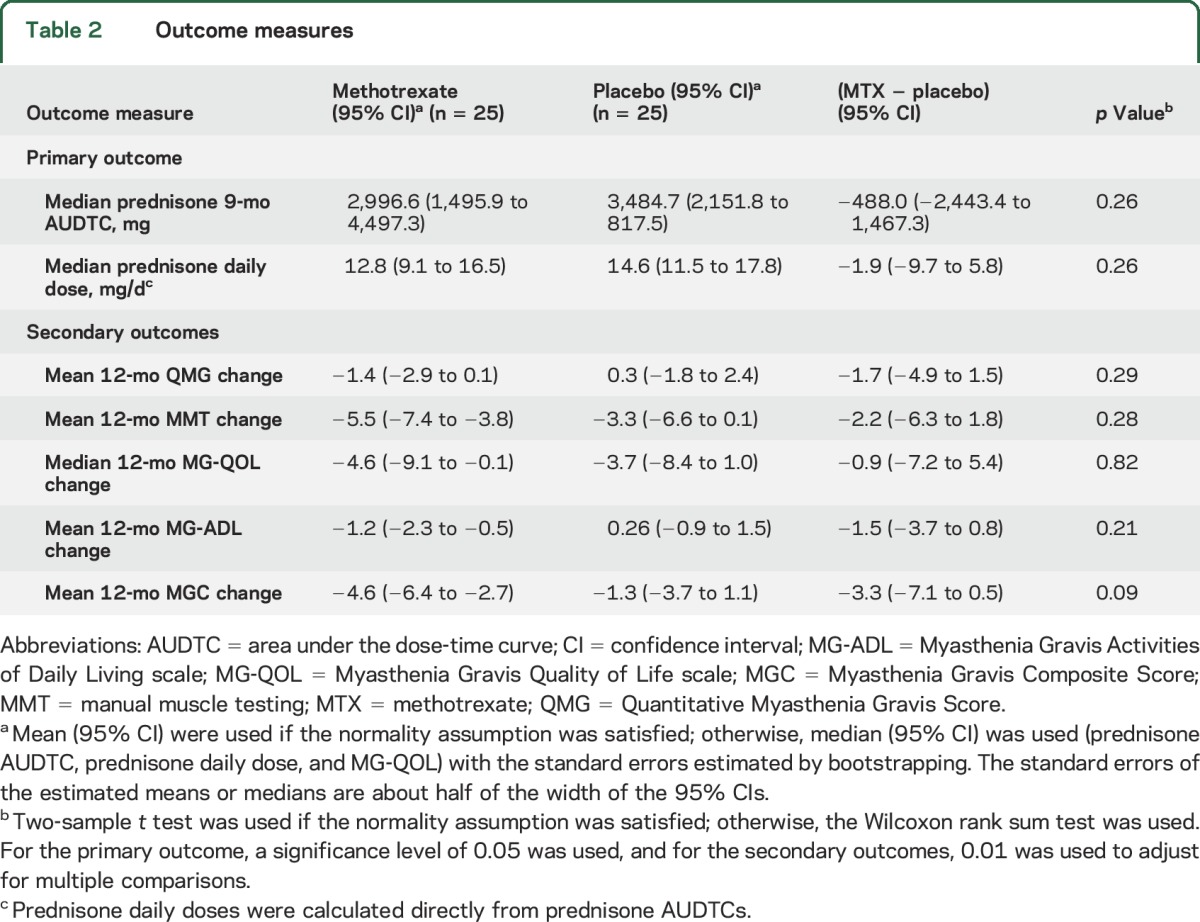
Secondary outcomes also did not show differences in 12-month change from baseline between MTX and placebo, although the estimated between-group differences (methotrexate/placebo) were mostly in favor of methotrexate (table 2, Supplemental figure e-1B). The estimated mean MGC difference of −3.3 (95% CI −7.1 to 0.5, p = 0.09) would be considered clinically meaningful.
Ancillary analysis.
Post hoc sensitivity analysis was performed using different imputation techniques as described in Methods (table 3, figure e-1, C and D). For prednisone AUDTC, LOCF (p = 0.27) or the highest prednisone dose carried forward (p = 0.20) showed no difference in the prednisone AUDTC between MTX and placebo participants. For secondary outcomes, sensitivity analysis suggested improvements with methotrexate vs placebo in QMG using both approaches (p < 0.01), and for MGC using worst scores carried forward (p < 0.01).
Table 3
Sensitivity analysis
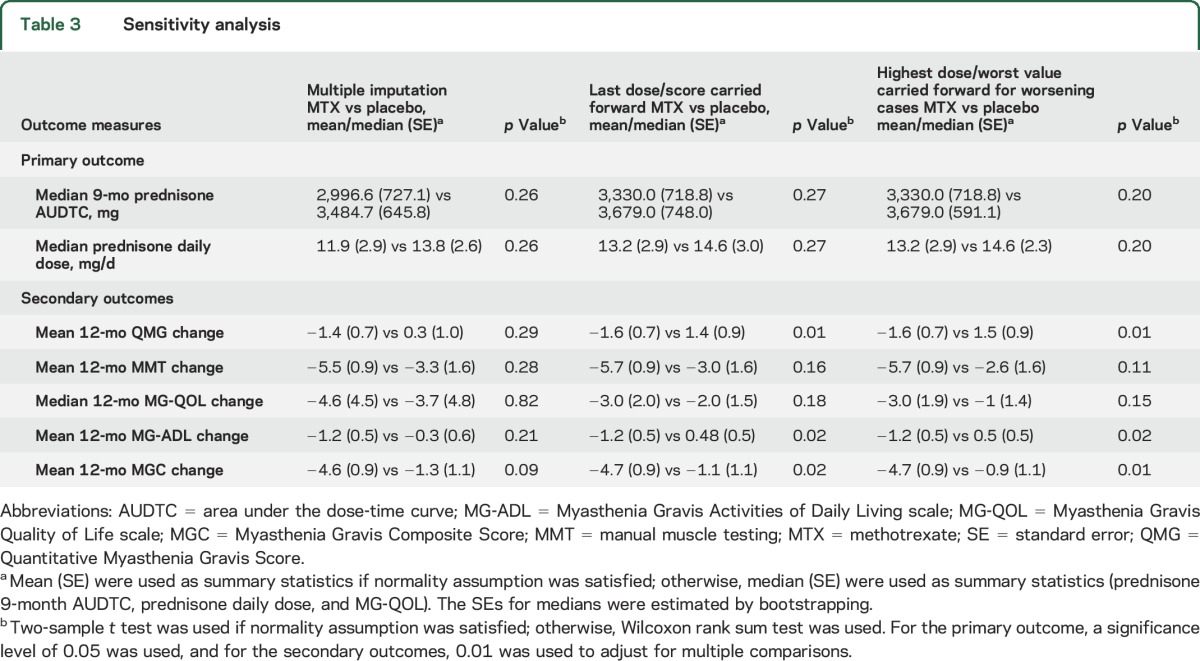
Exploratory analyses showed that 7/8 dropouts in the study had relatively low baseline prednisone doses (<30 mg per day), which might explain why the different imputation approaches did not affect the results for the prednisone AUDTC.
Analysis looking at treatment failures, defined as participants whose prednisone dose was increased during the study, showed 11 treatment failures in the MTX group vs 15 treatment failures in the placebo group (p = 0.26).
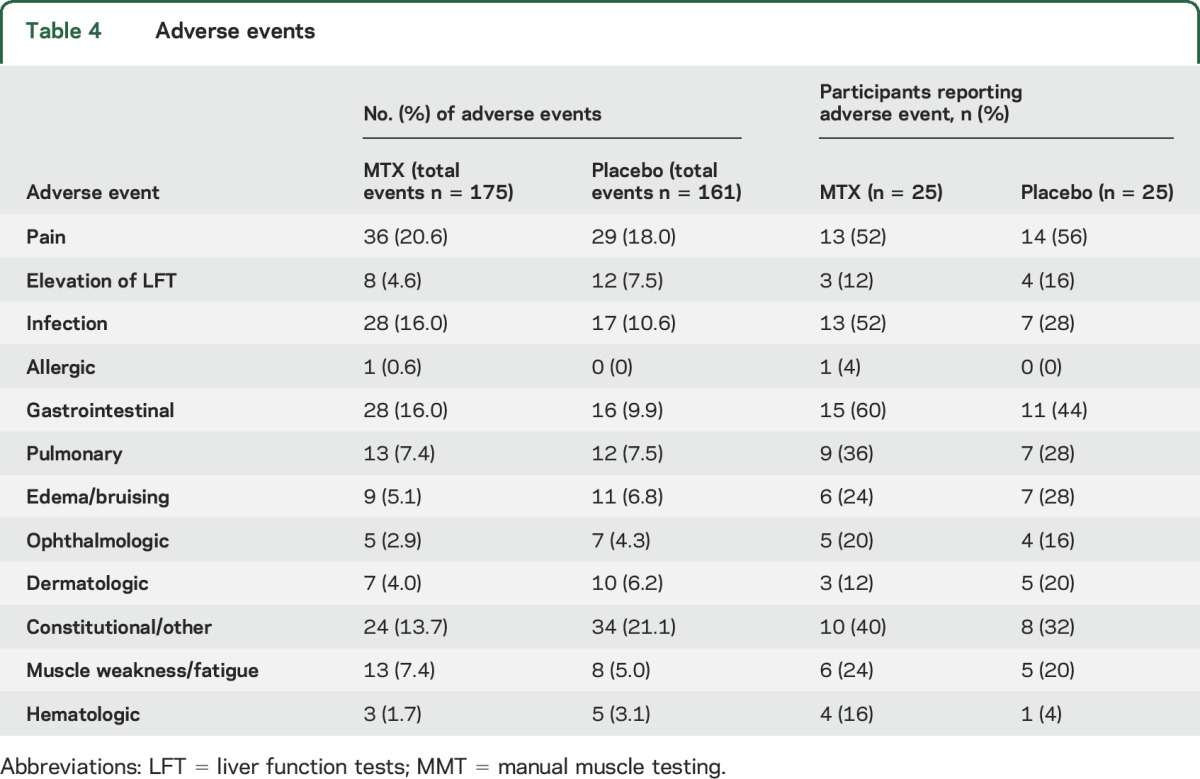
Adverse events.
There were 337 total adverse events reported (MTX group 176 events, placebo 161 events; table 4). Most of these were determined to be unrelated to treatment (66%). Seven patients had elevated liver function tests (3 in MTX, 4 in placebo). Two patients from the placebo group and 2 patients from the MTX group did not report any adverse events. There were no MTX-related serious adverse events. There were more gastrointestinal side effects and increased infections in the MTX group, but this did not result in any participant stopping the study. The most common adverse event was nonspecific pain (20.6% MTX vs 18.0% placebo).
Table 4
Adverse events
DISCUSSION
The primary endpoint of our study, month 4–12 prednisone AUDTC, was not significantly different between the MTX and placebo groups, so we conclude no steroid-sparing benefit for MTX in MG. Although it is tempting to conclude, due to the direction of change in the MTX/placebo differences for most outcomes, that MTX may have provided some benefit and this study was simply underpowered, it is also possible MTX does not work for MG. Although frustrating when studies produce indeterminate results, which was also the case with the mycophenolate studies, we have learned a number of important lessons that will help instruct future MG clinical trials.4,5
The variability of the prednisone AUDTC was greater than anticipated. We based our sample size estimates on the prednisolone AUDTC variability seen in a prior randomized control trial of azathioprine, which had a number of key differences from our study.2 (1) The azathioprine study included participants without prior immunosuppressant exposure; (2) all participants were started on high doses of prednisolone (1.5 mg/kg on alternate days, to a maximum of 100 mg); and (3) the primary outcome was the average prednisolone dosage evaluated at 12 and 36 months. Limiting cases to patients without prior immunosuppressant exposure is not feasible today, as most patients are started immediately on prednisone. Even at the time of the azathioprine study this prevented meeting the target enrollment of 100 participants (the authors were only able to recruit 34). Our participants were on lower initial doses of prednisone (median of 20 mg/d), which may have made it harder to detect a reduction in prednisone in our study. When planning our study, we chose 12 months based on our prior experience with the mycophenolate studies being too short, but the azathioprine study required >15 months before a reduction in prednisolone was detected, so our study may still have been too short.
Although broad inclusion criteria might seem intuitively appealing for MG trial generalizability, this may actually favor older participants with milder disease. Our study had an older average participant age, which may have enriched our population with late-onset MG cases. The older mean age may reflect a bias for older patients with MG to participate in clinical trials, or regional variation in the neuromuscular clinics included in the study. Our study had a male predominance in both treatment groups, which may reflect the older average age of participants (mid 60s), or regional variation in the ratio of sexes affected by MG. In the future, stratification strategies based on age at diagnosis may ensure an equal mix of patients with early- and late-diagnosed MG.
Participants on prednisone plus placebo may improve. Multiple recent studies have documented a better than expected clinical course for patients on prednisone plus placebo.4,5 We also saw patients on prednisone alone improve over the course of the study. However, prednisone itself has no randomized placebo-controlled studies supporting its use in isolation in MG, and long-term prednisone use is associated with many complications including osteoporosis, steroid-induced diabetes, poor wound healing, and infections.24 Even long-term low-dose glucocorticoid use is associated with typical steroid-related adverse events.25 Thus there has been interest in finding a steroid-sparing therapy for MG, but less success in demonstrating effectiveness of one.
Care must be taken when interpreting clinically meaningful changes. Although no change was statistically significant, the change in the MGC would be considered clinically meaningful.22 This clinically meaningful cutoff was determined in a cross-sectional correlation and reliability study, so does not account for the actual 12-month variability in the MGC. The variability estimates from our current study will help with future power and sample size calculations. The MGC and MG-ADL were the most sensitive measures seen here, and might improve our statistical power to see change in future studies.
MTX was well-tolerated in this study; however, a limitation would be whether the chosen dose of 20 mg weekly produced effective immunosuppression in all participants. One dropout occurred in the MTX group, and this was due to an inability to travel for study visits. Although the frequencies of gastrointestinal side effects or infections were higher in the MTX group, this did not result in any participant withdrawing from the study. There were no study drug–related serious adverse events.
Although this study did not show a steroid-sparing benefit for MG, we did learn important lessons that will instruct planning future studies, including: (1) a better handle on variability of a core set of outcome measures; (2) a better understanding of the expected change for participants on prednisone alone; (3) stratification strategies for participants with a prior history of immunosuppressant exposure; and (4) a better understanding of responsiveness across our outcome measures. As a field, the decision whether to test another off-label use of existing steroid-sparing immunosuppressant drugs needs to be weighed carefully, as such studies will likely require large numbers of participants with long follow-up periods. The MG community should consolidate future efforts towards studies of new investigational agents that have promise to do more than reduce the required dose of prednisone, but rather significantly alter the MG disease course.
GLOSSARY
| AUC | area under the curve |
| AUDTC | area under the dose-time curve |
| CI | confidence interval |
| LOCF | last observation carried forward |
| MG | myasthenia gravis |
| MG-ADL | Myasthenia Gravis Activities of Daily Living scale |
| MGC | Myasthenia Gravis Composite Score |
| MGFA | Myasthenia Gravis Foundation of America |
| MMT | manual muscle testing |
| MTX | methotrexate |
| QMG | Quantitative Myasthenia Gravis Score |
AUTHOR CONTRIBUTIONS
Drs. Pasnoor, Statland, Dimachkie, Burns, and Barohn and L. Herbelin contributed to drafting/revising the manuscript for content, study concept or design, analysis or interpretation of data, acquisition of data, and statistical analysis. Dr. He contributed to drafting/revising the manuscript for content, study concept or design, analysis or interpretation of data, and statistical analysis. Drs. Nations, Bril, Wang, Kissel, Saperstein, Rosenfeld, Shaibani, Jackson, Swenson, Howard, Goyal, Wicklund, Pulley, Mozaffar, David, Benatar, Pazcuzzi, and Simpson and Ms. Miller contributed to drafting/revising the manuscript for content, and acquisition of data.
STUDY FUNDING
This study was funded by Food and Drug Administration Orphan Products Division RO1 FD 003538 (M.P.). Additional funding was provided in part by grants UL1 RR 033179 (which is now UL1 TR 000001) from the University of Kansas Medical Center Clinical and Translational Science Awards (CTSA). Dr. Statland's work on this project was supported by a NCATS grant awarded to the University of Kansas Medical Center for Frontiers: The Heartland Institute for Clinical and Translational Research #KL2TR000119.
DISCLOSURE
M. Pasnoor, L. Herbelin, T. Burns, S. Nations, V. Bril, A. Wang, B. Elsheikh, J. Kissel, D. Saperstein, A. Shaibani, C. Jackson, A. Swenson, J. Howard, N. Goyal, W. David, M. Wicklund, M. Pulley, M. Becker, T. Mozaffar, M. Benatar, R. Pascuzzi, E. Simpson, J. Rosenfeld, and M. Dimachkie report no disclosures relevant to the manuscript. J. Statland's work on this project was supported by an NCATS grant awarded to the University of Kansas Medical Center for Frontiers: The Heartland Institute for Clinical and Translational Research #KL2TR000119. R. Barohn's work on the project was supported by Food and Drug Administration Orphan Products Division RO1 FD 003538. Go to Neurology.org for full disclosures.
REFERENCES
Articles from Neurology are provided here courtesy of American Academy of Neurology
Full text links
Read article at publisher's site: https://doi.org/10.1212/wnl.0000000000002795
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc4932232?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/8785493
Article citations
Efficacy and safety of immunosuppressants and monoclonal antibodies in adults with myasthenia gravis: a systematic review and network meta-analysis.
J Transl Med, 22(1):955, 21 Oct 2024
Cited by: 0 articles | PMID: 39434135 | PMCID: PMC11492773
Review Free full text in Europe PMC
Immune mediated myasthenia gravis in children, current concepts and new treatments: A narrative review article.
Iran J Child Neurol, 18(3):21-42, 22 Jun 2024
Cited by: 0 articles | PMID: 38988843 | PMCID: PMC11231678
Review Free full text in Europe PMC
Generalized myasthenia gravis with acetylcholine receptor antibodies: A guidance for treatment.
Eur J Neurol, 31(5):e16229, 06 Feb 2024
Cited by: 2 articles | PMID: 38321574 | PMCID: PMC11236053
Review Free full text in Europe PMC
Guideline for the management of myasthenic syndromes.
Ther Adv Neurol Disord, 16:17562864231213240, 26 Dec 2023
Cited by: 9 articles | PMID: 38152089 | PMCID: PMC10752078
Review Free full text in Europe PMC
Unlocking the Complexity of Neuromuscular Diseases: Insights from Human Pluripotent Stem Cell-Derived Neuromuscular Junctions.
Int J Mol Sci, 24(20):15291, 18 Oct 2023
Cited by: 3 articles | PMID: 37894969 | PMCID: PMC10607237
Review Free full text in Europe PMC
Go to all (52) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Clinical Trials
- (1 citation) ClinicalTrials.gov - NCT00814138
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
A Randomized Open-Labeled Trial of Methotrexate as a Steroid-Sparing Agent for Patients With Generalized Myasthenia Gravis.
Front Immunol, 13:839075, 18 Mar 2022
Cited by: 2 articles | PMID: 35371086 | PMCID: PMC8971191
A single-blinded trial of methotrexate versus azathioprine as steroid-sparing agents in generalized myasthenia gravis.
BMC Neurol, 11:97, 05 Aug 2011
Cited by: 42 articles | PMID: 21819556 | PMCID: PMC3170595
Randomized phase 2 study of FcRn antagonist efgartigimod in generalized myasthenia gravis.
Neurology, 92(23):e2661-e2673, 22 May 2019
Cited by: 101 articles | PMID: 31118245 | PMCID: PMC6556100
Methotrexate in generalized myasthenia gravis: a systematic review.
Acta Neurol Belg, 123(5):1679-1691, 27 Mar 2023
Cited by: 1 article | PMID: 36967437
Review
Funding
Funders who supported this work.
FDA HHS (1)
Grant ID: R01 FD003538
NCATS NIH HHS (2)
Grant ID: KL2 TR000119
Grant ID: UL1 TR001414
NCRR NIH HHS (1)
Grant ID: UL1 RR033179


